On-Substrate Preparation of a Poly(triphenylamino azomethine) for Electrochromic Devices
Abstract
1. Introduction
2. Experimental Procedure
2.1. General Procedures
2.2. Spectroscopic and Spectroelectrochemical Measurements
2.3. Electrochemical Measurements
2.4. On-Substrate Polymerization
2.5. Electrochromic Device
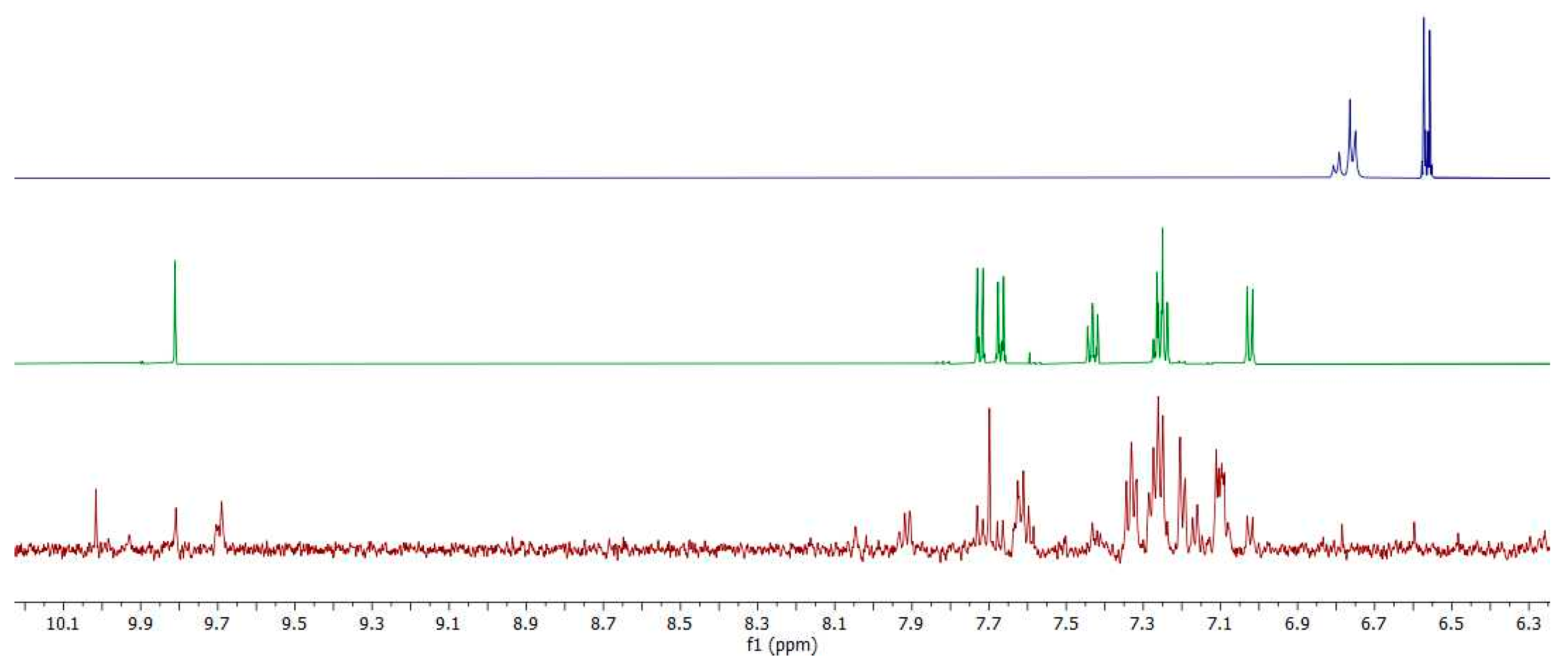
2.6. Synthesis
3. Results and Discussion
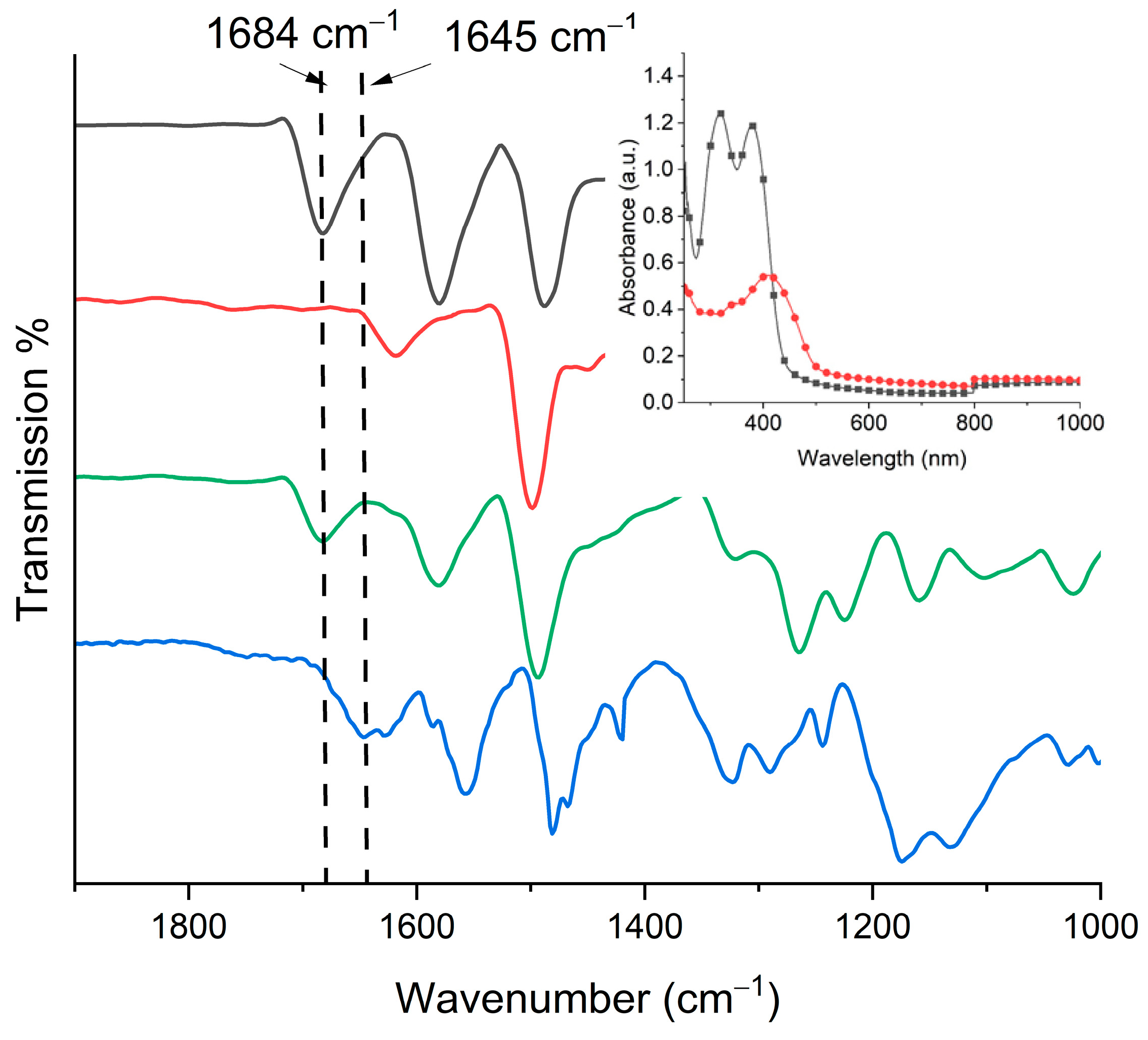
3.1. Polyazomethine Preparation
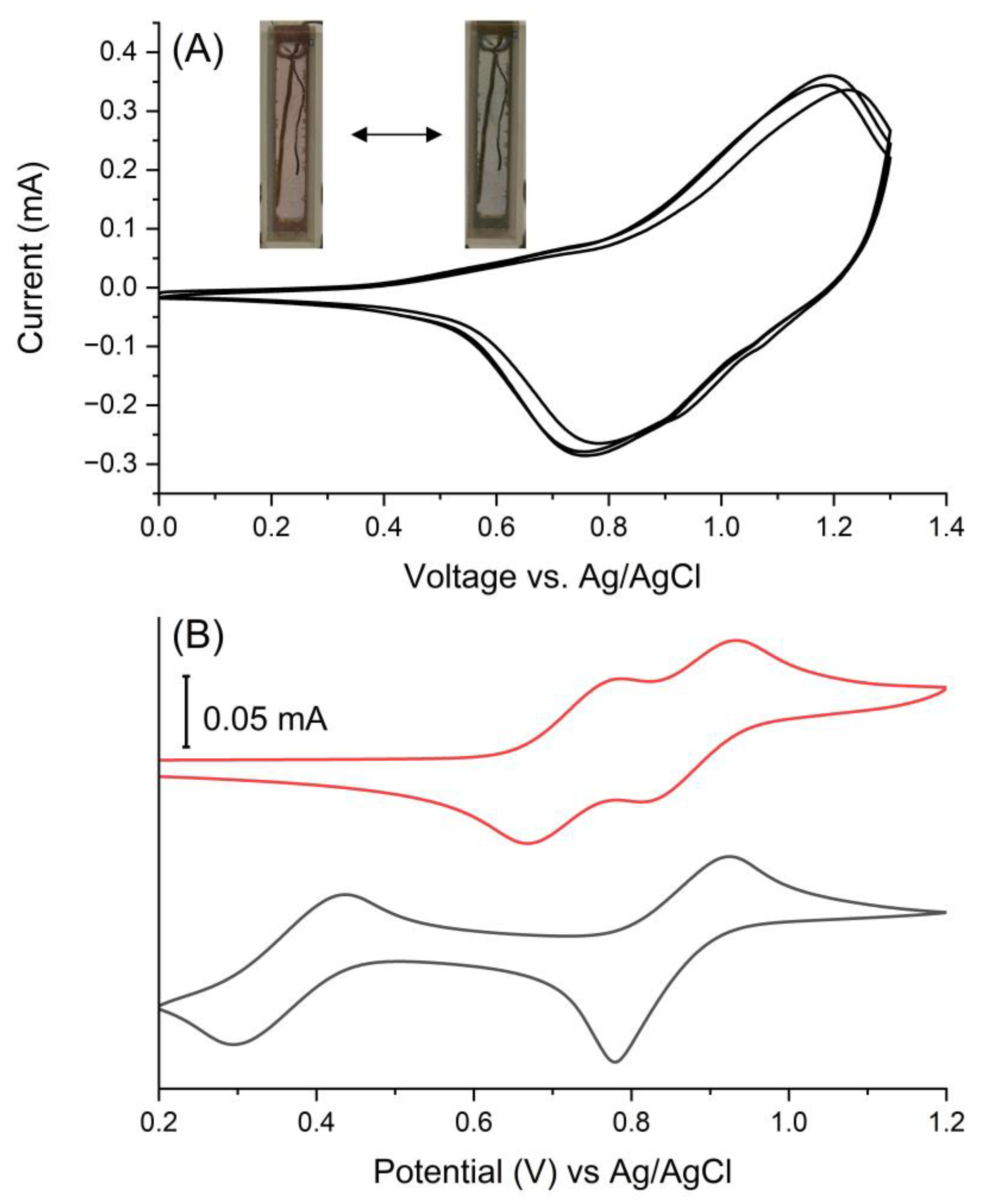
3.2. Electrochemistry and Electrochromism
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Müllen, K.; Scherf, U. Conjugated Polymers: Where We Come from, Where We Stand, and Where We Might Go. Macromol. Chem. Phys. 2023, 224, 2200337. [Google Scholar] [CrossRef]
- Neo, W.T.; Ye, Q.; Chua, S.-J.; Xu, J. Conjugated polymer-based electrochromics: Materials, device fabrication and application prospects. J. Mater. Chem. C 2016, 4, 7364–7376. [Google Scholar] [CrossRef]
- Kim, J.; Rémond, M.; Kim, D.; Jang, H.; Kim, E. Electrochromic Conjugated Polymers for Multifunctional Smart Windows with Integrative Functionalities. Adv. Mater. Technol. 2020, 5, 1900890. [Google Scholar] [CrossRef]
- Abidin, T.; Zhang, Q.; Wang, K.-L.; Liaw, D.-J. Recent advances in electrochromic polymers. Polymer 2014, 55, 5293–5304. [Google Scholar] [CrossRef]
- Jensen, J.; Madsen, M.V.; Krebs, F.C. Photochemical stability of electrochromic polymers and devices. J. Mater. Chem. C 2013, 1, 4826. [Google Scholar] [CrossRef]
- Zhang, S.; Ren, J.; Chen, S.; Luo, Y.; Bai, X.; Ye, L.; Yang, F.; Cao, Y. Large area electrochromic displays with ultrafast response speed and high contrast using solution-processable and patternable honeycomb-like polyaniline nanostructures. J. Electroanal. Chem. 2020, 870, 114248. [Google Scholar] [CrossRef]
- Macher, S.; Schott, M.; Dontigny, M.; Guerfi, A.; Zaghib, K.; Posset, U.; Löbmann, P. Large-Area Electrochromic Devices on Flexible Polymer Substrates with High Optical Contrast and Enhanced Cycling Stability. Adv. Mater. Technol. 2021, 6, 2000836. [Google Scholar] [CrossRef]
- Tsuda, Y.; Kojima, M.; Matsuda, T.; Oh, J.M. Soluble Polyimides Based on Long-chain Alkyl Groups via Amide Linkages. Polym. J. 2008, 40, 354–366. [Google Scholar] [CrossRef]
