1. Introduction
Islet allotransplantation is a promising cell therapy for the treatment of type 1 diabetes. To date, islet cells have been successfully transplanted into patients in clinical trials around the world. However, scaling up the islet transplantation technology to the general population of patients with type 1 diabetes remains a major challenge [
1]. The issue is that islet transplantation is limited by the shortage of organ donors, islet death after transplantation, and the need for lifelong immunosuppression to prevent immune rejection [
2]. The need for immunosuppressant use is a major concern as they can cause side effects such as kidney dysfunction, increased susceptibility to infections, and an increased risk of cancer. Additionally, immunosuppressive drugs may also have deleterious effects on the transplanted islets themselves, which in turn may lead to transplant rejection [
3]. Successful islet encapsulation can eliminate immunosuppressant use. The biocompatible, semi-permeable microcapsules allow the diffusion of oxygen, insulin, and nutrients while blocking immune cells. They must selectively permit low-molecular weight compounds and address challenges in engraftment, survival, rejection, and islet homeostasis [
2,
4].
There are three approaches to encapsulating pancreatic islets: macro-, micro-, and nano-encapsulation. Microencapsulation is the preferred approach for pancreatic islet transplantation as such microcapsules provide an optimal surface-to-volume ratio that aids the fast exchange of nutrients and hormones [
5]. The most common approach to the microencapsulation of pancreatic islets is the use of microcapsules based on polymer-coated alginate [
6]. The most frequently used polymers are poly-L-lysine (PLL) and poly-L-ornithine (PLO) [
7]. Although these capsules have been extensively studied, they do not meet the parameters for biocompatibility. The use of such capsules causes an inflammatory reaction to the transplant [
8,
9]. A range of other alginate-based systems have been explored, including ultra-high-viscosity alginate, Alg-cellulose sulfate)-poly(methylene-coguanidine), Alg-chitosan, Alg-PLL-poly(acrylic acid), Algpoly(L-ornithine)-Alg, Alg-PLL-poly(ethylene glycol), Algchitosan-poly(ethyleneglycol), and Alg-PLL-poly(ethyleneglycol)-Alg. Problems still facing the survival of encapsulated islets include immune rejection, death due to lack of nutrients, post-transplantation hypoxia, and loss of microcapsule stability [
10]. Survival after transplantation can be increased by improving the encapsulation technologies and through searches for new biocompatible materials and their combinations [
11]. Such microcapsules should have good biocompatibility, stable structural and mechanical properties, and be selectively permeable [
12].
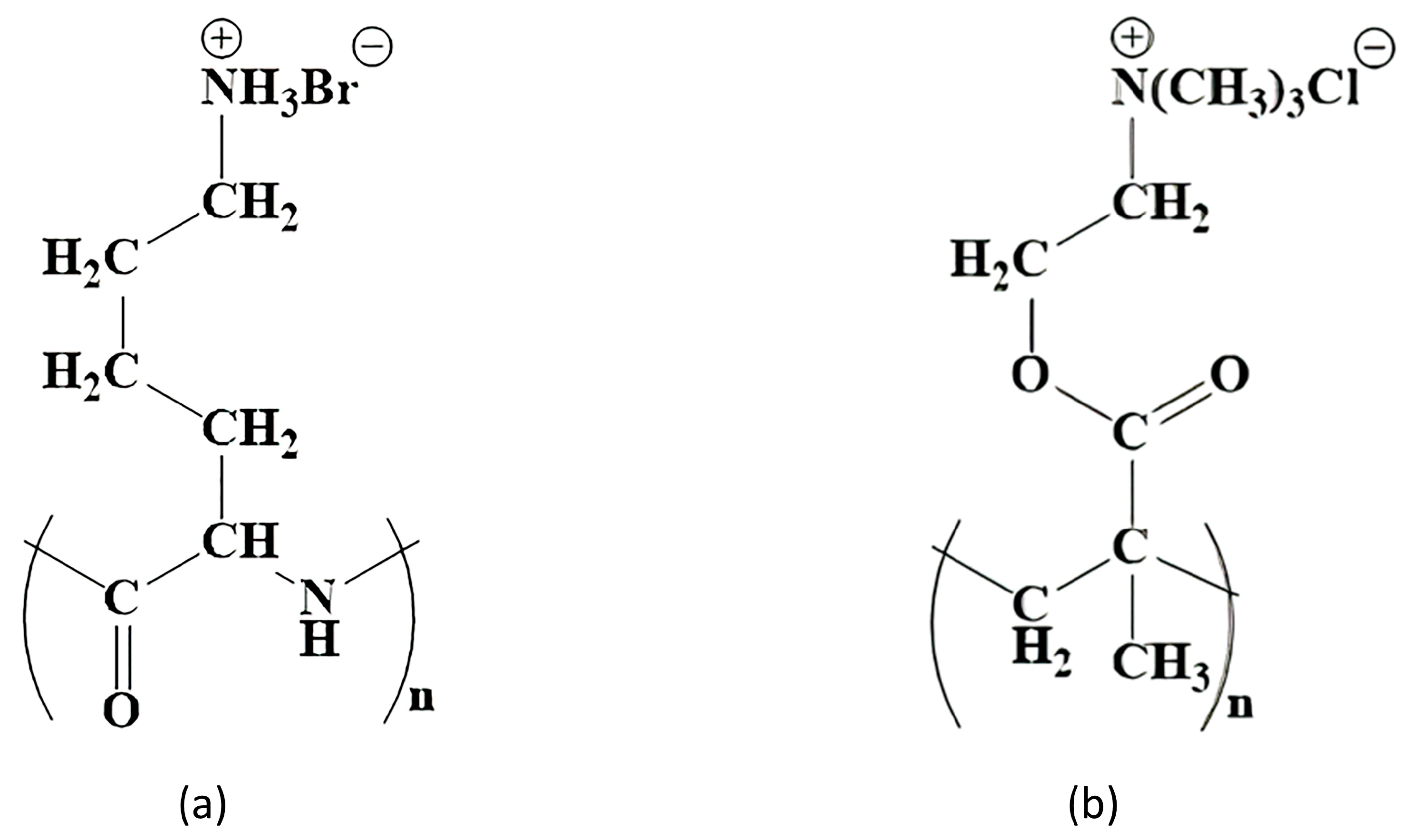
Potential polymer candidates for this include poly [2-(methacryloyloxy)ethyl]trimethylammonium chloride (PMETAC). PMETAC has a similar structure to PLL and is cationic, which is a necessary condition for the formation of the layer on the surface of the alginate microcapsule. Both polymers have fragments of the same length between the polymer chain and the nitrogen atom (
Figure 1).
In addition to there being an effective synthetic route and its sound physico-chemical characterization, the safety of PMETAC should be considered. The absence of a cytotoxic effect of PMETAC has already been evaluated using three different cell-based assays (MTT, Neutral Red, and LIVE/DEAD
®) and relevant immune cells, including two mouse cell lines (J774A.1 and BV2), as well as human peripheral blood mononuclear cells (PBMCs) [
13]. The use of PMETAC as a biocompatible material has also been proposed previously. It is known that modification of the surface of poly(etheretherketone) with PMETAC leads to a significant improvement in blood compatibility and reduces pericapsular fouling around the polymer implant [
14]. Furthermore, the inclusion of this polymer in a complex with poly(2-hydroxyethyl methacrylate) (HEMA) and fibroblast growth factor (bFGF) has been found to promote the regeneration of nervous tissue and functional recovery after spinal cord injury in rats [
15]. In another study, recipient rat axons, astrocytes, and blood vessels grew into an implant based on the NEMA–PMETAC complex, together with neural progenitor cells (iPSC-NPs), when transplanted into an area of spinal cord injury. This polymeric complex integrated into the injured spinal cord, reduced cavity formation, and maintained iPSC-NP cell survival [
16]. PMETAC has also been found to have improved biocompatibility and is a promising coating for medical devices [
17]. Previously, when PMETAC was used as a copolymer in the creation of alginate microcapsules for the encapsulation of C2C12 mouse cells, it was shown that the permeability of the obtained microcapsules had a cutoff at 70 kDa, potentially making these suitable for the encapsulation of islets. Shen et al., 2009 have described the possible formation of a polyelectrolyte complex between PMETAC and negatively charged proteins (e.g., albumin). Fortunately, the relatively simple measure of replacing the regular medium with a serum-free medium for incubation after fabrication eliminated any host reactions [
18]. We took this experience into account and synthesized a new type of capsule that has not been used before. Moreover, the PMETAC polymer has not previously been used to encapsulate the islets of Langerhans to compensate for insulin deficiency.
