Enhancing Selectivity with Molecularly Imprinted Polymers via Non-Thermal Dielectric Barrier Discharge Plasma
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. MIP Synthesis Using a Traditional Radical Reaction Process
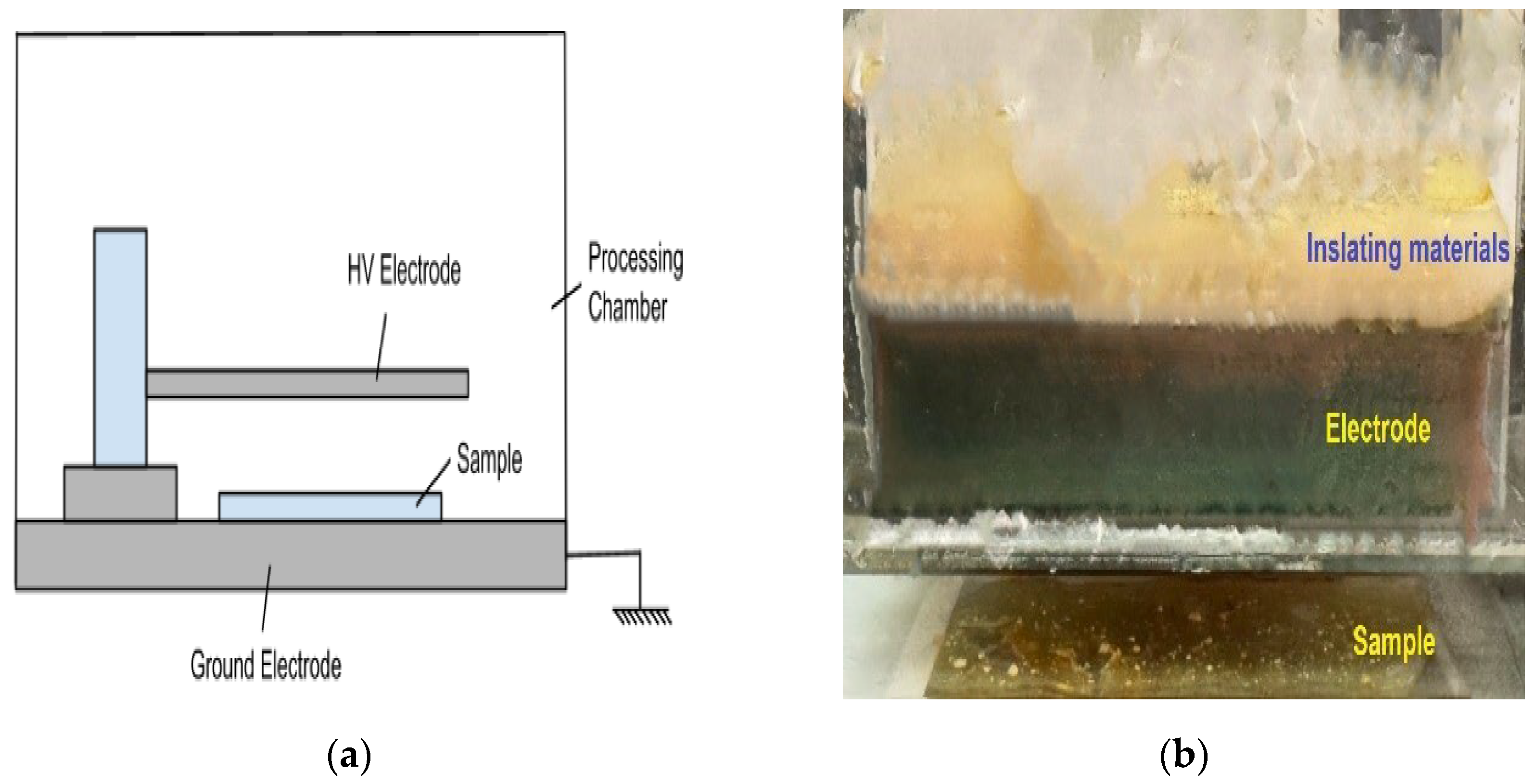
2.3. DBD Plasma Generation
2.4. MIP Synthesis Using the DBD Plasma Method
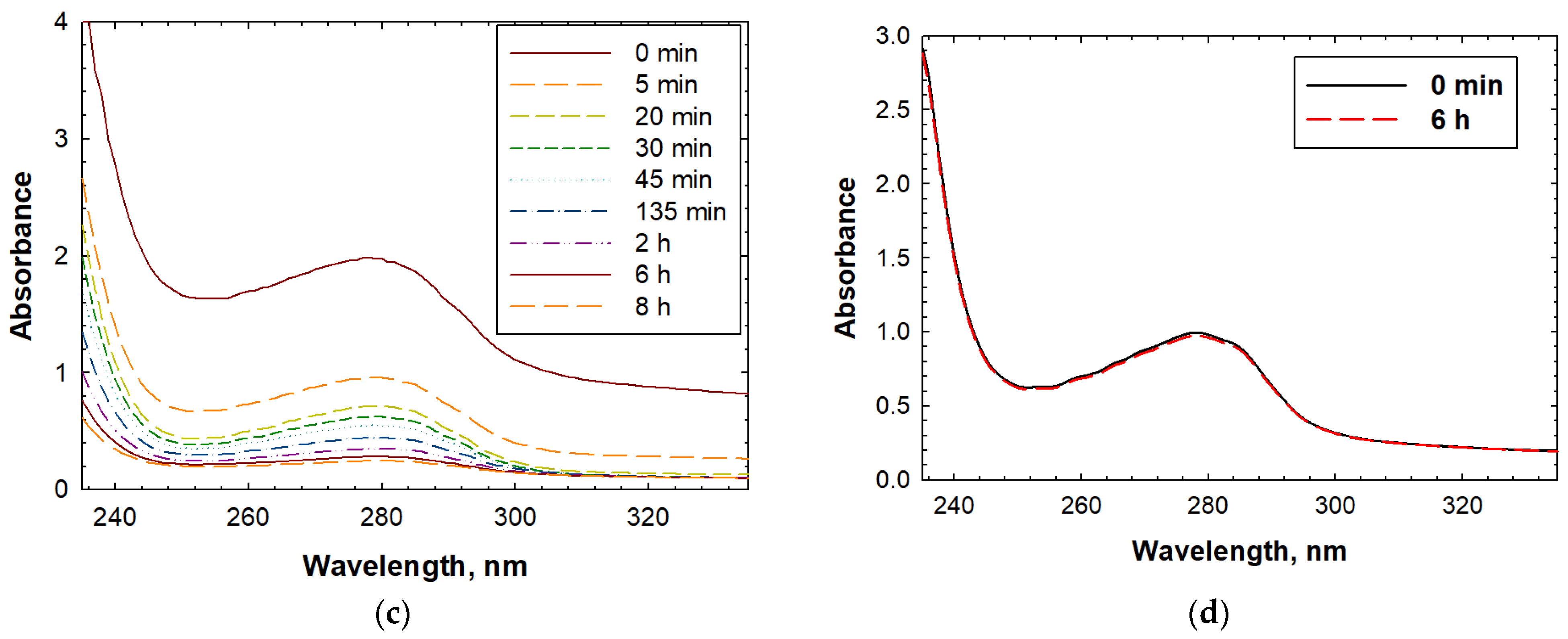
2.5. Removal of the CESA Template from the MIPs
2.6. Rebinding of CESA Using the MIPs
2.7. Rebinding of BSA and Selectivity towards CESA Using the MIPs
2.8. Characterization
3. Results and Discussion
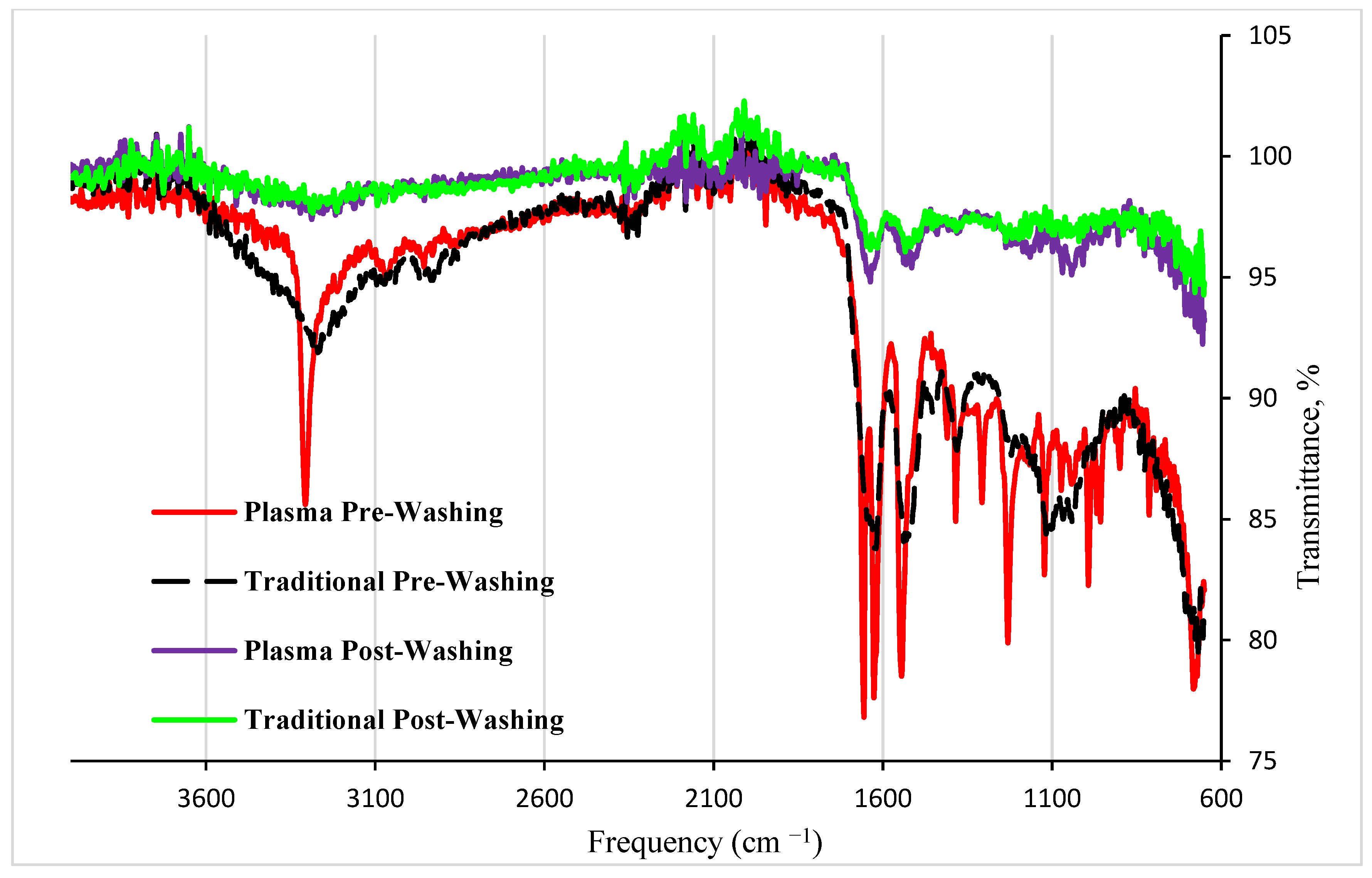
