Cytotoxicity of Doxorubicin-Curcumin Nanoparticles Conjugated with Two Different Peptides (CKR and EVQ) against FLT3 Protein in Leukemic Stem Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Chemotherapeutic Drugs and Curcuminoid Mixture
2.3. Leukemic Cell Lines and Cell Culture Conditions
2.4. Preparation and Physicochemical Characterization of Drug-Loaded Micelles
2.4.1. Method for DCM and CM Preparation
2.4.2. Optimization Conditions for DCM and CM Preparation
2.4.3. Characterization of DCM and CM Physicochemical Properties
2.4.4. Study of the Interaction between Dox, Cur, and P407 in DCM and CM Using XRD and DSC Analyses
2.4.5. In Vitro Dox and Cur Release Profile in DCM and CM
2.5. Production, Purification, and Assessment of P407-FLT3 Peptide Conjugation for the Drug-Micelle Conjugated FLT3 Peptide Preparation
2.5.1. Production and Purification of P407-FLT3 Peptide Conjugation
- P407-CHO and CA production
- CA-conjugated FLT3-peptide preparation
2.5.2. The Evaluation of P407-FLT3 Peptide Conjugation
2.6. DCM and CM Conjugated with FLT3 Peptide Preparation and Physicochemical Assessment
2.7. Evaluation of Cell Viability after Treatment with Dox Solution, Dox-Cur Solution, and DCM with or without FLT3 Peptides by MTT Assay
2.8. Statistical Analysis
3. Results
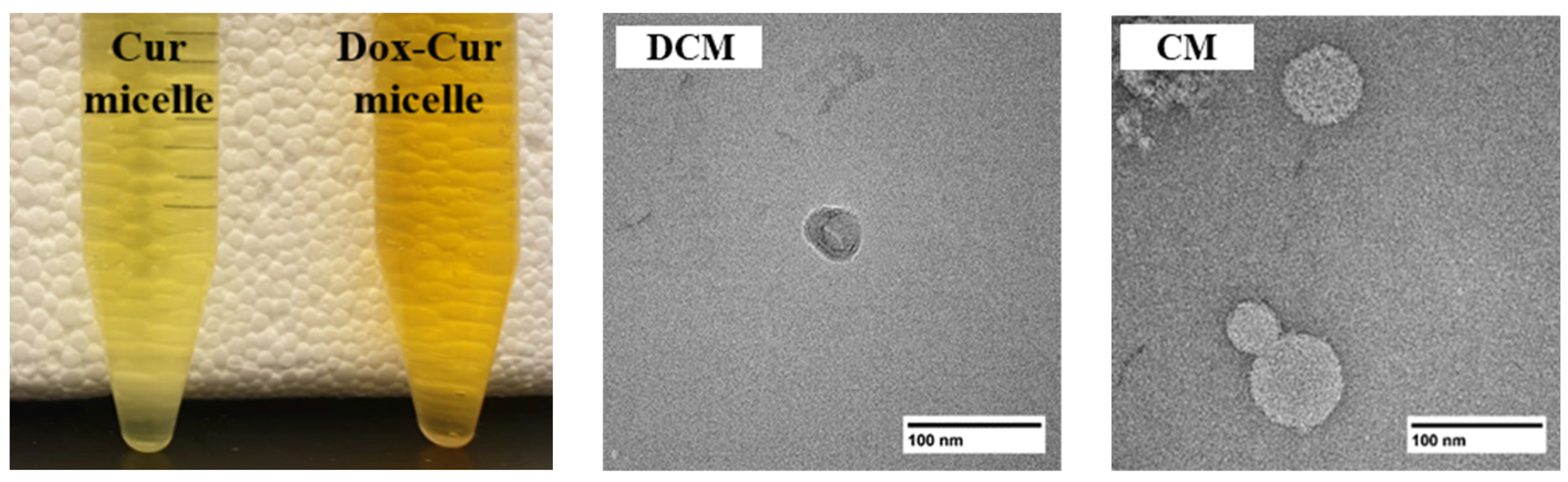
3.1. Preparation and Evaluation of the Physicochemical Properties of Drug-Micelles
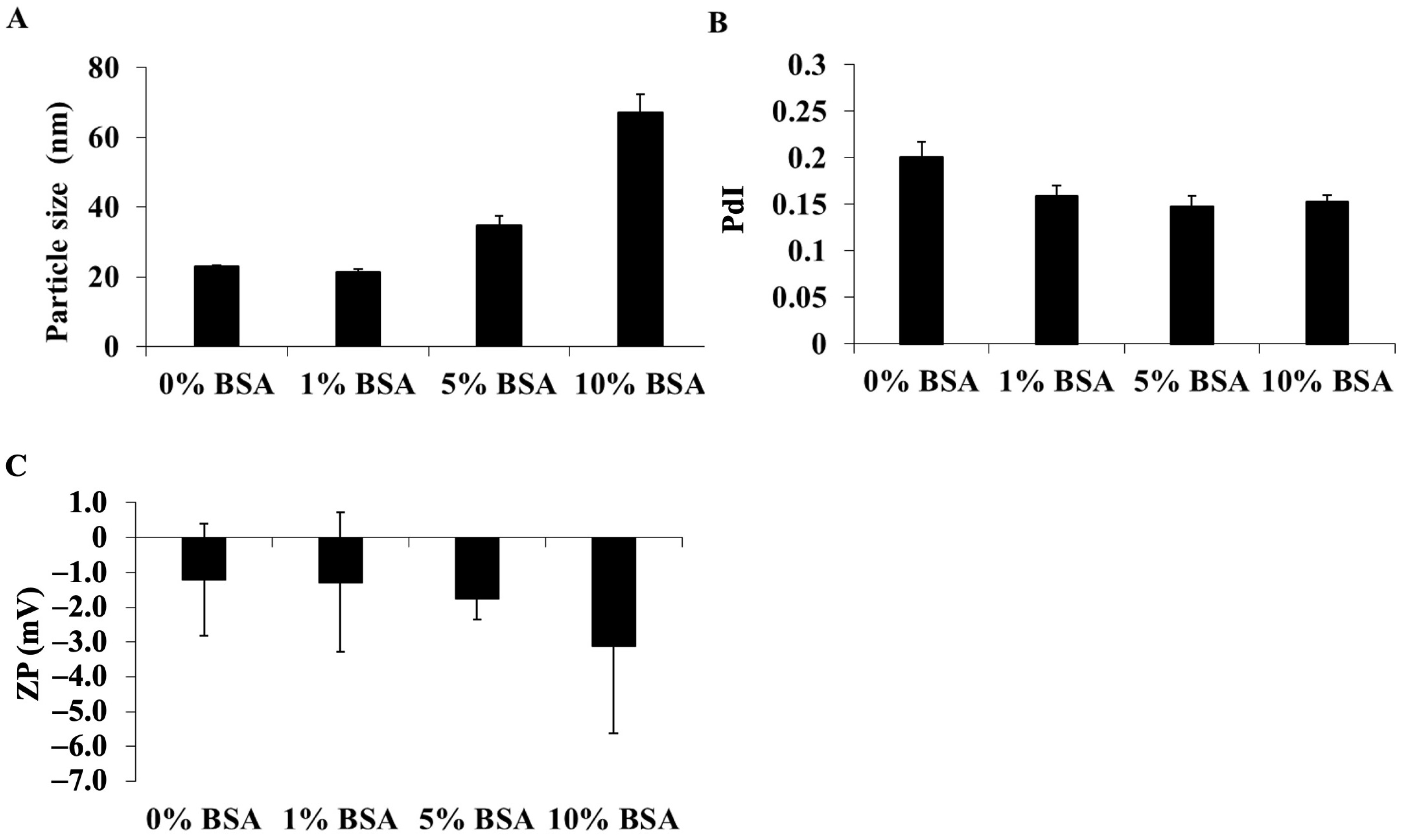
3.1.1. Formula Optimization and Characterization of DCM and CM
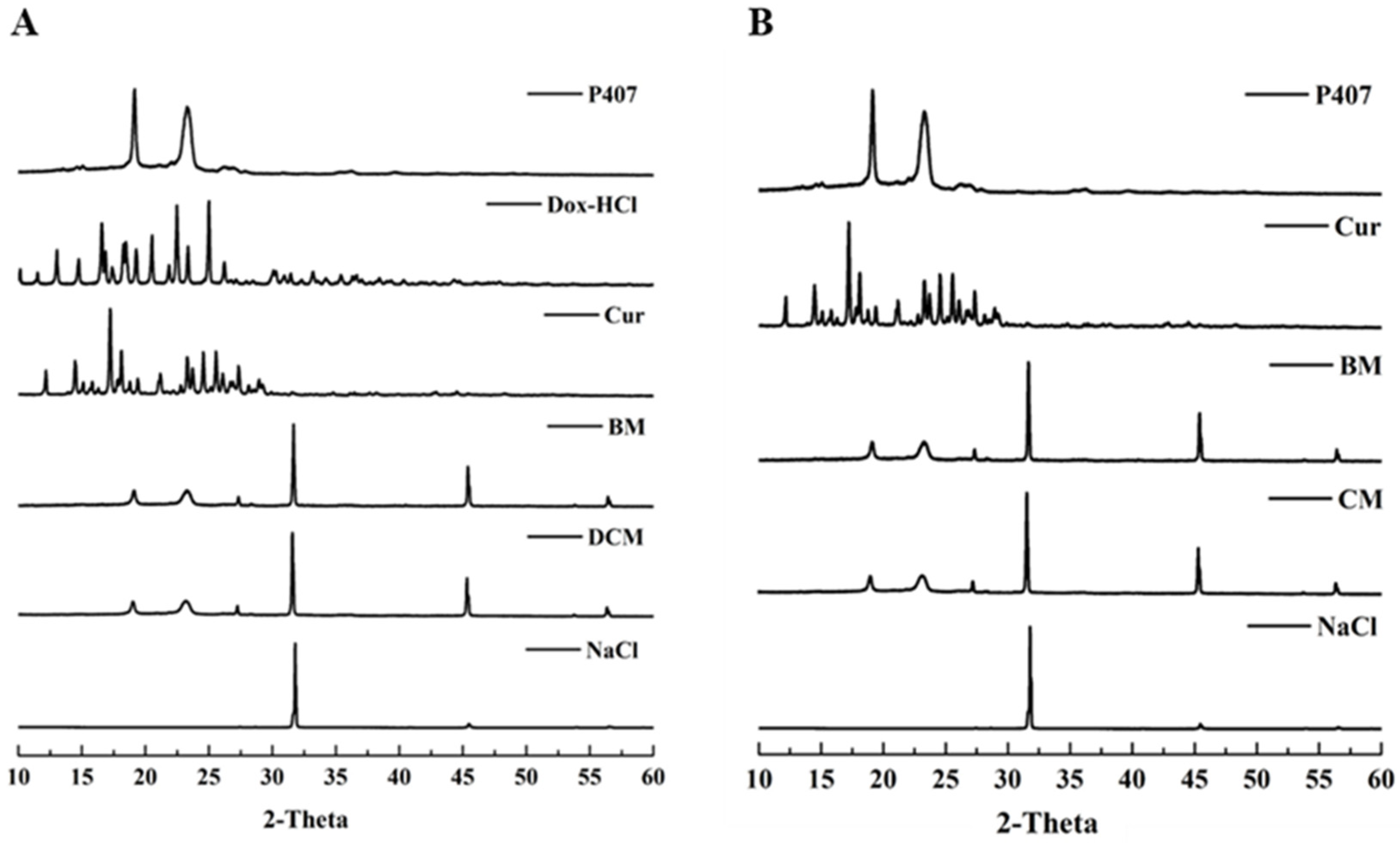
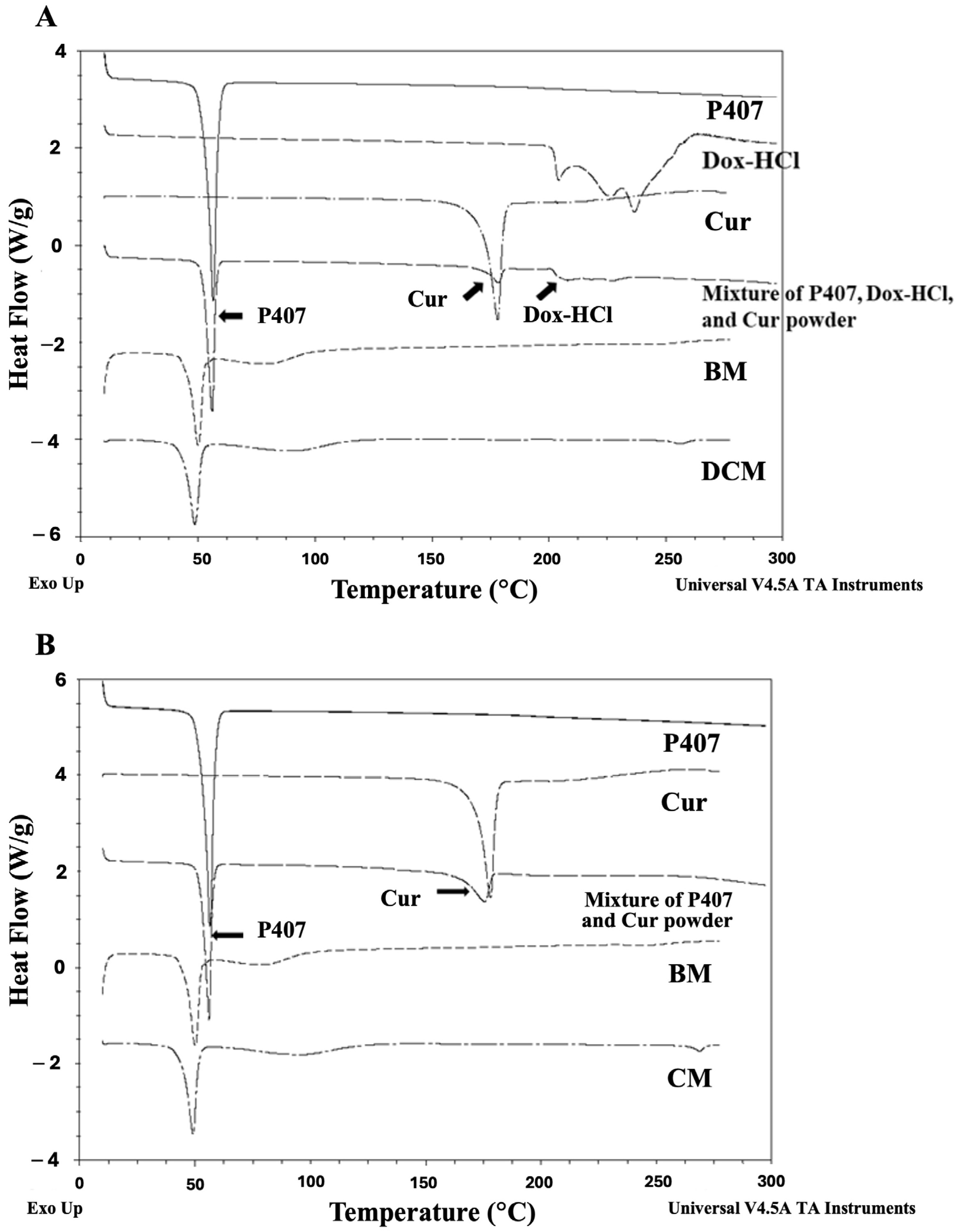
3.1.2. The Interaction between Dox-HCl, Cur, and P407 in DM, CM, and DCM
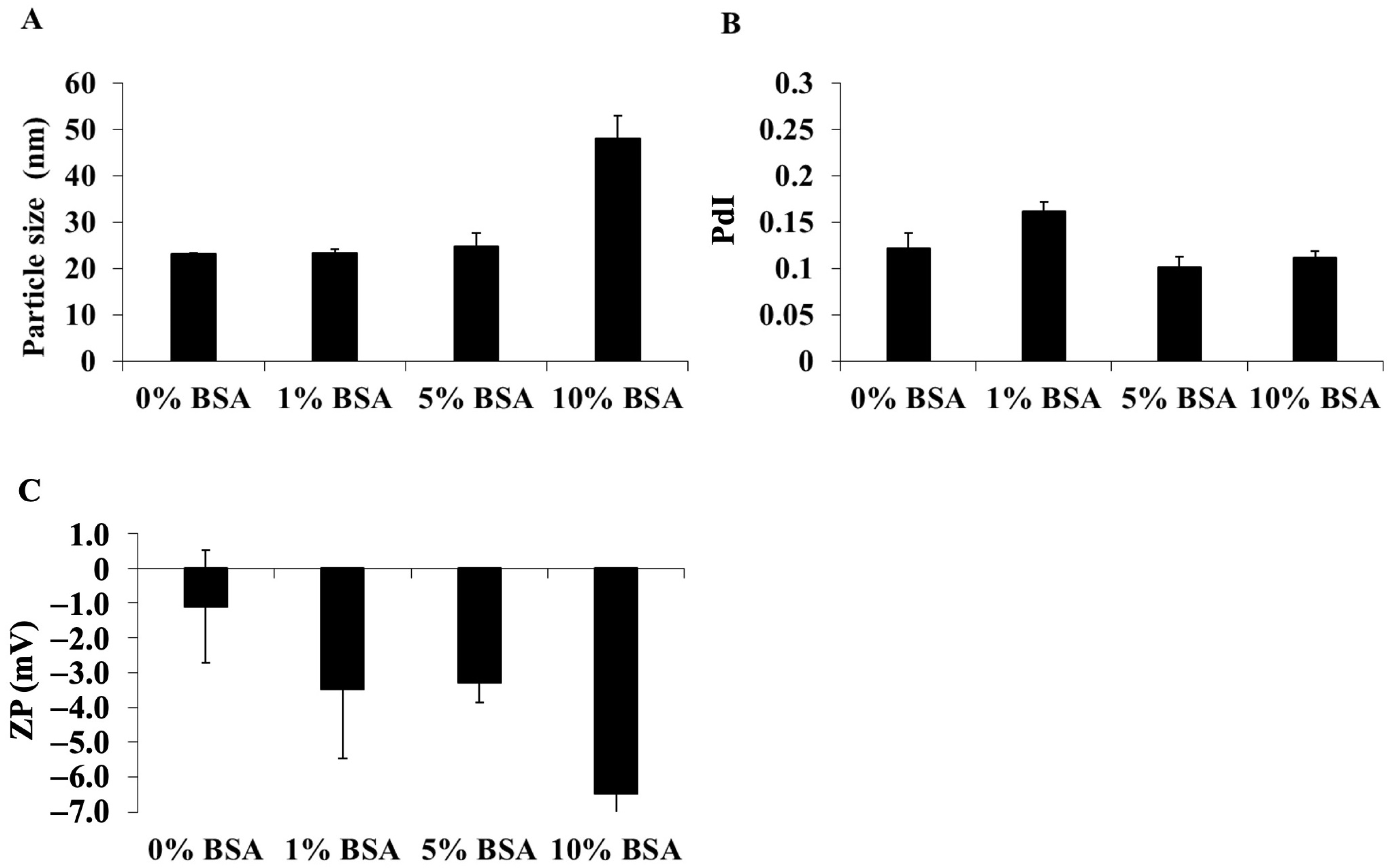
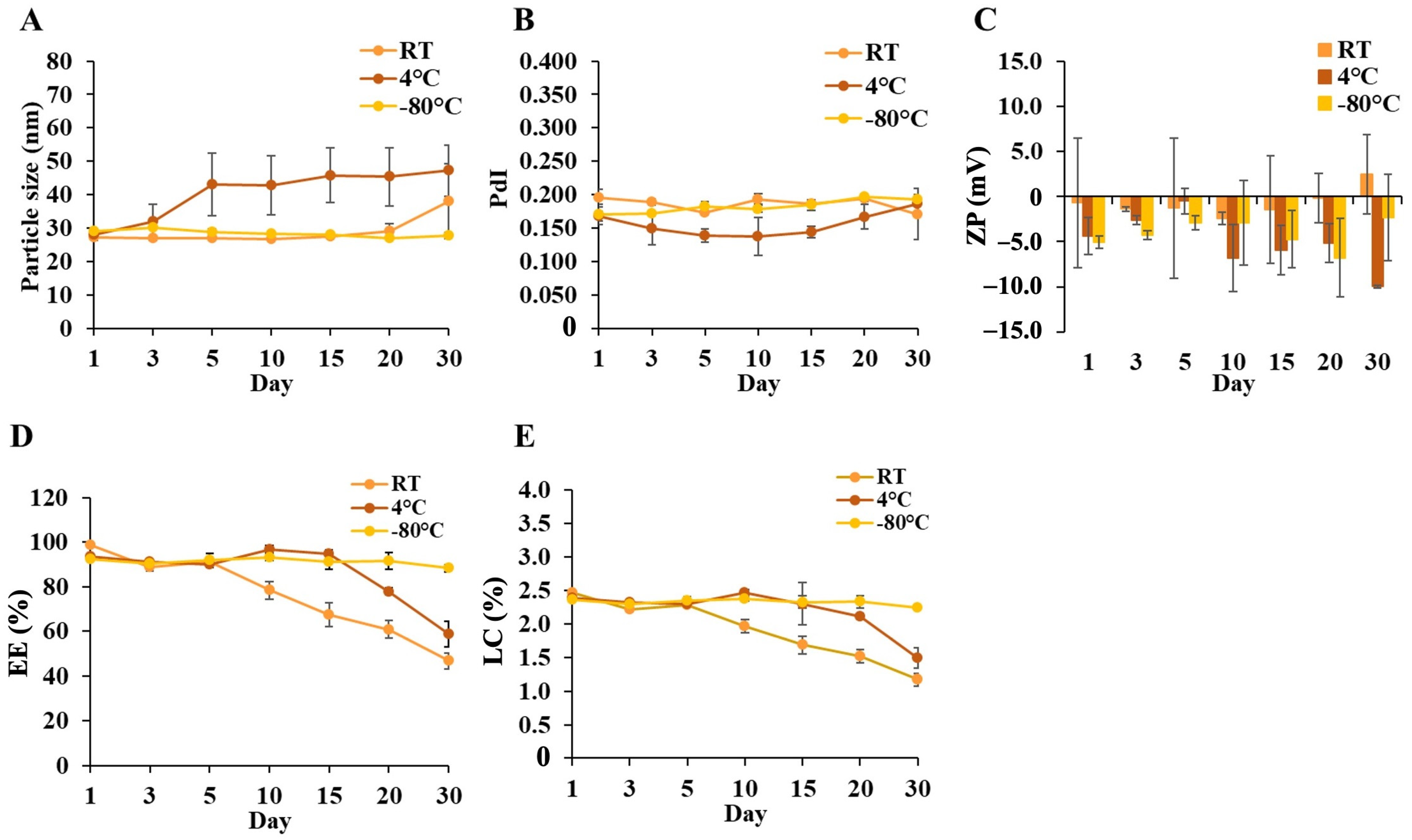
3.1.3. Effects of Colloidal, Temperature, and Time on DCM and CM Stability
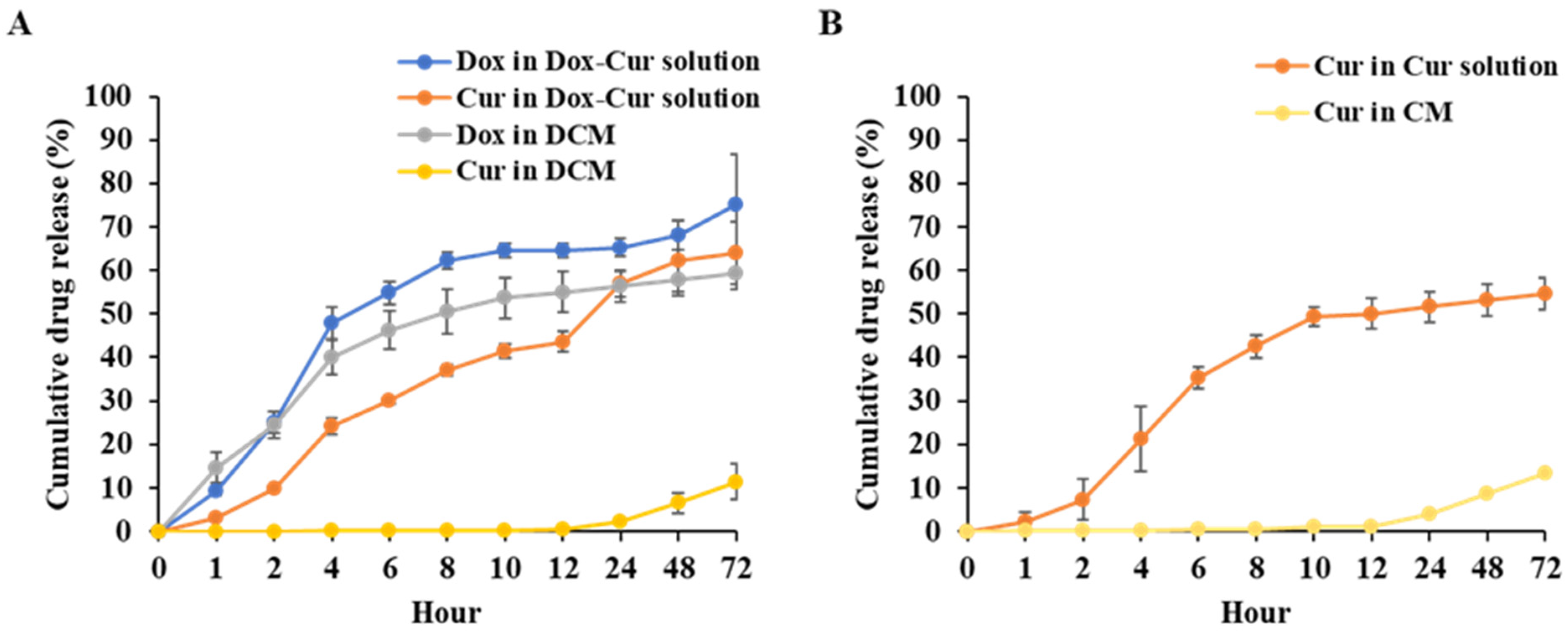
3.1.4. In Vitro Release Profile of DCM and CM
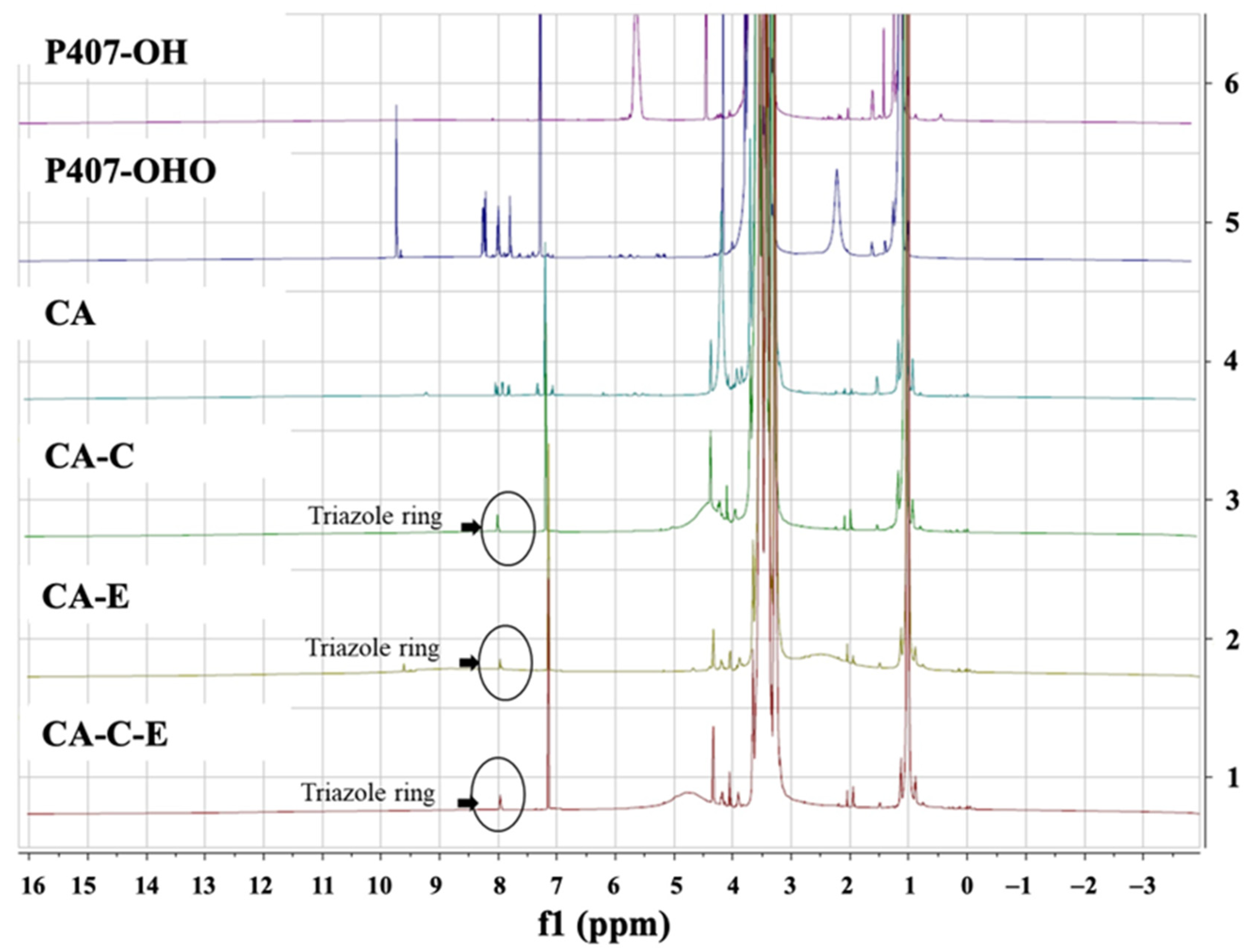
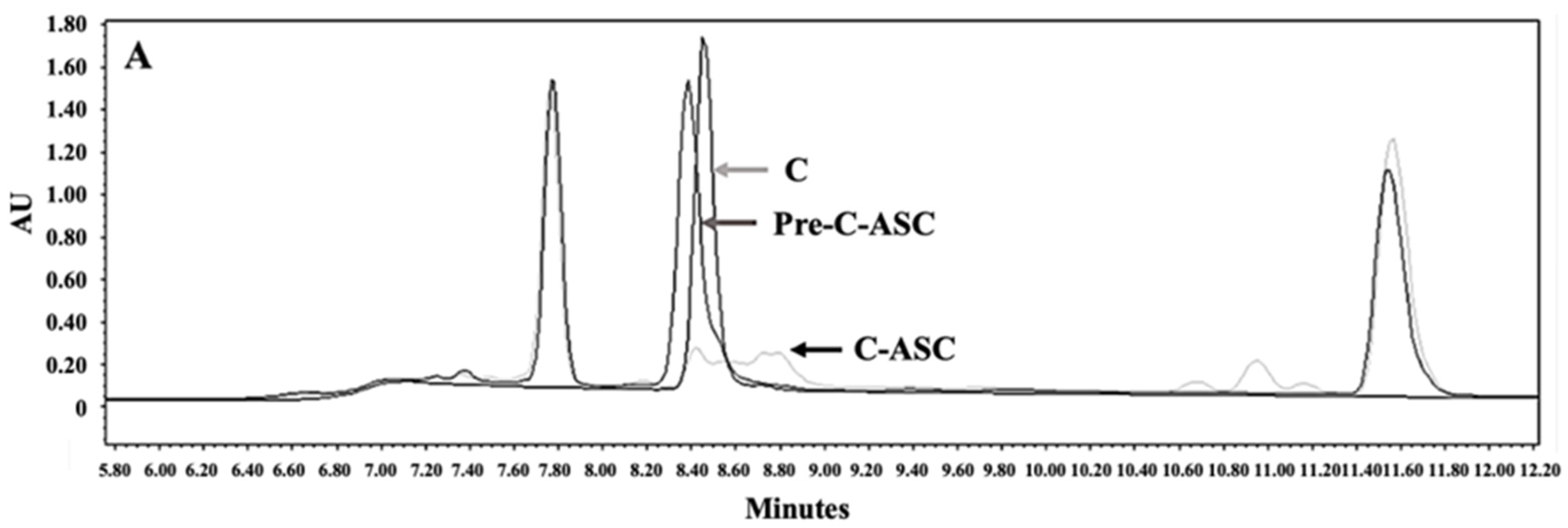
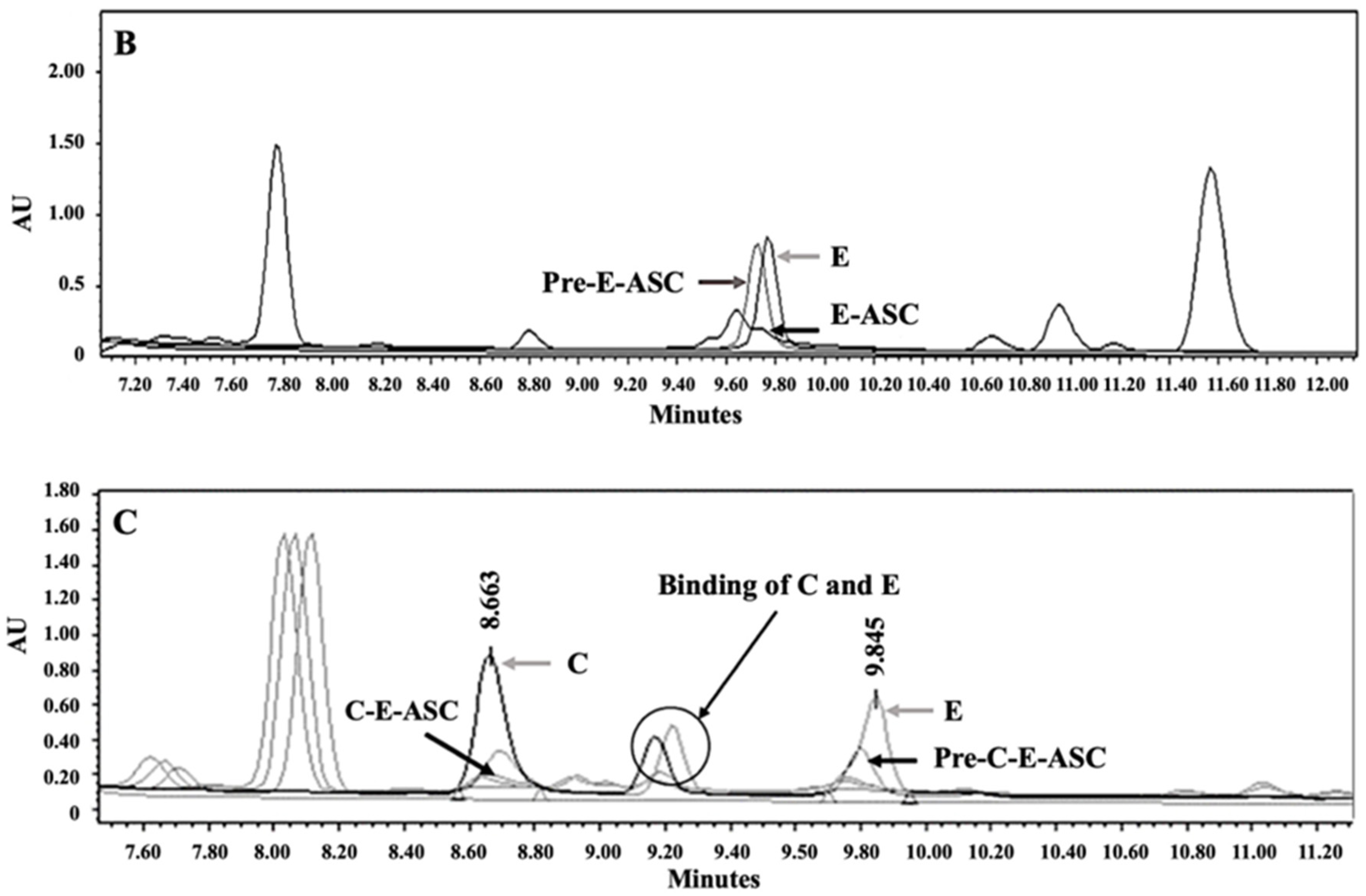
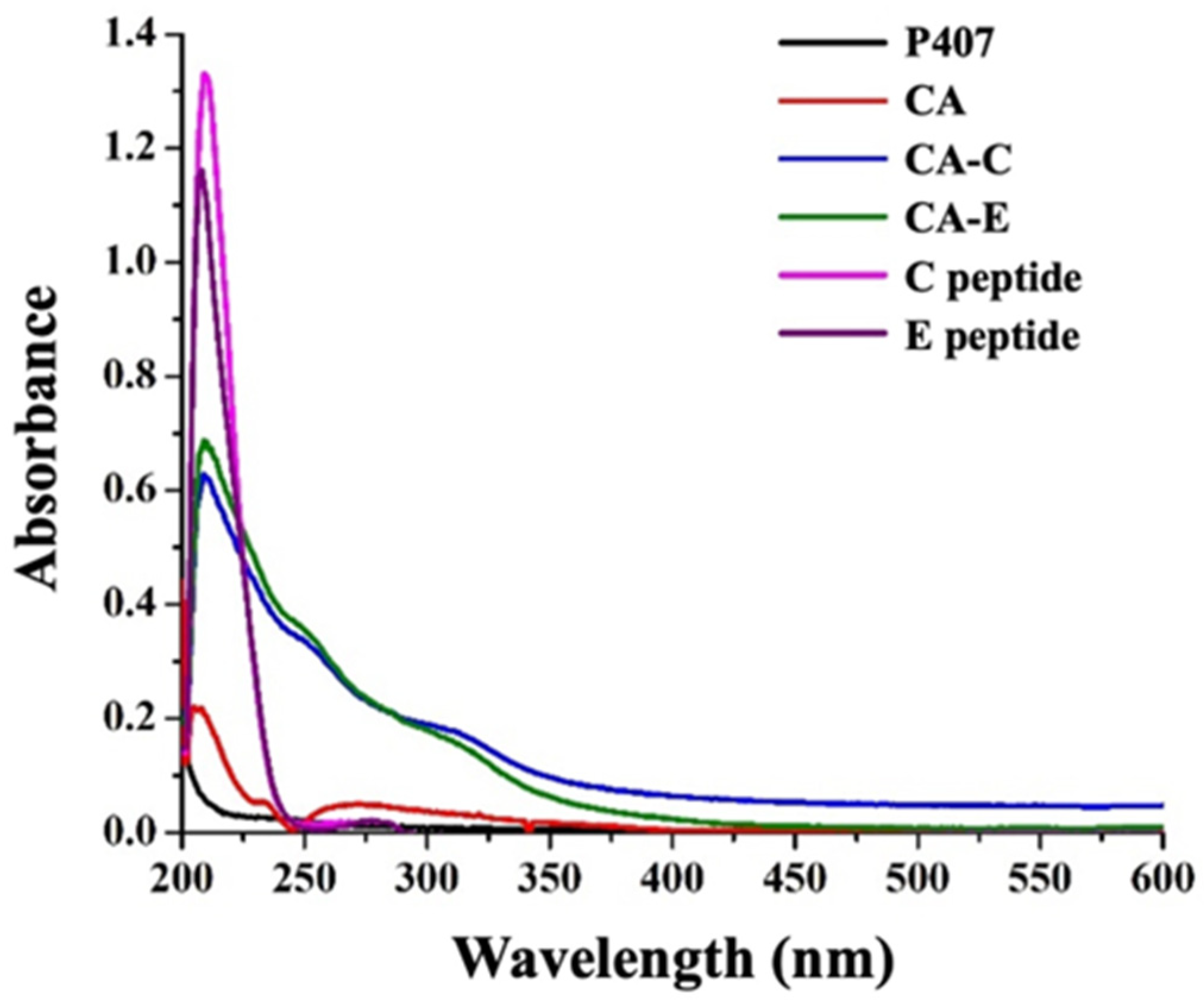
