Effect of the Addition of PLA on the Thermal and Mechanical Properties of Reprocessed HDPE
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Recycling Method
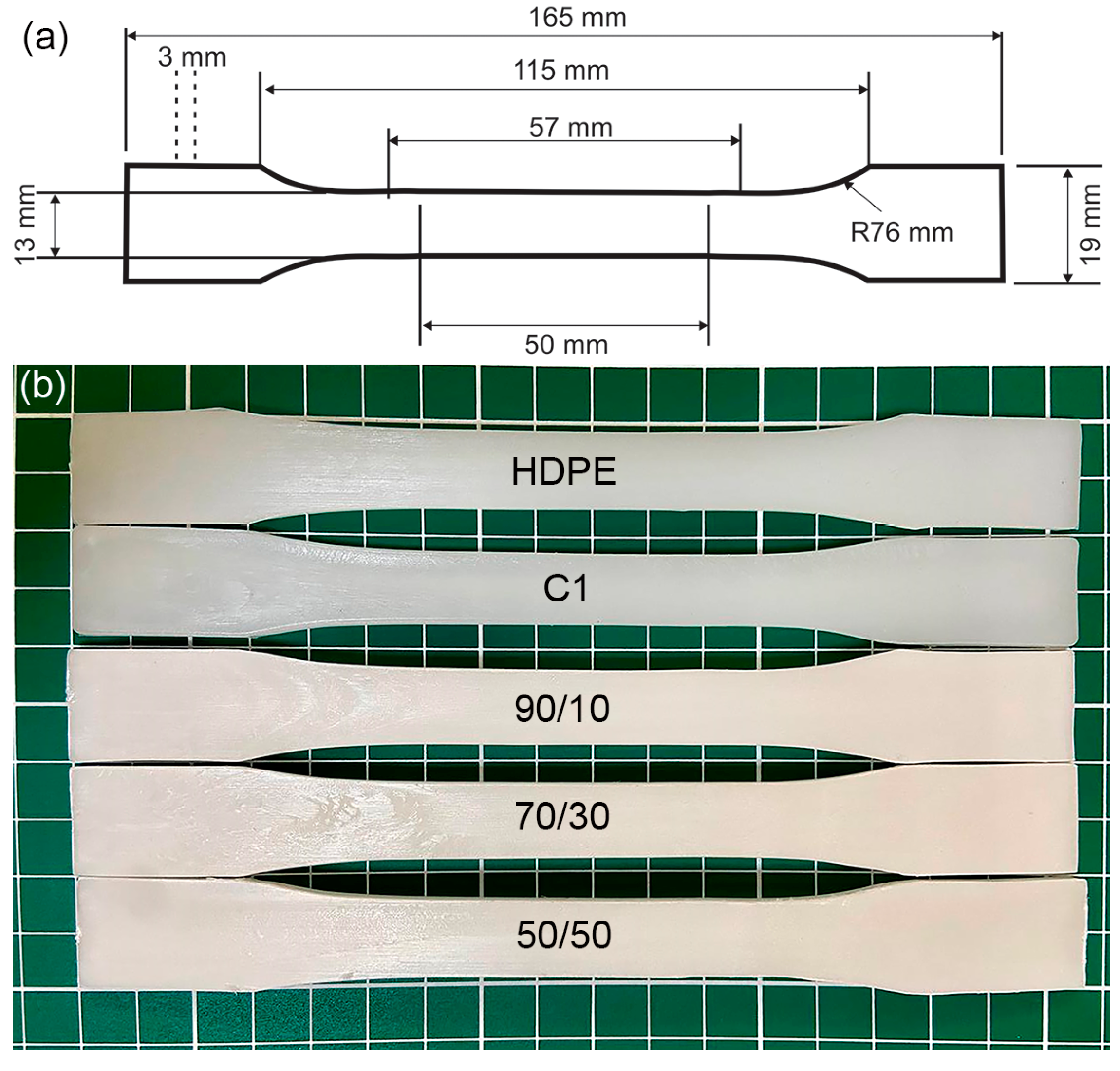
2.3. Characteristics of Samples
3. Results
3.1. Mechanical Properties
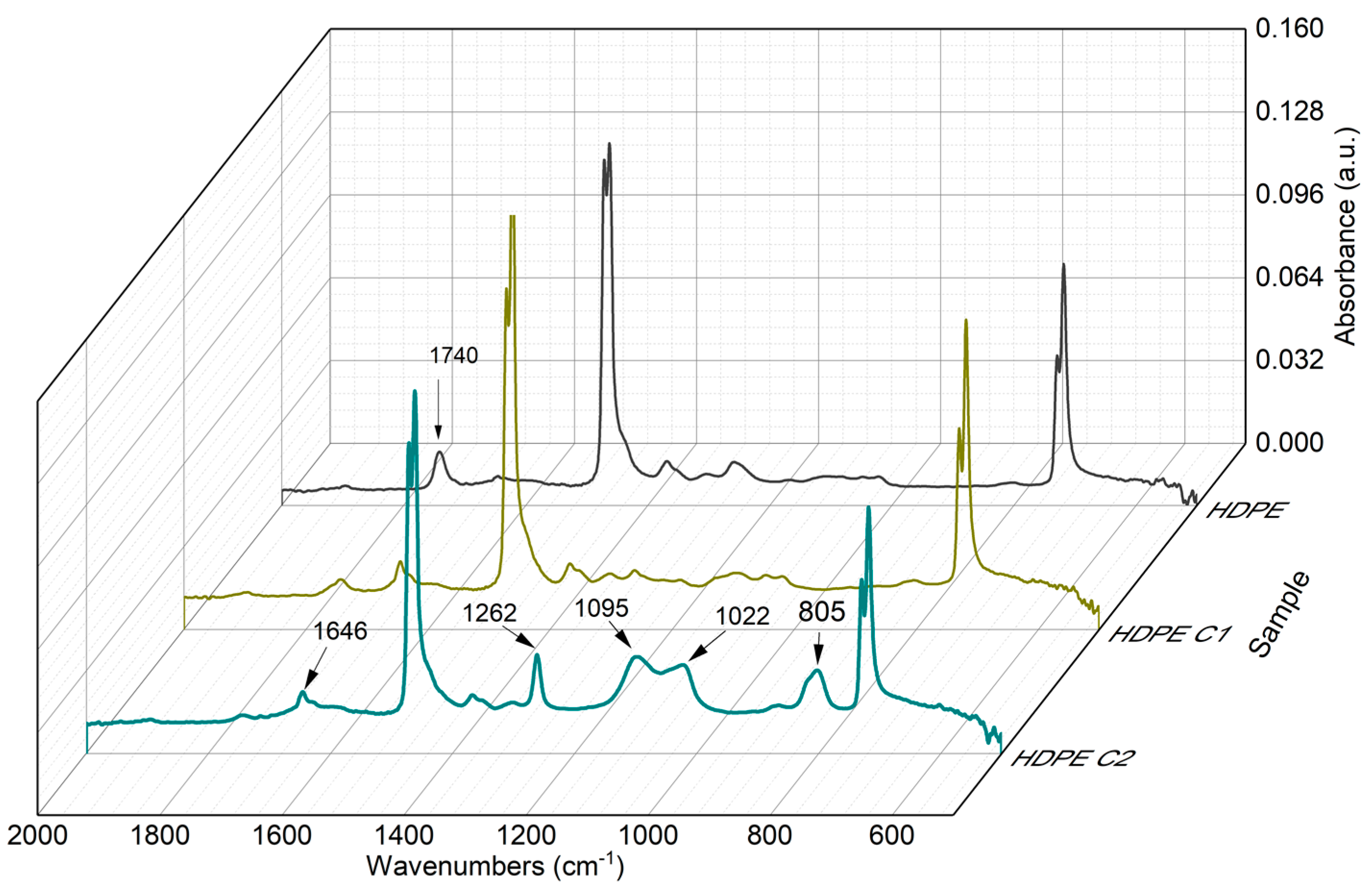
3.2. Functional Group Analysis