- The most characteristic peaks were observed at 1657 cm−1, corresponding to the amide I band (–C=O stretching), and at 1533 cm−1, corresponding to the amide II band (C-N stretch with N-H bending). These peaks significantly decreased after washing, indicating that most of these peaks are from the CESA protein, so the peaks diminished when CESA is removed from the MIP cavities. Additionally, the appearance of sharp peaks at 3300 cm−1 from the MIP prepared from DBD plasma, which was not observed from that prepared from traditional radical reactions, indicates more OH or NH groups in this MIP, which is the major difference between the two polymers. It is known that these groups can be generated in DBD plasma. The peak at around 890 cm−1 can be associated with the C-C stretching vibration in the polymer backbone.
- The peaks around 1040–1100 cm−1 indicate the presence of S=O stretching from sulfonate groups in the 1-(3-sulfopropyl)-2-vinylpyridinium monomer. The peaks around 1700–1725 cm−1 are attributed to the carboxylic acid in 4-vinylbenzoic acid. The peaks around 1735–1750 cm−1 are attributed to the ester C=O in 4-vinylphenyl acetate. The peaks around 1500–1600 cm−1 are due to the benzene rings in several monomers. The peaks at 3589–3602 cm−1 correspond to the O-H or N-H stretching vibrations. These peaks indicate that all six monomers have been involved in the polymerization.
