Abstract
In this study, chlorine-induced corrosion and blister formation on steel pipes (SPs) coated with modified polyethylene powder (MPP) were evaluated through various tests, including chlorine exposure, wet immersion, and temperature gradient experiments. The results confirmed that the extent of corrosion and iron leaching varied with the coating type as expected. In batch leaching tests, no corrosion was observed on modified polyethylene-coated steel pipes (MPCSPs) within a chlorine concentration range of 0 mg/L to 10 mg/L; similarly, there were no significant changes in specimen weight or iron levels. In contrast, the control group with uncoated SPs exhibited significant iron leaching and corrosion, a trend consistent in sequential leaching experiments. SEM analysis after a month of chlorine exposure revealed no significant corrosion on MPCSPs, and SEM-EDX confirmed no major changes in the carbon bond structure, indicating resistance to high chlorine concentrations. Comparative analysis of wet immersion and temperature gradient tests between MPCSP and conventional epoxy-coated SP (ECSP) specimens revealed that MPCSPs did not develop blisters even after 100 days of immersion, whereas ECSPs began showing blisters as early as 50 days. In temperature gradient tests, MPCSPs showed no blisters after 100 days, while ECSPs exhibited severe internal coating layer blisters.
1. Introduction
Steel pipes (SPs) are fundamental components of modern infrastructure, extensively used in transporting fluids across various industries, including oil and gas, water treatment, and chemical processing [1,2,3]. Despite their widespread utility, the longevity and performance of SPs are compromised by corrosion [4,5,6]. The economic implications of corrosion are substantial, requiring significant annual expenditures on the maintenance, repair, and replacement of infrastructure globally. To prevent corrosion, protective coatings are applied to SPs, enhancing their resistance to environmental and chemical aggressors. Among the variety of coatings available, polyethylene-based coatings have gained prominence due to their effective barrier properties, mechanical strength, and cost-efficiency [7,8,9]. Recent studies have significantly advanced our understanding of various aspects of pipeline corrosion, providing critical insights into prevention strategies, mitigation techniques, and the fundamental mechanisms driving corrosion processes [10,11,12,13,14,15,16]. However, the dynamic and aggressive conditions to which pipes are exposed—ranging from high temperatures and pressures to chemically active environments—demand continuous improvements in coating formulations to enhance protective capabilities.
In the Republic of Korea, most water treatment facilities use chlorine as a disinfectant, which can cause corrosion in pipes [17,18]. Moisture in contact with pipes acts as an electrolyte in electrochemical reactions, and the dissolution of acidic substances such as H2S and CO2 in water can accelerate the corrosion of SPs [19]. Previous research has shown that the durability of surface-coated SPs is greatly affected by the moisture penetration resistance of the coating material and environmental conditions such as temperature and humidity [20]. Additionally, exposure to high temperatures can significantly reduce the effective lifespan of the coating [21,22,23]. As a countermeasure, the interiors of SPs are coated with materials such as epoxy, urethane, and urea [3,24,25]. However, over time, the chlorine in tap water can deform these interior coatings, leading to internal corrosion. Therefore, the development of effective coating materials that can prevent pipe corrosion caused by chlorine, moisture penetration, exposure to temperature, and humidity is significant.
In the case of underground coated SPs, applied coatings should last at least 20 years to ensure the suitability of use and stability of the pipe system [26]. Surface coatings correctly applied are expected to protect the workability and fittings of SPs over the long term and effectively prevent corrosion [27]. Accordingly, polymeric coatings have been widely accepted to protect onshore and offshore pipelines [28]. Specifically, three-layer polypropylene coating (TLPC) and epoxy have been applied to SPs exposed to corrosive environments [29]. TLPC consists of a thin epoxy resin layer, a modified middle layer of polypropylene, and an external polypropylene layer. They demonstrate excellent adhesion, effectively isolating the pipe from harmful substances and preventing corrosion. Despite the effectiveness of TLPCs in preventing corrosion on SPs, further experimental study is necessary for several reasons. Firstly, improved coatings can significantly boost the lifespan and reliability of SPs by providing better corrosion resistance and mechanical wear properties. This not only helps by reducing maintenance costs but also minimizes the environmental risks associated with leaks and failures. Additionally, the development of innovative, cost-effective coatings can help industries meet increasingly stringent safety and environmental regulations. Furthermore, advancements in smart coating technologies, which integrate sensors to monitor the health and integrity of SPs, represent a forward leap in predictive maintenance strategies. This can lead to more timely interventions, preventing failures before they occur and ensuring safer operations. Consequently, continued research and development in this area is essential for enhancing the functionality and durability of SPs, making them more sustainable and efficient in their applications across various sectors.
In this study, the chlorine-induced corrosion of modified polyethylene-coated steel pipes (MPCSPs), which underwent extensive surface pretreatment including degreasing, acid treatment, phosphate coating, and heating, was investigated experimentally. The microstructural integrity of the coating surface was also examined to determine the extent of corrosion. Additionally, the occurrence of surface blisters on coated pipe specimens exposed to various conditions of temperature, moisture, and exposure duration was assessed through visual inspection. This research aims to develop an MPCSP variant with enhanced corrosion resistance and to evaluate its performance compared to conventional epoxy coatings.