- Li, M.; Leenaers, P.J.; Wienk, M.M.; Janssen, R.A.J. The effect of alkyl side chain length on the formation of two semi-crystalline phases in low band gap conjugated polymers. J. Mater. Chem. C 2020, 8, 5856–5867. [Google Scholar] [CrossRef]
- Sicard, L.; Navarathne, D.; Skalski, T.; Skene, W.G. On-Substrate Preparation of an Electroactive Conjugated Polyazomethine from Solution-Processable Monomers and its Application in Electrochromic Devices. Adv. Funct. Mater. 2013, 23, 3549–3559. [Google Scholar] [CrossRef]
- Leliège, A.; Barik, S.; Skene, W.G. Photopatternable Electrochromic Materials from Oxetane Precursors. ACS Appl. Mater. Interfaces 2014, 6, 6920–6929. [Google Scholar] [CrossRef]
- Wałęsa-Chorab, M.; Skene, W.G. Investigation of an electroactive immobilized azomethine for potential electrochromic use. Sol. Energy Mater. Sol. Cells 2019, 200, 109977. [Google Scholar] [CrossRef]
- Wałęsa-Chorab, M.; Skene, W.G. Extending the Color Retention of an Electrochromic Device by Immobilizing Color Switching and Ion-Storage Complementary Layers. Electron. Mater. 2020, 1, 40–53. [Google Scholar] [CrossRef]
- Wałęsa-Chorab, M.; Skene, W.G. Leveraging reversible bonds for property modification of electrochromes and their immobilization by dual modes: Thermal and electrochemical polymerization. Prog. Org. Coat. 2024, 187, 108113. [Google Scholar] [CrossRef]
- Søndergaard, R.R.; Hösel, M.; Krebs, F.C. Roll-to-Roll fabrication of large area functional organic materials. J. Polym. Sci. Part B Polym. Phys. 2013, 51, 16–34. [Google Scholar] [CrossRef]
- Budida, J.; Srinivasan, K. Review of thin film deposition and techniques. Mater. Today Proc. 2023, 92, 1030–1033. [Google Scholar] [CrossRef]
- Yunus, Y.; Mahadzir, N.A.; Mohamed Ansari, M.N.; Tg Abd Aziz, T.H.; Mohd Afdzaluddin, A.; Anwar, H.; Wang, M.; Ismail, A.G. Review of the Common Deposition Methods of Thin-Film Pentacene, Its Derivatives, and Their Performance. Polymers 2022, 14, 1112. [Google Scholar] [CrossRef]
- Bilger, D.; Homayounfar, S.Z.; Andrew, T.L. A critical review of reactive vapor deposition for conjugated polymer synthesis. J. Mater. Chem. C 2019, 7, 7159–7174. [Google Scholar] [CrossRef]
