Biocompatibility and Antibacterial Activity of Eugenol and Copaiba Essential Oil-Based Emulsions Loaded on Cotton Textile Materials
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Formulation of Oil/Water (O/W) Emulsions
2.3. Preliminary Treatments of Textile Materials
2.4. Immobilization of Emulsions on the Textile Materials
2.5. Analysis of Emulsions
2.5.1. Optical Microscopy
2.5.2. Creaming Index (CI)
2.5.3. Conductometric Analysis
2.5.4. Rheology Measurement
2.6. Characterization of the Functionalized Textile Materials
2.6.1. Measurement of Antibacterial Activity
2.6.2. In Vitro Cytotoxicity Determination of Fabrics Extracts
2.6.3. In Vivo Dermal Test of Emulsion-Treated Fabrics
3. Results and Discussion
3.1. Morphological Analysis of Emulsions
3.2. Physicochemical and Stability Analysis of Emulsions
3.3. Rheological Analysis of Emulsions
3.3.1. Amplitude Sweep
3.3.2. Frequency Sweep
3.3.3. Flow Curves
3.3.4. Time Tests
3.3.5. Temperature Tests
3.4. Analysis of Antibacterial Activity
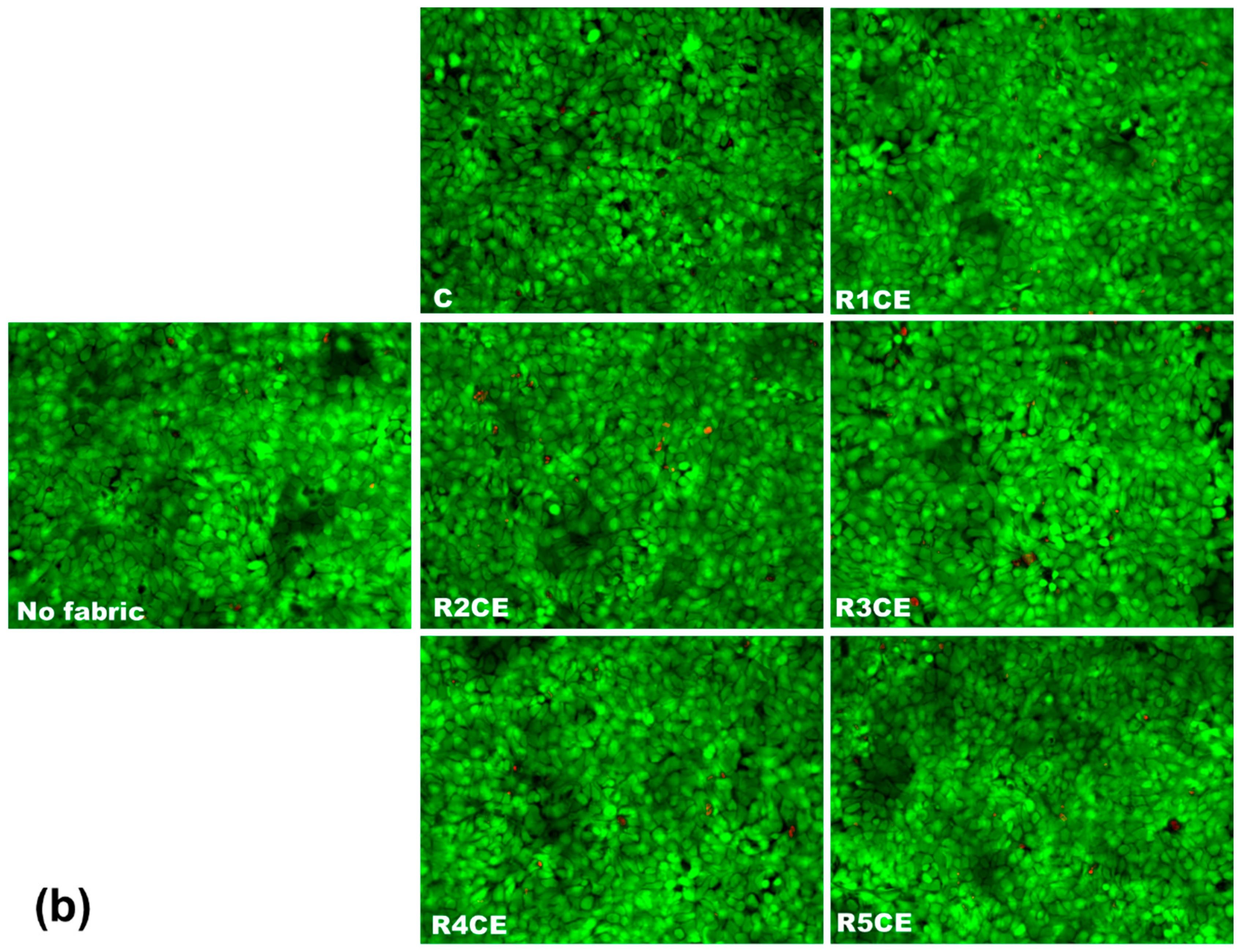
3.5. Analysis of Fabric Extracts’ Biocompatibility on Human Keratinocytes
3.6. In Vivo Evaluation of Emulsion-Treated Fabrics
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Reale, R.; Medeghini, L.; Botticelli, M. Stealing from Phytotherapy-Heritage Conservation with Essential Oils: A Review, from Remedy to Sustainable Restoration Product. Sustainability 2024, 16, 5110. [Google Scholar] [CrossRef]
- Tripa, S.; Indrie, L.; Tripa, F.; Mare, M. A Review on Deterioration of Textile Cultural Heritage Objects and Sustainable Solutions to Mitigate the Degradation. Ind. Textila 2023, 74, 555–563. [Google Scholar] [CrossRef]
- Habschied, K.; Nisevic, J.; Krstanovic, V.; Loncaric, A.; Lendic, K.V.; Mastanjevic, K. Formulation of a Wort-Based Beverage with the Addition of Chokeberry (Aronia melanocarpa) Juice and Mint Essential Oil. Appl. Sci. 2023, 13, 2334. [Google Scholar] [CrossRef]
- Childers, P.M.; Aleshire, M.E. Use of Essential Oils by Health Care Professionals for Health Maintenance. Holist. Nurs. Pract. 2020, 34, 91–102. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.C.; Liu, D.Q.; Jin, Y.; Li, D.H.; Qiao, Z.; Wang, G.L.; Xia, H.L.; Xu, L.L.; Li, E.Z. Screening of Essential Oils for the Inhibition of Enterobacter ludwigii Isolated from Tomato Fruits. J. Food Process. Preserv. 2024, 2024, 8852823. [Google Scholar] [CrossRef]