2. Research Significance
This study explores the potential of modified polyethylene powder (MPP) as a coating material that offers enhanced corrosion resistance compared to traditional coatings. This is vital in industries such as chemical processing and offshore oil drilling, where pipe durability is crucial for safety and operational efficiency. The research aims to provide empirical data on the effectiveness, durability, and commercial viability of MPP coatings. The results could lead to more reliable and durable pipe systems, reducing maintenance frequency and costs. Furthermore, this research could contribute to the development of coatings that are not only more effective but also environmentally friendly. This study is part of an ongoing research project on smart pipe systems equipped with sensors for improved maintenance strategies. Additional test results from MPCSPs exposed to harsh marine environments will be reported.
3. Experimental Design
An experimental campaign was designed to investigate the effect of chlorine on the corrosion of coated and uncoated SP samples. Surface inspection was also performed to check if blisters formed on the specimens after the wet immersion and thermal gradient experiments were completed. The details of the prepared test series, testing methods, and measurements are shown in Figure 1. It should be noted that the coating in Figure 1 refers to the internal coating, and the exterior has been coated entirely with TLPC. As mentioned previously, a conventional epoxy-coated steel pipe (ECSP) was chosen for the comparison purpose. The selection of MPP, phosphate film, epoxy primer, modified PE adhesive layer, and resin layer in this study was carefully made to maximize corrosion protection and adhesion, with consideration for field application. MPP provides an initial barrier against corrosion, while the phosphate film enhances adhesion and offers additional corrosion resistance. The epoxy primer ensures strong bonding and adds resistance to moisture and chemicals. The modified PE adhesive layer further strengthens the adhesion between layers, and the resin layer adds a smooth, durable, and environmentally resistant finish. Together, these materials create a robust, long-lasting protective piping system.
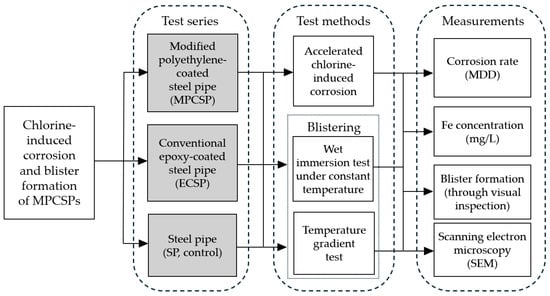
Figure 1.
Test series, methods, and measurements considered in this study.
3.1. Test Specimens and Coating Processes
Figure 2 displays the prepared MPCSP and ECSP specimens after undergoing several coating processes. MPP was applied inside the MPCSP specimens, while the exterior underwent treatment with a phosphate film. This was followed by an extrusion coating of an epoxy primer, a modified PE adhesive layer, and a resin layer. A corrosion experiment on conventional SPs was also conducted for comparison purposes.

Figure 2.
Representative specimens prepared through pretreatment process: (a) modified polyethylene-coated steel pipe (MPCSP); (b) epoxy-coated steel pipe (ECSP).
A chemical pretreatment process was applied to prevent the formation of an oxidation film layer and delamination (refer to Figure 3). The chemical pretreatment involves a series of reactions where Zn(H2PO4)2 decomposes into ZnHPO4 and H3PO4. Additionally, 3ZnHPO4 decomposes into Zn3(PO4)2 and H3PO4. Furthermore, 2H3PO4 reacts with Fe, producing Fe(H2PO4)2, H2, and the precipitate FePO4. Ultimately, Fe(H2PO4)2 reacts with 2Zn(H2PO4)2 to decompose into the main component of the phosphophyllite coating, Zn2Fe(PO4)2, and 4H3PO4.
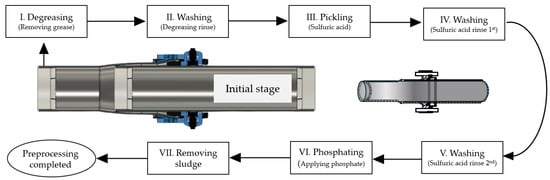
Figure 3.
Chemical pretreatment process applied in steel pipes investigated in this study.
After completing the chemical pretreatment, both the interior and exterior of the specimens were coated using a three-layer method. The interior coating involved heating the pipe to a predetermined temperature after pretreatment and applying a powder lining to thermally fuse modified PE powder, resulting in a corrosion-resistant coating consisting of an epoxy primer and modified PE powder lining. The exterior was coated by first applying an epoxy primer, followed by the co-extrusion of modified PE and high-density PE onto steel pipes that had undergone special pretreatment and heating. Thus, the exterior coating comprises an epoxy primer, modified PE, and high-density PE applied through extrusion.