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- BelBruno, J.J. Molecularly Imprinted Polymers. Chem. Rev. 2019, 119, 94–119. [Google Scholar] [CrossRef]
- Kang, M.S.; Cho, E.; Choi, H.E.; Amri, C.; Lee, J.-H.; Kim, K.S. Molecularly Imprinted Polymers (MIPs): Emerging Biomaterials for Cancer Theragnostic Applications. Biomater. Res. 2023, 27, 45. [Google Scholar] [CrossRef]
- Beltran, A.; Borrull, F.; Marcé, R.M.; Cormack, P.A.G. Molecularly-Imprinted Polymers: Useful Sorbents for Selective Extractions. TrAC Trends Anal. Chem. 2010, 29, 1363–1375. [Google Scholar] [CrossRef]
- Sellergren, B. (Ed.) Molecularly Imprinted Polymers: Man-Made Mimics of Antibodies and Their Application in Analytical Chemistry; Science Direct: Amsterdam, The Netherlands, 2001. [Google Scholar]
- Haupt, K.; Mosbach, K. Molecularly Imprinted Polymers and Their Use in Biomimetic Sensors. Chem. Rev. 2000, 100, 2495–2504. [Google Scholar] [CrossRef] [PubMed]
- Wulff, G.; Sarhan, A.; Sarhan, A.-W.; Sarhan, H. Use of Polymers with Enzyme-Analogous Structures for the Resolution of Racemates. Angew. Chem. Int. Ed. Engl. 1972, 11, 341. [Google Scholar]
- Piletsky, S.A.; Alcock, S.; Turner, A.P.F. Molecular Imprinting: At the Edge of the Third Millennium. Trends Biotechnol. 2001, 19, 9–12. [Google Scholar] [CrossRef]
- Yan, H.; Row, K.H. Characteristic and Synthetic Approach of Molecularly Imprinted Polymer. Int. J. Mol. Sci. 2006, 7, 155–178. [Google Scholar] [CrossRef]
- Liu, Z.; Xu, Z.; Wang, D.; Yang, Y.; Duan, Y.; Ma, L.; Lin, T.; Liu, H. A Review on Molecularly Imprinted Polymers Preparation by Computational Simulation-Aided Methods. Polymers 2021, 13, 2657. [Google Scholar] [CrossRef]
- Lu, W.; Wang, S.; Liu, R.; Guan, Y.; Zhang, Y. Human Serum Albumin-Imprinted Polymers with High Capacity and Selectivity for Abundant Protein Depletion. Acta Biomater. 2021, 126, 249–258. [Google Scholar] [CrossRef]
- Yang, Y.; He, X.; Xu, S.; Wang, D.; Liu, Z.; Xu, Z. Post-Imprinting Modification of Molecularly Imprinted Polymer for Proteins Detection: A Review. Int. J. Biol. Macromol. 2023, 253, 127104. [Google Scholar] [CrossRef]
- Cowie, J.M.G.; Arrighi, V. Polymers; CRC Press: Boca Raton, FL, USA, 2007; ISBN 9780429125546. [Google Scholar]
- Chen, K.; Cao, M.; Qiao, Z.; He, L.; Wei, Y.; Ji, H.-F. Polymerization of Solid-State 2,2′-Bithiophene Thin Film or Doped in Cellulose Paper Using DBD Plasma and Its Applications in Paper-Based Electronics. ACS Appl. Polym. Mater. 2020, 2, 1518–1527. [Google Scholar] [CrossRef]
- Fridman, G.; Brooks, A.D.; Balasubramanian, M.; Fridman, A.; Gutsol, A.; Vasilets, V.N.; Ayan, H.; Friedman, G. Comparison of Direct and Indirect Effects of Non-Thermal Atmospheric-Pressure Plasma on Bacteria. Plasma Process. Polym. 2007, 4, 370–375. [Google Scholar] [CrossRef]
- Joshua, B.; Smith, I.A.; Ji, H.-F. Biomolecule Response to Nonthermal Plasma. Plasma Med. 2017, 7, 427–443. [Google Scholar]