- Hao, Q.L.; Peng, H.; Zhao, R.C.; Wang, J.Z.; Lu, Z.G.; Wang, J.W.; Shen, J.; Xiao, Z.B.; Liu, G.Y.; Hao, J.F.; et al. Reactive Nano-essential Oils for Sustained Release of Essential Oils and Application to Wallpaper. Chin. Chem. Lett. 2022, 33, 320–323. [Google Scholar] [CrossRef]
- Teran, J.L.L.; Maldonado, E.V.C.; Rangel, J.D.A.; Otazo, J.P.; Rico, M.I.B.; Xie, F. Development of Antibacterial Thermoplastic Starch with Natural Oils and Extracts: Structural, Mechanical and Thermal Properties. Polymers 2024, 16, 180. [Google Scholar] [CrossRef]
- Iqhrammullah, M.; Rizki, D.R.; Purnama, A.; Duta, T.F.; Harapan, H.; Idroes, R.; Ginting, B. Antiviral Molecular Targets of Essential Oils against SARS-CoV-2: A Systematic Review. Sci. Pharm. 2023, 91, 15. [Google Scholar] [CrossRef]
- Baj, T.; Baryluk, A.; Sieniawska, E. Application of Mixture Design for Optimum Antioxidant Activity of Mixtures of Essential Oils from Ocimum basilicum L., Origanum majorana L. and Rosmarinus officinalis L. Ind. Crops Prod. 2018, 115, 52–61. [Google Scholar] [CrossRef]
- Moglad, E.H.; Abdellah, R.A.O.; Alfadhel, M.; Salkini, M.A.; Kamal, M.; Hassan, M.Z.; Yusufoglu, H.S. Chemical Composition, Antimicrobial and Anti-inflammatory Activities of Essential Oil from Cymbopogon proximus: In vitro, and in vivo Studies. Lat. Am. J. Pharm. 2021, 40, 597–602. [Google Scholar]
- Passos, G.F.; Fernandes, E.S.; da Cunha, F.M.; Ferreira, J.; Pianowski, L.F.; Campos, M.M.; Calixto, J.B. Anti-inflammatory and Anti-allergic Properties of the Essential Oil and Active Compounds from Cordia verbenacea. J. Ethnopharmacol. 2007, 110, 323–333. [Google Scholar] [CrossRef]
- Chen, Y.J.; Luo, J.X.; Zhang, N.; Yu, W.J.; Jiang, J.X.; Dai, G.H. Insecticidal activities of Salvia hispanica L. essential oil and combinations of their main compounds against the beet armyworm Spodoptera exigua. Ind. Crops Prod. 2021, 162, 113271. [Google Scholar] [CrossRef]
- Reichling, J. Antiviral and Virucidal Properties of Essential Oils and Isolated Compounds—A Scientific Approach. Planta Med. 2022, 88, 587–603. [Google Scholar] [CrossRef]