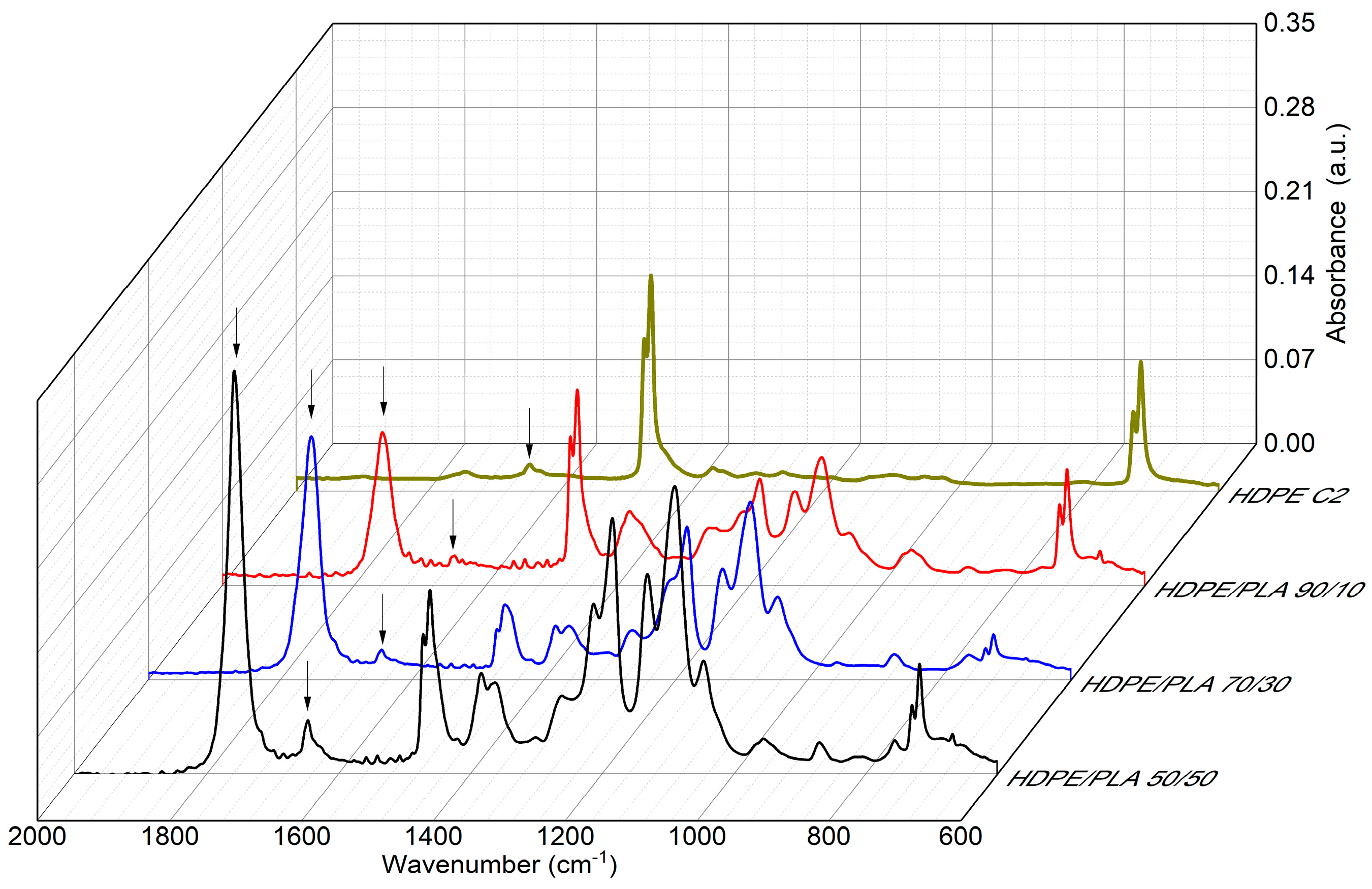
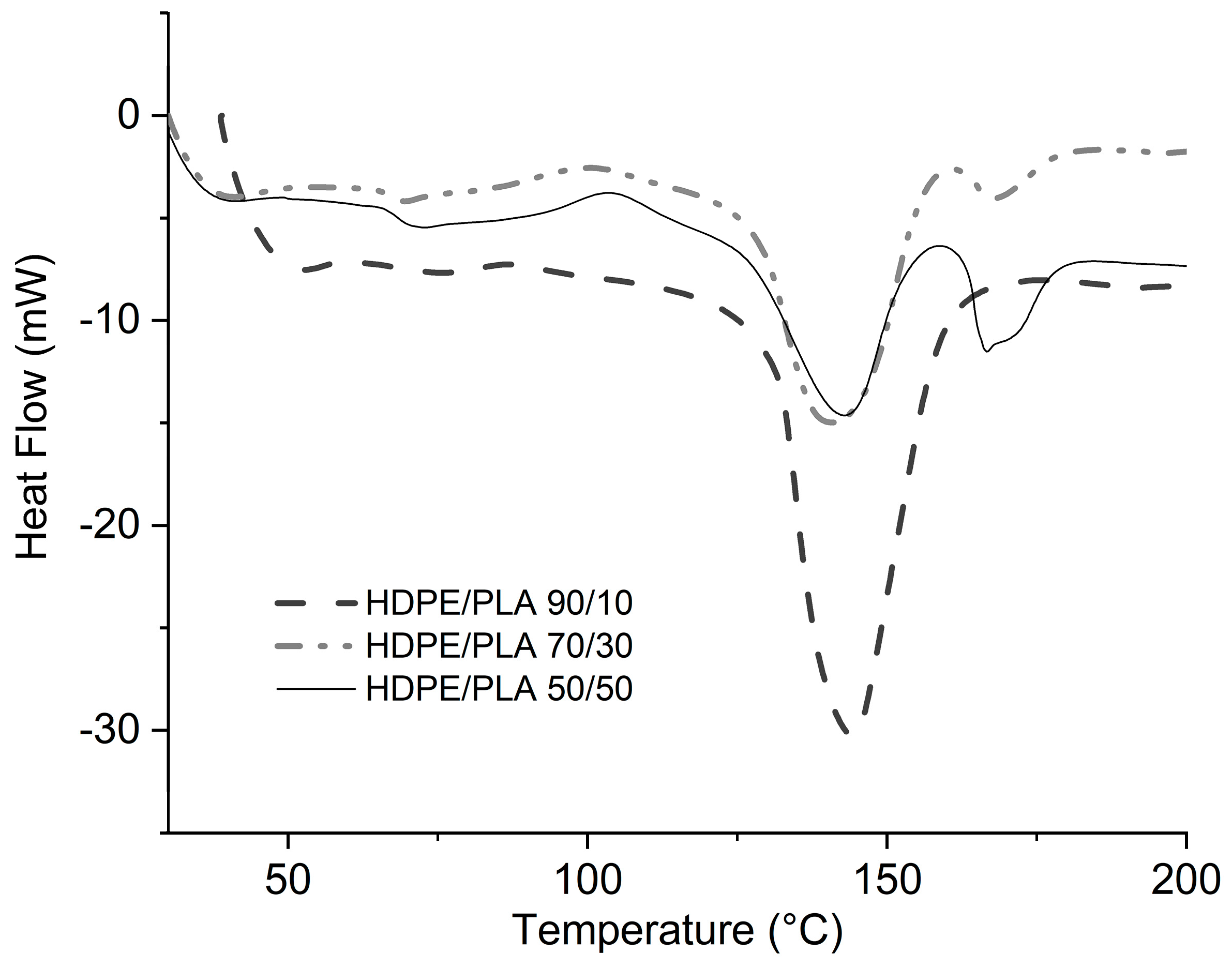
3.3. HDPE/PLA Blend Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Reciclaje de Plástico en México: El Potencial Del HDPE. Available online: https://ambienteplastico.com/reciclaje-de-plastico-en-mexico-el-potencial-del-hdpe/ (accessed on 10 July 2024).