Thus, we consider PMETAC to represent a simple and stable alternative to poly-L-lysine and propose it as a potential polymer coating for microcapsules for pancreatic islet encapsulation. Such microcapsules could provide higher biocompatibility and graft stability. Moreover, we believe that the new capsule based on alginate and PMETAC has the potential for widespread use in clinical practice to compensate for insulin deficiency conditions. Our work is the first time that a comprehensive analysis has been conducted into the stability (osmotic stability, thermal stability, and stability under culture conditions) and cytotoxicity of a new microencapsulating system based on alginate-PMETAC-alginate for potential future use in islet immunoisolation.
2. Materials and Methods
2.1. Animals
The pancreatic source material was obtained from 12–15-week-old Wistar rats. All our studies with experimental animals were approved by the local ethics committee of the Privolzhsky Research Medical University (protocol No. 10; date: 26 June 2020).
2.2. Islet Isolation
Perfusion of the pancreas was performed using a solution of collagenase V (Collagenase from Clostridium histolyticum, Sigma, Saint Louis, MI, USA) in a modified Hanks solution enriched with CaCl2. Then, the perfused pancreas was shaken on a shaker preheated to 37 °C for 11–15 min. During digestion, the pancreatic tissue gradually softened until the whole organ dispersed into small granules. The digestion was stopped by adding HBSS supplemented with 5% BSA. Purification of the OLs from the exocrine tissue was carried out by triple filtration through a metal sieve with a mesh diameter of 0.5 mm. Any remaining undigested tissue was then discarded. The suspension was centrifuged at 200× g for 3 min and the supernatant was removed. The pellet was then centrifuged on density gradients of Ficoll DL-400 (1.095, 1.084 and 1.072, 1.048 mg/L) (Sigma, Saint Louis, MI, USA) at 800× g for 15 min. After density gradient centrifugation, the islets were washed with MHBS. The isolated OLs were maintained in RPMI (Gibco, London, UK) culture medium with a low glucose level supplemented with L-glutamine (0.58 mg/mL) (PanEco, Moscow, Russia), 10% FBS (Gibco, London, UK), and antibiotic-antimycotic (Antibiotic-Antimycotic 100X (ThermoScientific, Waltham, MA, USA)) at 37 °C and 5% CO2. During isolation, dithizone staining was performed to identify the islet cells. Staining was visualized using a Leica DM2500 microscope (Leica, Berlin, Germany).
2.3. Polymer Synthesis
Poly [2-(methacryloyloxy)ethyl]trimethylammonium chloride (PMETAC) was synthesized by polymerization from [2-(methacryloyloxy)ethyl]trimethylammonium chloride (Aldrich, 408107, Saint Louis, MI, USA) according to the procedure described in [
19].
The IR spectrum of the polymer was recorded using an FT 801 IR Fourier spectrometer with an ATR attachment (OOO NPR Simeks, Novosibirsk, Russia). The polymer was placed on the diamond substrate of the ATR attachment and compressed with the built-in press. Determination of the molecular weight distribution of the polymer samples was carried out by gel permeation chromatography using a high-performance liquid chromatograph, the LC-20AD (Shimadzu, Kyoto, Japan). Analysis conditions: eluent 0.5 N acetic acid solution, flow rate 0.8 mL/min, Т = 30 °С, an ELSD detector (low-temperature evaporative light scattering detector). Column: TSK-GEL G3000SWXL, 7.8 mm ID × 30.0 cm L, 5 µm.
2.4. Microcapsule Synthesis
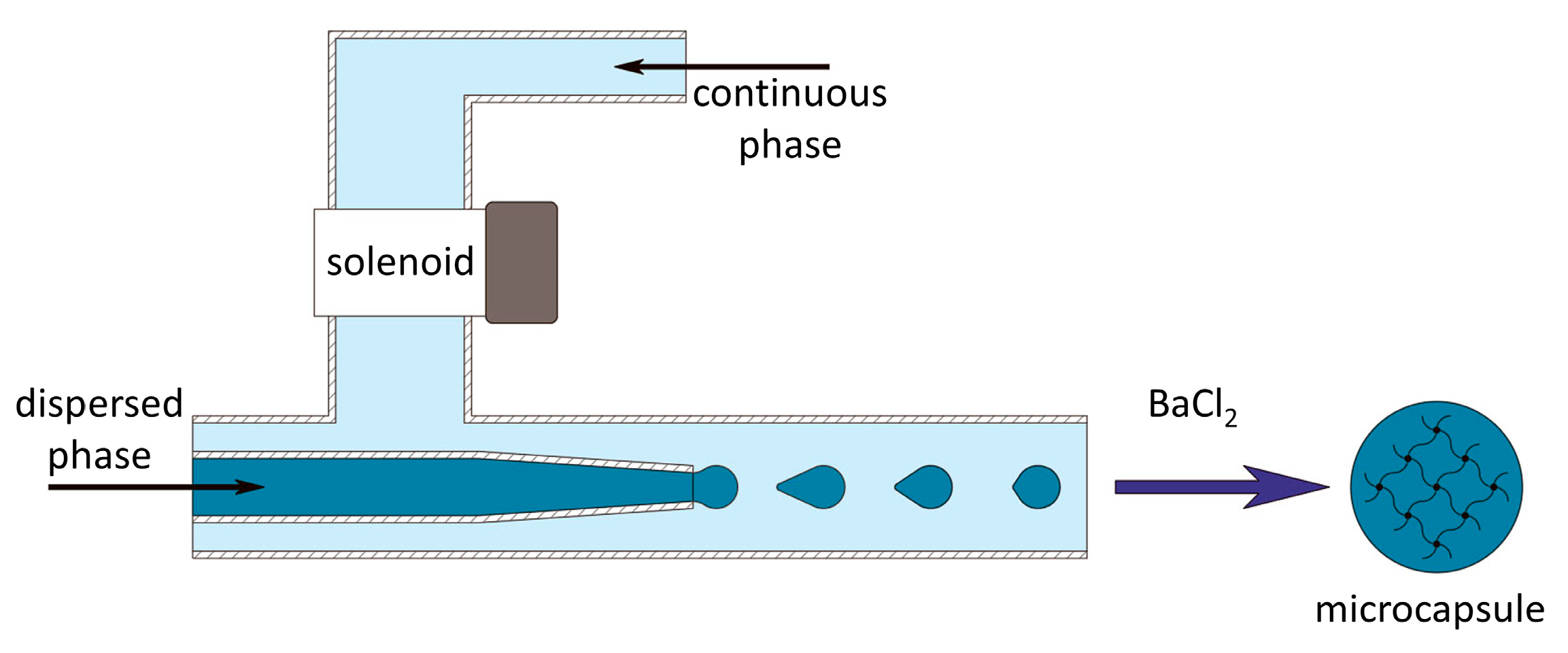
To conduct research on the development of the alginate microcapsules, a microfluidic installation (microfluidic device) was developed for the controlled formation of microdroplets, using an external influence on the flow of the continuous phase (
Figure 1). The microfluidic setup is protected by know-how order No. 249/Akhd (dated 09/02/2022). Prior to the experiment, the microfluidic device was cleaned with alcohol and UV-sterilized for 15 min, and all solutions for use were aseptically processed. When forming microcapsules that did not contain pancreatic islets, a low-viscosity solution of sodium alginate («Aldrich», A1112, Saint Louis, MI, USA) (2 wt.%) and dextran of molecular weight 20,000 Da (АО «Vector», Novosibirsk, Russia) (15 wt.%) in phosphate-buffered saline was used as the dispersed phase (material for future microcapsules). During the formation of the microcapsules containing pancreatic islets, the concentration of sodium alginate and dextran was 2.7 and 20 wt.%, respectively. Prior to encapsulation, a suspension comprising 1 part pancreatic islets in a nutrient medium was combined with 3 parts of the alginate composition, resulting in a volumetric ratio of 1:3. The concentration of islets in the final suspension for encapsulation was 15 thousand/mL. To visualize the process of microcapsule formation, the dispersed phase was stained with methylene blue (Khimreaktiv, Russia). A solution of polyethylene glycol of molecular weight 8000 Da (PEG-8000) (Nizhnekamskneftekhim, Russia) (30 wt.%) in distilled water was used as the continuous phase.