- Hegemann, D.; Nisol, B.; Watson, S.; Wertheimer, M.R. Energy Conversion Efficiency in Plasma Polymerization—A Comparison of Low- and Atmospheric-Pressure Processes. Plasma Process. Polym. 2016, 13, 834–842. [Google Scholar] [CrossRef]
- Chen, K.; Cao, M.; Feng, E.; Sohlberg, K.; Ji, H.-F. Polymerization of Solid-State Aminophenol to Polyaniline Derivative Using a Dielectric Barrier Discharge Plasma. Plasma 2020, 3, 187–195. [Google Scholar] [CrossRef]
- Mieles, M.; Stitt, C.; Ji, H.-F. Bulk Polymerization of PEGDA in Spruce Wood Using a DBD Plasma-Initiated Process to Improve the Flexural Strength of the Wood–Polymer Composite. Plasma 2022, 5, 146–153. [Google Scholar] [CrossRef]
- Mieles, M.; Harper, S.; Ji, H.-F. Bulk Polymerization of Acrylic Acid Using Dielectric-Barrier Discharge Plasma in a Mesoporous Material. Polymers 2023, 15, 2965. [Google Scholar] [CrossRef]
- Li, Y.; Atif, R.; Chen, K.; Cheng, J.; Chen, Q.; Qiao, Z.; Fridman, G.; Fridman, A.; Ji, H.-F. Polymerization of D-Ribose in Dielectric Barrier Discharge Plasma. Plasma 2018, 1, 144–149. [Google Scholar] [CrossRef]
- Abessolo Ondo, D.; Loyer, F.; Chemin, J.; Bulou, S.; Choquet, P.; Boscher, N.D. Atmospheric Plasma Oxidative Polymerization of Ethylene Dioxythiophene (EDOT) for the Large-scale Preparation of Highly Transparent Conducting Thin Films. Plasma Process. Polym. 2018, 15, 1700172. [Google Scholar] [CrossRef]
- Borra, J.; Valt, A.; Arefi-Khonsari, F.; Tatoulian, M. Atmospheric Pressure Deposition of Thin Functional Coatings: Polymer Surface Patterning by DBD and Post-Discharge Polymerization of Liquid Vinyl Monomer from Surface Radicals. Plasma Process. Polym. 2012, 9, 1104–1115. [Google Scholar] [CrossRef]
- Cools, P.; Van Vrekhem, S.; De Geyter, N.; Morent, R. The Use of DBD Plasma Treatment and Polymerization for the Enhancement of Biomedical UHMWPE. Thin Solid. Film. 2014, 572, 251–259. [Google Scholar] [CrossRef]
- Asandulesa, M.; Topala, I.; Pohoata, V.; Dumitrascu, N. Influence of Operational Parameters on Plasma Polymerization Process at Atmospheric Pressure. J. Appl. Phys. 2010, 108, 093310. [Google Scholar] [CrossRef]
- Martin, P.M. Handbook of Deposition Technologies for Films and Coatings; Elsevier: Amsterdam, The Netherlands, 2009. [Google Scholar]
- Sicherer, S.H. Food Allergy. Lancet 2002, 360, 701–710. [Google Scholar] [CrossRef]
- Mine, Y.; Yang, M. Recent Advances in the Understanding of Egg Allergens: Basic, Industrial, and Clinical Perspectives. J. Agric. Food Chem. 2008, 56, 4874–4900. [Google Scholar] [CrossRef]
- Wal, J.-M. Thermal Processing and Allergenicity of Foods. Allergy 2003, 58, 727–729. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, L.; Dang, Y.; Chen, Z.; Zhang, R.; Li, Y.; Ye, B.-C. A Robust Electrochemical Sensing of Molecularly Imprinted Polymer Prepared by Using Bifunctional Monomer and Its Application in Detection of Cypermethrin. Biosens. Bioelectron. 2019, 127, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Elaine, A.A.; Krisyanto, S.I.; Hasanah, A.N. Dual-Functional Monomer MIPs and Their Comparison to Mono-Functional Monomer MIPs for SPE and as Sensors. Polymers 2022, 14, 3498. [Google Scholar] [CrossRef]
