Extraction of Fungal Chitosan by Leveraging Pineapple Peel Substrate for Sustainable Biopolymer Production
Abstract
1. Introduction
2. Materials and Methods
2.1. Media and Chemicals
2.2. Fungal Biomass Production
2.2.1. Biomass Production Using Synthetic Media
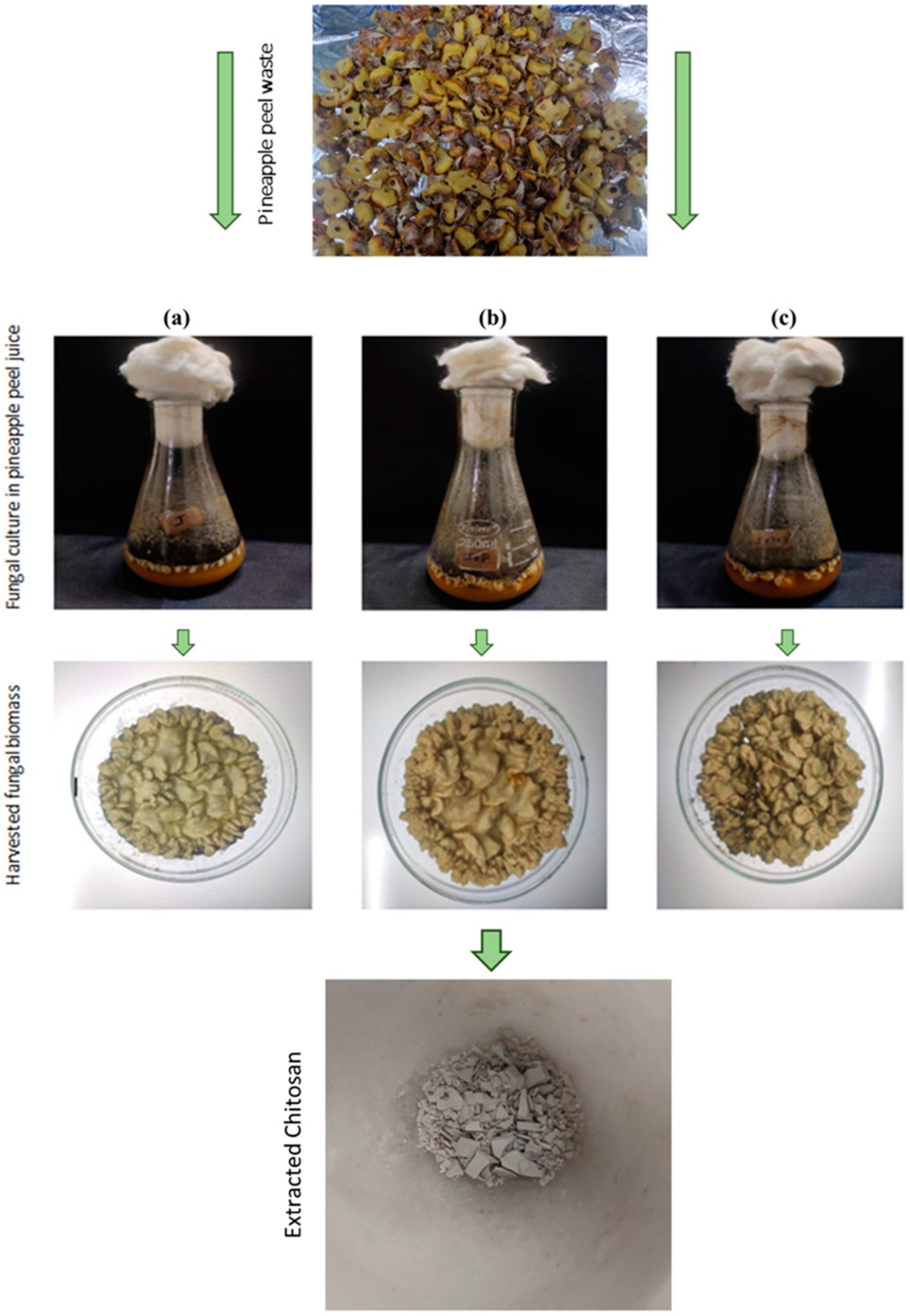
2.2.2. Biomass Production of Pineapple Peel Waste
2.3. Extraction of Fungal Chitosan
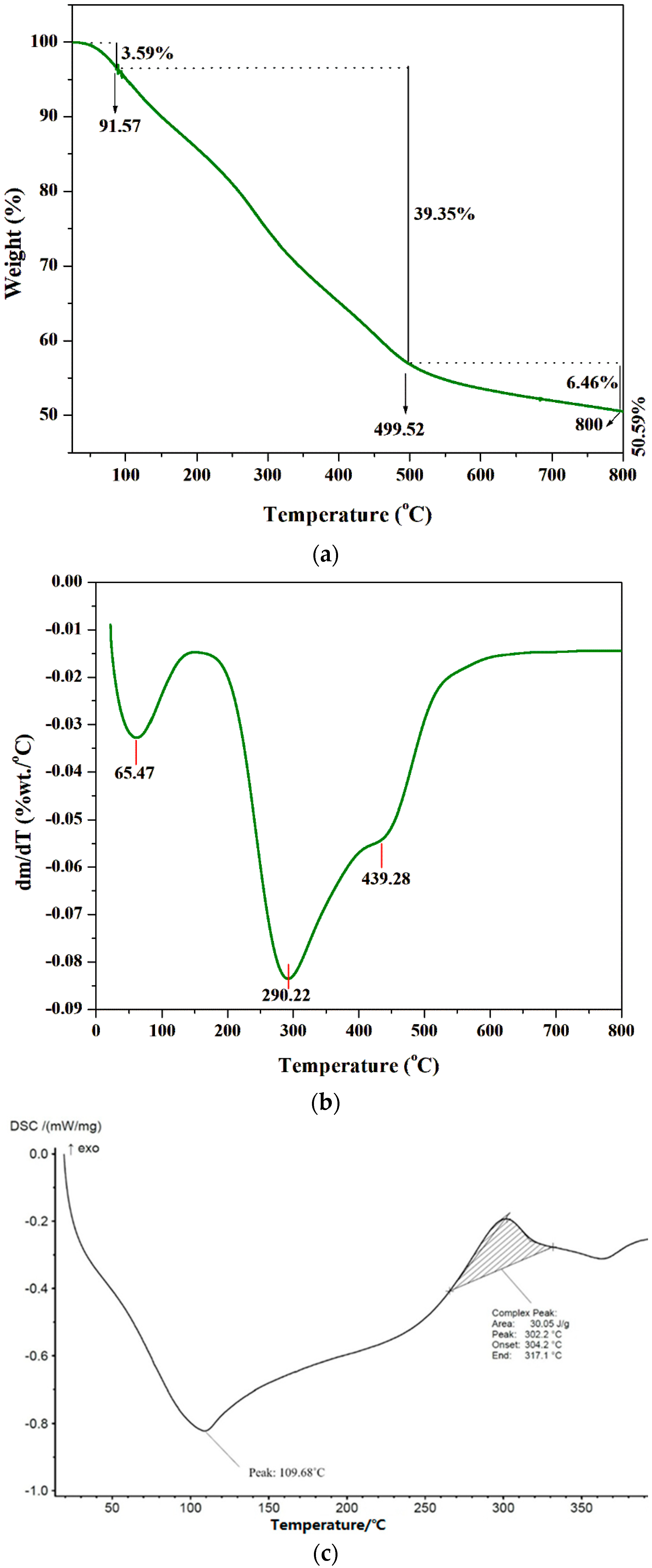
2.4. Characterization of Extracted Chitosan
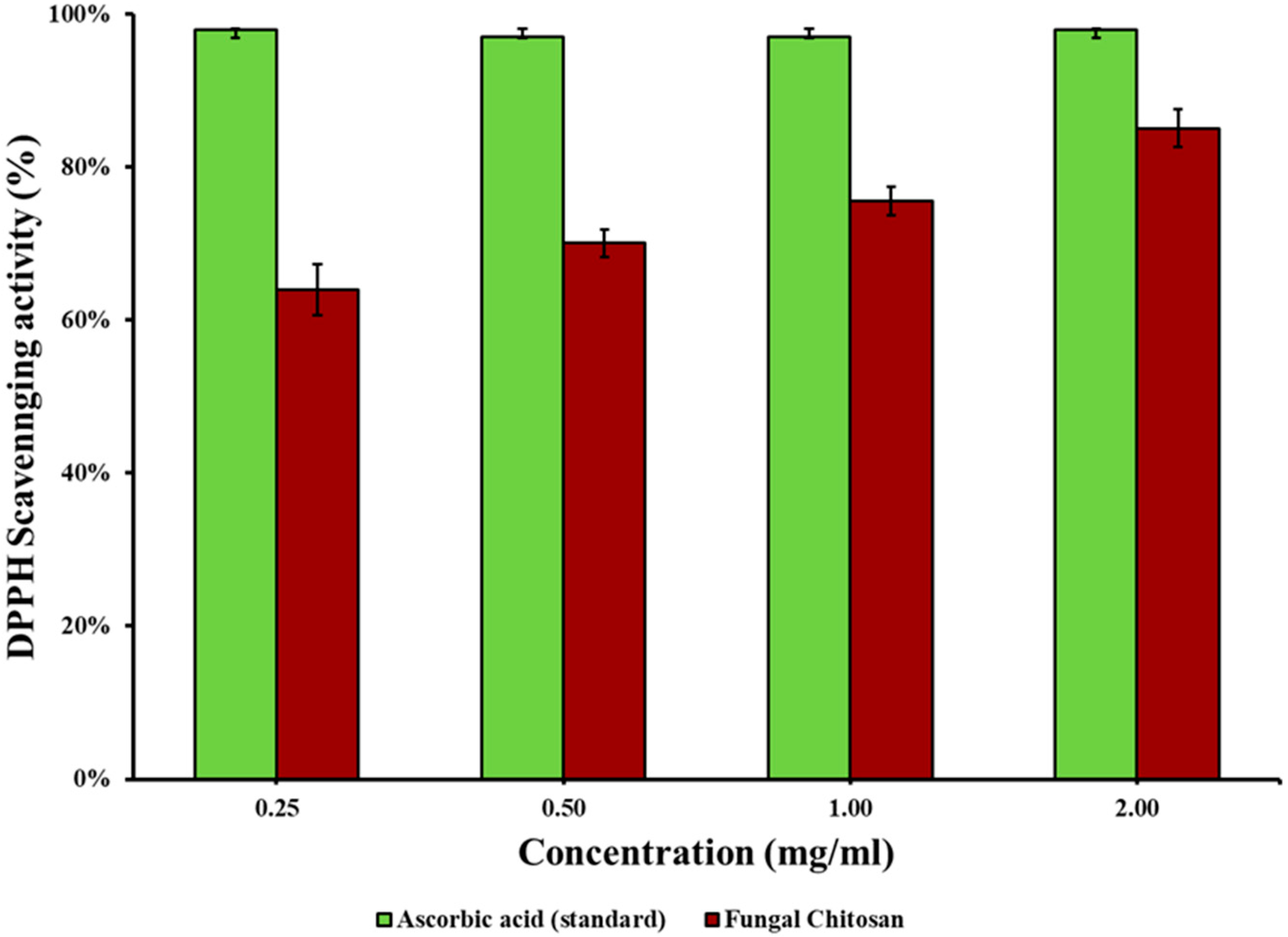
2.5. Antioxidant Activity of Extracted Chitosan
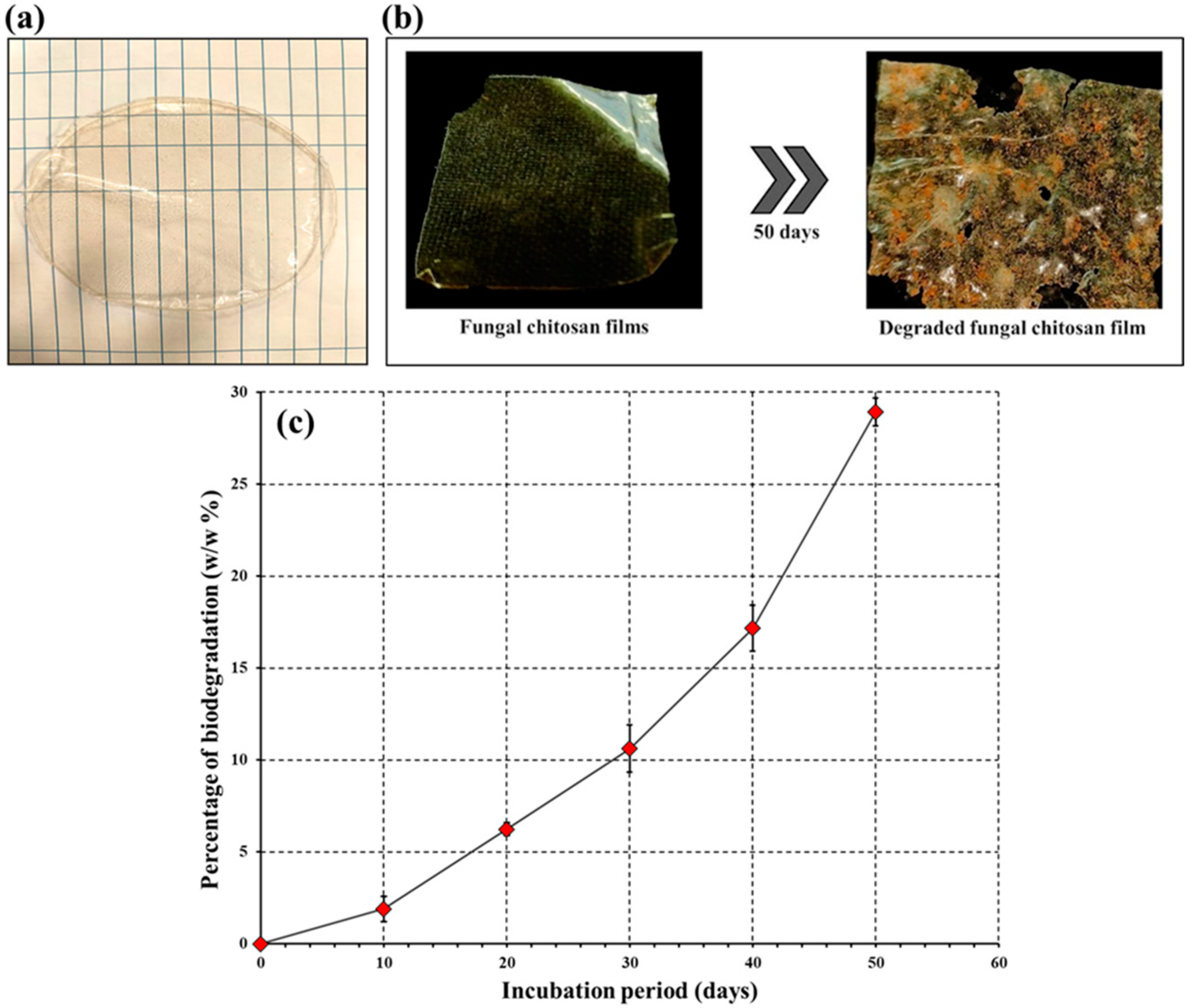
2.6. Fabrication of Chitosan Film
2.7. Biodegradation Study of the Chitosan Film
3. Results and Discussion
3.1. Extraction of Chitosan from Fungal Biomass
3.2. Characterization of Extracted Chitosan
3.3. Antioxidant Activity of the Extracted Chitosan
3.4. Fabrication of Chitosan Sheet
3.5. Biodegradation Study of the Chitosan Film
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gupta, N.; Poddar, K.; Sarkar, D.; Kumari, N.; Padhan, B.; Sarkar, A. Fruit waste management by pigment production and utilization of residual as bioadsorbent. J. Environ. Manag. 2019, 244, 138–143. [Google Scholar] [CrossRef]
- Sodhi, A.S.; Sharma, N.; Bhatia, S.; Verma, A.; Soni, S.; Batra, N. Insights on Sustainable Approaches for Production and Applications of Value Added Products. Chemosphere 2022, 286, 131623. [Google Scholar] [CrossRef] [PubMed]
- Roda, A.; Lambri, M. Food uses of pineapple waste and by-products: A review. Int. J. Food Sci. Technol. 2019, 54, 1009–1017. [Google Scholar] [CrossRef]
- Sepúlveda, L.; Romaní, A.; Aguilar, C.N.; Teixeira, J. Valorization of pineapple waste for the extraction of bioactive compounds and glycosides using autohydrolysis. Innov. Food Sci. Emerg. Technol. 2018, 47, 38–45. [Google Scholar] [CrossRef]
- Meena, L.; Sengar, A.S.; Neog, R.; Sunil, C.K. Pineapple processing waste (PPW): Bioactive compounds, their extraction, and utilisation: A review. J. Food Sci. Technol. 2022, 59, 4152–4164. [Google Scholar] [CrossRef] [PubMed]
- Sarangi, P.K.; Singh, A.K.; Srivastava, R.K.; Gupta, V.K. Recent Progress and Future Perspectives for Zero Agriculture Waste Technologies: Pineapple Waste as a Case Study. Sustain. Sci. Pract. Policy 2023, 15, 3575. [Google Scholar] [CrossRef]