3.2. Chlorine-Induced Corrosion Test
Batch and sequential leaching experiments were conducted on MPCSP and SP specimens to evaluate the corrosion rate when exposed to chlorine. The setup for the batch leaching experiment, depicted in Figure 4a, includes a temperature control device, a magnetic stirring bar, a stirring device, and a hot plate. Rectangular specimens (30 mm × 20 mm) were prepared with a 5.0 mm hole at the top to facilitate suspension inside the experimental apparatus. Each specimen was secured in the batch leaching experiment device and 1.0 L of tap water was added to a beaker. A 12% NaOCl solution was diluted with ultrapure distilled water to achieve a concentration of 1000 mg/L.

Figure 4.
Chloride-induced accelerated corrosion test setup: (a) Batch leaching; (b) Sequential leaching.
In this study, the chlorine injection concentration ranged from 0 to 10 mg/L (Cl2), with corrosion and iron leaching levels measured over a period of seven days. To determine the appropriate concentration levels and time intervals for the main experiment, a series of pre-tests were conducted. These pre-tests involved varying chlorine concentrations and monitoring their effects on corrosion and iron leaching over different time periods. The results from these preliminary tests allowed us to identify the specific concentration levels and time intervals that would provide the most accurate and reliable data for the main study. By analyzing the trends observed during the pre-tests, we ensured that the chosen parameters were sufficient to capture the onset and progression of corrosion and iron leaching, while also preventing any significant experimental errors or inconsistencies
The sequential leaching experiment, illustrated in Figure 4b, utilized specimens of the same size of 30 mm × 20 mm. Unlike the batch experiments, tap water in the sequential experiment was cycled with a 12% NaOCl solution diluted to 1000 mg/L, injecting a chlorine concentration of 10 mg/L to simulate extreme conditions. Corrosion and iron leaching concentrations were measured every five days over 30 days to assess corrosion resistance. The specimens were suspended in the sequential experiment device as shown in Figure 4b.
3.3. Blistering Test
Wet immersion testing at a constant temperature was performed to assess whether blisters formed on the coated specimens and to evaluate their permeability. This test aimed to identify the point at which blisters occur under constant water exposure at a specified temperature. MPCSP (150A, 600A) and ECSP (150A) specimens were immersed in a small water tank within an oven maintained at 68 °C.
A thermal gradient experiment was also conducted to examine the permeability and potential blister formation. Specimens of the same type and size were exposed in water tanks at varying temperatures. The experimental setup was specially designed to accommodate the curvature of the specimens, which affects their shape when cut from coated pipe products, as depicted in Figure 5 and Table 1.
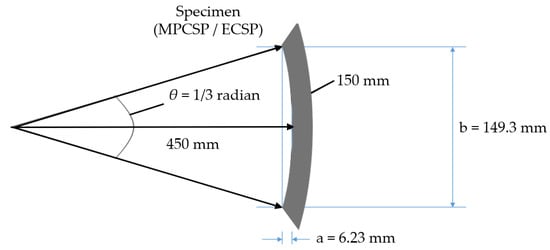
Figure 5.
Specimen (900A) details (for blistering test) to reflect the curvature.

Table 1.
Detailed sizes of specimens for blistering test (unit: mm).
As illustrated in Figure 6a,b, the setup included inner and outer tanks; the inner tank contained hot water at 50 °C, and the outer tank held cold water at 20 °C. The specimens were attached to the outer wall of the inner tank, as shown in Figure 6c. This arrangement exposed the outer steel portion to the 20 °C water and the inner coated portion to the 50 °C water, creating conditions conducive to water penetration. The presence and extent of blisters were visually inspected during the experiments.
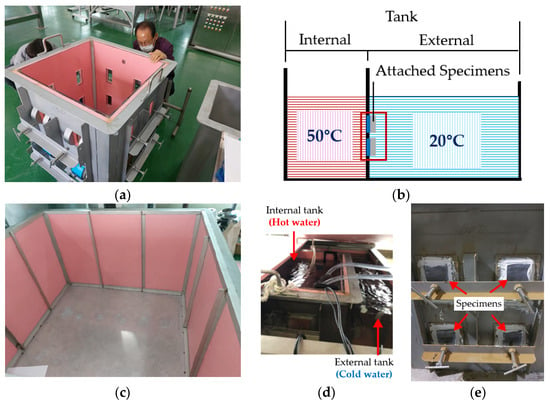
Figure 6.
Thermal gradient test: (a) internal tank; (b) schematic representation of test; (c) external tank; (d) photo of the test in progress; (e) test specimens attached between two tanks.
3.4. Measurements and Surface Analysis
The corrosion rate, expressed as MDD (milligrams per decimeter squared per day), was estimated based on changes in the measured weights of the specimens before and after the experiment. The weights were measured after all corrosion products and scale had been removed, and the specimens had been thoroughly dried. MDD can be determined using Equation (1) as follows:
The concentration of released Fe was measured using an atomic absorption spectrometer (Varian AA-600) equipped with a dedicated graphite furnace, model GTA100, and Zeeman background correction. Both the field emission scanning electron microscope (FESEM) and the scanning electron microscope–energy dispersive X-ray spectrometer (SEM–EDX) were used to analyze the specimens before the sequential experiment began and again one month later.
The SEM analyses were performed at the Koptri Institute (Seoul, Republic of Korea). Analytical conditions included an accelerating voltage of 5 kV and a beam current of 30 nA for FESEM, while an accelerating voltage of 15 kV and a beam current of 50 nA were used for SEM–EDX.
