Silybin-Functionalized PCL Electrospun Fibrous Membranes for Potential Pharmaceutical and Biomedical Applications
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Electrospinning
2.3. Physicochemical Characterization of the Electrospun Membranes
2.3.1. Morphological Characterization
2.3.2. Thermal Analysis
2.3.3. Encapsulation Efficiency
2.3.4. Drug Release Study
2.4. Biological Characterization of the Electrospun Membranes
2.4.1. Cell Culture
2.4.2. Cell Viability Assay
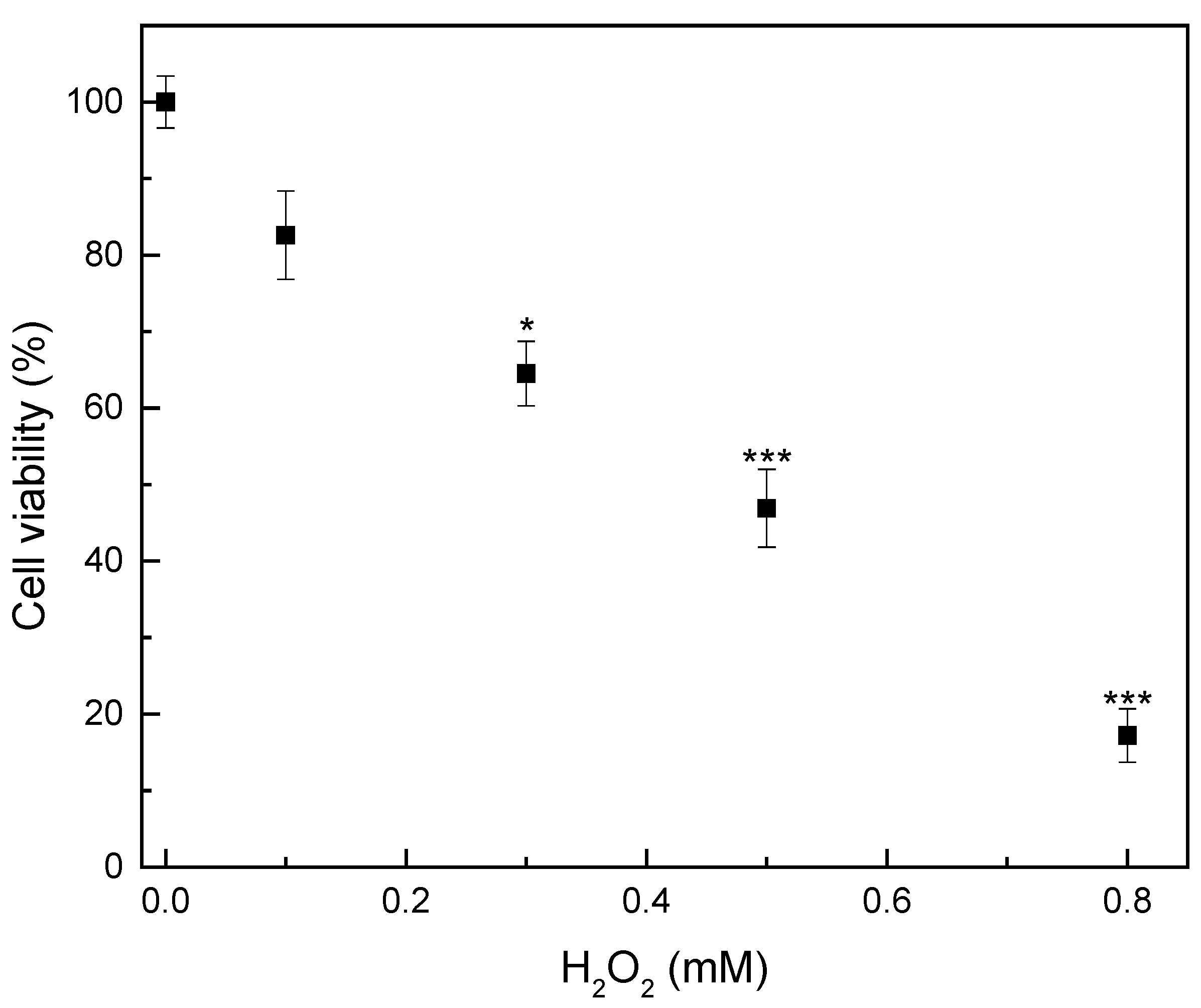
2.4.3. Investigation of Hydrogen Peroxide-Induced Oxidative Damage Pattern
2.4.4. Protective Effects of Silybin against H2O2-Induced Oxidative Stress in HEK-293 Cells