The resulting microcapsules were placed in a chloride gelling solution of BaCl2·2Н2O (Khimreaktiv, Russia) (2.9 wt.%). Then, the gelled hydrogel microcapsules were washed with a buffer solution based on Tris (Aldrich, Saint Louis, MI, USA) (0.45 wt. %), pH = 7.2. After that, to increase the stability and to ensure the desired permeability, the microcapsules were incubated in a solution of poly [2-(methacryloyloxy)ethyl]trimethylammonium chloride in buffer solution for 10 min. Next, the microcapsules were again washed in buffer solution. Then, to increase the biocompatibility of the microcapsules, an additional alginate shell was applied to the surface of the polymer membrane by incubating them in a 0.2 wt.% solution of sodium alginate in physiological saline. Finally, the microcapsules were washed in buffer solution and placed in Hank’s solution (HBSS) (PanEko, Moscow, Russia).
2.5. Permeability Measurements
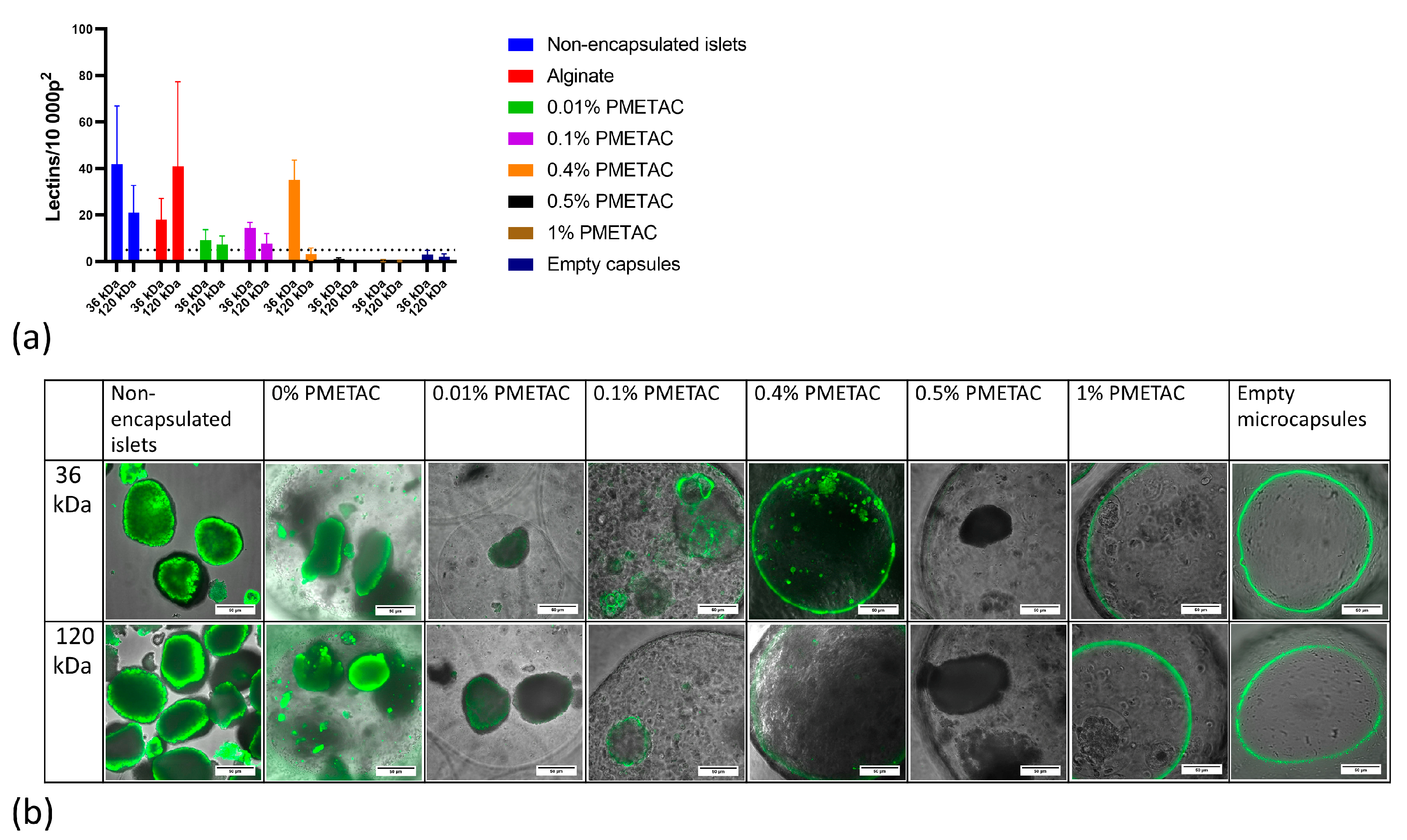
We evaluated the effectiveness of the сapsules at preventing penetration by macromolecules while permitting that of insulin, glucose, and other low-molecular weight molecules. Effective immunoisolation requires that the hydrogel layer excludes immunoglobulin G (IgG) and the complement molecule C3—both of which mediate the recognition of foreign cells by the immune system. These molecules are approximately 180 and 185 kDa in size, respectively.
The permeation test was carried out at 4 °C following established protocols [
20,
21,
22]. For characterization of their permeability, different microcapsules were incubated with FITC-coupled lectins of a range of molecular weights. Lectins bind to carbohydrates found on the surfaces of islet cells. The microcapsules were incubated for 48 h at 4 °C in 0.5 mL of the following lectin solution: 5 µL (10 mg/µL) FITC-Triticum Vulgare (WGA, molecular weight 36 kDa), 14 µL (2 mg/µL) FITC-Maackia amurensis I (MAL-I, molecular weight 75 kDa), 5 µL (10 mg/µL) FITC Ricinus communis (RCA-I, molecular weight 120 kDa), and FITC-Sambuca nigra (SNA, molecular weight 150 kDa). After incubation, the microcapsules were washed 3 times with HBSS solution and examined microscopically. Image analysis was performed with ImageJ 1.43u. The number of fluorescent lectins that had penetrated the microcapsules was counted over an area of 10,000 pixels
2 in the brightest part of the image.