4. Test Results
4.1. Effect of Coating Type on Measured Corrosion Rate and Fe Concentration
The results of the batch leaching experiments confirmed that MPCSP exhibits superior corrosion resistance and significantly lower MDD compared to the control (SP) when exposed to chlorine, as shown in Figure 7a,b. Based on the results of the 7-day chlorine-induced corrosion test (see Figure 7a), the SP specimens showed greater iron leaching and higher corrosion rates. In contrast, the MPCSP specimens, with their internal polyethylene coating, are believed to have prevented iron leaching from the underlying carbon steel pipe, thereby preserving corrosion resistance against chlorine. Figure 7b shows the test results under the assumed extreme water pipe conditions. A 10 mg/L chlorine solution was injected, and the specimens were subjected to a 30-day continuous circulation experiment. The specimens were removed at 5-day intervals to measure the corrosion rate, based on weight loss, and the iron leaching concentration to evaluate the corrosion resistance to chlorine. The results confirmed that both the corrosion rate and iron leaching increased over time. Specifically, MPCSP specimens showed no significant change in weight and maintained an approximately zero-corrosion level, regardless of variations in chlorine injection concentration. No iron leaching was detected from the specimens, though iron concentrations measured at 0.02 mg/L likely originated from the tap water. Thus, it is expected that the MPP investigated does not react with chlorine at the concentrations introduced from water treatment plants. In contrast, SP displayed significant iron leaching and high corrosion rates under the same conditions. The batch leaching experiments suggest that the MPP coating inside SP prevents iron leaching from carbon steel pipes and maintains corrosion resistance against chlorine.
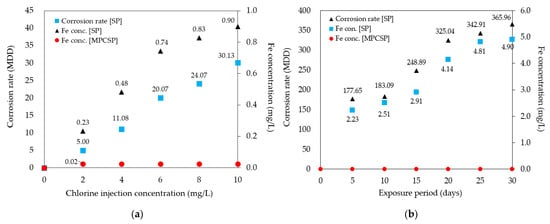
Figure 7.
Effect of coating on measured corrosion rate and Fe concentration: (a) depends on chlorine injection concentration; (b) depends on exposure period (days).
Similarly, sequential leaching experiments revealed that MPCSP specimens experienced no change in weight, maintained a zero-corrosion level, and showed no iron leaching for 30 days. Despite high concentrations of chlorine, the MPCSP exhibited no changes or surface corrosion, whereas the control suffered significant iron leaching and corrosion, leading to rust formation. Both the batch and sequential leaching experiments confirm that MPCSP possesses improved corrosion resistance.
The test results above can be attributed to the use of a thermal fusion method for lining the pipes with modified PE powder after special pretreatment. Additionally, the application of primer likely contributes to the strong adhesion of the coating material to the pipe, enhancing protection against corrosion. The synthetic resin PE coating prevented any lamination imperfections or defects on the coating surface during the experimental period, demonstrating low absorbency and improved corrosion resistance. Supporting this conclusion, the SEM results indicated no significant differences in surface corrosion between the MPCSP sample without chlorine injection and the sample after one month of injecting 1000 mg/L of chlorine. Both samples maintained uniform surfaces under magnification, as depicted in Figure 8b,d,f, with no detectable increase in porosity or surface roughness, confirming the absence of corrosion due to chlorine exposure. It should be noted that the three spots for each specimen, whether chlorine-injected or not, were randomly selected to investigate surface changes after the exposure test. In other words, the SEM images for Figure 8a,c,e and Figure 8b,d,f were taken from two separate samples, respectively.
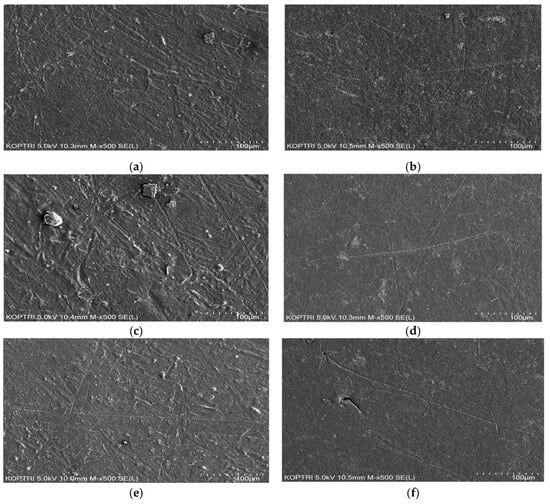
Figure 8.
SEM results for some representative samples: (a) Point 1 without chlorine injection; (b) Point 1 with 1000 mg/L of chlorine injection; (c) Point 2 without chlorine injection; (d) Point 2 with 1000 mg/L of chlorine injection; (e) Point 3 without chlorine injection; (f) Point 3 with 1000 mg/L of chlorine injection.