3.2. Synthesis and Characterization of P407-FLT3-Specific Peptide Conjugates
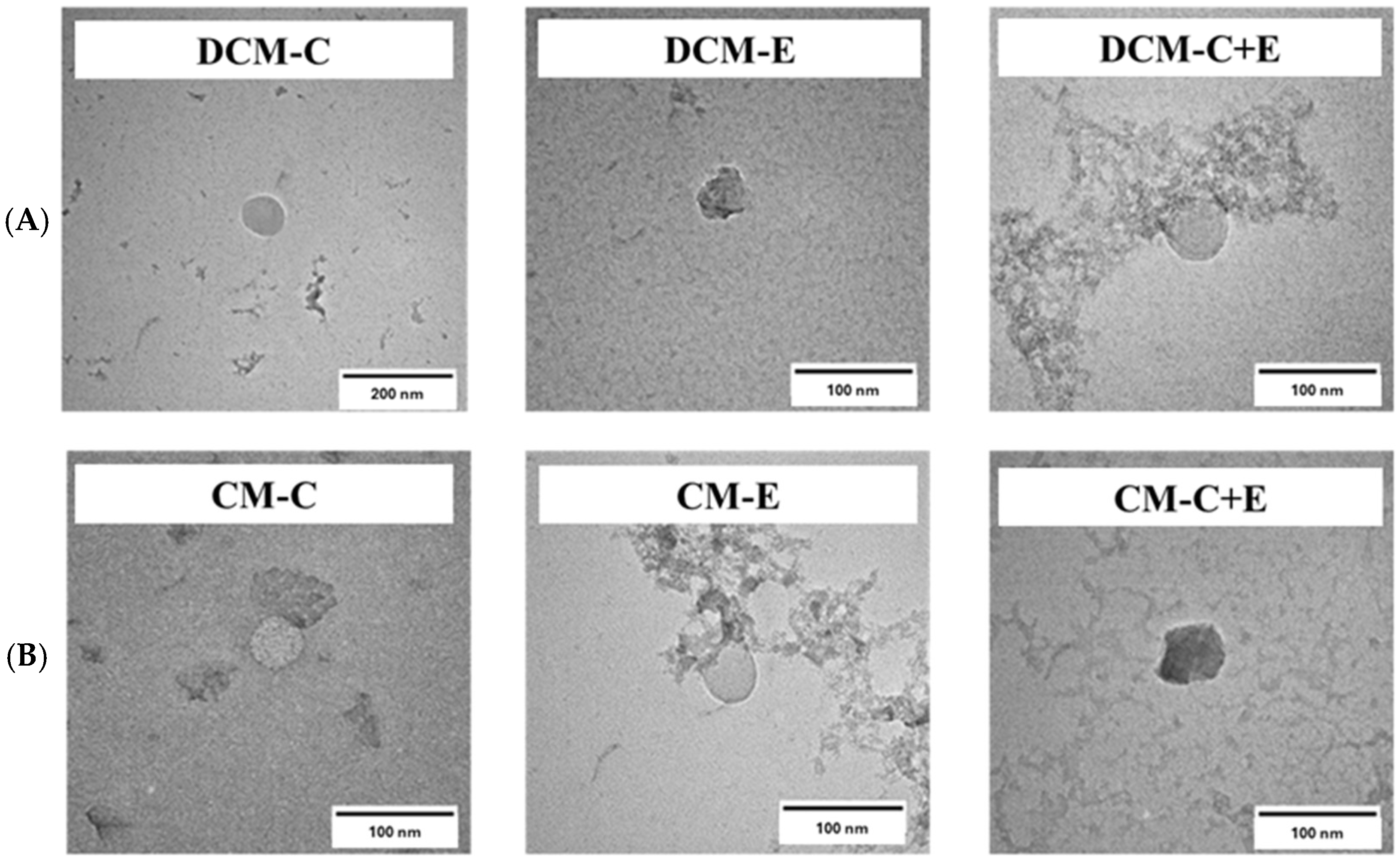
3.3. Preparation and Characterization of DCM and CM Conjugated with C, E, and Both C and E Peptides
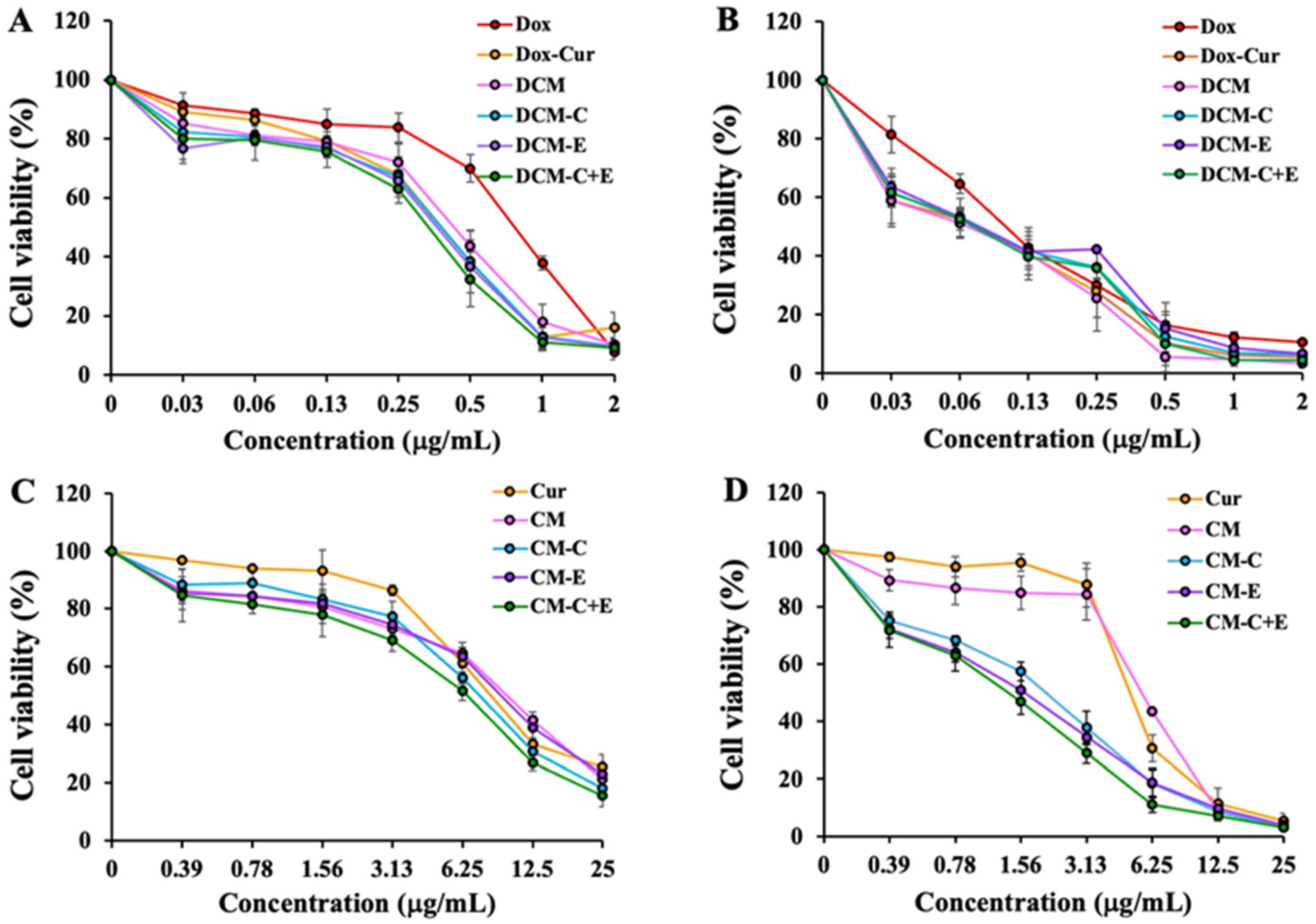
3.4. Determination of Cytotoxic Effects of Dox Solution, Dox-Cur Solution, and DCM Conjugated with or without FLT3 Peptides on Leukemic Cell Viability by MTT Assay
3.5. Determination of Cytotoxic Effects of Cur Solution and CM Conjugated with or without FLT3 Peptides on Leukemic Cell Viability by MTT Assay
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ferlay, J.; Soerjomataram, I.; Dikshit, R.; Eser, S.; Mathers, C.; Rebelo, M.; Parkin, D.M.; Forman, D.; Bray, F. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int. J. Cancer 2015, 136, E359–E386. [Google Scholar] [CrossRef]
- Long, N.A.; Golla, U.; Sharma, A.; Claxton, D.F. Acute Myeloid Leukemia Stem Cells: Origin, Characteristics, and Clinical Implications. Stem Cell Rev. Rep. 2022, 18, 1211–1226. [Google Scholar] [CrossRef] [PubMed]
- Juliusson, G.; Antunovic, P.; Derolf, A.; Lehmann, S.; Mollgard, L.; Stockelberg, D.; Tidefelt, U.; Wahlin, A.; Hoglund, M. Age and acute myeloid leukemia: Real world data on decision to treat and outcomes from the Swedish Acute Leukemia Registry. Blood 2009, 113, 4179–4187. [Google Scholar] [CrossRef]
- Bonnet, D.; Dick, J.E. Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nat. Med. 1997, 3, 730–737. [Google Scholar] [CrossRef] [PubMed]
- Luo, L.; Han, Z.C. Leukemia stem cells. Int. J. Hematol. 2006, 84, 123–127. [Google Scholar] [CrossRef] [PubMed]
- Matthews, W.; Jordan, C.T.; Wiegand, G.W.; Pardoll, D.; Lemischka, I.R. A receptor tyrosine kinase specific to hematopoietic stem and progenitor cell-enriched populations. Cell 1991, 65, 1143–1152. [Google Scholar] [CrossRef]
- Ray, R.J.; Paige, C.J.; Furlonger, C.; Lyman, S.D.; Rottapel, R. Flt3 ligand supports the differentiation of early B cell progenitors in the presence of interleukin-11 and interleukin-7. Eur. J. Immunol. 1996, 26, 1504–1510. [Google Scholar] [CrossRef]