3. Results and Discussion
3.1. Physiochemical Characterization of Silybin-Encapsulated Fibrous Membranes
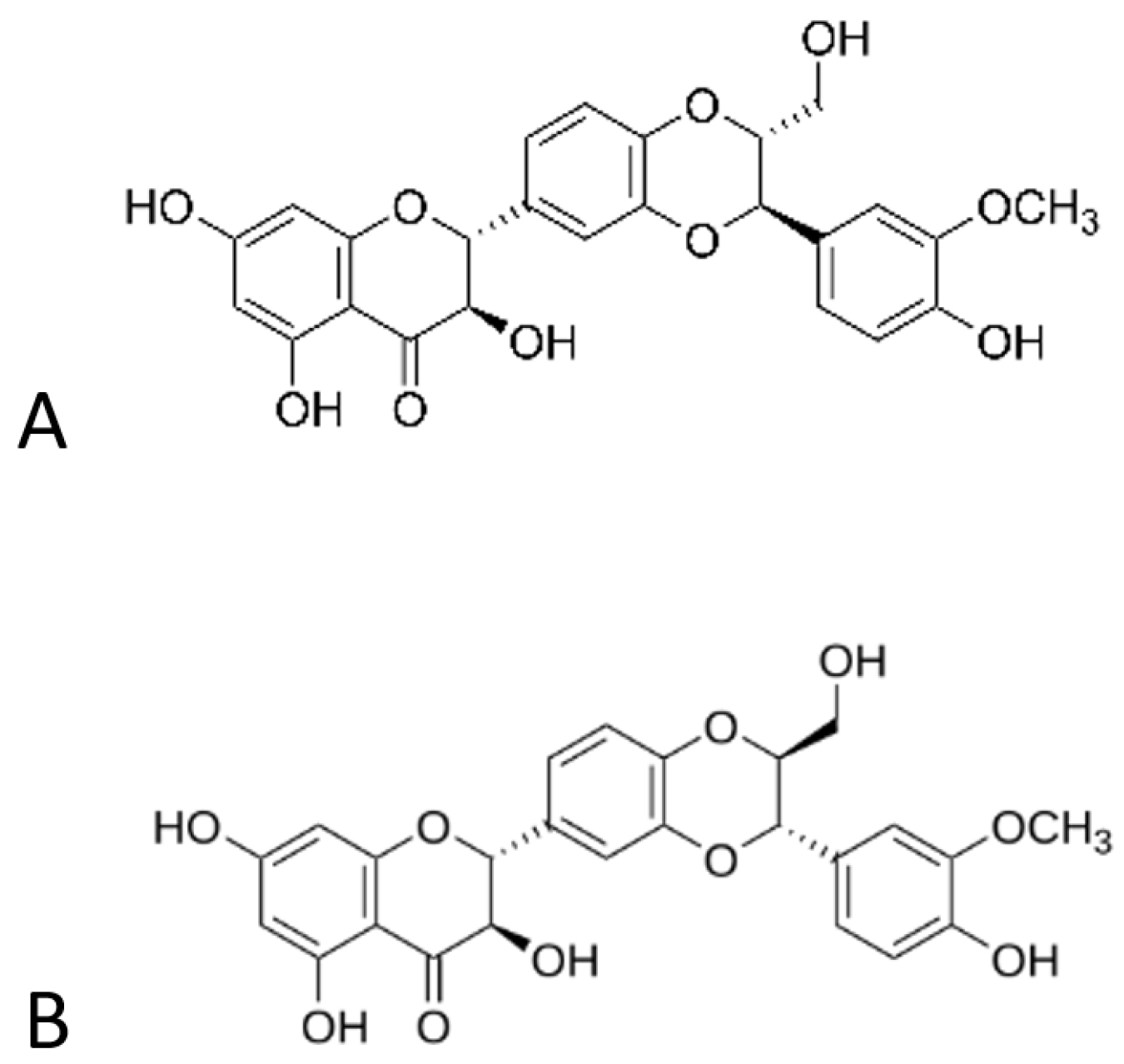
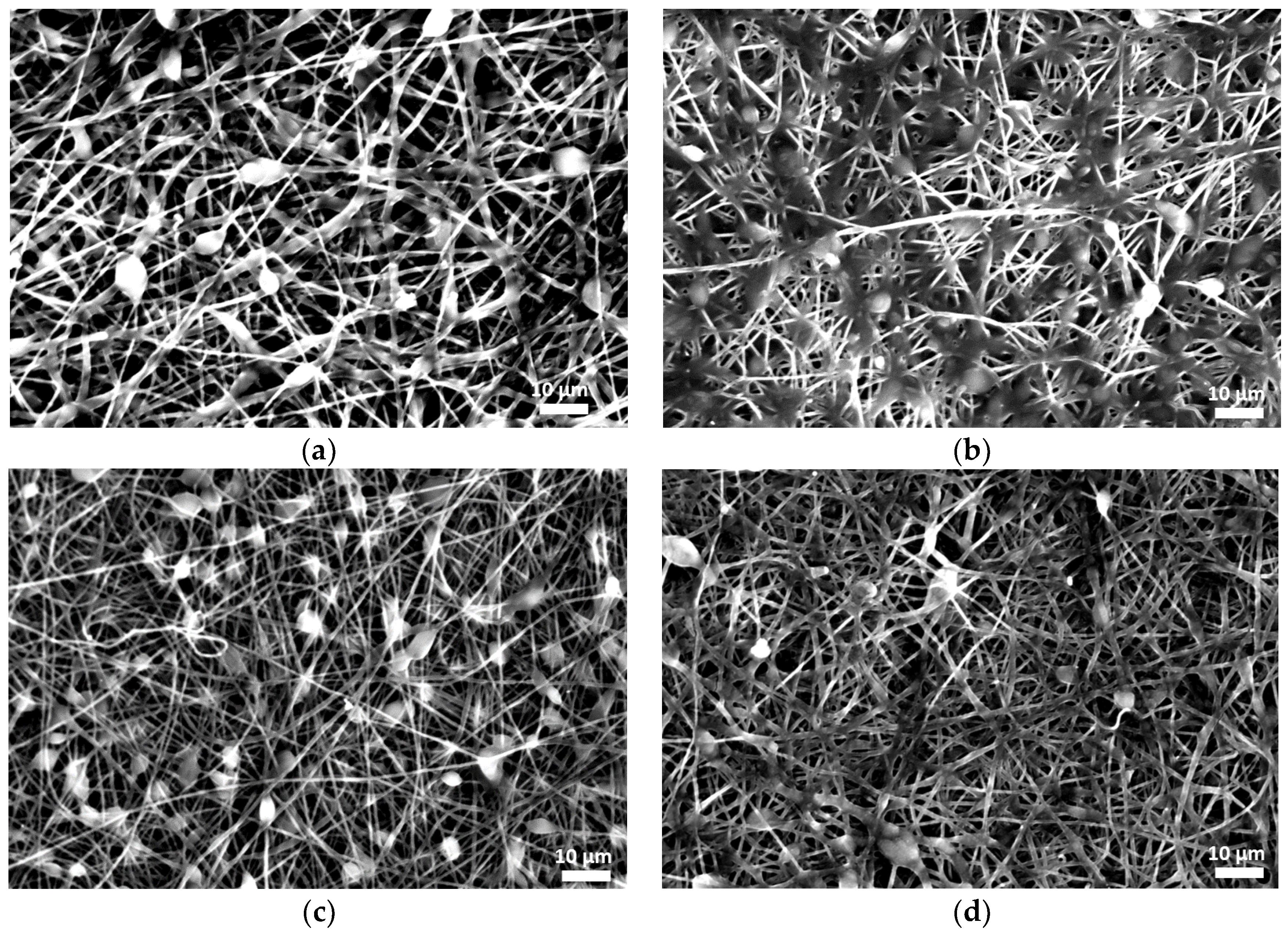