- Suresh, S.; Umesh, M.; Santosh, A.S. Biological extraction of chitin from fish scale waste using proteolytic bacteria Stenotrophomonas koreensis and its possible application as an active packaging material. Biomass Convers. Biorefin. 2023, 1–11. [Google Scholar] [CrossRef]
- Mridul, U.; Suma, S.; Thazeem, B.; Sreehari, S.; Adhithya Sankar, S.; Liya Merin, S.; Sneha, G.; Nilina, J. Sustainable biodegradable and bio-based materials. In Handbook of Sustainable Materials: Modelling, Characterization, and Optimization; CRC Press: Boca Raton, FL, USA, 2023; pp. 71–92. [Google Scholar]
- Meng, Q.; Sun, Y.; Cong, H.; Hu, H.; Xu, F.-J. An overview of chitosan and its application in infectious diseases. Drug Deliv. Transl. Res. 2021, 11, 1340–1351. [Google Scholar] [CrossRef]
- Umesh, M.; Basheer, T.; Sarojini, S.; Santhosh, A.S.; Suresh, S.; James, N. Waste Management for Waste Entrepreneurship: An Emerging Concept. In Trash or Treasure: Entrepreneurial Opportunities in Waste Management; Singh, P., Borthakur, A., Eds.; Springer Nature: Cham, Switzerland, 2024; pp. 231–253. [Google Scholar]
- Oladzadabbasabadi, N.; Mohammadi Nafchi, A.; Ariffin, F.; Wijekoon, M.M.J.O.; Al-Hassan, A.A.; Dheyab, M.A.; Ghasemlou, M. Recent advances in extraction, modification, and application of chitosan in packaging industry. Carbohydr. Polym. 2022, 277, 118876. [Google Scholar] [CrossRef]
- Umesh, M.; Sarojini, S.; Dutta Choudhury, D.; Sankar Santhosh, A.; Kariyadan, S. Food Waste Valorization for Bioplastic Production. In Waste Valorization for Value-Added Products; Bentham Science Publishers: Sharjah, United Arab Emirates, 2023; pp. 216–249. [Google Scholar]
- Huq, T.; Khan, A.; Brown, D.; Dhayagude, N.; He, Z.; Ni, Y. Sources, Production and Commercial Applications of Fungal Chitosan: A Review. J. Bioresour. Bioprod. 2022, 7, 85–98. [Google Scholar] [CrossRef]
- Kaur, S.; Dhillon, G.S. The Versatile Biopolymer Chitosan: Potential Sources, Evaluation of Extraction Methods and Applications. Crit. Rev. Microbiol. 2014, 40, 155–175. [Google Scholar] [CrossRef] [PubMed]
- Mourya, V.K.; Inamdar, N.N.; Choudhari, Y.M. Chitooligosaccharides: Synthesis, Characterization and Applications. Polym. Sci. Series A 2011, 53, 583–612. [Google Scholar] [CrossRef]
- Abo Elsoud, M.M.; El Kady, E.M. Current Trends in Fungal Biosynthesis of Chitin and Chitosan. Bull. Natl. Salmon Resour. Cent. 2019, 43, 59. [Google Scholar] [CrossRef]