2.6. Evaluation of Osmotic Stability
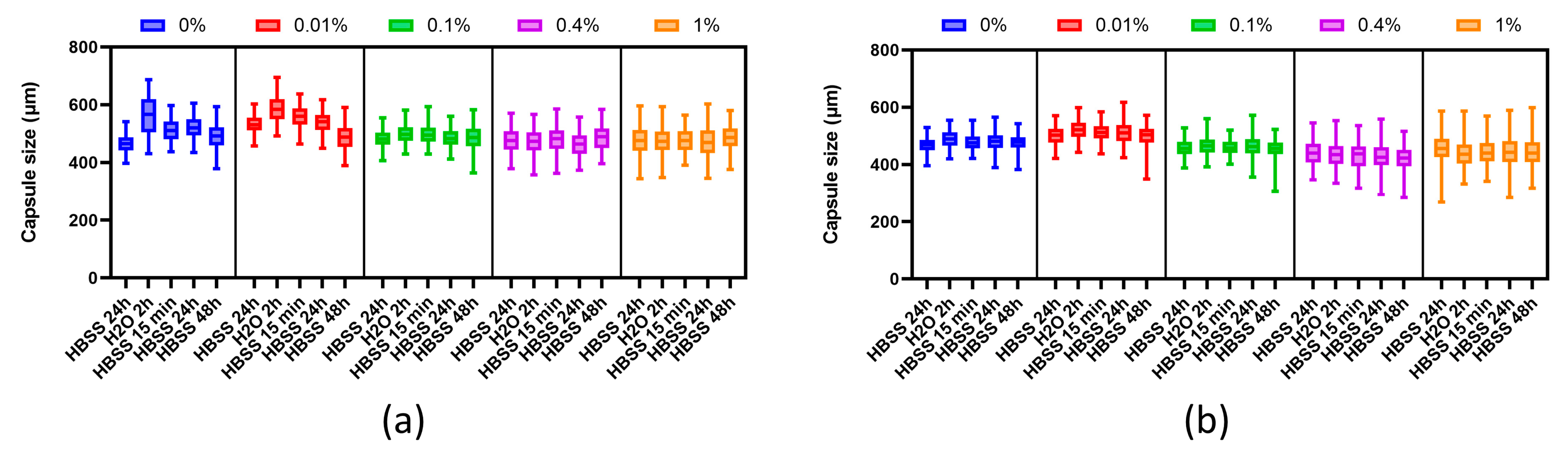
The resistance of the microcapsules to deformation and their elastic properties were determined using an osmotic pressure test. The test was performed according to the protocol described in Verheyen 2019. In this test, the microcapsules were subjected to sequential exposure to H2O (2 h, 0 mOsm) and HBSS++ (15 min, 24 h, and 72 h, 270–305 mOsm). Microcapsule resistance to swelling was defined by the maximum deformation attained at the end of the osmotic stress period. Microcapsule elasticity was determined by the diameter recovery achieved after the stress was removed. The tests were carried out at 2 and 7 days after manufacture.
2.7. Evaluation of Thermal Stability
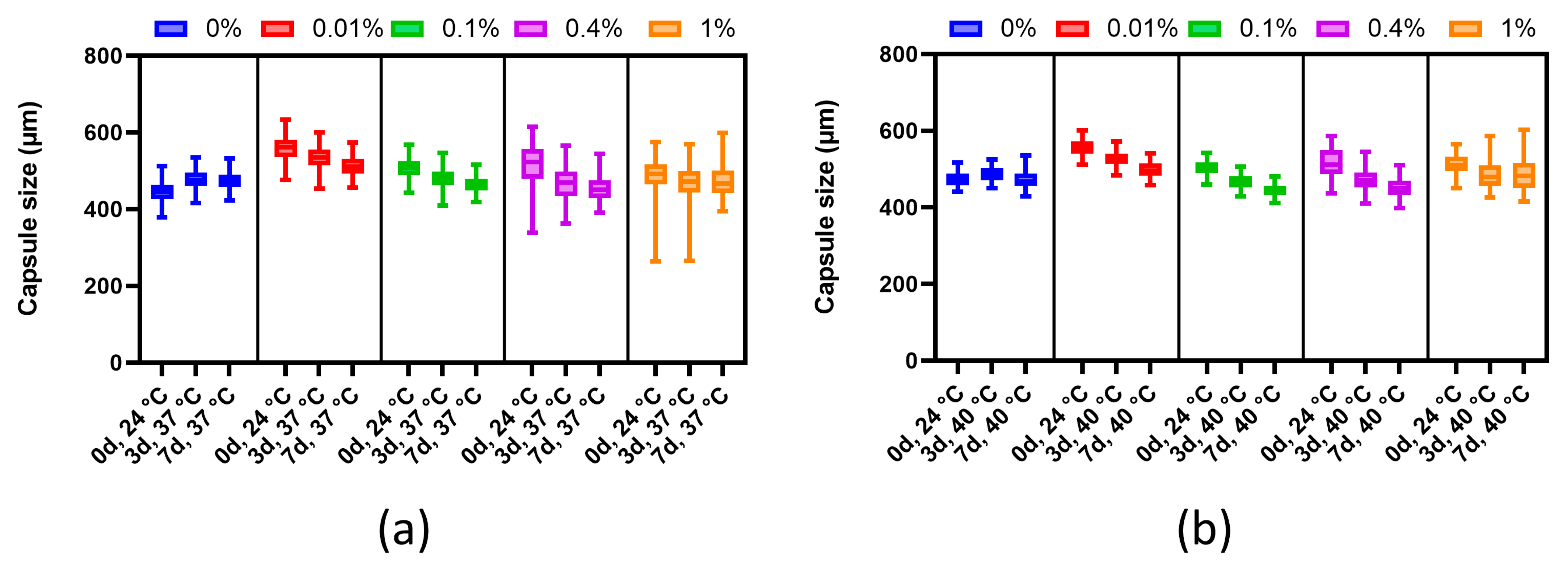
Before testing, the diameters of each group of microcapsules in the HBSS solution were measured. Next, the microcapsules were placed under temperature conditions (37 °C and 40 °C). To study the dynamics of changes, measurements of microcapsule sizes were carried out after 3 and 7 days. The HBBS solution was replaced every day.