- Dianatdar, A.; Bose, R.K. Oxidative chemical vapor deposition for synthesis and processing of conjugated polymers: A critical review. J. Mater. Chem. C 2023, 11, 11776–11802. [Google Scholar] [CrossRef]
- Shibata, M.; Sakai, Y.; Yokoyama, D. Advantages and disadvantages of vacuum-deposited and spin-coated amorphous organic semiconductor films for organic light-emitting diodes. J. Mater. Chem. C 2015, 3, 11178–11191. [Google Scholar] [CrossRef]
- Bhattacharyya, D.; Howden, R.M.; Borrelli, D.C.; Gleason, K.K. Vapor phase oxidative synthesis of conjugated polymers and applications. J. Polym. Sci. Part B Polym. Phys. 2012, 50, 1329–1351. [Google Scholar] [CrossRef]
- Sabouraud, G.; Sadki, S.; Brodie, N. The mechanisms of pyrrole electropolymerization. Chem. Soc. Rev. 2000, 29, 283–293. [Google Scholar] [CrossRef]
- Zhao, M.; Zhang, H.; Gu, C.; Ma, Y. Electrochemical polymerization: An emerging approach for fabricating high-quality luminescent films and super-resolution OLEDs. J. Mater. Chem. C 2020, 8, 5310–5320. [Google Scholar] [CrossRef]
- Audebert, P.; Hapiot, P.; Capdevielle, P.; Maumy, M. Electrochemical polymerization of several salen-type complexes. Kinetic studies in the microsecond time range. J. Electroanal. Chem. 1992, 338, 269–278. [Google Scholar] [CrossRef]
- Chou, M.-Y.; Leung, M.; Su, Y.O.; Chiang, C.L.; Lin, C.-C.; Liu, J.-H.; Kuo, C.-K.; Mou, C.-Y. Electropolymerization of Starburst Triarylamines and Their Application to Electrochromism and Electroluminescence. Chem. Mater. 2004, 16, 654–661. [Google Scholar] [CrossRef]
- Schaming, D.; Ahmed, I.; Hao, J.; Alain-Rizzo, V.; Farha, R.; Goldmann, M.; Xu, H.; Giraudeau, A.; Audebert, P.; Ruhlmann, L. Easy methods for the electropolymerization of porphyrins based on the oxidation of the macrocycles. Electrochim. Acta 2011, 56, 10454–10463. [Google Scholar] [CrossRef]
- Stöckle, B.; Ng, D.Y.W.; Meier, C.; Paust, T.; Bischoff, F.; Diemant, T.; Behm, R.J.; Gottschalk, K.; Ziener, U.; Weil, T. Precise Control of Polydopamine Film Formation by Electropolymerization. Macromol. Symp. 2014, 346, 73–81. [Google Scholar] [CrossRef]
- Odian, G. Principles of Polymerization; Wiley: Hoboken, NJ, USA, 2004; ISBN 9780471274001. [Google Scholar]