- Kong, M.G.; Kroesen, G.; Morfill, G.; Nosenko, T.; Shimizu, T.; van Dijk, J.; Zimmermann, J.L. Plasma Medicine: An Introductory Review. New J. Phys. 2009, 11, 115012. [Google Scholar] [CrossRef]
- Laroussi, M. Low-Temperature Plasmas for Medicine? IEEE Trans. Plasma Sci. 2009, 37, 714–725. [Google Scholar] [CrossRef]
- Stoffels, E.; Sakiyama, Y.; Graves, D.B. Cold Atmospheric Plasma: Charged Species and Their Interactions with Cells and Tissues. IEEE Trans. Plasma Sci. 2008, 36, 1441–1457. [Google Scholar] [CrossRef]
- Hegemann, D.; Nisol, B.; Gaiser, S.; Watson, S.; Wertheimer, M.R. Energy Conversion Efficiency in Low- and Atmos-pheric-Pressure Plasma Polymerization Processes with Hydrocarbons. Phys. Chem. Chem. Phys. 2019, 21, 8698–8708. [Google Scholar] [CrossRef] [PubMed]
- Hegemann, D.; Nisol, B.; Watson, S.; Wertheimer, M.R. Energy Conversion Efficiency in Low- and Atmospheric-Pressure Plasma Polymerization Processes, Part II: HMDSO. Plasma Chem. Plasma Process. 2017, 37, 257–271. [Google Scholar] [CrossRef]
- Mun, M.K.; Jang, Y.J.; Kim, J.E.; Yeom, G.Y.; Kim, D.W. Plasma Functional Polymerization of Dopamine Using Atmospheric Pressure Plasma and a Dopamine Solution Mist. RSC Adv. 2019, 9, 12814–12822. [Google Scholar] [CrossRef]
- Liu, S.; Liu, D.; Pan, Z. The Effect of Polyaniline (PANI) Coating via Dielectric-Barrier Discharge (DBD) Plasma on Conductivity and Air Drag of Polyethylene Terephthalate (PET) Yarn. Polymers 2018, 10, 351. [Google Scholar] [CrossRef] [PubMed]


| Name | Structure | Properties |
|---|---|---|
| 1-(3-Sulfopropyl)-2-vinylpyridinium Hydroxide Inner Salt | 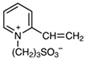 | Has both negative and positive components |
| 4-Vinylbenzoic Acid | 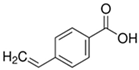 | Contains a COOH for hydrogen bonding |
| 4-Vinylphenylboronic Acid | 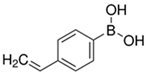 | Contains a COOH for hydrogel bonding |
| 3-Ethyl-1-vinyl-H-imidazol-3-ium bromide |  | Has a positive charge |
| 4-Methyl-5-vinylthiazole | 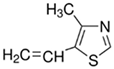 | Has a positive charge |
| 1-Vinylimidazole | 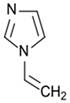 | Positive in pH 5.5 buffer solution |
| N-Vinylformamide | 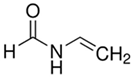 | Electrically neutral |
| 4-Vinylphenyl Acetate |  | Electrically neutral |
| 2-Methacryloyloxyethyl phosphorylcholine | 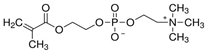 | Contains both positive and negative charges |
| Monomer Names | Mass of Monomers Used (g) |
|---|---|
| 4-Vinylbenzoic Acid | 0.03 |
| 4-Vinylphenylboronic Acid | 0.03 |
| N-Vinylformamide | 0.03 |
| 4-Vinylphenyl Acetate | 0.03 |
| 4-Vinylbenzoic Acid | 0.03 |
| 3-Ethyl-1-vinyl-H-imidazol-3-ium bromide | 0.03 |
| N-Vinylformamide | 0.03 |
| 1-(3-Sulfopropyl)-2-vinylpyridinium | 0.03 |
| N-Vinylformamide | 0.03 |
| 4-Vinylbenzoic Acid | 0.03 |
| N-Vinylformamide | 0.015 |