- Aishah, N.; Bijarimi, M.; Moshiul, A.K.M.; Desa, M.S.Z.M.; Norazmi, M.; Alhadadi, W.; Hafizah, F.; Nor, M.Z.M. Mechanical and thermal properties of binary blends poly lactic acid (PLA) and recycled high-density polyethylene (rHDPE). IOP Conf. Ser. Mater. Sci. Eng. 2020, 736, 052022. [Google Scholar] [CrossRef]
- Madhu, G.; Bhunia, H.; Bajpai, P.K.; Nando, G.B. Physico-mechanical properties and biodegradation of oxo-degradable HDPE/PLA blends. Polym. Sci. Ser. A 2016, 58, 57–75. [Google Scholar] [CrossRef]
- Quitadamo, A.; Massardier, V.; Santulli, C.; Valente, M. Optimization of Thermoplastic Blend Matrix HDPE/PLA with Different Types and Levels of Coupling Agents. Materials 2018, 11, 2527. [Google Scholar] [CrossRef] [PubMed]
- Rajan, K.P.; Thomas, S.P.; Gopanna, A.; Al-Ghamdi1, A.; Chavali, M. Polyblends and composites of poly (lactic acid) (PLA): A review on the state of the art. J. Polym. Sci. Eng. 2018, 1, 723. [Google Scholar] [CrossRef]
- ASTM D638-14; Standard Test Method for Tensile Properties of Plastics. ASTM: West Conshohocken, PA, USA, 2022.
- ASTM D256-23e1; Standard Test Methods for Determining the Izod Pendulum Impact Resistance of Plastics. ASTM: West Conshohocken, PA, USA, 2023.
- del Prever, E.B.; Crova, M.; Costa, L.; Dallera, A.; Camino, G.; Gallinaro, P. Unacceptable biodegradation of polyethylene in vivo. Biomaterials 1996, 17, 873–878. [Google Scholar] [CrossRef] [PubMed]
- Medel, F.J.; Rimnac, C.M.; Kurtz, S.M. On the assessment of oxidative and microstructural changes after in vivo degradation of historical UHMWPE knee components by means of vibrational spectroscopies and nanoindentation. J. Biomed. Mater. Res. Part A 2009, 89A, 530–538. [Google Scholar] [CrossRef] [PubMed]
- Slouf, M.; Vackova, T.; Nevoralova, M.; Pokorny, D. Micromechanical properties of one-step and sequentially crosslinked UHMWPEs for total joint replacements. Polym. Test. 2015, 41, 191–197. [Google Scholar] [CrossRef]
- Slouf, M.; Gajdosova, V.; Dybal, J.; Sticha, R.; Fulin, P.; Pokorny, D.; Mateo, J.; Panisello, J.J.; Canales, V.; Medel, F.; et al. European Database of Explanted UHMWPE Liners from Total Joint Replacements: Correlations among Polymer Modifications, Structure, Oxidation, Mechanical Properties and Lifetime In Vivo. Polymers 2023, 15, 568. [Google Scholar] [CrossRef]
- Kang, P.H.; Nho, Y.C. The effect of γ-irradiation on ultra-high molecular weight polyethylene recrystallized under different cooling conditions. Radiat. Phys. Chem. 2001, 60, 79–87. [Google Scholar] [CrossRef]
