A High-Methanol-Permeation Resistivity Polyamide-Based Proton Exchange Membrane Fabricated via a Hyperbranching Design
Abstract
1. Introduction
2. Experimental
2.1. Materials
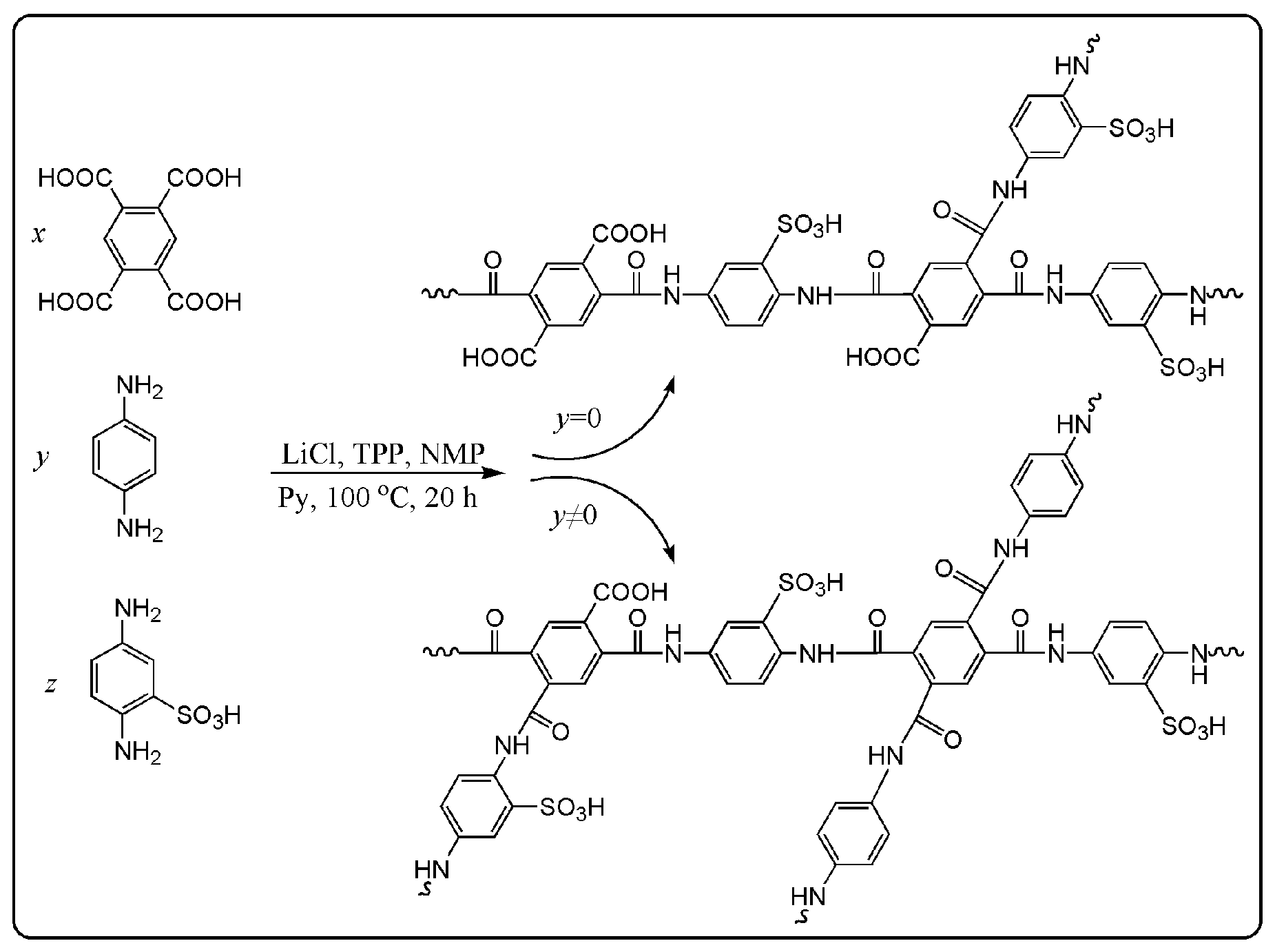
2.2. Synthesis
2.3. Membrane Preparation
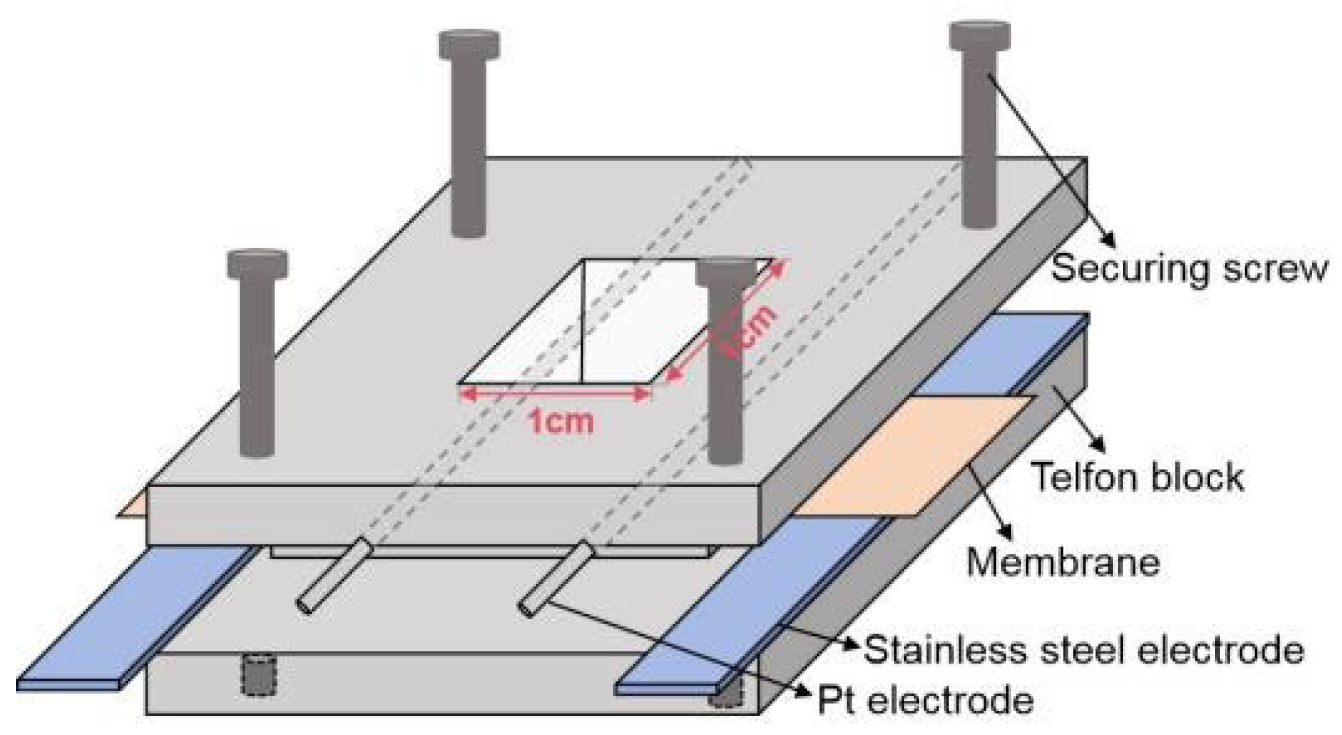
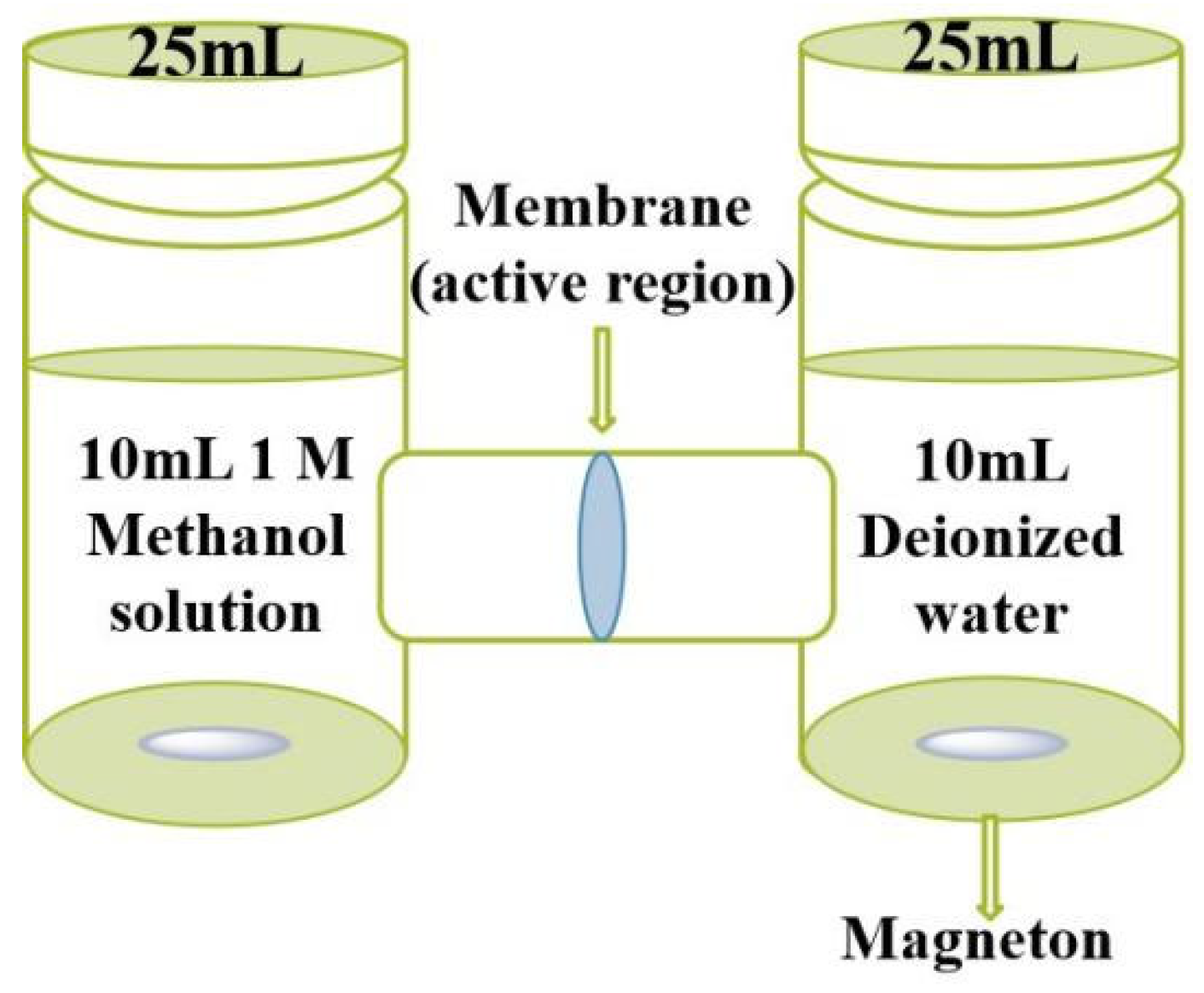
2.4. Characterizations and Measurements
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Kai, Z.; Yujun, Z.; Kun, Y.; Yibing, L.; Qixing, T. Study on the Concentration Inversion of NO & NO2 Gas from the Vehicle Exhaust Based on Weighted PLS. Sci. Res. Publ. 2017, 7, 106–115. [Google Scholar] [CrossRef]
- Chen, Z.; Higgins, D.; Yu, A.; Zhang, L.; Zhang, J. A review on non-precious metal electrocatalysts for PEM fuel cells. Energy Environ. Sci. 2011, 4, 3167. [Google Scholar] [CrossRef]
- Cheng, F.; Chen, J. Metal–air batteries: From oxygen reduction electrochemistry to cathode catalysts. Chem. Soc. Rev. 2012, 41, 2172. [Google Scholar] [CrossRef] [PubMed]
- Razmi, A.R.; Alirahmi, S.M.; Nabat, M.H.; Assareh, E.; Shahbakhti, M. A green hydrogen energy storage concept based on parabolic trough collector and proton exchange membrane electrolyzer/fuel cell: Thermodynamic and exergoeconomic analyses with multi-objective optimization. Int. J. Hydrogen Energy 2022, 47, 26468–26489. [Google Scholar] [CrossRef]
- Shamsi, M.; Obaid, A.A.; Farokhi, S.; Bayat, A. A novel process simulation model for hydrogen production via reforming of biomass gasification tar. Int. J. Hydrogen Energy 2022, 47, 772–781. [Google Scholar] [CrossRef]
- Alirahmi, S.M.; Assareh, E.; Chitsaz, A.; Holagh, S.G.; Jalilinasrabady, S. Electrolyzer-fuel cell combination for grid peak load management in a geothermal power plant: Power to hydrogen and hydrogen to power conversion. Int. J. Hydrogen Energy 2021, 46, 25650–25665. [Google Scholar] [CrossRef]
- Selim, A.; Szijjártó, G.P.; Románszki, L.; Tompos, A. Development of WO3-Nafion Based Membranes for Enabling Higher Water Retention at Low Humidity and Enhancing PEMFC Performance at Intermediate Temperature Operation. Polymers 2022, 14, 2492. [Google Scholar] [CrossRef]
- Wu, L.; Zhang, Z.; Ran, J.; Zhou, D.; Li, C.; Xu, T. Advances in proton-exchange membranes for fuel cells: An overview on proton conductive channels (PCCs). Phys. Chem. Chem. Phys. 2013, 15, 4870. [Google Scholar] [CrossRef]