- Kinash, O.V.; Lisachenko, O.D.; Kupriyan, K.V. Fungicidal and Inhibitory Effects of Monarda Fistulosa Essential Oil and Eugenol Against Fungi of Aspergillus Genus. Med. Biol. 2018, 1, 63. [Google Scholar] [CrossRef]
- Warnke, P.H.; Becker, S.T.; Podschun, R.; Sivananthan, S.; Springer, I.N.; Russo, P.A.J.; Wiltfang, J.; Fickenscher, H.; Sherry, E. The Battle Against Multi-Resistant Strains: Renaissance of Antimicrobial Essential Oils as a Promising Force to Fight Hospital-Acquired Infections. J. Maxillofac. Surg. 2009, 37, 392–397. [Google Scholar] [CrossRef]
- Yap, P.S.X.; Yiap, B.C.; Ping, H.C.; Lim, S.H.E. Essential Oils, A New Horizon in Combating Bacterial Antibiotic Resistance. Open Microbiol. J. 2014, 8, 6–14. [Google Scholar] [CrossRef] [PubMed]
- Bahr, T.; Allred, K.; Martinez, D.; Rodriguez, D.; Winterton, P. Effects of a massage-like essential oil application procedure using Copaiba and Deep Blue oils in individuals with hand arthritis. Complement. Ther. Clin. Pract. 2018, 33, 170–176. [Google Scholar] [CrossRef]
- da Trindade, R.; da Silva, J.K.; Setzer, W.N. Copaifera of the Neotropics: A Review of the Phytochemistry and Pharmacology. Int. J. Mol. Sci. 2018, 19, 1511. [Google Scholar] [CrossRef] [PubMed]
- Urasaki, Y.; Beaumont, C.; Workman, M.; Talbot, J.N.; Hill, D.K.; Le, T.T. Fast-Acting and Receptor-Mediated Regulation of Neuronal Signaling Pathways by Copaiba Essential Oil. Int. J. Mol. Sci. 2020, 21, 2259. [Google Scholar] [CrossRef]
- Urasaki, Y.; Beaumont, C.; Talbot, J.N.; Hill, D.K.; Le, T.T. Akt3 Regulates the Tissue-Specific Response to Copaiba Essential Oil. Int. J. Mol. Sci. 2020, 21, 2851. [Google Scholar] [CrossRef]
- Woranuch, S.; Yoksan, R. Eugenol-Loaded Chitosan Nanoparticles: I. Thermal Stability Improvement of Eugenol through Encapsulation. Carbohydr. Polym. 2013, 96, 578–585. [Google Scholar] [CrossRef]
- Prasad, S.N.; Srinivas Bharath, M.M. Muralidhara, Neurorestorative Effects of Eugenol, a Spice Bioactive: Evidence in Cell Model and its Efficacy as an Intervention Molecule to Abrogate Brain Oxidative Dysfunctions in the Streptozotocin Diabetic Rat. Neurochem. Int. 2016, 95, 24–36. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, Y.; Zhu, X.; Cao, P.; Wei, S.; Lu, Y. Antibacterial and antibiofilm activities of eugenol from essential oil of Syzygium aromaticum (L.) Merr. & L. M. Perry (clove) Leaf Against Periodontal Pathogen Porphyromonas gingivalis. Microb. Pathog. 2017, 113, 396–402. [Google Scholar] [CrossRef]