- Armstrong, S.A.; Kung, A.L.; Mabon, M.E.; Silverman, L.B.; Stam, R.W.; Den Boer, M.L.; Pieters, R.; Kersey, J.H.; Sallan, S.E.; Fletcher, J.A. Inhibition of FLT3 in MLL: Validation of a therapeutic target identified by gene expression based classification. Cancer Cell 2003, 3, 173–183. [Google Scholar] [CrossRef]
- Ozeki, K.; Kiyoi, H.; Hirose, Y.; Iwai, M.; Ninomiya, M.; Kodera, Y.; Miyawaki, S.; Kuriyama, K.; Shimazaki, C.; Akiyama, H. Biologic and clinical significance of the FLT3 transcript level in acute myeloid leukemia. Blood 2004, 103, 1901–1908. [Google Scholar] [CrossRef]
- Sudhindra, A.; Smith, C.C. FLT3 inhibitors in AML: Are we there yet? Curr. Hematol. Malig. Rep. 2014, 9, 174–185. [Google Scholar] [CrossRef]
- Tima, S.; Okonogi, S.; Ampasavate, C.; Pickens, C.; Berkland, C.; Anuchapreeda, S. Development and Characterization of FLT3-Specific Curcumin-Loaded Polymeric Micelles as a Drug Delivery System for Treating FLT3-Overexpressing Leukemic Cells. J. Pharm. Sci. 2016, 105, 3645–3657. [Google Scholar] [CrossRef] [PubMed]
- Hilmer, S.N.; Cogger, V.C.; Muller, M.; Le Couteur, D.G. The hepatic pharmacokinetics of doxorubicin and liposomal doxorubicin. Drug Metab. Dispos. 2004, 32, 794–799. [Google Scholar] [CrossRef]
- Gabizon, A.; Shmeeda, H.; Barenholz, Y. Pharmacokinetics of pegylated liposomal Doxorubicin: Review of animal and human studies. Clin. Pharmacokinet. 2003, 42, 419–436. [Google Scholar] [CrossRef] [PubMed]
- Minotti, G.; Menna, P.; Salvatorelli, E.; Cairo, G.; Gianni, L. Anthracyclines: Molecular advances and pharmacologic developments in antitumor activity and cardiotoxicity. Pharmacol. Rev. 2004, 56, 185–229. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, H.F.; Sun, Z.; Yao, X.; Litzow, M.R.; Luger, S.M.; Paietta, E.M.; Racevskis, J.; Dewald, G.W.; Ketterling, R.P.; Bennett, J.M. Anthracycline dose intensification in acute myeloid leukemia. N. Engl. J. Med. 2009, 361, 1249–1259. [Google Scholar] [CrossRef]