2.8. Stability of Microcapsules under Culture Conditions
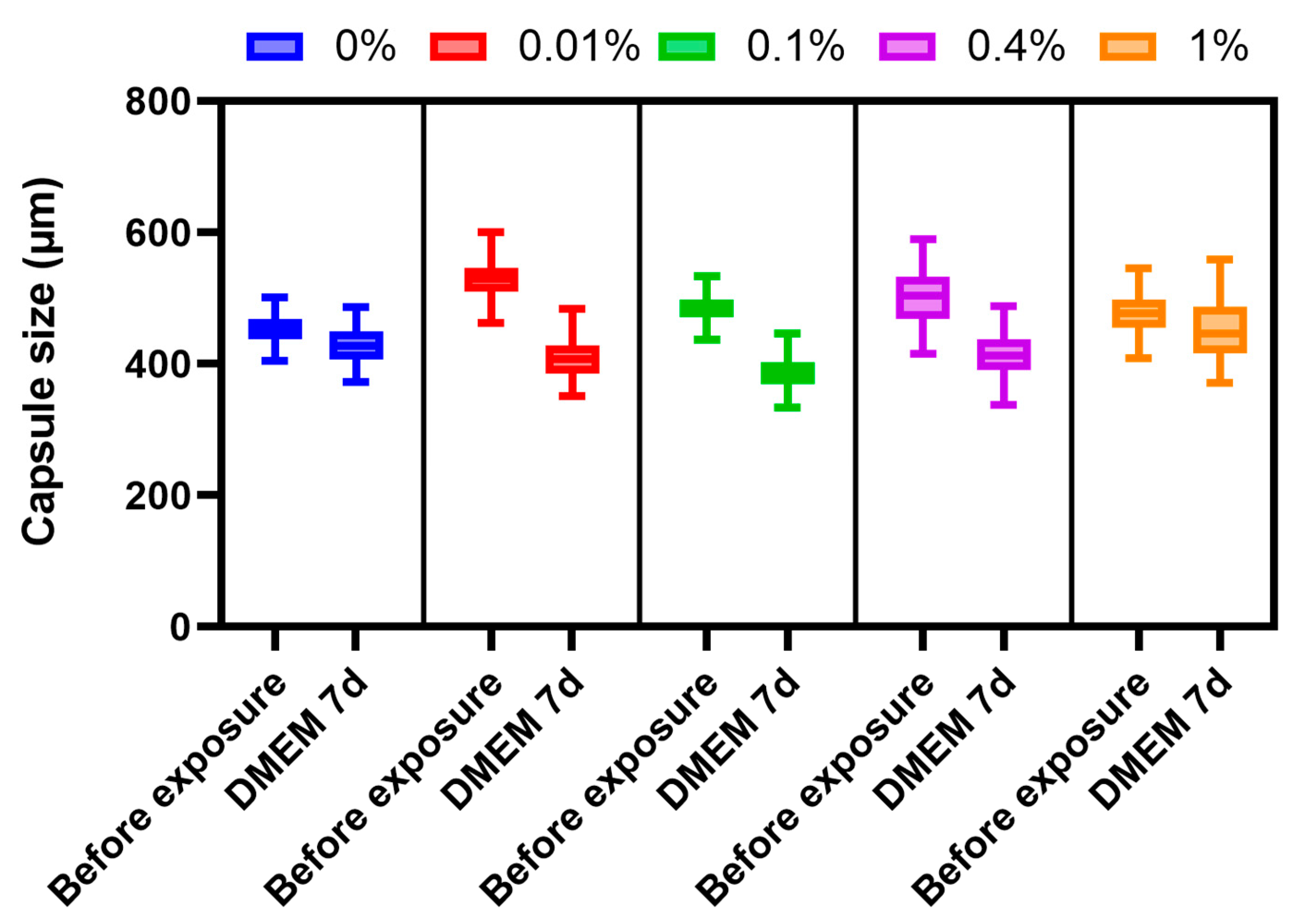
To study the stability of the microcapsules under cell culture conditions, they were placed in an RPMI culture medium containing 10% fetal bovine serum (FBS) and an antimycotic antibiotic at 37 °C in a 5% CO2 atmosphere. Microcapsule sizes were measured before the start of the experiment and after 7 days.
2.9. Stability of Microcapsules under Exposure to Saline
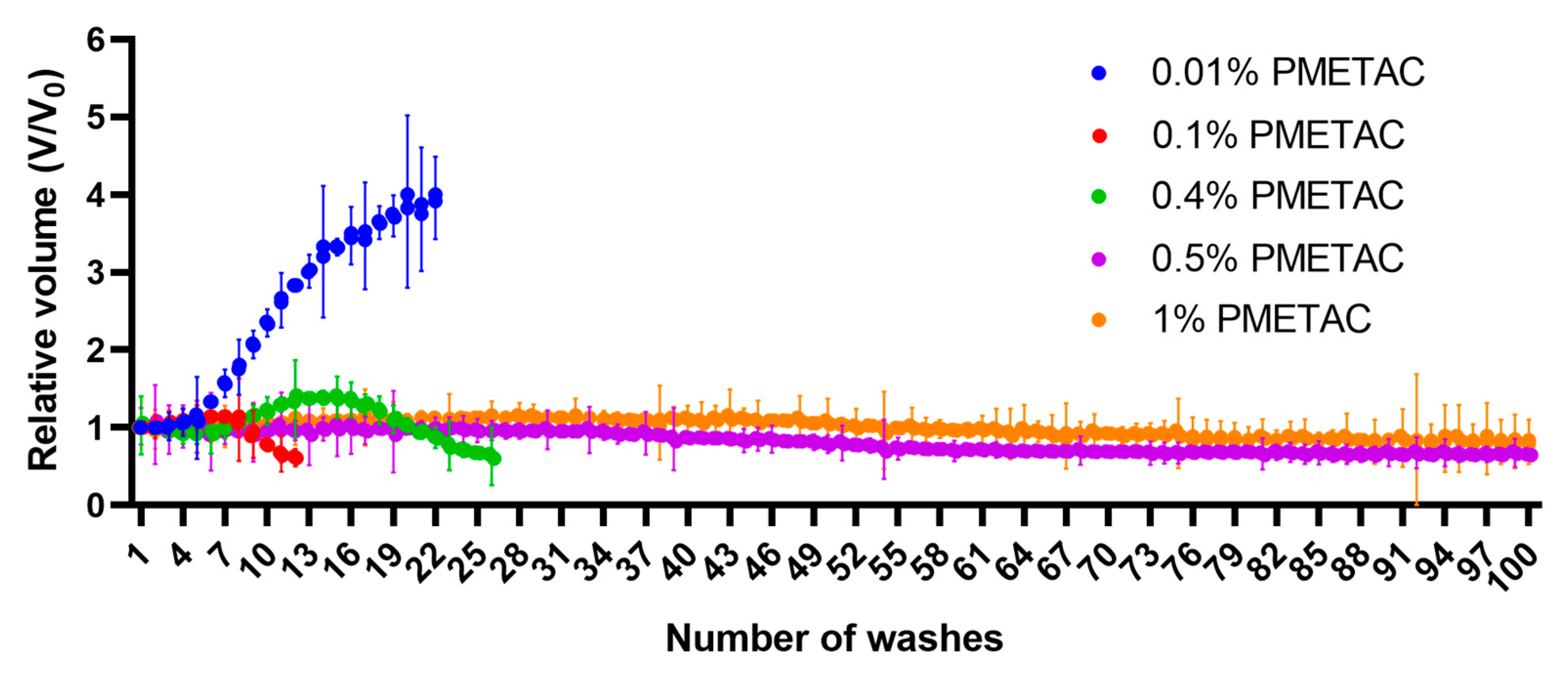
Assessment of the stability of the alginate microcapsules was carried out by repeated washing with saline solution in a 2 mL syringe. The initial number of microcapsules used was 4500. The microcapsules were deposited, then the volume they occupied was recorded, after which excess fluid was removed. After that, physiological saline was drawn into the syringe to a volume of 2 mL. The microcapsules in the resulting solution were mixed and kept for 5 min, then the process was repeated. Saline solution was used for this test since it contains sodium ions that can replace the cross-linking ions of the alginate microcapsule.