- Sebastián, D.; Serov, A.; Artyushkova, K.; Atanassov, P.; Aricò, A.S.; Baglio, V. Performance, methanol tolerance and stability of Fe-aminobenzimidazole derived catalyst for direct methanol fuel cells. J. Power Sources 2016, 319, 235–246. [Google Scholar] [CrossRef]
- Roelofs, K.S.; Hirth, T.; Schiestel, T. Sulfonated poly(ether ether ketone)-based silica nanocomposite membranes for direct ethanol fuel cells. J. Membr. Sci. 2010, 346, 215–226. [Google Scholar] [CrossRef]
- Na, Y.; Khadke, P.; Glüsen, A.; Kimiaie, N.; Müller, M.; Krewer, U. A robust methanol concentration sensing technique in direct methanol fuel cells and stacks using cell dynamics. Int. J. Hydrogen Energy 2022, 47, 6237–6246. [Google Scholar] [CrossRef]
- Beydaghi, H.; Bagheri, A.; Salarizadeh, P.; Kashefi, S.; Hooshyari, K.; Amoozadeh, A.; Shamsi, T.; Bonaccorso, F.; Pellegrini, V. Enhancing the Performance of Poly(phthalazinone ether ketone)-Based Membranes Using a New Type of Functionalized TiO2 with Superior Proton Conductivity. Ind. Eng. Chem. Res. 2020, 59, 6589–6599. [Google Scholar] [CrossRef]
- Shukla, A.; Dhanasekaran, P.; Sasikala, S.; Nagaraju, N.; Bhat, S.D.; Pillai, V.K. Covalent grafting of polystyrene sulfonic acid on graphene oxide nanoplatelets to form a composite membrane electrolyte with sulfonated poly(ether ether ketone) for direct methanol fuel cells. J. Membr. Sci. 2020, 595, 117484. [Google Scholar] [CrossRef]
- Ma, L.; Cai, W.; Li, J.; Fan, K.; Jiang, Y.; Ma, L.; Cheng, H. A high performance polyamide-based proton exchange membrane fabricated via construction of hierarchical proton conductive channels. J. Power Sources 2016, 302, 189–194. [Google Scholar] [CrossRef]
- Liu, H.; Song, C.; Zhang, L.; Zhang, J.; Wang, H.; Wilkinson, D.P. A review of anode catalysis in the direct methanol fuel cell. J. Power Sources 2006, 155, 95–110. [Google Scholar] [CrossRef]
- Al-Battya, S.; Dawsona, C.; Shanmukhama, S.P.; Robertsbs, E.P.L.; Holmesa, S.M. Improvement of direct methanol fuel cell performance using a novel mordenite barrier layer. J. Mater. Chem. A 2016, 4, 10850–10857. [Google Scholar] [CrossRef]