- Wunderlich, B.; Czornyj, G. A Study of Equilibrium Melting of Polyethylene. Macromolecules 1977, 10, 906–913. [Google Scholar] [CrossRef]
- Torres-Huerta, A.; Nacional, P.; Domínguez-Crespo, M.; Palma-Ramírez, D.; Flores-Vela, A.; Castellanos-Alvarez, E.; Del-Angel-López, D. Preparation and degradation study of hdpe/pla polymer blends for packaging applications. Rev. Mex. Ing. Quimica 2018, 19, 251–271. [Google Scholar] [CrossRef]
- Cárdenas-Giraldo, J.M.; Rojas-González, A.F.; Galviz-Garcia, B.C. Cambios en la estructura química del polietileno de alta densidad al experimentar múltiples reprocesamientos. Rev. Ing. Univ. Medellín 2019, 18, 111–124. [Google Scholar] [CrossRef]
- Krehula, L.K.; Katančić, Z.; Siročić, A.P.; Hrnjak-Murgić, Z. Weathering of High-Density Polyethylene-Wood Plastic Composites. J. Wood Chem. Technol. 2014, 34, 39–54. [Google Scholar] [CrossRef]
- Aguiar, M.I.; Sousa, A.F.; Teixeira, G.; Tavares, A.P.; Ferreira, A.M.; Coutinho, J.A. Enhancing plastic waste recycling: Evaluating the impact of additives on the enzymatic polymer degradation. Catal. Today 2024, 429, 114492. [Google Scholar] [CrossRef]
- Zhang, Y.; Lin, Y.; Gou, H.; Feng, X.; Zhang, X.; Yang, L. Screening of Polyethylene-Degrading Bacteria from Rhyzopertha Dominica and Evaluation of Its Key Enzymes Degrading Polyethylene. Polymers 2022, 14, 5127. [Google Scholar] [CrossRef]
- Yarahmadi, N.; Jakubowicz, I.; Enebro, J. Polylactic acid and its blends with petroleum-based resins: Effects of reprocessing and recycling on properties. J. Appl. Polym. Sci. 2016, 133, 43916. [Google Scholar] [CrossRef]
- Badia, J.; Strömberg, E.; Karlsson, S.; Ribes-Greus, A. The role of crystalline, mobile amorphous and rigid amorphous fractions in the performance of recycled poly (ethylene terephthalate) (PET). Polym. Degrad. Stab. 2011, 97, 98–107. [Google Scholar] [CrossRef]
- Czarnecka-Komorowska, D.; Chandra, S.; Kopeć, B.; Borowski, J.; Garbacz, T. Investigating the Effect of Photo-Oxidative Degradation on the Ageing Resistance of the Car Mudflaps Manufactured with Post-Production High-Density Polyethylene Wastes. Adv. Sci. Technol. Res. J. 2022, 16, 38–47. [Google Scholar] [CrossRef] [PubMed]
- Oudhuis, A.; Thiewes, H.; van Hutten, P.; Brinke, G.T. A comparison between the morphology of semicrystalline polymer blends of poly(ε-caprolactone)/poly(vinyl methyl ether) and poly(ε-caprolactone)/(styrene-acrylonitrile). Polymer 1994, 35, 3936–3942. [Google Scholar] [CrossRef]

| Blend (wt%) | Temperature (°C) | ||
|---|---|---|---|
| HDPE/PLA | Zone 1 | Zone 2 | Zone 3 |
| 100/0 | 190 | 200 | 210 |
| 90/10 | 190 | 200 | 210 |