As shown in Table 2, the SEM-EDX results confirmed that the primary components of MPCSP following the experiments were C and O, along with trace elements such as Na, Al, Cl, K, Ca, Ti, and Fe. The presence of chlorine might be attributed to the silicon treatment applied to the surface during sample preparation. One month after chlorine injection, elements other than C and O were detected at concentrations below 1% on average, indicating a minimal impact on corrosion. Based on previous research [30], the use of modified PE powder significantly influences the adhesion between the coating and the pipe, and resistance to water absorption can be improved through phosphate washing and chemical pretreatment. Moreover, three-layer PE coating improves the adhesion and chemical resistance of the pipe [31,32]. The corrosion prevention in these samples is largely due to the improved surface from chemical pretreatment and MPP application, alongside the superior corrosion resistance of the MPCSP test specimens. Overall, it can be concluded that the MPP coating effectively prevents the distribution of sediment and the attachment of limescale on the coating.

Table 2.
SEM-EDX results of MPCSP samples.
4.2. Effect of Coating Type on Blister Formation
Figure 9 displays the results of the wet immersion experiment on both MPCSP and ECSP specimens over a set period. Specifically, it presents test results from samples collected from MPCSPs (150A) and the same sizes of ECSP pipes. Samples from the MPCSPs showed no occurrence of blisters after 100 days. Conversely, specimens from ECSPs developed blisters after just 20 days, and by 100 days, most of the film was composed of blisters. As shown in Figure 10, the results from the temperature gradient test were quite similar to those from the wet immersion test. Namely, there was faster blister formation and propagation on the ECSP within the test period, as detected through visual inspection. The test results obtained in this study showed consistency with previous studies [32] that reported the improved mechanical strength and adhesion of three-layer polypropylene coatings applied to steel pipes.
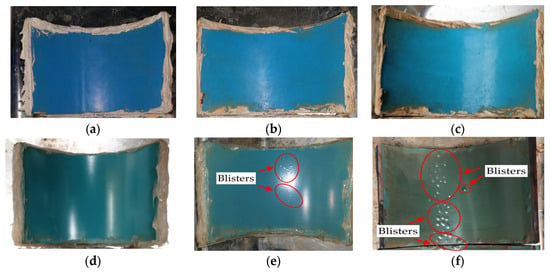
Figure 9.
Blister formation on coating surface after wet immersion test: (a) 20 days (MPCSP); (b) 50 days (MPCSP); (c) 100 days (MPCSP); (d) 20 days (ECSP); (e) 50 days (ESCP); (f) 100 days (ESCP).
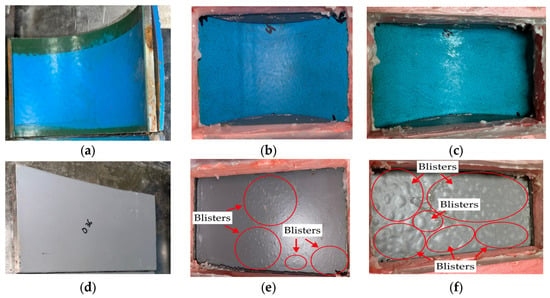
Figure 10.
Blister formation on surface after thermal gradient test: (a) 0 day (MPCSP); (b) 20 days (MPCSP); (c) 100 days (MPCSP); (d) 0 day (ECSP); (e) 20 days (ECSP); (f) 100 days (ECSP).
Another reason for the reduced blister formation on MPCSPs compared to that on ECSPs might be attributed to the chemical pretreatment adopted in this study. However, further study considering more coating types and exposure conditions might be necessary.
5. Limitations and Future Studies
This study demonstrated the enhanced corrosion resistance of steel pipes coated with MPP, indicating the potential to extend their operational lifespan and reduce maintenance costs. This could be particularly beneficial for industries reliant on pipelines, such as water supply and oil transportation. However, the effectiveness of PE as a protective barrier depends on the quality of its adhesion to the steel pipes, a concern highlighted by Lee et al. [33], who found that poor bonding could significantly undermine corrosion resistance. Moreover, our study did not fully explore the material’s behavior under varying temperature ranges, raising concerns about its performance in high-temperature environments—a limitation also noted by Kim et al. [34]. The physical durability of PE, particularly its resistance to abrasion, remains uncertain and was not addressed in this study, which aligns with gaps identified in similar research [35]. Additionally, the scope of our research was limited to specific corrosive environments and did not assess the long-term performance of lined pipes, a concern echoed in studies by Singh et al. [36].
The corrosion resistance of surface-coated steel pipes is significantly influenced by the choice of coating materials and technologies [37,38,39,40,41,42,43]. Common coatings include epoxy, known for its strong adhesion and chemical resistance [44], and PE and PP, which are preferred for their moisture barrier properties. Ceramic coatings are also increasingly utilized for their durability and resistance to harsh conditions. Innovative application techniques such as plasma spraying and thermal spraying have greatly improved the uniformity and adhesion of these coatings. Despite these advancements, significant challenges remain in coating materials and technologies.