- Tacar, O.; Sriamornsak, P.; Dass, C.R. Doxorubicin: An update on anticancer molecular action, toxicity and novel drug delivery systems. J. Pharm. Pharmacol. 2013, 65, 157–170. [Google Scholar] [CrossRef]
- Cho, K.; Wang, X.; Nie, S.; Chen, Z.; Shin, D.M. Therapeutic nanoparticles for drug delivery in cancer. Clin. Cancer Res. 2008, 14, 1310–1316. [Google Scholar] [CrossRef]
- Broxterman, H.J.; Georgopapadakou, N.H. Anticancer therapeutics: “Addictive” targets, multi-targeted drugs, new drug combinations. Drug Resist. Updates 2005, 8, 183–197. [Google Scholar] [CrossRef]
- Jonas, B.A.; Pollyea, D.A. How we use venetoclax with hypomethylating agents for the treatment of newly diagnosed patients with acute myeloid leukemia. Leukemia 2019, 33, 2795–2804. [Google Scholar] [CrossRef]
- Tan, B.L.; Norhaizan, M.E. Curcumin combination chemotherapy: The implication and efficacy in cancer. Molecules 2019, 24, 2527. [Google Scholar] [CrossRef]
- Anuchapreeda, S.; Tima, S.; Duangrat, C.; Limtrakul, P. Effect of pure curcumin, demethoxycurcumin, and bisdemethoxycurcumin on WT1 gene expression in leukemic cell lines. Cancer Chemother. Pharmacol. 2008, 62, 585–594. [Google Scholar] [CrossRef]
- Duvoix, A.; Morceau, F.; Delhalle, S.; Schmitz, M.; Schnekenburger, M.; Galteau, M.M.; Dicato, M.; Diederich, M. Induction of apoptosis by curcumin: Mediation by glutathione S-transferase P1-1 inhibition. Biochem. Pharmacol. 2003, 66, 1475–1483. [Google Scholar] [CrossRef]
- Sharma, R.A.; Gescher, A.J.; Steward, W.P. Curcumin: The story so far. Eur. J. Cancer 2005, 41, 1955–1968. [Google Scholar] [CrossRef]
- Tima, S.; Anuchapreeda, S.; Ampasavate, C.; Berkland, C.; Okonogi, S. Stable curcumin-loaded polymeric micellar formulation for enhancing cellular uptake and cytotoxicity to FLT3 overexpressing EoL-1 leukemic cells. Eur. J. Pharm. Biopharm. 2017, 114, 57–68. [Google Scholar] [CrossRef]
- Adams, M.L.; Lavasanifar, A.; Kwon, G.S. Amphiphilic block copolymers for drug delivery. J. Pharm. Sci. 2003, 92, 1343–1355. [Google Scholar] [CrossRef]