- Chuesutham, T.; Sirivat, A.; Paradee, N.; Changkhamchom, S.; Wattanakul, K.; Anumart, S.; Krathumkhet, N.; Khampim, J. Improvement of sulfonated poly(ether ether ketone)/Y zeolite-SO3H via organo-functionalization method for direct methanol fuel cell. Renew. Energy 2019, 138, 243–249. [Google Scholar] [CrossRef]
- Lai, Q.Z.; Yin, G.P.; Wang, Z.B.; Du, C.Y.; Zuo, P.J.; Cheng, X.Q. Influence of Methanol Crossover on the Fuel Utilization of Passive Direct Methanol Fuel Cell. Fuel Cells 2008, 8, 399–403. [Google Scholar] [CrossRef]
- Goor, M.; Menkin, S.; Peled, E. High power direct methanol fuel cell for mobility and portable applications. Int. J. Hydrogen Energy 2019, 44, 3138–3143. [Google Scholar] [CrossRef]
- Park, Y.-S.; Yamazaki, Y. Novel Nafion/Hydroxyapatite composite membrane with high crystallinity and low methanol crossover for DMFCs. Polym. Bull. 2004, 53, 181–192. [Google Scholar] [CrossRef]
- Munjewar, S.S.; Thombre, S.B.; Mallick, R.K. Approaches to overcome the barrier issues of passive direct methanol fuel cell—Review. Renew. Sustain. Energy Rev. 2017, 67, 1087–1104. [Google Scholar] [CrossRef]
- Chen, Z.; Holmberg, B.; Li, W.; Wang, X.; Deng, W.; Munoz, R.; Yan, Y. Nafion/Zeolite Nanocomposite Membrane by in Situ Crystallization for a Direct Methanol Fuel Cell. Chem. Mater. 2006, 18, 5669–5675. [Google Scholar] [CrossRef]
- Ozden, A.; Ercelik, M.; Devrim, Y.; Colpan, C.O.; Hamdullahpur, F. Evaluation of sulfonated polysulfone/zirconium hydrogen phosphate composite membranes for direct methanol fuel cells. Electrochim. Acta 2017, 256, 196–210. [Google Scholar] [CrossRef]
- Ozden, A.; Ercelik, M.; Ozdemir, Y.; Devrim, Y.; Colpan, C.O. Enhancement of direct methanol fuel cell performance through the inclusion of zirconium phosphate. Int. J. Hydrogen Energy 2017, 42, 21501–21517. [Google Scholar] [CrossRef]
- Ercelik, M.; Ozden, A.; Devrim, Y.; Colpan, C.O. Investigation of Nafion based composite membranes on the performance of DMFCs. Int. J. Hydrogen Energy 2017, 42, 2658–2668. [Google Scholar] [CrossRef]
- Heinzel, A.; Barragán, V.M. A review of the state-of-the-art of the methanol crossover in direct methanol fuel cells. J. Power Sources 1999, 84, 70–74. [Google Scholar] [CrossRef]
- Xie, X.; Yu, H.; Deng, H.; Zhang, G.; Guo, T.; Sun, J.; Jiao, K. Modeling of passive vapor feed alkaline membrane direct methanol fuel cell. Appl. Therm. Eng. 2018, 131, 920–932. [Google Scholar] [CrossRef]