Further research is necessary to refine materials science and surface coatings to develop more resilient solutions and improve application technologies. Integrating sensors and monitoring technologies into coated pipes can facilitate early detection and mitigation of corrosion, enabling more effective maintenance strategies. Priority areas include developing new coating materials that withstand extreme environmental conditions—such as high temperatures, variable pH levels, and high salinity. Emerging materials like advanced polymer composites and ceramic-based coatings show promise for enhancing durability and reducing permeability. Additionally, innovative application techniques that ensure consistent coating thickness and improved substrate adhesion could significantly reduce the risks of blistering and subsequent corrosion. The environmental impact of current coating materials and processes also necessitates a continued focus on sustainability to minimize the ecological footprint of protective coatings.
This comprehensive approach advances corrosion science and supports industries reliant on these critical infrastructures, ensuring safer, more reliable, and cost-effective operations. This study contributes to an ongoing research project focused on developing smart pipe systems equipped with sensors for enhanced maintenance strategies. Further results from MPCSPs exposed to harsh marine environments will be reported in subsequent publications.
6. Conclusions
An experimental study on the chlorine-induced corrosion and blister formation on modified polyethylene-coated steel pipes (MPCSPs) was conducted with comparisons to conventional epoxy coated steel pipes (ECSPs). The following conclusions can be drawn:
- MPCSPs demonstrated improved corrosion resistance compared to standard steel pipes (SPs), effectively preventing iron leaching and maintaining structural integrity under chlorine exposure.
- The modified polyethylene coating exhibited robust resistance to environmental stressors such as moisture and temperature variations, ensuring stable performance without significant degradation.
- MPCSPs did not develop blisters even after 100 days of immersion, whereas ECSPs began showing blisters as early as 50 days.
- The three-layer coating system effectively prevented blister formation, outperforming conventional epoxy resin coatings.
- The study highlights the potential of modified polyethylene to enhance both the chemical and physical properties of coatings, promoting more advanced and sustainable solutions.
Ongoing research into advanced coating technologies, including smart coatings with integrated sensors, is essential for enhancing predictive maintenance strategies and safety. Furthermore, improved coating technologies can significantly reduce both the economic costs and environmental risks associated with steel pipe corrosion. These findings suggest that further investigation into the long-term performance and environmental impact of innovative coating technologies is warranted.
Author Contributions
Conceptualization, M.K.L.; methodology, M.K.L.; software, M.O.K.; validation, M.K.L. and M.O.K.; formal analysis, M.K.L. and M.O.K.; investigation, M.K.L. and M.O.K.; resources, M.K.L.; data curation, D.K. and M.O.K.; writing—original draft preparation, D.K. and M.O.K.; writing—review and editing, M.O.K.; visualization, D.K. and M.O.K.; supervision, M.K.L.; project administration, M.K.L.; funding acquisition, M.K.L. All authors have read and agreed to the published version of the manuscript.
Funding
The funding source of this research is the Ministry of Environment of the Republic of Korea.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
Data are contained within the article.
Acknowledgments
This work was supported by a grant from Coating Korea Co., Ltd., funded by the Ministry of Environment of the Republic of Korea (RS-2021-KE001717).
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Thomson, I.; Saithala, J.R. Review of pipeline coating systems from an operator’s perspective. Corros. Eng. Sci. Technol. 2016, 51, 118–135. [Google Scholar] [CrossRef]
- Chiong, S.; Goh, P.; Ismail, A. Novel hydrophobic PVDF/APTES-GO nanocomposite for natural gas pipelines coating. J. Nat. Gas Sci. Eng. 2017, 42, 190–202. [Google Scholar] [CrossRef]
- Samimi, A. Use of Polyurethane Coating to Prevent Corrosion in Oil and Gas Pipelines Transfer. Int. J. Innov. Appl. Stud. 2012, 1, 186–193. [Google Scholar]