- Guntero, V.A.; Ferretti, C.A.; Mancini, P.M.E.; Kneeteman, M.N. Synthesis and Encapsulation of bis-eugenol in a Mesoporous Solid Material: Enhancement of the Antioxidant Activity of a Natural Compound from Clove Oil. Chem. Sci. Int. J. 2018, 22, 1–10. [Google Scholar] [CrossRef]
- Ruiz-Gonzalez, N.; Lopez-Malo, A.; Palou, E.; Ramirez-Corona, N.; Jimenez-Munguia, M. TAntimicrobial Activity and Physicochemical Characterization of Oregano, Thyme and Clove Leave Essential Oils, Nonencapsulated and Nanoencapsulated, Using Emulsification. Appl. Food Biotechnol. 2019, 6, 237–246. [Google Scholar] [CrossRef]
- Chirila, L.; Popescu, A.; Cerempei, A.; Constantinescu, R.R.; Olaru, S.; Stan, M. Eco-Friendly Antibacterial and Biocompatible Coatings by Applying Cinnamon Essential Oil and Propolis Based Emulsions on Cotton Textiles. J. Nat. Fibers 2022, 19, 14435–14448. [Google Scholar] [CrossRef]
- Chirilă, L.; Stan, M.S.; Olaru, S.; Popescu, A.; Lite, M.-C.; Toma, D.; Voinea, I.C. Novel Collagen-Based Emulsions Embedded with Palmarosa Essential Oil, and Chamomile and Calendula Tinctures, for Skin-Friendly Textile Materials. Materials 2024, 17, 3867. [Google Scholar] [CrossRef] [PubMed]
- Rosu, G.; Muresan, E.I.; Spac, A.F.; Diaconu, M.; Ciolacu, D.E.; Danila, A.; Tita, C.; Muresan, A. Aromatherapeutic and Antibacterial Properties of Cotton Materials Treated with Emulsions Containing Peppermint Essential Oil (Menthae piperitae aetheroleum). Polymers 2023, 15, 2348. [Google Scholar] [CrossRef] [PubMed]
- ISO 20645:2004; Textile Fabrics—Determination of The Antibacterial Activity—Agar Diffusion Plate Test. International Organization for Standardization: Geneva, Switzerland, 2004.
- Fanizza, C.; Stefanelli, M.; Risuglia, A.; Bruni, E.; Ietto, F.; Incoronato, F.; Marra, F.; Preziosi, A.; Mancini, P.; Sarto, M.S.; et al. In Vitro and In Vivo Biocompatibility Studies on Engineered Fabric with Graphene Nanoplatelets. Nanomaterials 2022, 12, 1405. [Google Scholar] [CrossRef]
- ISO 10993-10; Biological Evaluation of Medical Devices–Part 10: Tests for Irritation and Skin Sensitization. International Organization for Standardization: Geneva, Switzerland, 2021.