- Parthiban, V.; Akula, S.; Sahu, A.K. Surfactant templated nanoporous carbon-Nafion hybrid membranes for direct methanol fuel cells with reduced methanol crossover. J. Membr. Sci. 2017, 541, 127–136. [Google Scholar] [CrossRef]
- Bang, H.S.; Kim, D.; Hwang, S.S.; Won, J. Surface-modified porous membranes with electrospun Nafion/PVA fibres for non-aqueous redox flow battery. J. Membr. Sci. 2016, 514, 186–194. [Google Scholar] [CrossRef]
- Cai, W.; Fan, K.; Li, J.; Ma, L.; Xu, G.; Xu, S.; Ma, L.; Cheng, H. A bi-functional polymeric nano-sieve Nafion composite membrane: Improved performance for direct methanol fuel cell applications. Int. J. Hydrogen Energy 2016, 41, 17102–17111. [Google Scholar] [CrossRef]
- Jin, J.; Hao, R.; He, X.; Li, G. Sulfonated poly(phenylsulfone)/fluorinated polybenzoxazole nanofiber composite membranes for proton exchange membrane fuel cells. Int. J. Hydrogen Energy 2015, 40, 14421–14427. [Google Scholar] [CrossRef]
- Shin, D.W.; Guiver, M.D.; Lee, Y.M. Hydrocarbon-Based Polymer Electrolyte Membranes: Importance of Morphology on Ion Transport and Membrane Stability. Chem. Rev. 2017, 117, 4759–4805. [Google Scholar] [CrossRef]
- Colpan, C.O.; Ouellette, D.; Glüsen, A.; Müller, M.; Stolten, D. Reduction of methanol crossover in a flowing electrolyte-direct methanol fuel cell. Int. J. Hydrogen Energy 2017, 42, 21530–21545. [Google Scholar] [CrossRef]
- Guo, T.; Sun, J.; Zhang, J.; Deng, H.; Xie, X.; Jiao, K.; Huang, X. Transient analysis of passive vapor-feed DMFC fed with neat methanol. Int. J. Hydrogen Energy 2017, 42, 3222–3239. [Google Scholar] [CrossRef]
- Stanis, R.J.; Lambert, T.N.; Yaklin, M.A. Poly(3,4-ethylenedioxythiphene) (PEDOT)-Modified Anodes: Reduced Methanol Crossover in Direct Methanol Fuel Cells. Energy Fuels 2010, 24, 3125–3129. [Google Scholar] [CrossRef]
- Wang, C.; Shin, D.W.; Lee, S.Y.; Kang, N.R.; Lee, Y.M.; Guiver, M.D. Poly(arylene ether sulfone) proton exchange membranes with flexible acid side chains. J. Membr. Sci. 2012, 405–406, 68–78. [Google Scholar] [CrossRef]
- Tsai, J.-C.; Lin, C.-K. Acid–base blend membranes based on Nafion®/aminated SPEEK for reducing methanol permeability. J. Taiwan Inst. Chem. Eng. 2011, 42, 281–285. [Google Scholar] [CrossRef]