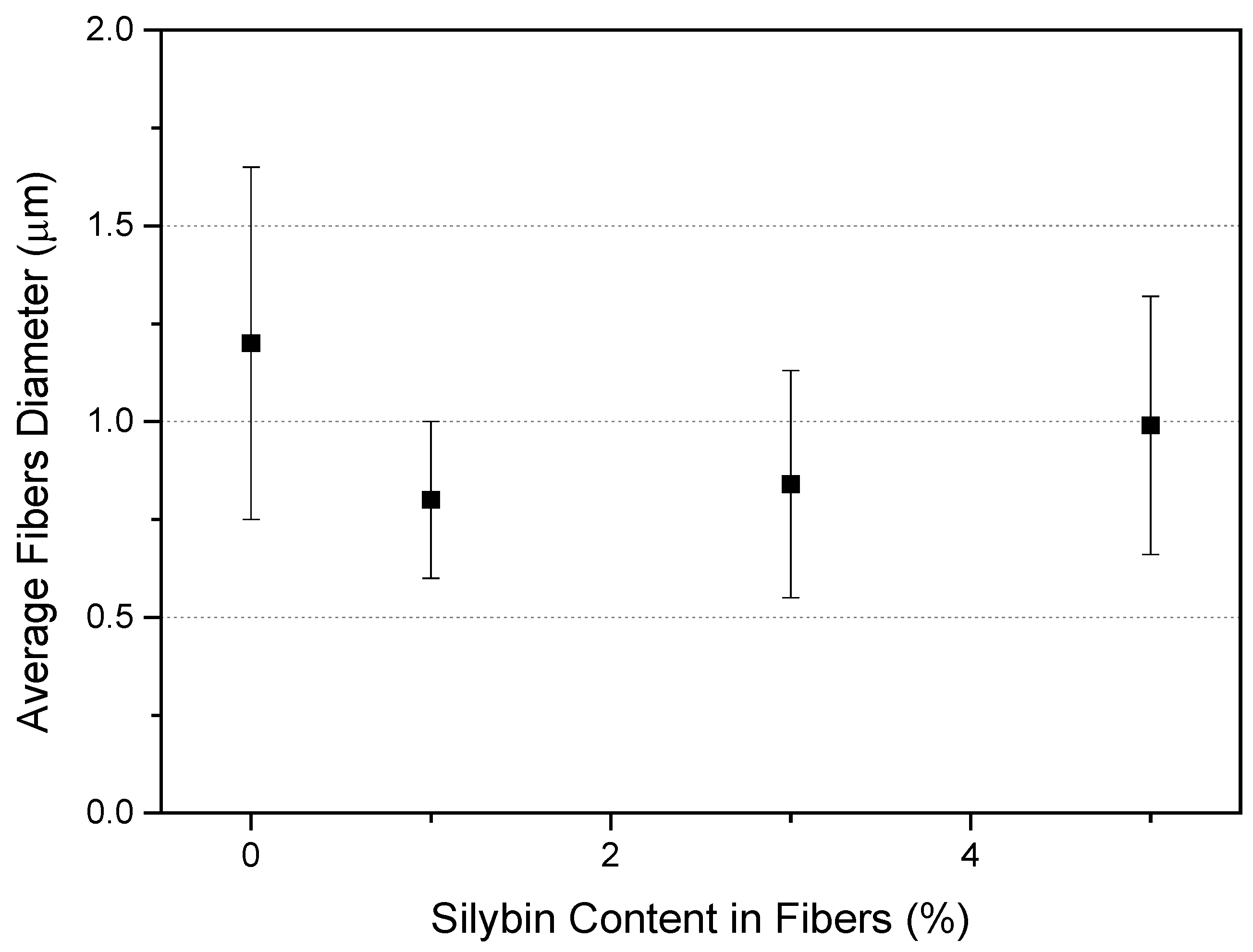
3.1.1. Morphology of Fibrous Membranes
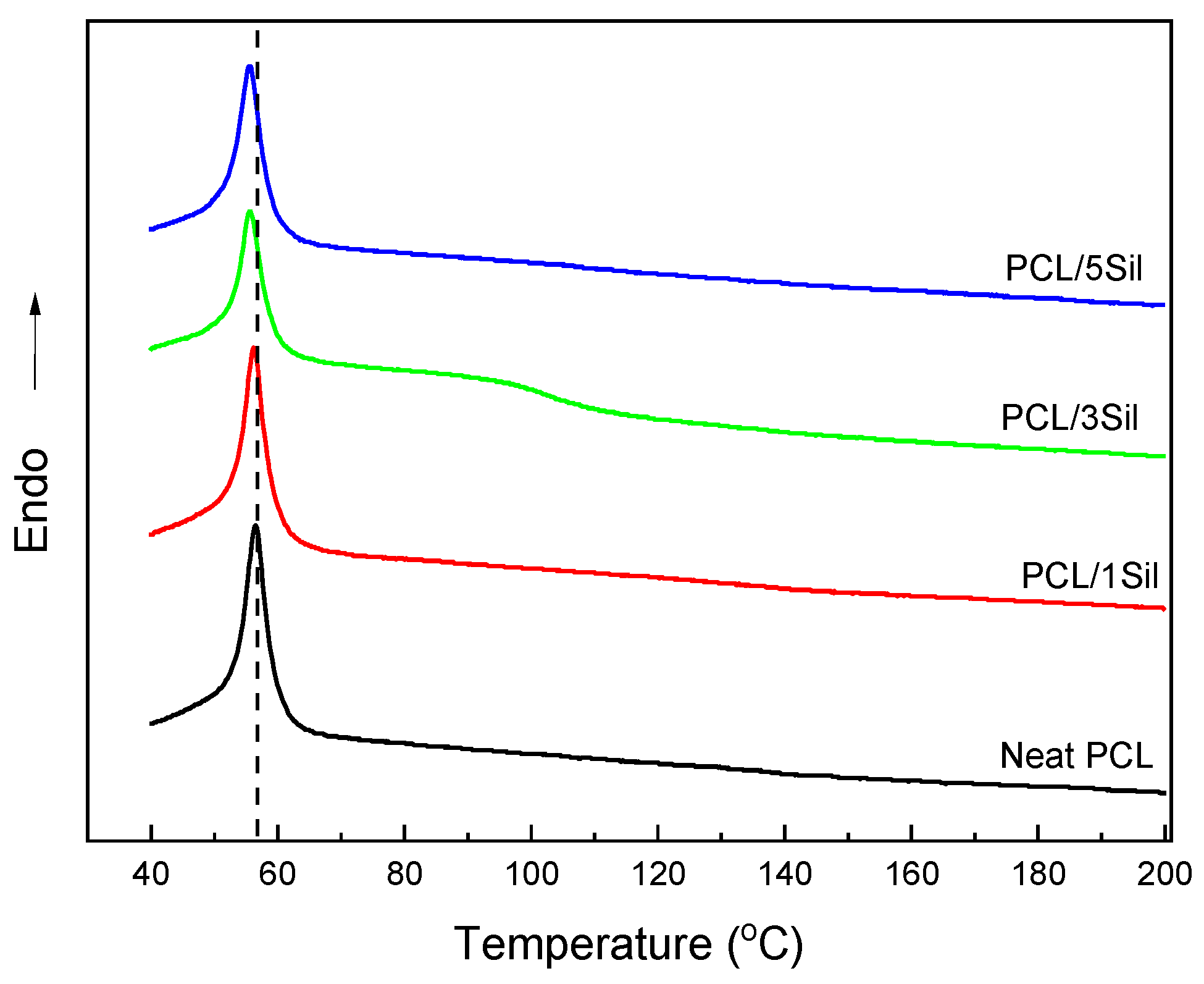
3.1.2. Thermal Behavior of Fibrous Membranes
3.1.3. Encapsulation Efficiency and Drug Loading