2.10. Viability and Functional Activity of Encapsulated Islets
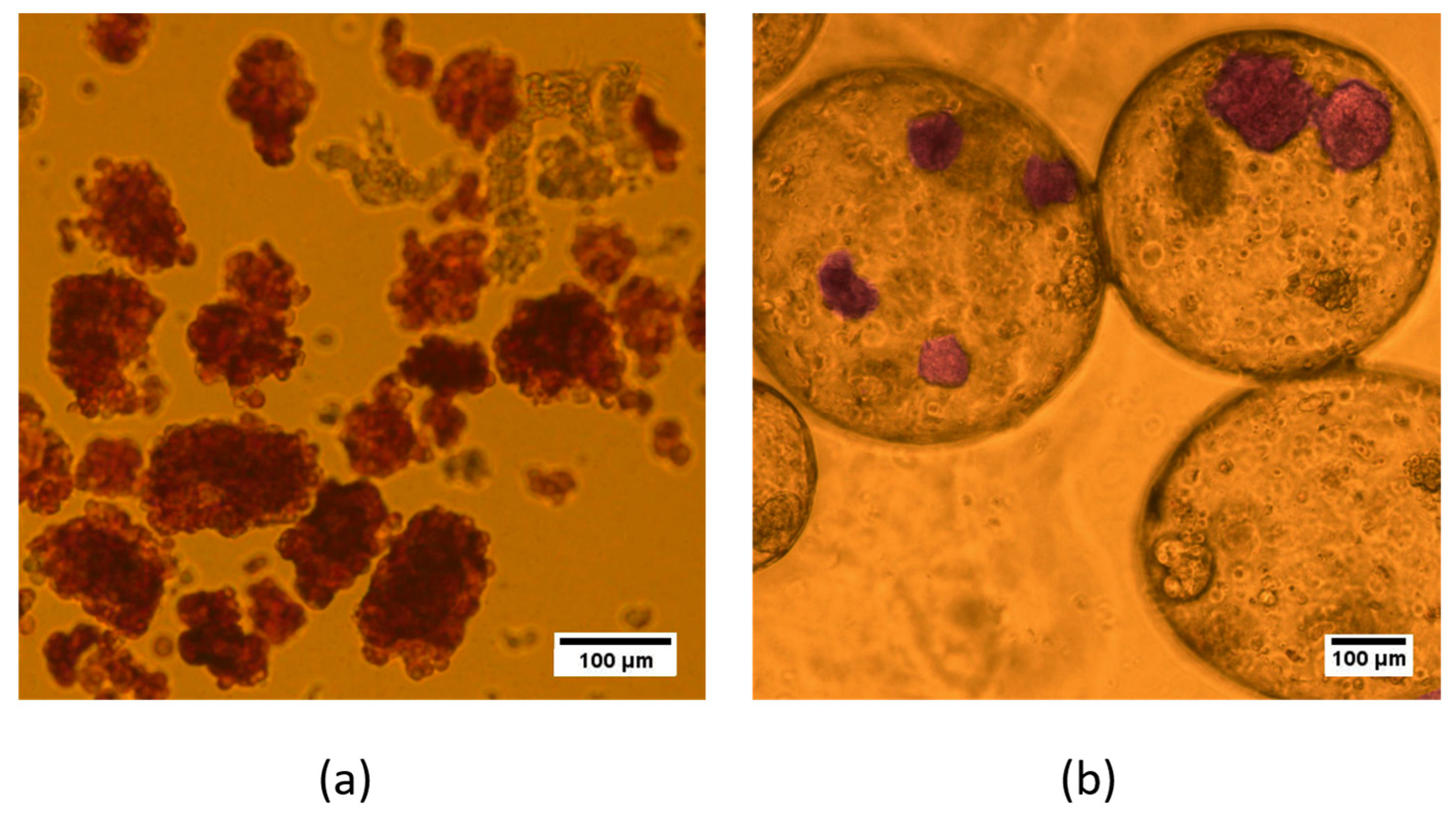
To identify the morphology and insulin synthesis of islets before and after encapsulation, we used dithizone staining (DTZ).
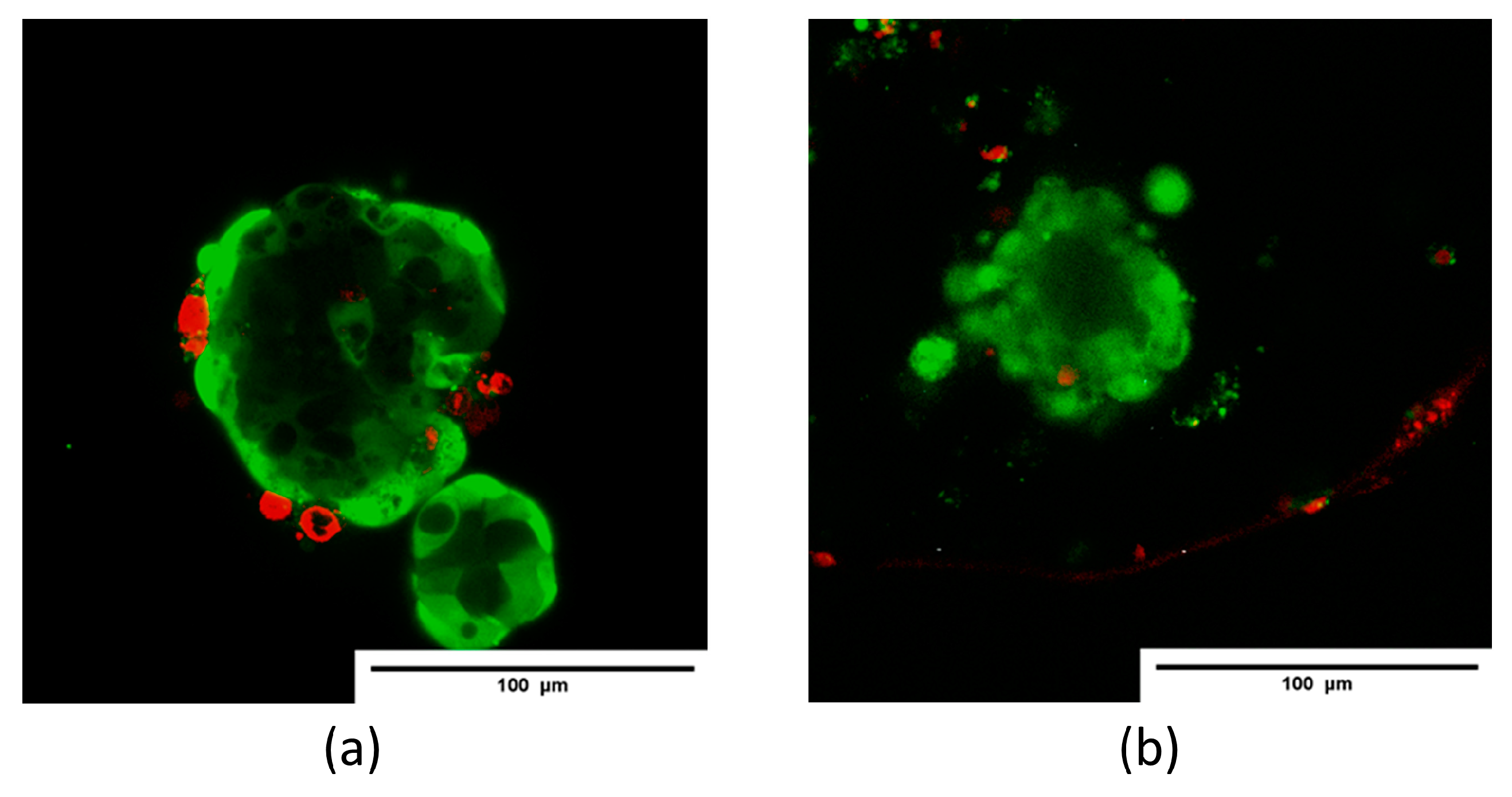
The survival or death of isolated islet cells was assessed using the Live/Dead Cell Double Staining Kit (Sigma-Aldrich, Saint Louis, MI, USA) at 24 h after isolation and after encapsulation, according to the standard protocol. A 500 μL sample of an islet suspension was incubated in a mixture of 2 μL calcein-AM (live cell, green) and 1 μL ethidium homodimer-1 (dead cell, red) for 15 min at room temperature. Visualization was performed on an LSM 880 confocal fluorescence microscope (Carl Zeiss, Mainz, Germany). For calcium-AM detection, fluorescence excitation at 488 nm was used, with detection in the range of 500–549 nm; propidium iodide fluorescence was excited at 543 nm, with detection in the range of 611–700 nm. The ratio of the green-colored area to the total cell area (%) was calculated from the confocal images of the aggregates in 3–5 randomly selected fields per sample using ImageJ software.