- Priyanka, K.; Umesh, M.; Preethi, K. Banana peels as a cost effective substrate for fungal chitosan synthesis: Optimisation and characterisation. Environ. Technol. 2023; ahead of print. [Google Scholar]
- Ishak, K.A.; Zahid, N.I.; Velayutham, T.S.; Khyasudeen, M.F.; Annuar, M.S.M. Corroborative studies on chain packing characteristics of biological medium-chain-length poly-3-hydroxyalkanoates with different monomeric composition. Int. J. Biol. Macromol. 2024, 269 Pt 1, 131973. [Google Scholar] [CrossRef]
- Pradeep, F.S.; Begam, M.; Palaniswamy, M.; Pradeep, B. Influence of culture media on growth and pigment production by Fusarium moniliforme KUMBF1201 isolated from paddy field soil. World Appl. Sci., J. 2013, 22, 70–77. [Google Scholar]
- Chatterjee, S.; Das, S. Developmental stages of biofilm and characterization of extracellular matrix of manglicolous fungus Aspergillus niger BSC-1. J. Basic Microbiol. 2020, 60, 231–242. [Google Scholar] [CrossRef]
- Cicciù, M.; Fiorillo, L.; Cervino, G. Chitosan Use in Dentistry: A Systematic Review of Recent Clinical Studies. Mar. Drugs 2019, 17, 417. [Google Scholar] [CrossRef]
- Samson, R.A.; Varga, J. Aspergillus Systematics in the Genomic Era; CBS Fungal Biodiversity Centre: Utrecht, The Netherlands, 2007; Volume 59. [Google Scholar]
- Nyongesa, B.W.; Okoth, S.; Ayugi, V. Identification Key for Aspergillus Species Isolated from Maize and Soil of Nandi County, Kenya. Adv. Microbiol. 2015, 5, 205. [Google Scholar] [CrossRef]
- Mohy Eldin, A.; Al-Sharnouby, S.F.S.; ElGabry, K.I.M.; Ramadan, A.I. Aspergillus terreus, Penicillium sp. and Bacillus sp. isolated from mangrove soil having laccase and peroxidase role in depolymerization of polyethylene bags. Process Biochem. 2022, 118, 215–226. [Google Scholar] [CrossRef]
- Manivel, G.; Raj, D.M.L.; Prathiviraj, R.; Senthilraja, P. Distribution of phylogenetic proximity upon species-rich marine Ascomycetes with reference to Pichavaram mangrove soil sediment of southern India. Gene Rep. 2020, 21, 100878. [Google Scholar] [CrossRef]
- Santhosh, A.S.; Umesh, M. Valorization of waste chilli stalks (Capsicum annuum) as a sustainable substrate for cellulose extraction: Insights into its thermomechanical, film forming and biodegradation properties. Biomass Convers. Biorefin. 2024, 1–14. [Google Scholar] [CrossRef]