- Sridhar, N.; Dunn, D.; Anderko, A.; Lencka, M.; Schutt, H. Effects of water and gas compositions on the internal corrosion of gas pipelines-modeling and experimental studies. Corrosion 2001, 57, 221–235. [Google Scholar] [CrossRef]
- García-Ávila, F.; Del Pino, L.F.; Bonifaz, G.A.; Zhindòn-Arévalo, C.; Ramos-Fernàndez, L.; Garcìa-Altamirano, D.; Sanchez, C. Effect of chlorine residual on copper pipes in drinking water systems. J. Eng. Sci. Technol. Rev. 2019, 12, 119–126. [Google Scholar] [CrossRef]
- Cantor, A.F.; Park, J.K.; Vaiyavatjamai, P. The effect of chlorine on corrosion in drinking water systems. J. Am. Water Work. Assoc. AWWA 2003, 95, 112–123. [Google Scholar] [CrossRef]
- Guidetti, G.P.; Rigosi, G.L.; Marzola, R. The use of polypropylene in pipeline coatings. Prog. Org. Coat. 1996, 27, 79–85. [Google Scholar] [CrossRef]
- Li, R.; Liu, J.; Shi, A.; Luo, X.; Lin, J.; Zheng, R.; Fan, H.; Selasie, S.V.; Lin, H. A Facile Method to Modify Polypropylene Membrane by Polydopamine Coating via Inkjet Printing Technique for Superior Performance. J. Colloid Interface Sci. 2019, 552, 719–727. [Google Scholar] [CrossRef]
- Himma, N.F.; Anisah, S.; Prasetya, N.; Wenten, I.G. Advances in Preparation, Modification, and Application of Polypropylene Membrane. J. Polym. Eng. 2016, 36, 329–362. [Google Scholar] [CrossRef]
- Wasim, M.; Djukic, M.B. External corrosion of oil and gas pipelines: A review of failure mechanisms and predictive preventions. J. Nat. Gas Sci. Eng. 2022, 100, 104467. [Google Scholar] [CrossRef]
- Sani, F.M.; Brown, B.; Nesic, S. An Electrochemical Study of the Effect of High Salt Concentration on Uniform Corrosion of Carbon Steel in Aqueous CO2 Solutions. J. Electrochem. Soc. 2021, 168, 051501. [Google Scholar] [CrossRef]
- Tang, Z.; Wang, Z.; Lu, Y.; Sun, P. Cause analysis and preventive measures of pipeline corrosion and leakage accident in alkylation unit. Eng. Fail. Anal. 2021, 128, 105623. [Google Scholar] [CrossRef]
- Ashrafriahi, A.; Carcea, A.G.; Newman, R.C. An Inhibitive Effect of Aeration on the Pitting Corrosion of Steels in Ethanolic Environments. Corrosion 2022, 78, 181–188. [Google Scholar] [CrossRef]
- Zheng, R.; Zhao, X.; Dong, L.; Liu, G.; Huang, Y.; Xu, Y. On the cavitation erosion-corrosion of pipeline steel at different locations of Venturi pipe. Eng. Fail. Anal. 2022, 138, 106333. [Google Scholar] [CrossRef]
- Ormellese, M.; Beretta, S.; Brugnetti, F.; Brenna, A. Effects of non-stationary stray current on carbon steel buried pipelines under cathodic protection. Constr. Build. Mater. 2021, 281, 122645. [Google Scholar] [CrossRef]
- Kang, S.J.; Hong, M.S.; Kim, J.G. Method for Mitigating Stray Current Corrosion in Buried Pipelines Using Calcareous Deposits. Materials 2021, 14, 7905. [Google Scholar] [CrossRef]
- Han, K.-S.; Park, J.-H.; Park, Y.-B.; Kim, S.-J.; Kim, H.-D.; Choi, Y.-J.; Choi, I.-c.; Hong, S.-H. Effect of residual chlorine concentration on water pipe corrosion and corrosion control plan. Corros. Sci. Technol. 2018, 17, 12–19. [Google Scholar]
- Kim, K.; Kang, H.; Kim, T.; Iseley, D.T.; Choi, J.; Koo, J. Influencing factors analysis for drinking water steel pipe pitting corrosion using artificial neural network. Urban Water J. 2023, 20, 550–563. [Google Scholar] [CrossRef]
- Shein, A. Corrosion-electrochemical behavior of iron family silicides in various electrolytes. Prot. Met. Phys. Chem. 2010, 46, 479–488. [Google Scholar] [CrossRef]
- Kong, L.; Qi, D.; Li, H.; Ding, N.; Ge, P.; Xu, Y.; Zhang, C.; Pan, C.; Fan, X. Aging of polyethylene of raised temperature resistance pipe liner after a four-year service in a crude oil gathering system. J. Fail. Anal. Prev. 2021, 21, 1323–1330. [Google Scholar] [CrossRef]
- Yin, X.; Liang, J.; Gao, Y.; Lin, Z.; Chen, S.; Liu, C.; Tian, K.; Zhang, H.; Tang, G. Effects of LaB6 on the high-temperature oxidation behavior of TiC+TiBx reinforced titanium matrix composite coatings fabricated by laser cladding. Surf. Coat. Technol. 2021, 421, 127445. [Google Scholar] [CrossRef]
- Du, J.; Li, F.; Li, Y.; Lu, H.; Qi, X.; Yang, B.; Li, C.; Yu, P.; Wang, J.; Gao, L. The influence of nano-CeO2 on tribological properties and microstructure evolution of Cr3C2-NiCrCoMo composite coatings at high temperature. Surf. Coat. Technol. 2021, 428, 127913. [Google Scholar] [CrossRef]
- Yang, Z.-Z.; Hao, H.; Gao, Q.; Cao, Y.-B.; Han, R.-H.; Qi, H.-B. Strengthening mechanism and high-temperature properties of H13 + WC/Y2O3 laser-cladding coatings. Surf. Coat. Technol. 2021, 405, 126544. [Google Scholar] [CrossRef]