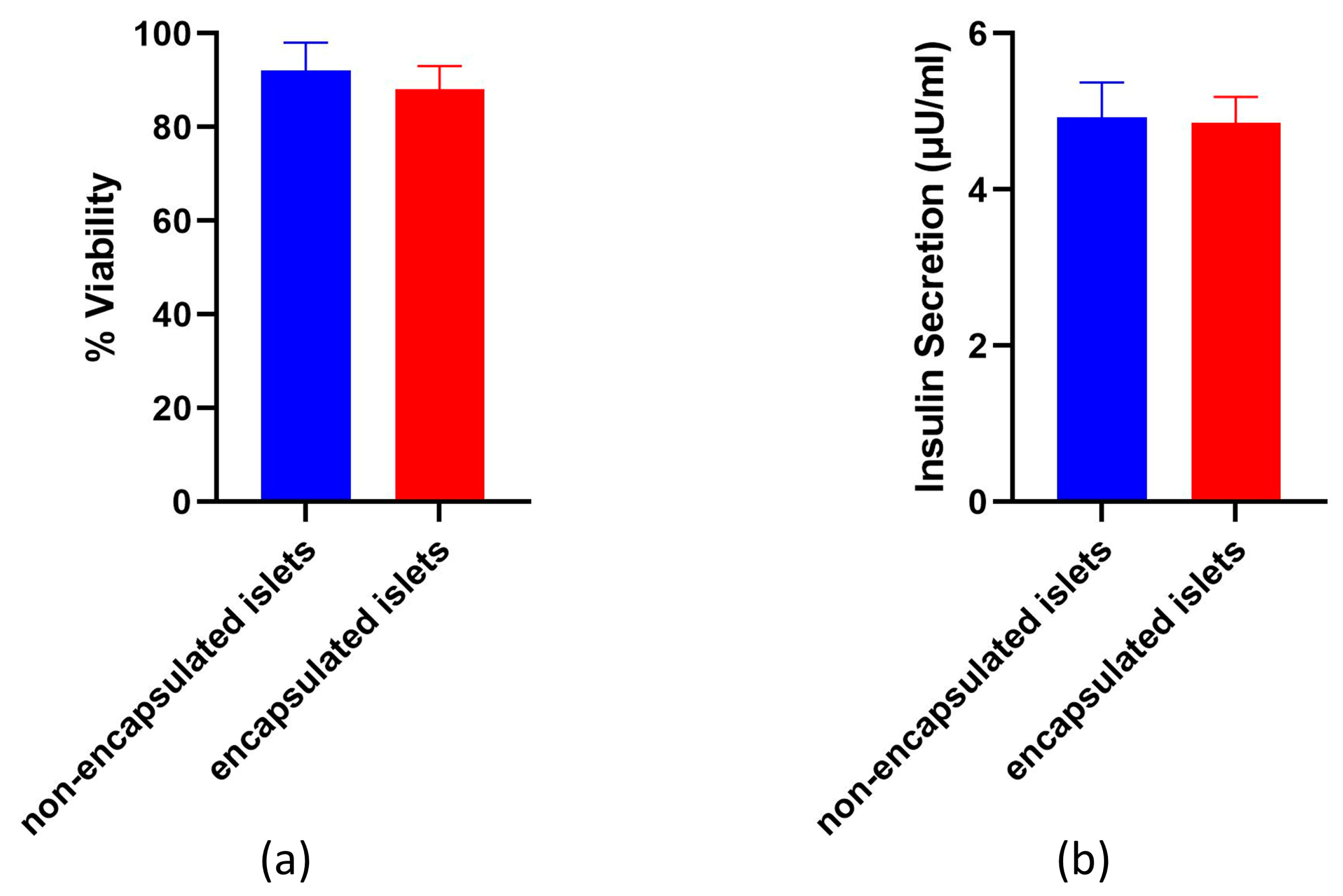
For functional activity studies, samples containing 350 islets/mL each were incubated at 37 °C and 5% CO2 for 24 h in cultural medium in standard conditions. The supernatant was then collected and stored at −20 °C for analysis. The concentration of the insulin released during incubation was detected using an insulin enzyme-linked immunosorbent assay (Cloud-clonecorp, Houston, TX, USA). Absorbance was measured using a Microplate reader with a 450 nm wavelength filter and expressed as (μg/mL). An assay was performed on non-encapsulated islets to serve as a control for the assay on the encapsulated islets.
2.11. Statistical Analysis
The permeability studies were performed in 2 independent tests, each of which analyzed at least 10 images. The stability studies involved 3 independent tests, each divided into 3 technical replicates, with 50 microcapsules from each repetition being analyzed; thus, the total number of microcapsules analyzed for each time point and each microcapsule variant was 450. The data are plotted as the medians ± the 25th and 75th quartiles. The distribution of all the data was first checked for normality using the Kolmogorov–Smirnov and Shapiro–Wilk tests. Statistical analysis of islet cell data was performed using the Mann–Whitney U test.
4. Discussion
In this study, we developed and tested a new microcapsule for islet encapsulation to address insulin deficiency. Our approach is innovative in two key ways. First, we applied the polymer PMETAC, which has not been previously used specifically for encapsulating islets of Langerhans. Second, unlike previous methods where PMETAC was combined with copolymers, we utilized PMETAC independently, creating a simpler and more efficient microcapsule.
By combining alginate with PMETAC, we optimized the capsule’s composition and preparation process. PMETAC’s non-cytotoxicity and excellent biocompatibility make it an ideal alternative to traditional anionic coatings. This simplified approach not only reduces production costs but also enhances the microcapsules’ potential for broader clinical application.
Alginate microcapsules coated using various concentrations of PMETAC have been tested for their permeability, stability, and cytotoxicity. The best result was shown by alginate microcapsules coated with 0.4% PMETAC. The islets remained viable and functionally active. Microcapsules coated using 0.4% polymer have the necessary permeability, are resistant to deformation by osmotic pressure, are thermally stable, change in volume by less than 15% when incubated in culture medium, and do not collapse when washed with physiological media.
The core of the microcapsule we worked with was alginate. Alginate is one of the well-studied natural polysaccharides that are most commonly used for the encapsulation of biological objects. Alginate hydrogels exhibit good biocompatibility and are often used to create shells around pancreatic islets of Langerhans [
32]. However, in other studies, alginate microcapsules have shown only limited success where their long-term survival is needed. Alginate microcapsules are unstable and rapidly biodegrade [
36] and are also sensitive to chelating agents, such as phosphate and citrate, and gel-degrading agents such as sodium and magnesium ions. In physiological solution, such replacement of ions results in osmotic swelling of the microcapsules, inevitably leading to increased pore size, destabilization, and rupture of the gel. Thus, the high porosity of the alginate network has promoted the development of different coating techniques [
37]. In our studies, we found that uncoated alginate microcapsules were also unstable and permeable to high-molecular weight compounds, which limited their effectiveness.