- Geris, R.; Teles de Jesus, V.E.; Ferreira da Silva, A.; Malta, M. Exploring Culture Media Diversity to Produce Fungal Secondary Metabolites and Cyborg Cells. Chem. Biodivers. 2024, 21, e202302066. [Google Scholar] [CrossRef]
- Umesh, M.; Suresh, S.; Santosh, A.S.; Prasad, S.; Chinnathambi, A.; Al Obaid, S.; Jhanani, G.K.; Shanmugam, S. Valorization of pineapple peel waste for fungal pigment production using Talaromyces albobiverticillius: Insights into antibacterial, antioxidant and textile dyeing properties. Environ. Res. 2023, 229, 115973. [Google Scholar] [CrossRef] [PubMed]
- Rakshit, S.; Mondal, S.; Pal, K.; Jana, A.; Soren, J.P.; Barman, P.; Mondal, K.C.; Halder, S.K. Extraction of chitin from Litopenaeus vannamei shell and its subsequent characterization: An approach of waste valorization through microbial bioprocessing. Bioprocess Biosyst. Eng. 2021, 44, 1943–1956. [Google Scholar] [CrossRef] [PubMed]
- Jantzen da Silva Lucas, A.; Quadro Oreste, E.; Leão Gouveia Costa, H.; Martín López, H.; Dias Medeiros Saad, C.; Prentice, C. Extraction, physicochemical characterization, and morphological properties of chitin and chitosan from cuticles of edible insects. Food Chem. 2021, 343, 128550. [Google Scholar] [CrossRef]
- Hisham, F.; Maziati Akmal, M.H.; Ahmad, F.B.; Ahmad, K. Facile extraction of chitin and chitosan from shrimp shell. Mater. Today Proc. 2021, 42, 2369–2373. [Google Scholar] [CrossRef]
- Anbu, S.; Padma, J.; Punithavalli, K.; Saranraj, P. Fruits peel waste as a novel media for the growth of economically important fungi. J. Pharmacogn. Phytochem. 2017, 6, 426–428. [Google Scholar]
- Rasmussen, S.; Parsons, A.J.; Bassett, S.; Christensen, M.J.; Hume, D.E.; Johnson, L.J.; Johnson, R.D.; Simpson, W.R.; Stacke, C.; Voisey, C.R.; et al. High nitrogen supply and carbohydrate content reduce fungal endophyte and alkaloid concentration in Lolium perenne. New Phytol. 2007, 173, 787–797. [Google Scholar] [CrossRef] [PubMed]
- Abasian, L.; Shafiei Alavijeh, R.; Satari, B.; Karimi, K. Sustainable and Effective Chitosan Production by Dimorphic Fungus Mucor Rouxii via Replacing Yeast Extract with Fungal Extract. Appl. Biochem. Biotechnol. 2020, 191, 666–678. [Google Scholar] [CrossRef]