- Seo, D.W.; Lim, Y.D.; Lee, S.H.; Hossain, M.A.; Islam, M.M.; Lee, H.C.; Jang, H.H.; Kim, W.G. Preparation and characterization of block copolymers containing multi-sulfonated unit for proton exchange membrane fuel cell. Electrochim. Acta 2012, 86, 352–359. [Google Scholar] [CrossRef]
- Liu, J.; Yu, L.; Cai, X.; Khan, U.; Cai, Z.; Xi, J.; Liu, B.; Kang, F. Sandwiching h-BN Monolayer Films between Sulfonated Poly(ether ether ketone) and Nafion for Proton Exchange Membranes with Improved Ion Selectivity. ACS Nano 2019, 13, 2094–2102. [Google Scholar] [CrossRef]
- Wan Mohd Noral Azman, W.N.E.; Jaafar, J.; Salleh, W.N.W.; Ismail, A.F.; Othman, M.H.D.; Rahman, M.A.; Rasdi, F.R.M. Highly selective SPEEK/ENR blended polymer electrolyte membranes for direct methanol fuel cell. Mater. Today Energy 2020, 17, 100427. [Google Scholar] [CrossRef]
- Li, J.; Cai, W.; Ma, L.; Zhang, Y.; Chen, Z.; Cheng, H. Towards Neat Methanol Operation of Direct Methanol Fuel Cells: A Novel Self-Assembled Proton Exchange Membrane. Chem. Commun. 2015, 51, 6556–6559. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, C.; Liu, X.; Yang, Z.; Dong, J.; Liu, Y.; Cai, W.; Cheng, H. Fabrication of a polymer electrolyte membrane with uneven side chains for enhancing proton conductivity. RSC Adv. 2016, 6, 79593–79601. [Google Scholar] [CrossRef]
- Pandey, R.P.; Thakur, A.K.; Shahi, V.K. Sulfonated Polyimide/Acid-Functionalized Graphene Oxide Composite Polymer Electrolyte Membranes with Improved Proton Conductivity and Water-Retention Properties. ACS Appl. Mater. Interfaces 2014, 6, 16993–17002. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Xu, G.; Li, S.; Ma, J.; Li, J.; Cai, W. Design and Optimization of a Hyper-Branched Polyimide Proton Exchange Membrane with Ultra-High Methanol-Permeation Resistivity for Direct Methanol Fuel Cells Applications. Polymers 2018, 10, 1175. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Yang, Z.; Zhang, Y.; Li, C.; Dong, J.; Liu, Y.; Cheng, H. Electrospun multifunctional sulfonated carbon nanofibers for design and fabrication of SPEEK composite proton exchange membranes for direct methanol fuel cell application. Int. J. Hydrogen Energy 2017, 42, 10275–10284. [Google Scholar] [CrossRef]