| 70/30 | 190 | 200 | 210 |
| 50/50 | 190 | 200 | 210 |
| 0/100 | 190 | 200 | 210 |
| HDPE C1 * | 190 | 200 | 210 |
| HDPE C2 ** | 190 | 200 | 210 |
| Material | Tensile Strength (MPa) |
|---|---|
| Virgin PLA | 22.41 ± 1.20 |
| Virgin HDPE | 23.69 ± 0.65 |
| HDPE C1 | 23.66 ± 0.67 |
| HDPE C2 | 23.44 ± 0.66 |
| Cycle 1 | |
| HDPE/PLA 90/10 | 23.38 ± 0.64 |
| HDPE/PLA 70/30 | 23.58 ± 0.85 |
| HDPE/PLA 50/50 | 23.28 ± 0.85 |
| Cycle 2 | |
| HDPE/PLA 90/10 | 23.32 ± 1.16 |
| HDPE/PLA 70/30 | 22.79 ± 0.67 |
| HDPE/PLA 50/50 | 21.50 ± 1.21 |
| Material | CI | χ (%) | OI | VI (×103) | Tm |
|---|---|---|---|---|---|
| HDPE | 0.67 | 57.8 | 0.001 | 2.77 | 132 |
| C1 | 0.65 | 57.6 | 0.38 | 3.60 | 135 |
| C2 | 0.59 | 57.4 | 0.90 | 4.46 | 135 |
| Material | Cycle 1 | Cycle 2 | ||||
|---|---|---|---|---|---|---|
| CI | χ (%) | OI | CI | χ (%) | OI | |
| HDPE | 0.65 | 58.6 | 0.38 | 0.59 | 58.4 | 0.90 |
| HDPE/PLA 90/10 | 0.22 | 43.9 | 3.8 | 0.98 | 42.2 | 16.4 |
| HDPE/PLA 70/30 | 0.94 | 68.5 | 5.9 | 0.98 | 52.4 | 18.9 |
| HDPE/PLA 50/50 | 0.98 | 61.6 | 7.6 | 0.96 | 57.1 | 25.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Estrada-Monje, A.; Silva-Goujon, M.A.; Rodríguez-Sánchez, I.; Conejo-Dávila, A.S.; Piñón-Balderrama, C.I.; Zaragoza-Estrada, A.; Baldenegro-Pérez, L.A.; Zaragoza-Contreras, E.A. Effect of the Addition of PLA on the Thermal and Mechanical Properties of Reprocessed HDPE. Polymers 2024, 16, 2387. https://doi.org/10.3390/polym16162387
Estrada-Monje A, Silva-Goujon MA, Rodríguez-Sánchez I, Conejo-Dávila AS, Piñón-Balderrama CI, Zaragoza-Estrada A, Baldenegro-Pérez LA, Zaragoza-Contreras EA. Effect of the Addition of PLA on the Thermal and Mechanical Properties of Reprocessed HDPE. Polymers. 2024; 16(16):2387. https://doi.org/10.3390/polym16162387
Chicago/Turabian StyleEstrada-Monje, Anayansi, Miroslava Alejandra Silva-Goujon, Isis Rodríguez-Sánchez, Alain Salvador Conejo-Dávila, Claudia Ivone Piñón-Balderrama, Anayansi Zaragoza-Estrada, Leonardo Aurelio Baldenegro-Pérez, and Erasto Armando Zaragoza-Contreras. 2024. "Effect of the Addition of PLA on the Thermal and Mechanical Properties of Reprocessed HDPE" Polymers 16, no. 16: 2387. https://doi.org/10.3390/polym16162387
APA StyleEstrada-Monje, A., Silva-Goujon, M. A., Rodríguez-Sánchez, I., Conejo-Dávila, A. S., Piñón-Balderrama, C. I., Zaragoza-Estrada, A., Baldenegro-Pérez, L. A., & Zaragoza-Contreras, E. A. (2024). Effect of the Addition of PLA on the Thermal and Mechanical Properties of Reprocessed HDPE. Polymers, 16(16), 2387. https://doi.org/10.3390/polym16162387