- Rajasärkkä, J.; Pernica, M.; Kuta, J.; Lašňák, J.; Šimek, Z.; Bláha, L. Drinking water contaminants from epoxy resin-coated pipes: A field study. Water Res. 2016, 103, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Verma, C.; Olasunkanmi, L.O.; Akpan, E.D.; Quraishi, M.A.; Dagdag, O.; el Gouri, M.; Sherif, E.-S.M.; Ebenso, E.E. Epoxy Resins as Anticorrosive Polymeric Materials: A Review. React. Funct. Polym. 2020, 156, 104741. [Google Scholar] [CrossRef]
- Samimi, A.; Dokhani, S.; Neshat, N.; Almasinia, B.; Setoudeh, M. The application and new mechanism of universal produce the 3-layer polyethylene coating. Int. J. Adv. Sci. Tech. Res. India 2012, 2, 465–473. [Google Scholar]
- Sastri, V.S.; Ghali, E.; Elboujdaini, M. Corrosion Prevention and Protection; Wiley and Sons: Hoboken, NJ, USA, 2007. [Google Scholar]
- Fu, A.Q.; Cheng, Y.F. Characterization of the permeability of a high performance composite coating to cathodic protection and its implications on pipeline integrity. Prog. Org. Coat. 2011, 72, 423–428. [Google Scholar] [CrossRef]
- Suárez-Vega, A.; Berriozabal, G.; Urbegain, A.; Minudri, D.; Somers, A.; Forsyth, M.; Marinova, N. Exploring Sustainable Coating Solutions for Applications in Highly Corrosive Environments. Coatings 2024, 14, 521. [Google Scholar] [CrossRef]
- Papavinasam, S.; Attard, M.; Revie, R.W. Evolution of External Pipeline Coatings for Corrosion Protection—A Review. Corros. Rev. 2008, 26, 373–438. [Google Scholar] [CrossRef]
- Samimi, A.; Zarinabadi, S. An Analysis of Polyethylene Coating Corrosion in Oil and Gas Pipelines. Am. J. Sci. 2011, 7, 103201036. [Google Scholar]
- Branch, M.; Mahshahr, I. Study an Analysis and Suggest new Mechanism of 3 layer polyethylene coating corrosion cooling water pipeline in oil refinery in Iran. Int. J. Innov. Appl. Stud. 2012, 1, 216–225. [Google Scholar]
- Lee, S.; Oh, W.; Kim, J. Acceleration and quantitative evaluation of degradation for corrosion protective coatings on buried pipeline: Part II. Application to the evaluation of polyethylene and coal-tar enamel coatings. Prog. Org. Coat. 2013, 76, 784–789. [Google Scholar] [CrossRef]
- Kim, H.-G.; Kim, I.-H.; Jung, Y.-I.; Park, D.-J.; Park, J.-Y.; Koo, Y.-H. Adhesion property and high-temperature oxidation behavior of Cr-coated Zircaloy-4 cladding tube prepared by 3D laser coating. J. Nucl. Mater. 2015, 465, 531–539. [Google Scholar] [CrossRef]
- Lee, M.K.; Cho, S.; Kim, M.O. Experimental Study on the Adhesion and Performance Evaluation of Joints for Modified Polyethylene Coated Steel Pipes. Compos. Res. 2024, 37, 238–245. [Google Scholar]
- Singh, R. Pipeline Integrity: Management and Risk Evaluation; Gulf Professional Publishing: Houston, TX, USA, 2017. [Google Scholar]
- Wang, L.L.; Chen, H.J.; Hao, L.; Lin, A.; Gan, F.X. Electrochemical corrosion behavior of electroless Ni-P coating in NaCl and H2SO4 solutions. Mater. Corros. 2010, 62, 1003–1007. [Google Scholar] [CrossRef]
- Eliyan, F.F.; Mahdi, E.-S.; Alfantazi, A. Electrochemical evaluation of the corrosion behaviour of API-X100 pipeline steel in aerated bicarbonate solutions. Corros. Sci. 2012, 58, 181–191. [Google Scholar] [CrossRef]
- Kumar, S.A.; Alagar, M.; Mohan, V. Studies on corrosion-resistant behavior of siliconized epoxy interpenetrating coatings over mild steel surface by electrochemical methods. J. Mater. Eng. Perform. 2002, 11, 123–129. [Google Scholar] [CrossRef]
- Won, B.; Kim, M.O.; Park, S.; Yi, J.H. Effects of Water Exposure on the Interfacial Bond between an Epoxy Resin Coating and a Concrete Substrate. Materials 2019, 12, 3715. [Google Scholar] [CrossRef]
- Kim, S.; Hong, H.; Han, T.H.; Kim, M.O. Early-age tensile bond characteristics of epoxy coatings for underwater applications. Coatings 2019, 9, 757. [Google Scholar] [CrossRef]
- Kim, S.; Hong, H.; Park, J.K.; Park, S.; Choi, S.I.; Kim, M.O. Effect of exposure conditions on the interfacial bond properties of SS400 plate coated with various epoxy resins. Coatings 2020, 10, 1159. [Google Scholar] [CrossRef]
- Kim, M.O.; Jeong, Y.; Kang, S.H.; Moon, J.; Yi, J.H. Tensile bond characteristics between underwater coating materials and concrete substrate. J. Korean Soc. Coast. Ocean Eng. 2018, 30, 298–305. [Google Scholar] [CrossRef]
- Zargarnezhad, H.; Asselin, E.; Wong, D.; Lam, C.C. A critical review of the time-dependent performance of polymeric pipeline coatings: Focus on hydration of epoxy-based coatings. Polymers 2021, 13, 1517. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).