- Lv, L.; Qiu, K.; Yu, X.; Chen, C.; Qin, F.; Shi, Y.; Ou, J.; Zhang, T.; Zhu, H.; Wu, J. Amphiphilic copolymeric micelles for doxorubicin and curcumin co-delivery to reverse multidrug resistance in breast cancer. J. Biomed. Nanotechnol. 2016, 12, 973–985. [Google Scholar] [CrossRef]
- Wang, J.; Ma, W.; Tu, P. Synergistically improved anti-tumor efficacy by co-delivery doxorubicin and curcumin polymeric micelles. Macromol. Biosci. 2015, 15, 1252–1261. [Google Scholar] [CrossRef]
- Sabzi, A.; Rahmani, A.; Edalati, M.; Kahroba, H.; Dadpour, M.R.; Salehi, R.; Zarebkohan, A. Targeted co-delivery of curcumin and doxorubicin by citric acid functionalized Poly (ε-caprolactone) based micelle in MDA-MB-231 cell. Colloids Surf. B Biointerfaces 2020, 194, 111225. [Google Scholar] [CrossRef]
- Woo Jung, Y.; Lee, H.; Yeon Kim, J.; Jin Koo, E.; Sang Oh, K.; Hong Yuk, S. Pluronic-based core/shell nanoparticles for drug delivery and diagnosis. Curr. Med. Chem. 2013, 20, 3488–3499. [Google Scholar] [CrossRef]
- Giuliano, E.; Paolino, D.; Fresta, M.; Cosco, D. Mucosal applications of poloxamer 407-based hydrogels: An overview. Pharmaceutics 2018, 10, 159. [Google Scholar] [CrossRef]
- Chueahongthong, F.; Tima, S.; Chiampanichayakul, S.; Dejkriengkraikul, P.; Okonogi, S.; Sasarom, M.; Rodwattanagul, S.; Berkland, C.; Anuchapreeda, S. Doxorubicin-Loaded Polymeric Micelles Conjugated with CKR- and EVQ-FLT3 Peptides for Cytotoxicity in Leukemic Stem Cells. Pharmaceutics 2022, 14, 2115. [Google Scholar] [CrossRef]
- Presolski, S.I.; Hong, V.P.; Finn, M.G. Copper-Catalyzed Azide-Alkyne Click Chemistry for Bioconjugation. Curr. Protoc. Chem. Biol. 2011, 3, 153–162. [Google Scholar] [CrossRef]
- Chueahongthong, F.; Tima, S.; Chiampanichayakul, S.; Berkland, C.; Anuchapreeda, S. Co-Treatments of Edible Curcumin from Turmeric Rhizomes and Chemotherapeutic Drugs on Cytotoxicity and FLT3 Protein Expression in Leukemic Stem Cells. Molecules 2021, 26, 5785. [Google Scholar] [CrossRef]
- Ghafary, S.M.; Rahimjazi, E.; Hamzehil, H.; Mousavi, S.M.M.; Nikkhah, M.; Hosseinkhani, S. Design and preparation of a theranostic peptideticle for targeted cancer therapy: Peptide-based codelivery of doxorubicin/curcumin and graphene quantum dots. Nanomed. Nanotechnol. Biol. Med. 2022, 42, 102544. [Google Scholar]