Traditionally, poly-L-lysine (PLL) and poly-L-ornithine (PLO) have been used to strengthen the surfaces of alginate microcapsules. These cationic polymers can form a shell around such microcapsules due to ionic bonding between unbound calcium/barium and the carboxylate alginate anions. Although both capsule types demonstrated the required permeability, it was found that PLO capsules were much more stable and swelled less in saline than PLL capsules [
7]. Our PMETAC-coated capsules demonstrated a similar improved stability compared to PLL capsules, similar to the stability observed with PLO capsules. In the next step, a layer of alginate is then deposited on the PLL or PLO layer. The outer alginate shell seals the cationic PLL from the tissues of the microcapsule recipient and thus makes the microcapsules biocompatible. Although microcapsules with this type of structure meet many of the requirements for cell immunoisolation, they have insufficient strength when implanted in large animals [
38].
Recent work has demonstrated the value of PMETAC for biocompatible implants. The application of PMETAC forms coatings that can be used successfully with biomaterials [
39]. Initially, PMETAC was used as a copolymer in the creation of alginate microcapsules for cell culture encapsulation [
18,
40]. The resulting microcapsules provided sufficient permeability and indicated that this polymer may be suitable for encapsulating the islet apparatus.
One of the most important parameters of microcapsules used for cell immunoisolation is their selective permeability. While the microcapsule must protect cells from destruction by the immune system, it must simultaneously permit the permeation of oxygen and biological compounds required for the viability and normal functioning of the cells inside. The final permeability of the microcapsules can depend on the conditions for their synthesis, the molecular weight of the components of the microcapsules, the duration of coating of the alginate with PMETAC, and its concentration in the coating solution [
41]. For polymers such as poly-L-lysine and poly-L-ornithine, a concentration of 0.05% is considered sufficient to create immunoisolation of the content of the microcapsules [
42]. In this work, we have shown the dependence of coating formation on PMETAC concentration and the molecular weight permeability cut-off of PMETAC-coated alginate microcapsules. We have created microcapsules that ensure the efficient diffusion of insulin molecules and prevent the diffusion of larger molecules. After being exposed to PMETAC polymers at a concentration of 0.4% for 10 min, alginate microcapsules develop the required permeability. We have proven that our microcapsules do not allow molecules larger than 120 kDa to pass, which is in line with other studies [
11].
The successful application of such microcapsules is dependent on their durability and stability. The microcapsules should be mechanically able to withstand internal sheering forces without compromising their permselectivity or biocompatibility. To address this key requirement, the microcapsules must withstand the internal sheer stresses and osmotic pressures generated both during microcapsule formation and implantation [
43]. Under the influence of low osmotic pressure conditions in the medium on the day after synthesis, the microcapsules we obtained increased by no more than 20–30% in volume. In other studies, researchers have also demonstrated the stability of their microcapsules, there being no more than a 30% dimensional change after exposing the microcapsules to water [
11]. With increased PMETAC concentration, the stability of the microcapsules also increases. Our results indicated that microcapsules using 0.4% PMETAC were both resistant to deformation by changing osmotic pressure and showed elastic properties. We followed the protocol for testing the osmotic stability of alginate microcapsules, but the difference was that the capsules in this study were reinforced with polyethylene glycol (MicroMix) [
31]. This study showed that MicroMix capsules demonstrated improved resistance to bulk osmotic stress compared to alginate-only capsules (ALG-only). Similarly, our PMETAC-coated capsules demonstrated osmotic stability comparable to MicroMix capsules. Moreover, we observed that higher concentrations of PMETAC further enhanced the stability of the capsules. Thus, we found that the osmotic stability of PMETAC-coated capsules is similar to that of MicroMix capsules.
Microcapsules must be able to withstand both the temperature range within the recipient’s body and the conditions used for cell incubation before transplantation. To analyze such thermal stability, we increased the microcapsule incubation time compared to the works of other authors and found that there was a decrease in microcapsule diameter when using PMETAC at a concentration below 1%. However, this reduction in microcapsule size did not critically affect the pore size. Mathematical analysis showed (data not included) that although the permeability of the microcapsules might change from 35 kDa to 30–32 kDa, nutrients and insulin could still continue to diffuse through the microcapsule. Analysis of the stability of microcapsules under long-term culture conditions showed a decrease in the sizes of alginate microcapsules coated with PMETAC, with a dependence of the shrinkage on the mass fraction of the polymer. Our findings indicate that PMETAC-coated capsules have mechanical properties comparable to PolyLN-Biodritin and LN-Biodritin capsules in similar tests [
33]. Thus, microcapsules coated using 0.4% PMETAC exhibit sufficient long-term stability when compared to other polymers.
A test model using multiple rinsings with saline indicated the resistance of alginate microcapsules coated with 0.4% PMETAC to such an impact. This test showed that the change in the size of the microcapsules is limited. Thus, we obtained microcapsules by using 0.4% PMETAC, which, despite a 5% reduction in their size, maintained their shape. This test suggested that these microcapsules should show long-term stability in vivo. Together, these tests demonstrated that the initial gel strength is largely due to the alginate gel cores, while the long-term strength is solely due to the polymer shells [
44]. Increasing the concentration of PMETAC increased the stability of the microcapsules but decreased their permeability. These data correlate with the results of alginate microcapsules coated with poly-L-lysine. An increase in the thickness of the poly-L-lysine polymer coating from 35 µm to 120 µm has been found to cause changes in their resistance to loads from 0.1 ± 0.01 g/microcapsule to 2 ± 0.2 g/microcapsule and a change in permeability from 150 kDa to 40 kDa [
45]. The use of PMETAC at a concentration of 0.4% leads to the formation of microcapsules that are optimal in terms of stability and permeability.
The viability and functional activity of encapsulated islets are key to successful transplantation. The encapsulation process can potentially stress insulin-producing cells, causing them to lose functionality and their properties. The cells may be affected by the mechanical effects of encapsulation, the temporary presence of a viscous alginate around them, or the cytotoxic properties of the encapsulation solutions. Therefore, it is essential to evaluate the impact of encapsulation systems on the islets of Langerhans. This is supported by the results of a study that demonstrated that encapsulation in Alginate-Ca
2+/Ba
2+ microbeads did not compromise the viability or insulin secretion of human islets during glucose stimulation [
20]. Moreover, certain microcapsules can enhance the viability and functional activity of islets by incorporating additional cells or molecules. When mesenchymal stem cells and arg-gly-asp tripeptides were added to microcapsules, this resulted in a 29.9% ± 5.7% increase in islet viability (
p = 0.02) and a two-fold increase in functional activity compared to unencapsulated islets (
p = 0.017) [
46]. Cells that were encapsulated in an alginate microcapsule coated using 0.4% PMETAC remained viable and functionally active, thus indicating that such a microcapsule has a minimal effect on the cells.
Therefore, microcapsules coated using 0.4% PMETAC have the necessary permeability, are resistant to osmotic deformation, are thermally stable, and change volume by less than 15% when incubated in culture medium. Furthermore, the repeated washing of such microcapsules does not destroy them, and the microcapsules, themselves, do not have a cytotoxic effect on the islet cells. Changes in microcapsule size during long-term storage can be avoided by using PMETAC at higher concentrations. However, an increase in the mass fraction of PMETAC leads to a sharp decrease in permeability. Another option for solving the problem of microcapsule shrinkage is the use of an additional layer to create a strong bond between the PMETAC and another polymer, for example, an anionic polymer. In this way, the stiffness of the microcapsule membrane could be increased, although the extent of selective permeability would need to be reassessed. Our unprecedented study of microcapsules based on alginate, coated only with PMETAC, has indicated that the use of a 0.4% coating does exhibit appropriate long-term stability.
Alginate microcapsules coated using 0.4% PMETAC are therefore promising for the development of an encapsulating system for the immunoisolation of pancreatic islets. For the further use of such microcapsules, additional studies are needed to investigate their properties, including their biocompatibility.