- de Gans, B.-J.; Duineveld, P.C.; Schubert, U.S. Inkjet Printing of Polymers: State of the Art and Future Developments. Adv. Mater. 2004, 16, 203–213. [Google Scholar] [CrossRef]
- Jeon, S.; Park, S.; Nam, J.; Kang, Y.; Kim, J.-M. Creating Patterned Conjugated Polymer Images Using Water-Compatible Reactive Inkjet Printing. ACS Appl. Mater. Interfaces 2016, 8, 1813–1818. [Google Scholar] [CrossRef]
- Basak, I.; Nowicki, G.; Ruttens, B.; Desta, D.; Prooth, J.; Jose, M.; Nagels, S.; Boyen, H.-G.; D’Haen, J.; Buntinx, M.; et al. Inkjet Printing of PEDOT:PSS Based Conductive Patterns for 3D Forming Applications. Polymers 2020, 12, 2915. [Google Scholar] [CrossRef]
- Nayak, L.; Mohanty, S.; Nayak, S.K.; Ramadoss, A. A review on inkjet printing of nanoparticle inks for flexible electronics. J. Mater. Chem. C 2019, 7, 8771–8795. [Google Scholar] [CrossRef]
- Choi, S.; Stassi, S.; Pisano, A.P.; Zohdi, T.I. Coffee-Ring Effect-Based Three Dimensional Patterning of Micro/Nanoparticle Assembly with a Single Droplet. Langmuir 2010, 26, 11690–11698. [Google Scholar] [CrossRef] [PubMed]
- Li, D.-W.; Wang, H.-Y.; Liu, Y.; Wei, D.-S.; Zhao, Z.-X. Large-scale fabrication of durable and robust super-hydrophobic spray coatings with excellent repairable and anti-corrosion performance. Chem. Eng. J. 2019, 367, 169–179. [Google Scholar] [CrossRef]
- Alanazi, T.I. Current spray-coating approaches to manufacture perovskite solar cells. Results Phys. 2023, 44, 106144. [Google Scholar] [CrossRef]
- Xie, Y.; Gao, S.; Eslamian, M. Fundamental Study on the Effect of Spray Parameters on Characteristics of P3HT:PCBM Active Layers Made by Spray Coating. Coatings 2015, 5, 488–510. [Google Scholar] [CrossRef]
- Wei, J.; Liu, D.; Sun, K.; Tian, W.; Jiang, W.; Sun, Y. Enhanced performances of fully solution-processed OLEDs via introducing flexible chains into thermally cross-linked thermally activated delayed fluorescent materials. Dye. Pigment. 2020, 182, 108624. [Google Scholar] [CrossRef]
- Burns, S.; MacLeod, J.; Trang Do, T.; Sonar, P.; Yambem, S.D. Effect of thermal annealing Super Yellow emissive layer on efficiency of OLEDs. Sci. Rep. 2017, 7, 40805. [Google Scholar] [CrossRef]