- Shukri, N.A.; Wahit, M.U.; Hilmi, F.F.; Othman, S.N.K. Investigation of the Stability and Homogeneity of Nigella Sativa Oil-in-Water Emulsion. Mater. Today Proc. 2020, 29, 58–62. [Google Scholar] [CrossRef]
- Lv, P.; Wang, D.; Chen, Y.; Zhu, S.; Zhang, J.; Mao, L.; Gao, Y.; Yuan, F. Pickering Emulsion Gels Stabilized By Novel Complex Particles of High-Pressure-Induced WPI Gel and Chitosan: Fabrication, Characterization and Encapsulation. Food Hydrocoll. 2020, 108, 105992. [Google Scholar] [CrossRef]
- Stojiljkovic, D.; Arsic, I.; Kostov, M.T.; Jovanovic, Z.; Tadic, V.; Đorđevic, S. Investigation of the effects of different emollients on the Structure and Skin Moisturizing Potential of the Cosmetic Creams. Acta Fac. Med. Naissensis 2013, 30, 193–200. [Google Scholar] [CrossRef]
- Rahaman, S.M.; Khatun, N.; Pal, P.; Mandal, T.; Patra, A.; Nandi, M.; Saha, B. A Deeper Insight into the Evaluation of Water-In-Oil Amicroemulsion Templated Samarium Sulfide Nanospheres: Exploring Its Role in Pickering Emulsion Formulation for Photocatalytic Dye Degradation and Synthesis of PANI@Sm2S3 Nanocomposites. Nanoscale Adv. 2024, 6, 1688–1703. [Google Scholar] [CrossRef]
- Lotos, E.D.; Danila, A.; Vasiliu, A.L.; Rosca, I.; Stroian, D.V.; Simionescu, B.C.; Mihai, M. The Potential Emulsions of Xanthan Gum and Daucus Carota Macerated Oil in Functional Textiles for Skincare Applications: Formulation, Characterization, and Performance Evaluation. Colloids Surf. A Physicochem. Eng. Asp. 2024, 682, 132960. [Google Scholar] [CrossRef]
- Calero, N.; Munoz, J.; Cox, P.W.; Heuer, A.; Guerrero, A. Influence of chitosan concentration on the stability, microstructure and rheological properties of O/W emulsions formulated with high-oleic sunflower oil and potato protein. Food Hydrocoll. 2013, 33, 152–162. [Google Scholar] [CrossRef]
- Mwangi, W.W.; Ho, K.-W.; Tey, B.T.; Chan, E.S. Effects of environmental factors on the physical stability of pickering-emulsions stabilized by chitosan particles. Food Hydrocoll. 2016, 60, 543–550. [Google Scholar] [CrossRef]
- Klinkeson, U. The role of chitosan in emulsion formation and stabilization. Food Rev. Int. 2013, 29, 371–393. [Google Scholar] [CrossRef]
- Zhang, F.; Cai, X.; Ding, L.; Wang, S. Effect of pH, ionic strength, chitosan deacetylation on the stability and rheological properties of O/W emulsions formulated with chitosan/casein complexes. Food Hydrocoll. 2021, 111, 1048–1054. [Google Scholar] [CrossRef]
- Speer, S.; Amin, S. Sustainable thermoresponsive whey protein- and chitosan-based oil-in-water emulsions for cosmetic applications. Inter. J. Cosmet. Sci. 2022, 44, 30–41. [Google Scholar] [CrossRef]
- Sharkawy, A.; Barreiro, M.F.; Rodrigues, A.E. Chitosan-based Pickering emulsions and their applications: A review. Carbohydr. Polym. 2020, 250, 116885. [Google Scholar] [CrossRef] [PubMed]
- de Souza Soares, L.; de Faria, J.T.; Amorim, M.L.; de Araujo, J.M.; Minim, L.A.; dos Reis Coimbra, J.S.; de Carvalho Teixeira, A.V.M.; de Oliveira, E.B. Rheological and physicochemical studies on emulsions formulated with chitosan previously dispersed in aqueous solutions of lactic acid. Food Biophys. 2017, 12, 109–118. [Google Scholar] [CrossRef]
- Ribeiro, E.F.; de Barros-Alexandrino, T.T.; Assis, O.B.G.; Junior, A.C.; Quiles, A.; Hernando, I.; Nicoletti, V.R. Chitosan and crosslinked nanoparticles: Synthesis, characterization and their role as Pickering emulsifiers. Carbohydr. Polym. 2020, 250, 116878. [Google Scholar] [CrossRef]