- Namboodiri, M.M.T.; Manikandan, A.; Paul, T.; Pakshirajan, K.; Pugazhenthi, G. Chitosan Production by Penicillium Citrinum Using Paper Mill Wastewater and Rice Straw Hydrolysate as Low-Cost Substrates in a Continuous Stirred Tank Reactor. Environ. Technol. 2023, 44, 2254–2269. [Google Scholar] [CrossRef]
- Affes, S.; Aranaz, I.; Acosta, N.; Heras, Á.; Nasri, M.; Maalej, H. Chitosan derivatives-based films as pH-sensitive drug delivery systems with enhanced antioxidant and antibacterial properties. Int. J. Biol. Macromol. 2021, 182, 730–742. [Google Scholar] [CrossRef]
- Lam, I.L.J.; Mohd Affandy, M.A.; Aqilah, N.M.N.; Vonnie, J.M.; Felicia, W.X.L.; Rovina, K. Physicochemical Characterization and Antimicrobial Analysis of Vegetal Chitosan Extracted from Distinct Forest Fungi Species. Polymers 2023, 15, 2328. [Google Scholar] [CrossRef]
- Kaya, M.; Baran, T.; Asan-Ozusaglam, M.; Cakmak, Y.S.; Tozak, K.O.; Mol, A.; Mentes, A.; Sezen, G. Extraction and characterization of chitin and chitosan with antimicrobial and antioxidant activities from cosmopolitan Orthoptera species (Insecta). Biotechnol. Bioprocess Eng. 2015, 20, 168–179. [Google Scholar] [CrossRef]
- Salama, H.E.; Saad, G.R.; Sabaa, M.W. Synthesis, characterization and biological activity of Schiff bases based on chitosan and arylpyrazole moiety. Int. J. Biol. Macromol. 2015, 79, 996–1003. [Google Scholar] [CrossRef] [PubMed]
- Koc, B.; Akyuz, L.; Cakmak, Y.S.; Sargin, I.; Salaberria, A.M.; Labidi, J.; Ilk, S.; Cekic, F.O.; Akata, I.; Kaya, M. Production and characterization of chitosan-fungal extract films. Food Biosci. 2020, 35, 100545. [Google Scholar] [CrossRef]
- El-araby, A.; El Ghadraoui, L.; Errachidi, F. Usage of biological chitosan against the contamination of post-harvest treatment of strawberries by Aspergillus niger. Front. Sustain. Food Syst. 2022, 6, 881434. [Google Scholar] [CrossRef]
- Kuyyogsuy, A. Preparation and Characterization of Chitosan obtained from Pacific White Shrimp Shells and its in vitro Antifungal Activity. Asian J. Chem. 2020, 32, 2515–2519. [Google Scholar] [CrossRef]
- Qiao, C.; Ma, X.; Wang, X.; Liu, L. Structure and properties of chitosan films: Effect of the type of solvent acid. LWT 2021, 135, 109984. [Google Scholar] [CrossRef]
- Kaya, M.; Akata, I.; Baran, T.; Menteş, A. Physicochemical Properties of Chitin and Chitosan Produced from Medicinal Fungus (Fomitopsis pinicola). Food Biophys. 2015, 10, 162–168. [Google Scholar] [CrossRef]