- Wałȩsa-Chorab, M.; Skene, W.G. On-substrate polymerization-a versatile approach for preparing conjugated polymers suitable as electrochromes and for metal ion sensing. RSC Adv. 2014, 4, 19053–19060. [Google Scholar] [CrossRef]
- Napierała, S.; Kubicki, M.; Wałęsa-Chorab, M. Toward Electrochromic Metallopolymers: Synthesis and Properties of Polyazomethines Based on Complexes of Transition-Metal Ions. Inorg. Chem. 2021, 60, 14011–14021. [Google Scholar] [CrossRef]
- Gautier, Y.; Skene, W.G. Effect of azomethine structural modification of electrochromic performance. J. Mater. Chem. C 2024, 12, 3589–3603. [Google Scholar] [CrossRef]
- Cai, J.; Niu, H.; Wang, C.; Ma, L.; Bai, X.; Wang, W. Tuning the bandgaps of polyazomethines containing triphenylamine by different linkage sites of dialdhyde monomers. Electrochim. Acta 2012, 76, 229–241. [Google Scholar] [CrossRef]
- Hu, Y.; Guo, D.; He, Z.; Dong, L.; Hou, Y.; Ma, D. Polyschiff bases containing triphenylamine groups as novel bifunctional materials for electrochromic and photodetector devices. Org. Electron. 2023, 120, 106830. [Google Scholar] [CrossRef]
- Iwan, A. An overview of LC polyazomethines with aliphatic–aromatic moieties: Thermal, optical, electrical and photovoltaic properties. Renew. Sustain. Energy Rev. 2015, 52, 65–79. [Google Scholar] [CrossRef]
- Wesley Jeevadason, A.; Kalidasa Murugavel, K.; Neelakantan, M.A. Review on Schiff bases and their metal complexes as organic photovoltaic materials. Renew. Sustain. Energy Rev. 2014, 36, 220–227. [Google Scholar] [CrossRef]
- Petrus, M.L.; Bouwer, R.K.M.; Lafont, U.; Murthy, D.H.K.; Kist, R.J.P.; Böhm, M.L.; Olivier, Y.; Savenije, T.J.; Siebbeles, L.D.A.; Greenham, N.C.; et al. Conjugated poly(azomethine)s via simple one-step polycondensation chemistry: Synthesis, thermal and optoelectronic properties. Polym. Chem. 2013, 4, 4182. [Google Scholar] [CrossRef]
- Hu, B.; Zhu, X.; Chen, X.; Pan, L.; Peng, S.; Wu, Y.; Shang, J.; Liu, G.; Yan, Q.; Li, R.-W. A Multilevel Memory Based on Proton-Doped Polyazomethine with an Excellent Uniformity in Resistive Switching. J. Am. Chem. Soc. 2012, 134, 17408–17411. [Google Scholar] [CrossRef]