- Yuan, D.B.; Hu, Y.Q.; Zeng, T.; Yin, S.W.; Tang, C.H.; Yang, X.Q. Development of stable Pickering emulsions/oil powders and Pickering HIPEs stabilized by gliadin/chitosan complex particles. Food Funct. 2017, 8, 2220–2230. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Pei, Y.; Qiao, M.; Ma, F.; Ren, H.; Zhao, Q. Preparation and characterizations of Pickering emulsions stabilized by hydrophobic starch particles. Food Hydrocoll. 2015, 45, 256–263. [Google Scholar] [CrossRef]
- Tzoumaki, M.V.; Moschakis, T.; Kiosseoglu, V.; Biliaderis, C.G. Oil-in-water emulsions stabilized by chitin nanocrystal particles. Food Hydrocoll. 2011, 25, 1521–1529. [Google Scholar] [CrossRef]
- Li, F.; Li, X.; Huang, K.; Luo, Y.; Mei, X. Preparation and characterization of pickering emulsion stabilized by hordein-chitosan complex particles. J. Food Eng. 2021, 292, 110275. [Google Scholar] [CrossRef]
- Bi, C.; Gao, F.; Zhu, Y.; Ji, F.; Zhang, Y.; Li, D.; Huang, Z. Effects of xanthan gum on the rheological properties of soy protein dispersion. Int. J. Agric. Biol. Eng. 2018, 11, 208–213. [Google Scholar] [CrossRef]
- Wong, S.K.; Low, L.E.; Supramanian, J.; Manickam, S.; Wong, T.W.; Pang, C.H.; Tang, S.Y. Physical stability and rheological behavior of Pickering emulsions stabilized by protein-polysaccharide hybrid nanoconjugates. Nanotechnology 2021, 10, 1293–1305. [Google Scholar] [CrossRef]
- Asfour, M.H.; Elmotasem, H.; Mostafa, D.M.; Salama, A.A.A. Chitosan based Pickering emulsion as a promising approach for topical application of rutin in a solubilized form intended for wound healing: In vitro and in vivo study. Int. J. Pharm. 2017, 534, 325–338. [Google Scholar] [CrossRef]
- Calero, N.; Munoz, J.; Ramirez, P.; Guerrero, A. Flow behaviour, linear viscoelastic and surface properties of chitosan aqueous solutions. Food Hydrocoll. 2010, 24, 659–666. [Google Scholar] [CrossRef]
- Gasbarro, N.M.; Solomon, M.J. Yield stress and rheology of a self-associating chitosan solution. Rheol. Acta 2019, 58, 729–739. [Google Scholar] [CrossRef]
- Roman, L.; Martinez, M.M.; Gomez, M. Assessing of the potential of extruded flour paste as fat replacer in O/W emulsion: A rheological and microstructural study. Food Res. Int. 2015, 74, 72–79. [Google Scholar] [CrossRef]
- Graca, C.; Raymundo, A.; Sousa, I. Rheology changes in oil-in-water emulsions stabilized by a complex system of animal and vegetable proteins induced by thermal processing. LWT-Food Sci. Technol. 2016, 74, 263–270. [Google Scholar] [CrossRef]
- Espert, M.; Salvador, A.; Sanz, T.; Hernandez, M.J. Cellulose ether emulsions as fat source in cocoa creams: Thermorheological properties (flow and viscoelasticity). LWT-Food Sci. Technol. 2020, 117, 108640. [Google Scholar] [CrossRef]
- Niknam, R.; Ghanbarzadeh, B.; Ayaseh, A.; Rezagholi, F. The effects of Plantago majer seed gum on steady and dynamic oscillatory shear rheology of sunflower oil-in-water emulsions. J. Texture Stud. 2018, 49, 536–547. [Google Scholar] [CrossRef]
- El-hefian, E.A.; Yahaya, A.H. Rheological study of chitosan and its blends: An overview. Maeja Int. J. Sci. Technol. 2010, 4, 210–220. Available online: http://www.mijst.mju.ac.th/vol4/210-220.pdf (accessed on 25 July 2024).