- Ssekatawa, K.; Byarugaba, D.K.; Wampande, E.M.; Moja, T.N.; Nxumalo, E.; Maaza, M.; Sackey, J.; Ejobi, F.; Kirabira, J.B. Isolation and characterization of chitosan from Ugandan edible mushrooms, Nile perch scales and banana weevils for biomedical applications. Sci. Rep. 2021, 11, 4116. [Google Scholar]
- Han, X.; Zheng, Z.; Yu, C.; Deng, Y.; Ye, Q.; Niu, F.; Chen, Q.; Pan, W.; Wang, Y. Preparation, characterization and antibacterial activity of new ionized chitosan. Carbohydr. Polym. 2022, 290, 119490. [Google Scholar] [CrossRef]
- Ablouh, E.-H.; Jalal, R.; Rhazi, M.; Taourirte, M. Surface modification of α-chitin using an acidic treatment followed by ultrasonication: Measurements of their sorption properties. Int. J. Biol. Macromol. 2020, 151, 492–498. [Google Scholar] [CrossRef]
- Moussout, H.; Ahlafi, H.; Aazza, M.; Bourakhouadar, M. Kinetics and mechanism of the thermal degradation of biopolymers chitin and chitosan using thermogravimetric analysis. Polym. Degrad. Stab. 2016, 130, 1–9. [Google Scholar] [CrossRef]
- Rodrigues, C.; de Mello, J.M.M.; Dalcanton, F.; Macuvele, D.L.P.; Padoin, N.; Fiori, M.A.; Soares, C.; Riella, H.G. Mechanical, Thermal and Antimicrobial Properties of Chitosan-Based-Nanocomposite with Potential Applications for Food Packaging. J. Polym. Environ. 2020, 28, 1216–1236. [Google Scholar] [CrossRef]
- Umesh, M.; Santhosh, A.S.; Shanmugam, S.; Thazeem, B.; Alharbi, S.A.; Almoallim, H.S.; Chi, N.T.L.; Pugazhendhi, A. Extraction, characterization, and fabrication of cellulose biopolymer sheets from Pistia stratiotes as a biodegradative coating material: An unique strategy for the conversion of invasive weeds into value-added products. J. Polym. Environ. 2022, 30, 5057–5068. [Google Scholar] [CrossRef]
- Yoshida, C.M.P.; Pacheco, M.S.; de Moraes, M.A.; Lopes, P.S.; Severino, P.; Souto, E.B.; da Silva, C.F. Effect of Chitosan and Aloe Vera Extract Concentrations on the Physicochemical Properties of Chitosan Biofilms. Polymers 2021, 13, 1187. [Google Scholar] [CrossRef]
- Guinesi, L.S.; Cavalheiro, É.T.G. The use of DSC curves to determine the acetylation degree of chitin/chitosan samples. Thermochim. Acta 2006, 444, 128–133. [Google Scholar] [CrossRef]