- Jebnouni, A.; Leclerc, N.; Teka, S.; Mansour, D.; Jaballah, N.S. Vinylene-versus azomethine-bridged carbazole-based polymers for light emission and sensor applications. J. Mol. Struct. 2021, 1244, 130994. [Google Scholar] [CrossRef]
- Marin, L.; Bejan, A.; Ailincai, D.; Belei, D. Poly(azomethine-phenothiazine)s with efficient emission in solid state. Eur. Polym. J. 2017, 95, 127–137. [Google Scholar] [CrossRef]
- Yeh, L.-C.; Huang, T.-C.; Lai, F.-Y.; Lai, G.-H.; Lo, A.-Y.; Hsu, S.-C.; Yang, T.-I.; Yeh, J.-M. Synthesis of electroactive polyazomethine and its application in electrochromic property and electrochemical sensor. Surf. Coat. Technol. 2016, 303, 154–161. [Google Scholar] [CrossRef]
- Ma, X.; Wu, Y.; Wen, H.; Niu, H.; Wang, C.; Qin, C.; Bai, X.; Lei, L.; Wang, W. Immobilized polyazomethines containing triphenylamine groups on ITO: Synthesis and acidochromic, electrochemical, electrochromic and photoelectronic properties. RSC Adv. 2016, 6, 4564–4575. [Google Scholar] [CrossRef]
- Garbay, G.; Giraud, L.; Gali, S.M.; Hadziioannou, G.; Grau, E.; Grelier, S.; Cloutet, E.; Cramail, H.; Brochon, C. Divanillin-Based Polyazomethines: Toward Biobased and Metal-Free π-Conjugated Polymers. ACS Omega 2020, 5, 5176–5181. [Google Scholar] [CrossRef]
- Li, G.; Yu, K.; Noordijk, J.; Meeusen-Wierts, M.H.M.; Gebben, B.; oude Lohuis, P.A.M.; Schotman, A.H.M.; Bernaerts, K.V. Hydrothermal polymerization towards fully biobased polyazomethines. Chem. Commun. 2020, 56, 9194–9197. [Google Scholar] [CrossRef]
- Connelly, N.G.; Geiger, W.E. Chemical Redox Agents for Organometallic Chemistry. Chem. Rev. 1996, 96, 877–910. [Google Scholar] [CrossRef]
- Wałęsa-Chorab, M.; Muras, K.; Filiatrault, H.L.; Skene, W.G. Suitability of alkyne donor-π-donor-π-donor scaffolds for electrofluorochromic and electrochromic use. J. Mater. Chem. C 2022, 10, 3691–3703. [Google Scholar] [CrossRef]
- Chang, C.-W.; Liou, G.-S.; Hsiao, S.-H. Highly stable anodic green electrochromic aromatic polyamides: Synthesis and electrochromic properties. J. Mater. Chem. 2007, 17, 1007–1015. [Google Scholar] [CrossRef]
- Chang, C.-W.; Liou, G.-S. Stably anodic green electrochromic aromatic poly(amine–amide–imide)s: Synthesis and electrochromic properties. Org. Electron. 2007, 8, 662–672. [Google Scholar] [CrossRef]