- Ellis, A.L.; Norton, A.B.; Mills, T.B.; Norton, I.T. Stabilisation of foams by agar particles. Food Hydrocoll. 2017, 73, 222–228. [Google Scholar] [CrossRef]
- Rinaudo, M. Chitin and chitosan: Properties and applications. Prog. Polym. Sci. 2006, 31, 603–632. [Google Scholar] [CrossRef]
- Speiciene, V.; Guilmineau, F.; Kulozik, U.; Leskauskaite, D. The effect of chitosan on the properties of emulsions stabilized by whey proteins. Food Chem. 2007, 102, 1048–1054. [Google Scholar] [CrossRef]
- Pinto, E.P.; Menezes, R.P.; Tavares, W.d.S.; Ferreira, A.M.; de Sousa, F.F.O.; da Silva, G.A.; Zamora, R.R.; Araújo, R.S.; de Souza, T.M. Copaiba essential oil loaded-nanocapsules film as a potential candidate for treating skin disorders: Preparation, characterization, and antibacterial properties. Int. J. Pharm. 2023, 633, 122608. [Google Scholar] [CrossRef]
- Rodrigues, V.M.; Oliveira, W.N.; Pereira, D.T.; Alencar, É.N.; Porto, D.L.; Aragão, C.F.S.; Moreira, S.M.G.; Rocha, H.A.O.; Amaral-Machado, L.; Egito, E.S.T. Copaiba Oil-Loaded Polymeric Nanocapsules: Production and In Vitro Biosafety Evaluation on Lung Cells as a Pre-Formulation Step to Produce Phytotherapeutic Medicine. Pharmaceutics 2023, 15, 161. [Google Scholar] [CrossRef]
- Nigro, F.; Cerqueira, C.; Rossi, A.; Cardoso, V.; Vermelho, A.B.; Ricci, E., Jr.; Santos, E.P.; Mansur, C.R.E. Development, characterization and in vitro toxicity evaluation of nanoemulsion-loaded hydrogel based on copaiba oil and coenzyme Q10. Colloid Surf. A Physicochem. Eng. Asp. 2020, 586, 124132. [Google Scholar] [CrossRef]
- de Araújo Lopes, A.; da Fonseca, F.N.; Rocha, T.M.; de Freitas, L.B.; Araújo, E.V.O.; Wong, D.V.T.; Lima Júnior, R.C.P.; Leal, L.K.A.M. Eugenol as a Promising Molecule for the Treatment of Dermatitis: Antioxidant and Anti-inflammatory Activities and Its Nanoformulation. Oxid. Med. Cell Longev. 2018, 2018, 8194849. [Google Scholar] [CrossRef]










| Code | Chitosan | Tween 80 | Glycerol | Hamamelis Water | Eugenol | Copaiba Essential Oil | Droplet Diameter (µm) |
|---|---|---|---|---|---|---|---|
| R1CE | 30 | 1.67 | 9 | 53.33 | 3 | 3 | 2.26 ± 0.56 |
| R2CE | 40 | 1.67 | 9 | 43.33 | 3 | 3 | 2.38 ± 0.92 |
| R3CE | 50 | 1.67 | 9 | 33.33 | 3 | 3 | 2.61 ± 0.77 |
| R4CE | 50 | 1.67 | 9 | 37.33 | 1 | 1 | 2.19 ± 0.68 |
| R5CE | 50 | 1.67 | 9 | 35.33 | 2 | 2 | 2.15 ± 0.54 |