- Soon, C.Y.; Tee, Y.B.; Tan, C.H.; Rosnita, A.T.; Khalina, A. Extraction and physicochemical characterization of chitin and chitosan from Zophobas morio larvae in varying sodium hydroxide concentration. Int. J. Biol. Macromol. 2018, 108, 135–142. [Google Scholar] [CrossRef]
- Li, Q.; Mi, Y.; Tan, W.; Guo, Z. Highly efficient free radical-scavenging property of phenolic-functionalized chitosan derivatives: Chemical modification and activity assessment. Int. J. Biol. Macromol. 2020, 164, 4279–4288. [Google Scholar] [CrossRef]
- Beyazit, N.; Çakran, H.S.; Cabir, A.; Akışcan, Y.; Demetgül, C. Synthesis, characterization and antioxidant activity of chitosan Schiff base derivatives bearing (-)-gossypol. Carbohydr. Polym. 2020, 240, 116333. [Google Scholar] [CrossRef]
- Savin, S.; Craciunescu, O.; Oancea, A.; Ilie, D.; Ciucan, T.; Antohi, L.S.; Toma, A.; Nicolescu, A.; Deleanu, C.; Oancea, F. Antioxidant, Cytotoxic and Antimicrobial Activity of Chitosan Preparations Extracted from Ganoderma Lucidum Mushroom. Chem. Biodivers. 2020, 17, e2000175. [Google Scholar] [CrossRef]
- Ai, H.; Wang, F.; Yang, Q.; Zhu, F.; Lei, C. Preparation and biological activities of chitosan from the larvae of housefly, Musca domestica. Carbohydr. Polym. 2008, 72, 419–423. [Google Scholar] [CrossRef]
- Zhang, W.; Jiang, W. Antioxidant and antibacterial chitosan film with tea polyphenols-mediated green synthesis silver nanoparticle via a novel one-pot method. Int. J. Biol. Macromol. 2020, 155, 1252–1261. [Google Scholar] [CrossRef] [PubMed]
- Hasan, M.; Gopakumar, D.A.; Olaiya, N.G.; Zarlaida, F.; Alfian, A.; Aprinasari, C.; Alfatah, T.; Rizal, S.; Khalil, H.P.S.A. Evaluation of the thermomechanical properties and biodegradation of brown rice starch-based chitosan biodegradable composite films. Int. J. Biol. Macromol. 2020, 156, 896–905. [Google Scholar] [CrossRef]
- Awasthi, S.K.; Kumar, M.; Kumar, V.; Sarsaiya, S.; Anerao, P.; Ghosh, P.; Singh, L.; Liu, H.; Zhang, Z.; Awasthi, M.K. A comprehensive review on recent advancements in biodegradation and sustainable management of biopolymers. Environ. Pollut. 2022, 307, 119600. [Google Scholar] [CrossRef]
- Lucas, N.; Bienaime, C.; Belloy, C.; Queneudec, M.; Silvestre, F.; Nava-Saucedo, J.-E. Polymer Biodegradation: Mechanisms and Estimation Techniques. Chemosphere 2008, 73, 429–442. [Google Scholar] [CrossRef] [PubMed]