- Blanchard, P.; Malacrida, C.; Cabanetos, C.; Roncali, J.; Ludwigs, S. Triphenylamine and some of its derivatives as versatile building blocks for organic electronic applications. Polym. Int. 2019, 68, 589–606. [Google Scholar] [CrossRef]
- Yen, H.J.; Liou, G.S. Recent advances in triphenylamine-based electrochromic derivatives and polymers. Polym. Chem. 2018, 9, 3001–3018. [Google Scholar] [CrossRef]
- Yen, H.-J.; Kung, Y.-R.; Hsiao, S.-H.; Liou, G.-S. Chapter 11. Arylamine-based High Performance Polymers for Electrochromic Applications. In Electrochromic Smart Materials: Fabrication and Applications; Xu, J.W., Chua, M.H., Shah, K.W., Eds.; The Royal Society of Chemistry: London, UK, 2019; pp. 323–371. ISBN 978-1-78801-143-3. [Google Scholar]
- Santra, D.C.; Nad, S.; Malik, S. Electrochemical polymerization of triphenylamine end-capped dendron: Electrochromic and electrofluorochromic switching behaviors. J. Electroanal. Chem. 2018, 823, 203–212. [Google Scholar] [CrossRef]
- Hsiao, S.-H.; Wang, H.-M.; Liao, S.-H. Redox-stable and visible/near-infrared electrochromic aramids with main-chain triphenylamine and pendent 3,6-di-tert-butylcarbazole units. Polym. Chem. 2014, 5, 2473. [Google Scholar] [CrossRef]
- Holze, R. Overoxidation of Intrinsically Conducting Polymers. Polymers 2022, 14, 1584. [Google Scholar] [CrossRef]
- Balakrishnan, S.; Venugopal, R.; Harikumar, A.; Deb, B.; Joseph, J. Effect of Hyper-Cross-Linking on the Electrochromic Device Properties of Cross-Linkable Carbazole–Diphenylamine Derivatives. ACS Appl. Polym. Mater. 2023, 5, 4170–4179. [Google Scholar] [CrossRef]
- Sun, Y.; Zhu, G.; Zhao, X.; Kang, W.; Li, M.; Zhang, X.; Yang, H.; Guo, L.; Lin, B. Solution-processable, hypercrosslinked polymer via post-crosslinking for electrochromic supercapacitor with outstanding electrochemical stability. Sol. Energy Mater. Sol. Cells 2020, 215, 110661. [Google Scholar] [CrossRef]
- Liu, F.; Bai, J.; Yu, G.; Ma, F.; Hou, Y.; Niu, H. Synthesis, electrochromic properties and flash memory behaviors of novel D-A-D polyazomethines containing EDOT and thiophene units. Org. Electron. 2020, 77, 105538. [Google Scholar] [CrossRef]