- Xie, H.; Tao, D.; Xiang, X.; Ou, Y.; Bai, X.; Wang, L. Synthesis and properties of highly branched star-shaped sulfonated block poly(arylene ether)s as proton exchange membranes. J. Membr. Sci. 2015, 473, 226–236. [Google Scholar] [CrossRef]
- Duan, Y.; Ru, C.; Pang, Y.; Li, J.; Liu, B.; Zhao, C. Crosslinked PAEK-based nanofiber reinforced Nafion membrane with ion-paired interfaces towards high-concentration DMFC. J. Membr. Sci. 2022, 655, 120589. [Google Scholar] [CrossRef]
- Ru, C.; Gu, Y.; Duan, Y.; Zhao, C.; Na, H. Enhancement in proton conductivity and methanol resistance of Nafion membrane induced by blending sulfonated poly(arylene ether ketones) for direct methanol fuel cells. J. Membr. Sci. 2019, 573, 439–447. [Google Scholar] [CrossRef]
- Wang, B.; Hong, L.; Li, Y.; Zhao, L.; Zhao, C.; Na, H. Property Enhancement Effects of Side-Chain-Type Naphthalene-Based Sulfonated Poly(arylene ether ketone) on Nafion Composite Membranes for Direct Methanol Fuel Cells. ACS Appl. Mater. Interfaces 2017, 9, 32227–32236. [Google Scholar] [CrossRef]
- Wang, C.; Zhou, Y.; Shen, B.; Zhao, X.; Li, J.; Ren, Q. Proton-conducting poly(ether sulfone ketone)s containing a high density of pendant sulfonic groups by a convenient and mild postsulfonation. Polym. Chem. 2018, 9, 4984–4993. [Google Scholar] [CrossRef]
- Oh, B.H.; Kim, A.R.; Yoo, D.J. Profile of extended chemical stability and mechanical integrity and high hydroxide ion conductivity of poly(ether imide) based membranes for anion exchange membrane fuel cells. Int. J. Hydrogen Energy 2019, 44, 4281–4292. [Google Scholar] [CrossRef]
- Li, X.; Bu, F.; Zhang, H.; Zhao, C. Facile synthesis of poly (arylene ether ketone)s containing flexible sulfoalkyl groups with enhanced oxidative stability for DMFCs. Int. J. Hydrogen Energy 2020, 45, 27632–27643. [Google Scholar] [CrossRef]
- Divya, K.; Rana, D.; Sri Abirami Saraswathi, M.S.; Bhat, S.D.; Shukla, A.; Nagendran, A. Investigation of the versatility of SPES membranes customized with sulfonated molybdenum disulfide nanosheets for DMFC applications. Int. J. Hydrogen Energy 2020, 45, 15507–15520. [Google Scholar] [CrossRef]
- Chikumba, F.T.; Tamer, M.; Akyalçın, L.; Kaytakoğlu, S. The development of sulfonated polyether ether ketone (sPEEK) and titanium silicon oxide (TiSiO4) composite membranes for DMFC applications. Int. J. Hydrogen Energy 2023, 48, 14038–14052. [Google Scholar] [CrossRef]