| Parameters | Emulsions | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| R1CE | R2CE | R3CE | R4CE | R5CE | ||||||
| Time of Storage | 0 h | 4 h | 0 h | 4 h | 0 h | 4 h | 0 h | 4 h | 0 h | 4 h |
| Conductivity (μS/cm) | 356.7 | 353.9 | 340.2 | 339.1 | 331.6 | 329.4 | 373.6 | 372.5 | 345.2 | 344.3 |
| Main Fitting Parameters | Emulsions | ||||
|---|---|---|---|---|---|
| R1CE | R2CE | R3CE | R4CE | R5CE | |
| η0 (Pa·s) | 0.2408 | 0.5686 | 0.7613 | 0.8692 | 1.106 |
| η∞·108 (Pa·s) | 93.3 | 2.18 | 2.59 | 2.93 | 3.27 |
| p | 0.068 | 0.149 | 0.152 | 0.151 | 0.175 |
| R2 | 0.942 | 0.979 | 0.936 | 0.972 | 0.986 |
| Standard deviation | ±0.06 | ±0.02 | ±0.04 | ±0.03 | ±0.03 |
| Diameter of Inhibition Zone (mm) | ||||||
|---|---|---|---|---|---|---|
| C | R1CE | R2CE | R3CE | R4CE | R5CE | |
| E. coli | 0 | 9.2 ± 1.194 | 8.7 ± 0.760 | 13.1 ± 1.563 | 7.6 ± 1.207 | 11.2 ± 1.689 |
| S. aureus | 0 | 9.4 ± 0.987 | 9.3 ± 2.209 | 12.7 ± 1.232 | 11.1 ± 1.563 | 12.4 ± 1.447 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chirilă, L.; Stan, M.S.; Voinea, I.C.; Popescu, A.; Ene, A.-G.; Danu, M.; Ibănescu, C.; Lite, M.-C. Biocompatibility and Antibacterial Activity of Eugenol and Copaiba Essential Oil-Based Emulsions Loaded on Cotton Textile Materials. Polymers 2024, 16, 2367. https://doi.org/10.3390/polym16162367
Chirilă L, Stan MS, Voinea IC, Popescu A, Ene A-G, Danu M, Ibănescu C, Lite M-C. Biocompatibility and Antibacterial Activity of Eugenol and Copaiba Essential Oil-Based Emulsions Loaded on Cotton Textile Materials. Polymers. 2024; 16(16):2367. https://doi.org/10.3390/polym16162367
Chicago/Turabian StyleChirilă, Laura, Miruna S. Stan, Ionela C. Voinea, Alina Popescu, Alexandra-Gabriela Ene, Maricel Danu, Constanța Ibănescu, and Mihaela-Cristina Lite. 2024. "Biocompatibility and Antibacterial Activity of Eugenol and Copaiba Essential Oil-Based Emulsions Loaded on Cotton Textile Materials" Polymers 16, no. 16: 2367. https://doi.org/10.3390/polym16162367
APA StyleChirilă, L., Stan, M. S., Voinea, I. C., Popescu, A., Ene, A.-G., Danu, M., Ibănescu, C., & Lite, M.-C. (2024). Biocompatibility and Antibacterial Activity of Eugenol and Copaiba Essential Oil-Based Emulsions Loaded on Cotton Textile Materials. Polymers, 16(16), 2367. https://doi.org/10.3390/polym16162367