- Abdelmoez, W.; Dahab, I.; Ragab, E.M.; Abdelsalam, O.A.; Mustafa, A. Bio- and oxo-degradable plastics: Insights on facts and challenges. Polym. Adv. Technol. 2021, 32, 1981–1996. [Google Scholar] [CrossRef]
- Silva, R.R.A.; Marques, C.S.; Arruda, T.R.; Teixeira, S.C.; de Oliveira, T.V. Biodegradation of Polymers: Stages, Measurement, Standards and Prospects. Macromolecules. 2023, 3, 371–399. [Google Scholar] [CrossRef]
- Pavoni, J.M.F.; dos Santos, N.Z.; May, I.C.; Pollo, L.D.; Tessaro, I.C. Impact of acid type and glutaraldehyde crosslinking in the physicochemical and mechanical properties and biodegradability of chitosan films. Polym. Bull. 2021, 78, 981–1000. [Google Scholar] [CrossRef]
- Deshmukh, A.R.; Aloui, H.; Khomlaem, C.; Negi, A.; Yun, J.-H.; Kim, H.-S.; Kim, B.S. Biodegradable films based on chitosan and defatted Chlorella biomass: Functional and physical characterization. Food Chem. 2021, 337, 127777. [Google Scholar] [CrossRef]


| Fungal Strain | Media | Yield |
|---|---|---|
| Aspergillus niger DEL01 (accession number: PP792611) | J (pineapple peel juice) | 63.4 ± 0.17 mg/L |
| J + P (pineapple peel juice + peptone) | 139 ± 0.25 mg/L | |
| J + P + D (pineapple peel juice + peptone + dextrose) | 98 ± 0.33 mg/L | |
| SDB | 24 ± 0.8 mg/L |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Davis, D.; Umesh, M.; Santhosh, A.S.; Suresh, S.; Shanmugam, S.; Kikas, T. Extraction of Fungal Chitosan by Leveraging Pineapple Peel Substrate for Sustainable Biopolymer Production. Polymers 2024, 16, 2455. https://doi.org/10.3390/polym16172455
Davis D, Umesh M, Santhosh AS, Suresh S, Shanmugam S, Kikas T. Extraction of Fungal Chitosan by Leveraging Pineapple Peel Substrate for Sustainable Biopolymer Production. Polymers. 2024; 16(17):2455. https://doi.org/10.3390/polym16172455
Chicago/Turabian StyleDavis, Delwin, Mridul Umesh, Adhithya Sankar Santhosh, Sreehari Suresh, Sabarathinam Shanmugam, and Timo Kikas. 2024. "Extraction of Fungal Chitosan by Leveraging Pineapple Peel Substrate for Sustainable Biopolymer Production" Polymers 16, no. 17: 2455. https://doi.org/10.3390/polym16172455
APA StyleDavis, D., Umesh, M., Santhosh, A. S., Suresh, S., Shanmugam, S., & Kikas, T. (2024). Extraction of Fungal Chitosan by Leveraging Pineapple Peel Substrate for Sustainable Biopolymer Production. Polymers, 16(17), 2455. https://doi.org/10.3390/polym16172455