- Rodgers, M.P.; Bonville, L.J.; Kunz, H.R.; Slattery, D.K.; Fenton, J.M. Fuel cell perfluorinated sulfonic acid membrane degradation correlating accelerated stress testing and lifetime. Chem. Rev. 2012, 112, 6075–6103. [Google Scholar] [CrossRef]
- Wang, Y.; Xing, C.; Guan, J.; Li, Y. Towards Flexible Dielectric Materials with High Dielectric Constant and Low Loss: PVDF Nanocomposites with both Homogenously Dispersed CNTs and Ionic Liquids Nanodomains. Polymers 2017, 9, 562. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, Y.; Chen, Y.; Li, C.; Dong, J.; Zhang, Q.; Wang, J.; Yang, Z.; Cheng, H. A superhydrophobic bromomethylated poly(phenylene oxide) as a multifunctional polymer filler in SPEEK membrane towards neat methanol operation of direct methanol fuel cells. J. Membr. Sci. 2017, 544, 58–67. [Google Scholar] [CrossRef]
- Li, H.; Wu, Y.; Sato, H.; Kong, L.; Zhang, C.; Huang, K.; Tao, D. A Nwe Facile Method for Preparation of Nylon-with High Crystallinity and Special Morphology. Macromolecules 2009, 42, 1175–1179. [Google Scholar] [CrossRef]
- Kreuer, K.D. On the development of proton conducting polymer membranes for hydrogen and methanol fuel cells. J. Membr. Sci. 2001, 185, 29–39. [Google Scholar] [CrossRef]
- Karlsson, L.E.; Wesslen, B.; Jannasch, P. Water absorption and proton conductivity of sulfonated acrylamide copolymers. Electrochim. Acta 2002, 47, 3269–3275. [Google Scholar] [CrossRef]
- Li, Q.; He, R.; Jensen, J.O.; Bjerrum, N.J. Approaches and Recent Development of Polymer Electrolyte Membranes for Fuel Cells Operating above 100 °C. Chem. Mater. 2003, 15, 4896–4915. [Google Scholar] [CrossRef]
- Ansari, Y.; Tucker, T.G.; Huang, W.; Klein, I.S.; Lee, S.Y.; Yarger, J.L.; Angell, C.A. A flexible all-inorganic fuel cell membrane with conductivity above Nafion, and durable operation at 150 °C. J. Power Sources 2016, 303, 142–149. [Google Scholar] [CrossRef]
- Sasikala, S.; Meenakshi, S.; Bhat, S.D.; Sahu, A.K. Functionalized Bentonite clay-sPEEK based composite membranes for direct methanol fuel cells. Electrochim. Acta 2014, 135, 232–241. [Google Scholar] [CrossRef]
- Sha Wang, L.; Nan Lai, A.; Xiao Lin, C.; Gen Zhang, Q.; Mei Zhu, A.; Lin Liu, Q. Orderly sandwich-shaped graphene oxide/Nafion composite membranes for direct methanol fuel cells. J. Membr. Sci. 2015, 492, 58–66. [Google Scholar] [CrossRef]