- Zhang, Y.; Li, T.; Hu, Y.; Chen, J.; He, Y.; Gao, X.; Zhang, Y. Co-delivery of doxorubicin and curcumin via cRGD-peptide modified PEG-PLA self-assembly nanomicelles for lung cancer therapy. Chin. Chem. Lett. 2022, 33, 2507–2511. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, Y.; Sun, X.; Wang, Y.; Du, C.; Bai, J. Morphologically transformable peptide nanocarriers coloaded with doxorubicin and curcumin inhibit the growth and metastasis of hepatocellular carcinoma. Mater. Today Bio 2024, 24, 100903. [Google Scholar] [CrossRef]
- Tang, H.; Li, L.; Wang, B.; Guangxi Scientific Research Center of Traditional Chinese Medicine. Observation of antitumor mechanism of GE11-modified paclitaxel and curcumin liposomes based on cellular morphology changes. AAPS Open 2024, 10, 1. [Google Scholar] [CrossRef]
- Chen, M.; Fang, X.; Du, R.; Meng, J.; Liu, J.; Liu, M.; Yang, Y.; Wang, C. A Nucleus-Targeting WT1 Antagonistic Peptide Encapsulated in Polymeric Nanomicelles Combats Refractory Chronic Myeloid Leukemia. Pharmaceutics 2023, 15, 2305. [Google Scholar] [CrossRef]
- Almeida, M.; Magalhães, M.; Veiga, F.; Figueiras, A. Poloxamers, poloxamines and polymeric micelles: Definition, structure and therapeutic applications in cancer. J. Polym. Res. 2018, 25, 31. [Google Scholar] [CrossRef]
- Alvarez-Lorenzo, C.; Sosnik, A.; Concheiro, A. PEO-PPO block copolymers for passive micellar targeting and overcoming multidrug resistance in cancer therapy. Curr. Drug Targets 2011, 12, 1112–1130. [Google Scholar] [CrossRef]
- Fuchs, D.; Daniel, V.; Sadeghi, M.; Opelz, G.; Naujokat, C. Salinomycin overcomes ABC transporter-mediated multidrug and apoptosis resistance in human leukemia stem cell-like KG-1a cells. Biochem. Biophys. Res. Commun. 2010, 394, 1098–1104. [Google Scholar] [CrossRef]
- Zhang, D.; Xu, Q.; Wang, N.; Yang, Y.; Liu, J.; Yu, G.; Yang, X.; Xu, H.; Wang, H. A complex micellar system co-delivering curcumin with doxorubicin against cardiotoxicity and tumor growth. Int. J. Nanomed. 2018, 13, 4549–4561. [Google Scholar] [CrossRef]