| 4-Vinylphenylboronic Acid | 0.015 |
| 3-Ethyl-1-vinyl-H-imidazol-3-ium Bromide | 0.015 |
| 4-Methyl-5-vinylthiazole | 0.015 |
| N-Vinylformamide | 0.02 |
| 4-Vinylbenzoic Acid | 0.02 |
| 3-Ethyl-1-vinyl-H-imidazol-3-ium bromide | 0.02 |
| 4-Vinylbenzoic Acid | 0.02 |
| 4-Methyl-5-vinylthiazole | 0.02 |
| 1-Vinylimidazole | 0.02 |
| 1-(3-Sulfopropyl)-2-vinylpyridinium | 0.01 |
| 4-Vinylbenzoic Acid | 0.01 |
| N-Vinylformamide | 0.01 |
| 1-Vinylimidazole | 0.01 |
| 4-Vinylphenyl Acetate | 0.01 |
| 4-Vinylphenylboronic Acid | 0.01 |
| 1-(3-Sulfopropyl)-2-vinylpyridinium | 0.01 |
| 4-Vinylbenzoic Acid | 0.01 |
| N-Vinylformamide | 0.01 |
| 1-Vinylimidazole | 0.01 |
| 4-Vinylphenyl Acetate | 0.01 |
| 4-Vinylphenylboronic Acid | 0.01 |
| 2-Methacryloyloxyethyl phosphorylcholine | 0.01 |
| Traditional Radical Polymerization | DBD Plasma Polymerization | |||||
|---|---|---|---|---|---|---|
| Monomers | Efficiency | Nonspecific Binding | Selectivity | Efficiency | Nonspecific Binding | Selectivity |
| 4-Vinylbenzoic Acid 4-Vinylphenylboronic Acid (A) | 68% | 20% | 3.4 | 72% | 12% | 6 |
| N-Vinylformamide 1-(3-Sulfopropyl)-2-vinylpyridinium (B) | 79% | 17% | 4.6 | 80% | 9% | 8.8 |
| N-Vinylformamide 4-Vinylphenylboronic Acid 3-Ethyl-1-vinyl-H-imidazol-3-ium Bromide 4-Methyl-5-vinylthiazole (C) | 62% | 12% | 5.1 | 65% | 7% | 9.2 |
| N-Vinylformamide 4-Vinylbenzoic Acid 3-Ethyl-1-vinyl-H-imidazol-3-ium Bromide (D) | 71% | 11% | 6.5 | 78% | 6% | 13 |
| 4-Vinylbenzoic Acid 4-Methyl-5-vinylthiazole 1-Vinylimidazole (E) | 63% | 11% | 5.72 | 81% | 7% | 11.6 |
| 1-(3-sulfopropyl)-2-vinylpyridinium 4-vinylbenzoic acid N-vinylformamide 1-vinylimidazole 4-vinylphenyl acetate 4-vinylphenylboronic acid (F) | 82% | 7% | 12.1 | 88% | 4% | 20 |
| 2-Methacryloyloxyethyl phosphorylcholine 1-(3-sulfopropyl)-2-vinylpyridinium 4-vinylbenzoic acid N-vinylformamide 1-vinylimidazole 4-vinylphenyl acetate 4-vinylphenylboronic acid (G) | 83% | 7% | 11.9 | 78% | 2% | 39 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amiri Khoshkar Vandani, S.; Liu, Q.; Lam, Y.; Ji, H.-F. Enhancing Selectivity with Molecularly Imprinted Polymers via Non-Thermal Dielectric Barrier Discharge Plasma. Polymers 2024, 16, 2380. https://doi.org/10.3390/polym16162380
Amiri Khoshkar Vandani S, Liu Q, Lam Y, Ji H-F. Enhancing Selectivity with Molecularly Imprinted Polymers via Non-Thermal Dielectric Barrier Discharge Plasma. Polymers. 2024; 16(16):2380. https://doi.org/10.3390/polym16162380
Chicago/Turabian StyleAmiri Khoshkar Vandani, Samira, Qianwei Liu, Yuki Lam, and Hai-Feng Ji. 2024. "Enhancing Selectivity with Molecularly Imprinted Polymers via Non-Thermal Dielectric Barrier Discharge Plasma" Polymers 16, no. 16: 2380. https://doi.org/10.3390/polym16162380
APA StyleAmiri Khoshkar Vandani, S., Liu, Q., Lam, Y., & Ji, H.-F. (2024). Enhancing Selectivity with Molecularly Imprinted Polymers via Non-Thermal Dielectric Barrier Discharge Plasma. Polymers, 16(16), 2380. https://doi.org/10.3390/polym16162380