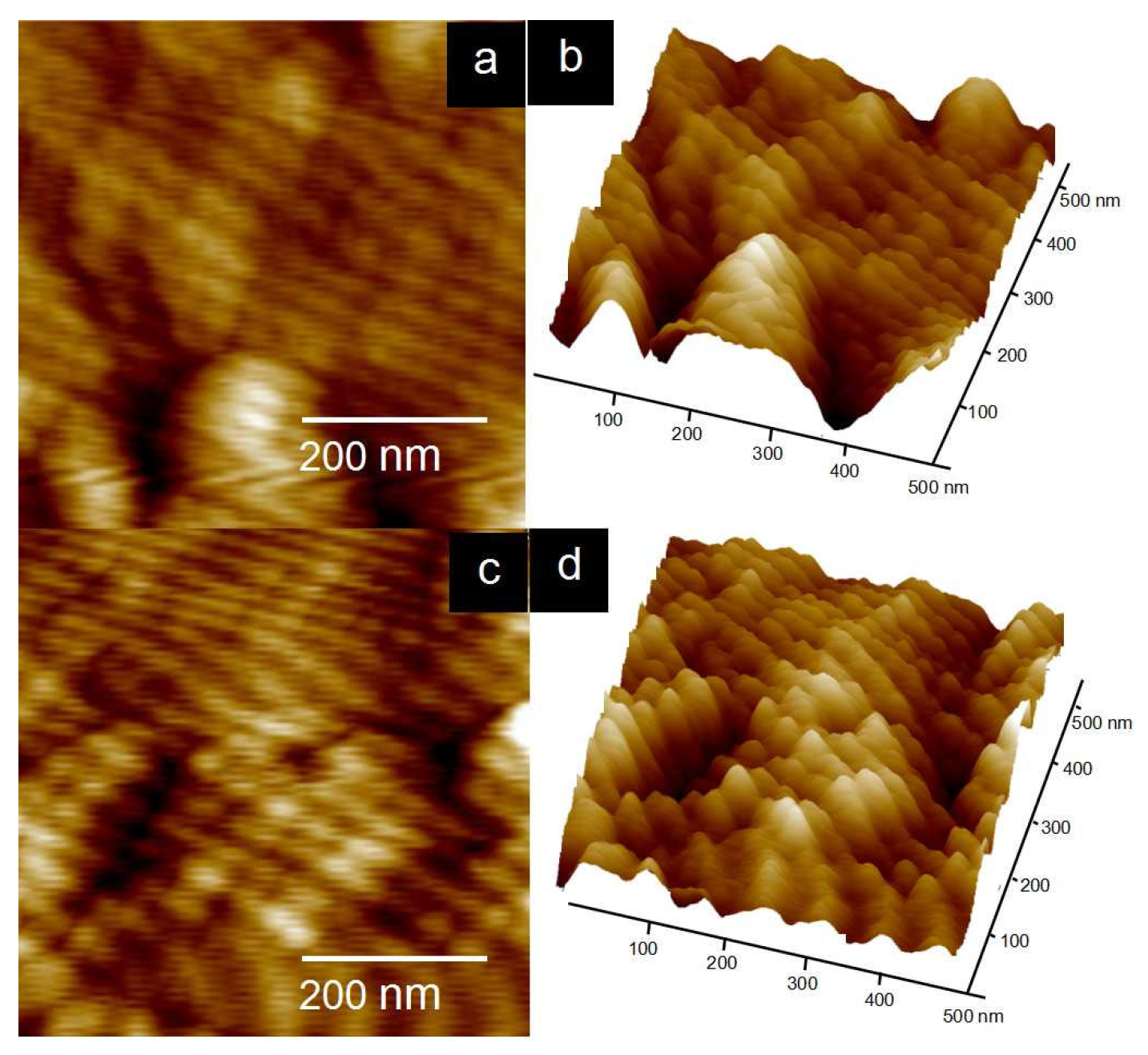

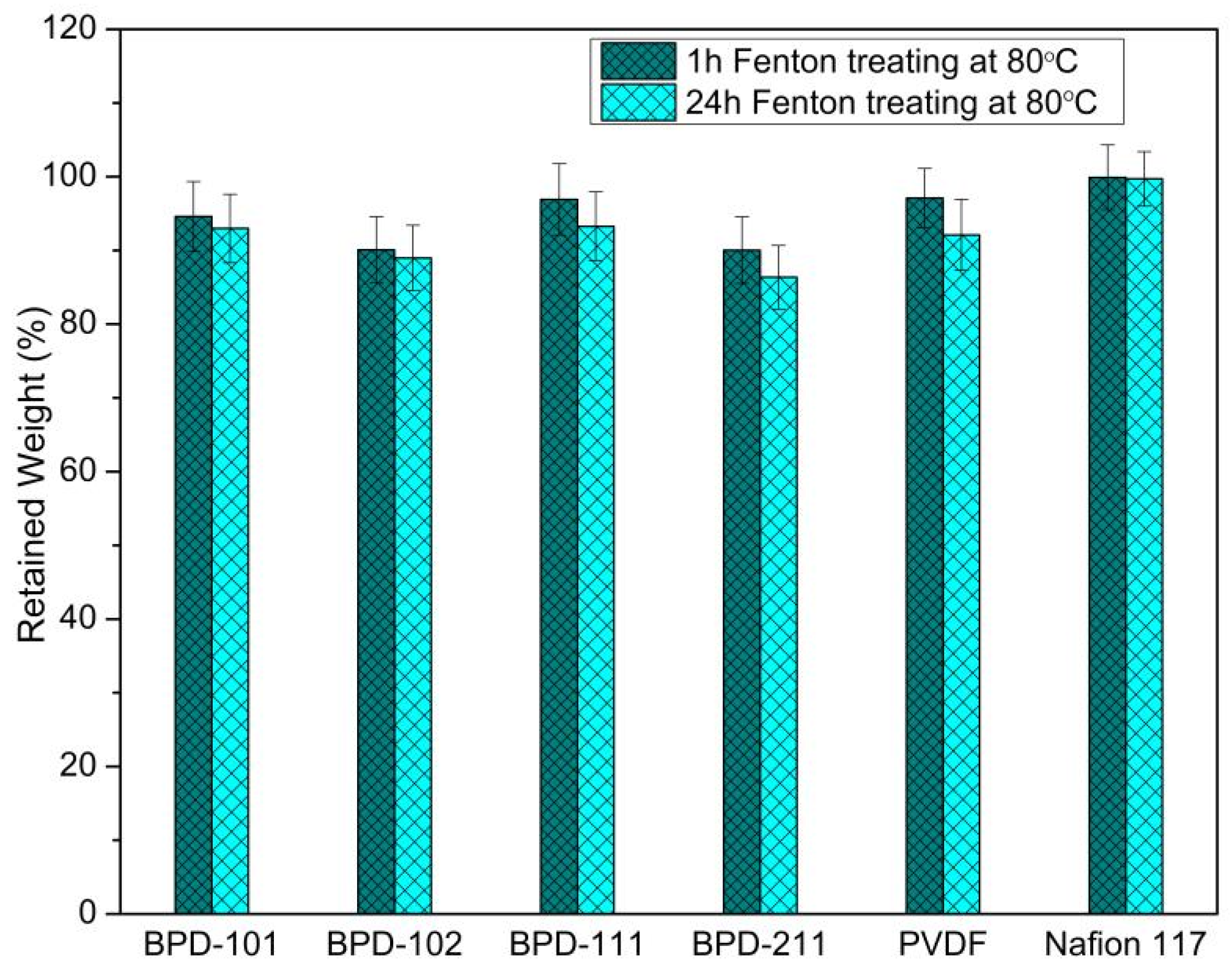
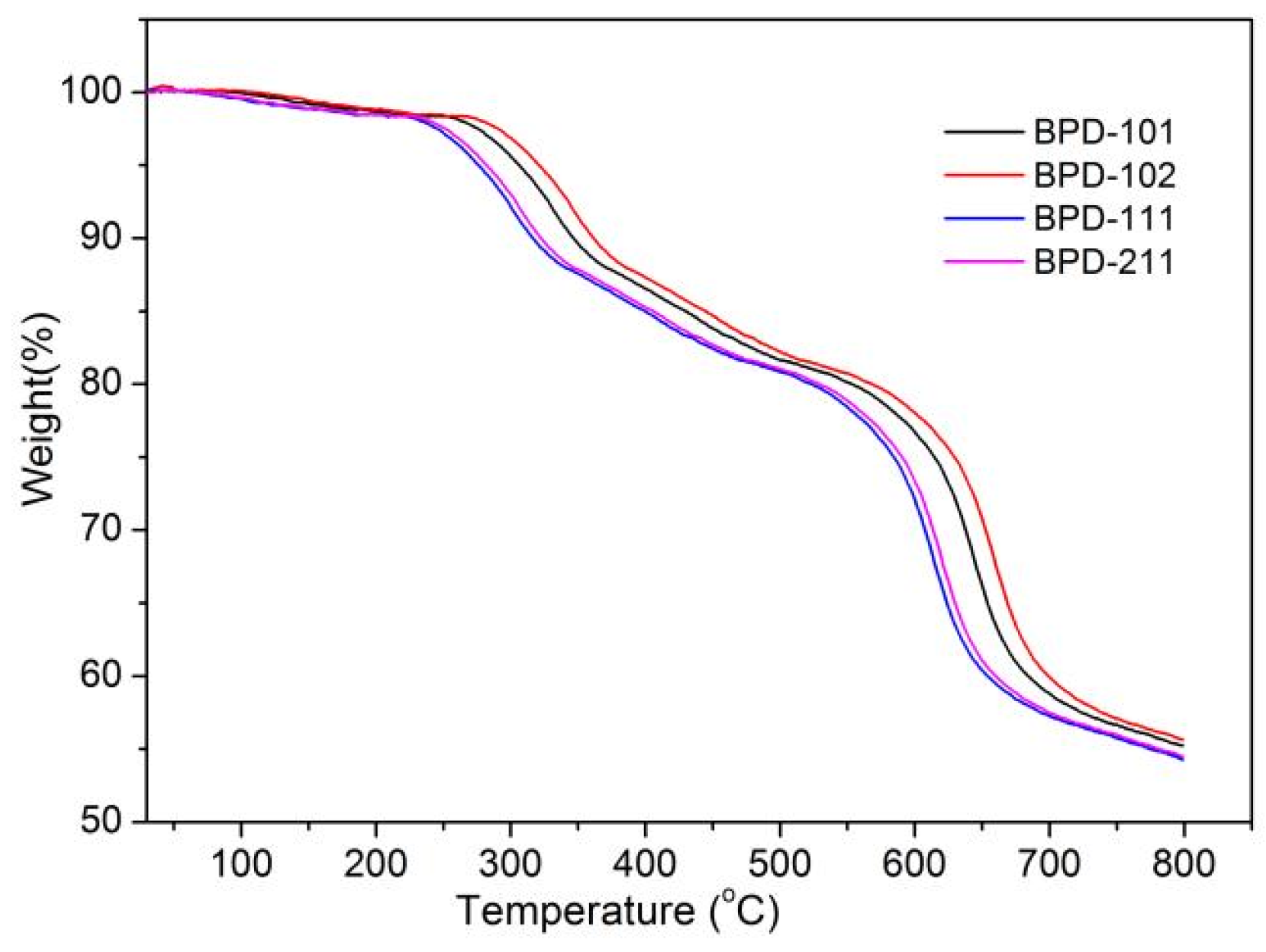
| Polymers | Mw (104 g/mol) | PDI | DS (%) |
|---|---|---|---|
| BPD-101 | 10.21 | 1.04 | 53 |
| BPD-102 | 10.51 | 1.11 | 55 |
| BPD-111 | 11.23 | 1.18 | 30 |
| BPD-211 | 13.32 | 1.24 | 47 |
| Membrane | BPD-101 | BPD-102 | BPD-111 | BPD-211 | Nafion 117 |
|---|---|---|---|---|---|
| Water uptake (%) | 36.4 ± 0.03 | 42.9 ± 0.04 | 10.7 ± 0.04 | 18.6 ± 0.03 | 21 ± 0.05 |
| Volume swelling (%) | 17.3 ± 0.05 | 22.2 ± 0.05 | 8.6 ± 0.04 | 10.5 ± 0.04 | 36.3 ± 0.05 |
| IEC (mmol/g) | 0.29 ± 0.03 | 0.36 ± 0.04 | 0.14 ± 0.03 | 0.25 ± 0.04 | 0.91 ± 0.04 |
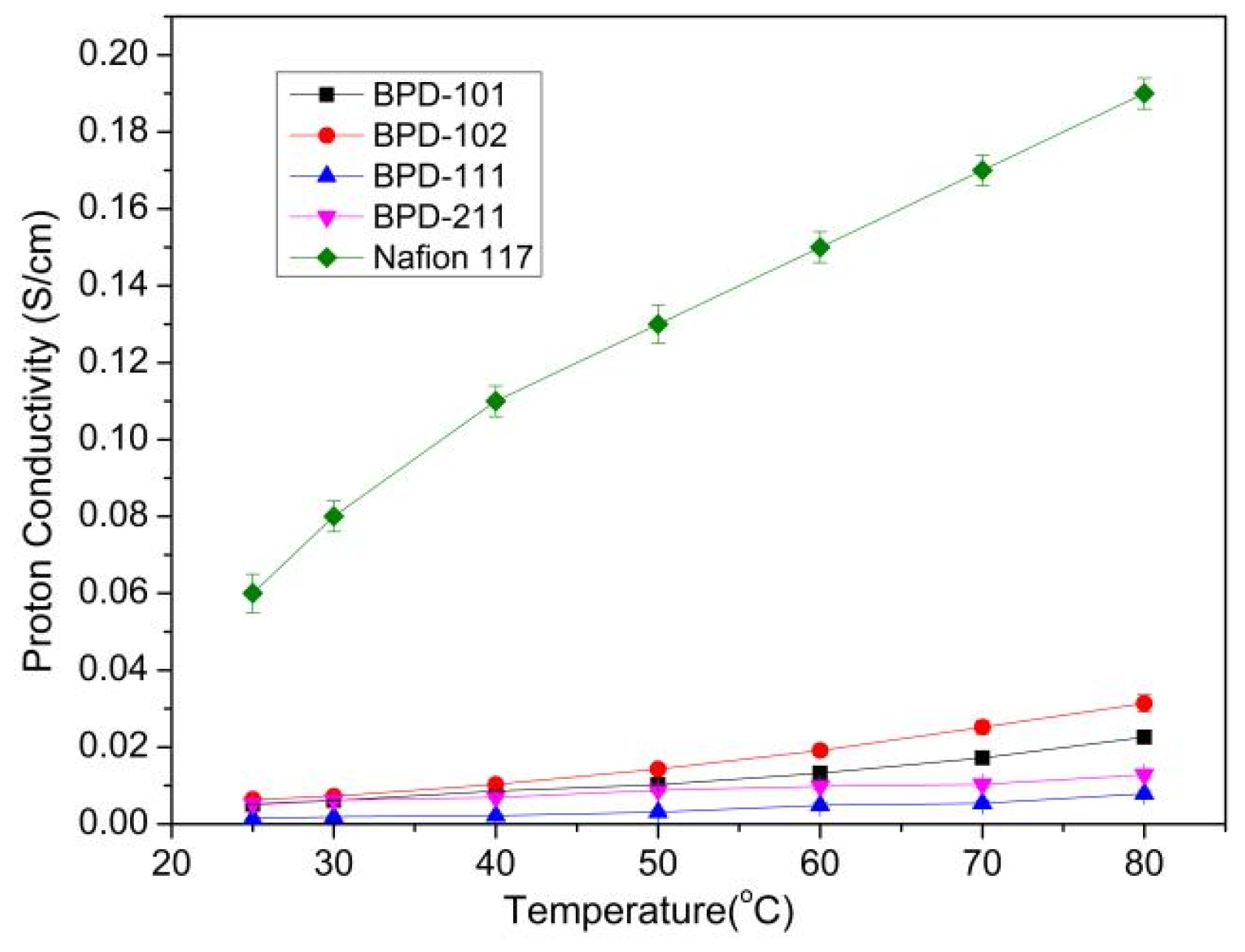
| Membrane | Proton Conductivity at 25 °C/σ (S/cm) | Methanol Permeability/p (×10−7 cm2/s) | Selectivity/s (×103) (S·s/cm3) |
|---|---|---|---|
| BPD-101 | 0.005 | 14.8 | 3.4 |
| BPD-102 | 0.006 | 17.4 | 3.4 |
| BPD-111 | 0.002 | 8.13 | 2.5 |
| BPD-211 | 0.006 | 10.5 | 5.7 |
| Nafion 117 | 0.07 | 60 | 11.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, L.; Song, H.; Gong, X.; Chen, L.; Gong, J.; Chen, Z.; Shen, J.; Gu, M. A High-Methanol-Permeation Resistivity Polyamide-Based Proton Exchange Membrane Fabricated via a Hyperbranching Design. Polymers 2024, 16, 2480. https://doi.org/10.3390/polym16172480
Ma L, Song H, Gong X, Chen L, Gong J, Chen Z, Shen J, Gu M. A High-Methanol-Permeation Resistivity Polyamide-Based Proton Exchange Membrane Fabricated via a Hyperbranching Design. Polymers. 2024; 16(17):2480. https://doi.org/10.3390/polym16172480
Chicago/Turabian StyleMa, Liying, Hongxia Song, Xiaofei Gong, Lu Chen, Jiangning Gong, Zhijiao Chen, Jing Shen, and Manqi Gu. 2024. "A High-Methanol-Permeation Resistivity Polyamide-Based Proton Exchange Membrane Fabricated via a Hyperbranching Design" Polymers 16, no. 17: 2480. https://doi.org/10.3390/polym16172480
APA StyleMa, L., Song, H., Gong, X., Chen, L., Gong, J., Chen, Z., Shen, J., & Gu, M. (2024). A High-Methanol-Permeation Resistivity Polyamide-Based Proton Exchange Membrane Fabricated via a Hyperbranching Design. Polymers, 16(17), 2480. https://doi.org/10.3390/polym16172480