- Hauser, P.V.; Chang, H.-M.; Yanagawa, N.; Hamon, M. Nanotechnology, nanomedicine, and the kidney. Appl. Sci. 2021, 11, 7187. [Google Scholar] [CrossRef]
- Owen, S.C.; Chan, D.P.; Shoichet, M.S. Polymeric micelle stability. Nano Today 2012, 7, 53–65. [Google Scholar] [CrossRef]
- Clogston, J.D.; Patri, A.K. Zeta potential measurement. In Characterization of Nanoparticles Intended for Drug Delivery; Springer: Berlin/Heidelberg, Germany, 2011; pp. 63–70. [Google Scholar]
- Zhang, C.; Zhang, J.; Qin, Y.; Song, H.; Huang, P.; Wang, W.; Wang, C.; Li, C.; Wang, Y.; Kong, D. Co-delivery of doxorubicin and pheophorbide A by pluronic F127 micelles for chemo-photodynamic combination therapy of melanoma. J. Mater. Chem. B 2018, 6, 3305–3314. [Google Scholar] [CrossRef]
- Wang, B.-L.; Shen, Y.-M.; Zhang, Q.-W.; Li, Y.-L.; Luo, M.; Liu, Z.; Li, Y.; Qian, Z.-Y.; Gao, X.; Shi, H.-S. Codelivery of curcumin and doxorubicin by MPEG-PCL results in improved efficacy of systemically administered chemotherapy in mice with lung cancer. Int. J. Nanomed. 2013, 8, 3521–3531. [Google Scholar]
- Göppert, T.; Müller, R. Adsorption kinetics of plasma proteins on solid lipid nanoparticles for drug targeting. Int. J. Pharm. 2005, 302, 172–186. [Google Scholar] [CrossRef]
- Song, S.; Liu, D.; Peng, J.; Sun, Y.; Li, Z.; Gu, J.-R.; Xu, Y. Peptide ligand-mediated liposome distribution and targeting to EGFR expressing tumor in vivo. Int. J. Pharm. 2008, 363, 155–161. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, C.; Wang, W.; Liu, J.; Liu, Q.; Huang, F.; Chu, L.; Gao, H.; Li, C.; Kong, D. Co-delivery of doxorubicin and curcumin by pH-sensitive prodrug nanoparticle for combination therapy of cancer. Sci. Rep. 2016, 6, 21225. [Google Scholar] [CrossRef]



| Sample | KG-1a Cells | EoL-1 Cells | ||
|---|---|---|---|---|
| IC50 of Dox (μg/mL) | IC50 of Cur (µg/mL) | IC50 of Dox (µg/mL) | IC50 of Cur (µg/mL) | |
| Dox | 0.81 ± 0.05 | - | 0.10 ± 0.00 | - |
| Dox-Cur | 0.40 ± 0.03 a | 3.61 ± 0.25 | 0.07 ± 0.01 | 0.67 ± 0.05 |