- Yen, H.J.; Liou, G.S. Novel blue and red electrochromic poly(azomethine ether)s based on electroactive triphenylamine moieties. Org. Electron. 2010, 11, 299–310. [Google Scholar] [CrossRef]
- Liu, F.; Cong, Z.; Yu, G.; Niu, H.; Hou, Y.; Wang, C.; Wang, S. Novel D-A-D conjugated polymers based on tetraphenylethylene monomer for electrochromism. Opt. Mater. 2020, 100, 109658. [Google Scholar] [CrossRef]



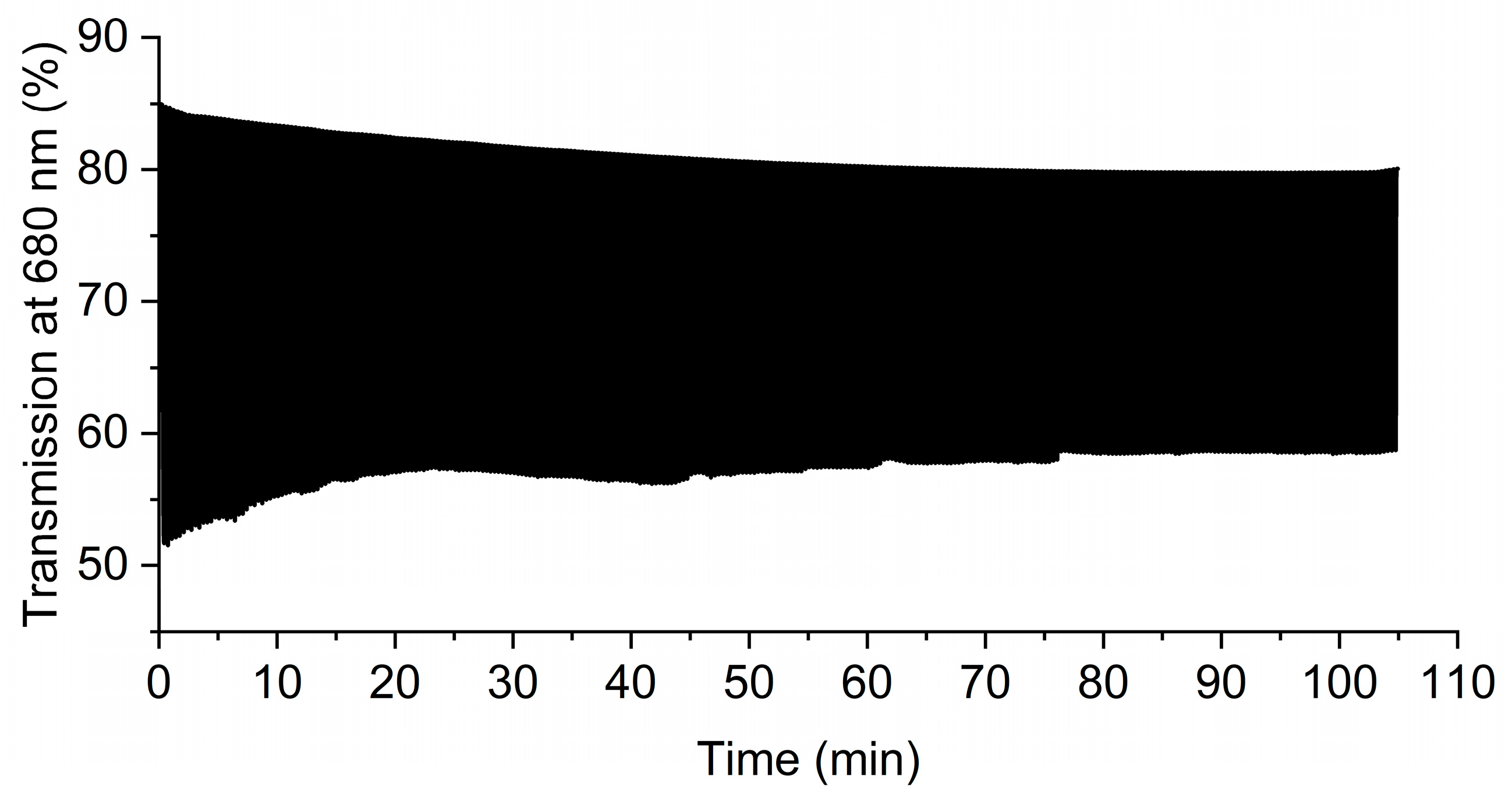
 ), −1.0 V (□). Inset: photographs of the device at 2.6 (left), and −1.0 (right) V.
), −1.0 V (□). Inset: photographs of the device at 2.6 (left), and −1.0 (right) V.
 ), −1.0 V (□). Inset: photographs of the device at 2.6 (left), and −1.0 (right) V.
), −1.0 V (□). Inset: photographs of the device at 2.6 (left), and −1.0 (right) V.


| Polyazomethine | Perceived Color Change | λ (nm) a | Response Time (s) | Ref. | |
|---|---|---|---|---|---|
| Tc | Tb | ||||
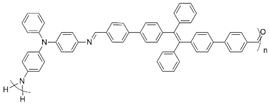
 | Yellow → red ↔ blue | 680 nm | 3.3 | 1.2 | This work |
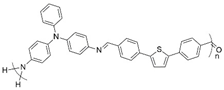 | Yellow ↔ yellowish-green | 428 nm | 4.54 | 3.88 | [66] |
 | Yellow ↔ bluish-green | 411 nm | 2.23 | 1.90 | [66] |
 | Yellow ↔ red | 717 nm | 5.17 | 4.01 | [67] |
 | Yellow ↔ blue | 725 nm | 2.01 | 3.15 | [67] |
 | Orange ↔ light purple | 905 nm | 6.45 | 1.07 | [68] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Filiatrault, H.L.; Muras, K.; Wałęsa-Chorab, M.; Skene, W.G. On-Substrate Preparation of a Poly(triphenylamino azomethine) for Electrochromic Devices. Polymers 2024, 16, 2440. https://doi.org/10.3390/polym16172440
Filiatrault HL, Muras K, Wałęsa-Chorab M, Skene WG. On-Substrate Preparation of a Poly(triphenylamino azomethine) for Electrochromic Devices. Polymers. 2024; 16(17):2440. https://doi.org/10.3390/polym16172440
Chicago/Turabian StyleFiliatrault, Heather L., Kacper Muras, Monika Wałęsa-Chorab, and W. G. Skene. 2024. "On-Substrate Preparation of a Poly(triphenylamino azomethine) for Electrochromic Devices" Polymers 16, no. 17: 2440. https://doi.org/10.3390/polym16172440
APA StyleFiliatrault, H. L., Muras, K., Wałęsa-Chorab, M., & Skene, W. G. (2024). On-Substrate Preparation of a Poly(triphenylamino azomethine) for Electrochromic Devices. Polymers, 16(17), 2440. https://doi.org/10.3390/polym16172440