| DCM | 0.45 ± 0.03 a | 4.70 ± 0.28 | 0.06 ± 0.02 | 0.65 ± 0.18 |
| DCM-C | 0.41 ± 0.09 a | 3.38 ± 0.74 | 0.08 ± 0.01 | 0.66 ± 0.11 |
| DCM-E | 0.39 ± 0.06 a | 2.80 ± 0.44 | 0.07 ± 0.02 | 0.53 ± 0.15 |
| DCM-C + E | 0.36 ± 0.05 a | 2.71 ± 0.35 | 0.07 ± 0.01 a | 0.53 ± 0.08 |
| Sample | IC50 (µg/mL) | |
|---|---|---|
| KG-1a Cells | EoL-1 Cells | |
| Cur | 8.78 ± 0.40 b,e | 5.18 ± 0.31 b,c,d,e |
| CM | 10.19 ± 0.38 a,c,e | 5.75 ± 0.13 a,c,d,e |
| CM-C | 7.74 ± 0.52 b,d | 2.19 ± 0.30 a,b,d,e |
| CM-E | 9.68 ± 1.29 c,e | 1.55 ± 0.44 a,b,c |
| CM-C + E | 6.69 ± 0.75 a,b,d | 1.43 ± 0.27 a,b,c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chueahongthong, F.; Chiampanichayakul, S.; Viriyaadhammaa, N.; Dejkriengkraikul, P.; Okonogi, S.; Berkland, C.; Anuchapreeda, S. Cytotoxicity of Doxorubicin-Curcumin Nanoparticles Conjugated with Two Different Peptides (CKR and EVQ) against FLT3 Protein in Leukemic Stem Cells. Polymers 2024, 16, 2498. https://doi.org/10.3390/polym16172498
Chueahongthong F, Chiampanichayakul S, Viriyaadhammaa N, Dejkriengkraikul P, Okonogi S, Berkland C, Anuchapreeda S. Cytotoxicity of Doxorubicin-Curcumin Nanoparticles Conjugated with Two Different Peptides (CKR and EVQ) against FLT3 Protein in Leukemic Stem Cells. Polymers. 2024; 16(17):2498. https://doi.org/10.3390/polym16172498
Chicago/Turabian StyleChueahongthong, Fah, Sawitree Chiampanichayakul, Natsima Viriyaadhammaa, Pornngarm Dejkriengkraikul, Siriporn Okonogi, Cory Berkland, and Songyot Anuchapreeda. 2024. "Cytotoxicity of Doxorubicin-Curcumin Nanoparticles Conjugated with Two Different Peptides (CKR and EVQ) against FLT3 Protein in Leukemic Stem Cells" Polymers 16, no. 17: 2498. https://doi.org/10.3390/polym16172498
APA StyleChueahongthong, F., Chiampanichayakul, S., Viriyaadhammaa, N., Dejkriengkraikul, P., Okonogi, S., Berkland, C., & Anuchapreeda, S. (2024). Cytotoxicity of Doxorubicin-Curcumin Nanoparticles Conjugated with Two Different Peptides (CKR and EVQ) against FLT3 Protein in Leukemic Stem Cells. Polymers, 16(17), 2498. https://doi.org/10.3390/polym16172498