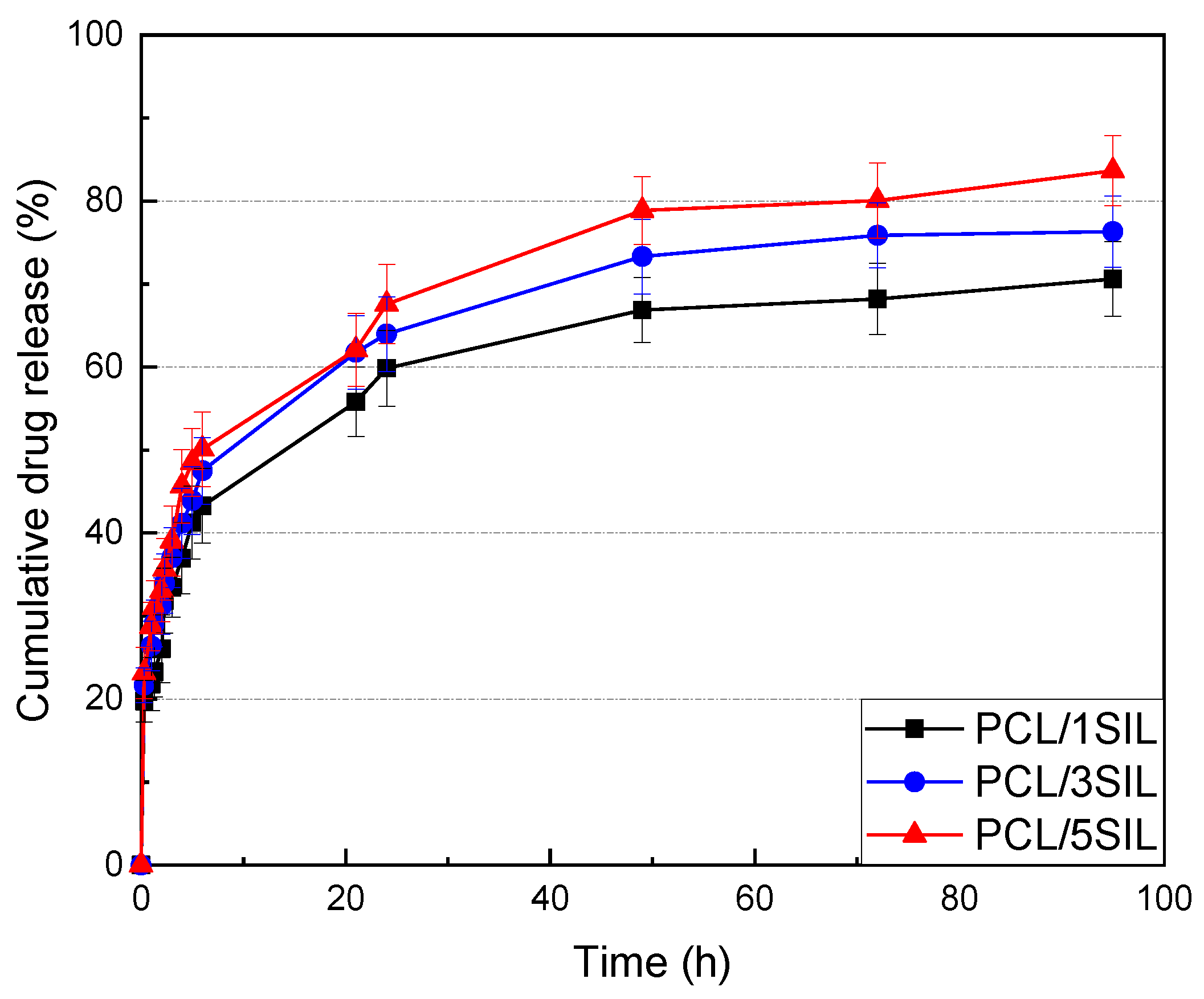
3.1.4. In Vitro Release of Silybin
3.2. Biological Characterization of Silybin-Encapsulated Fibrous Membranes
3.2.1. Biocompatibility Evaluation
3.2.2. In Vitro Antitumor Activity
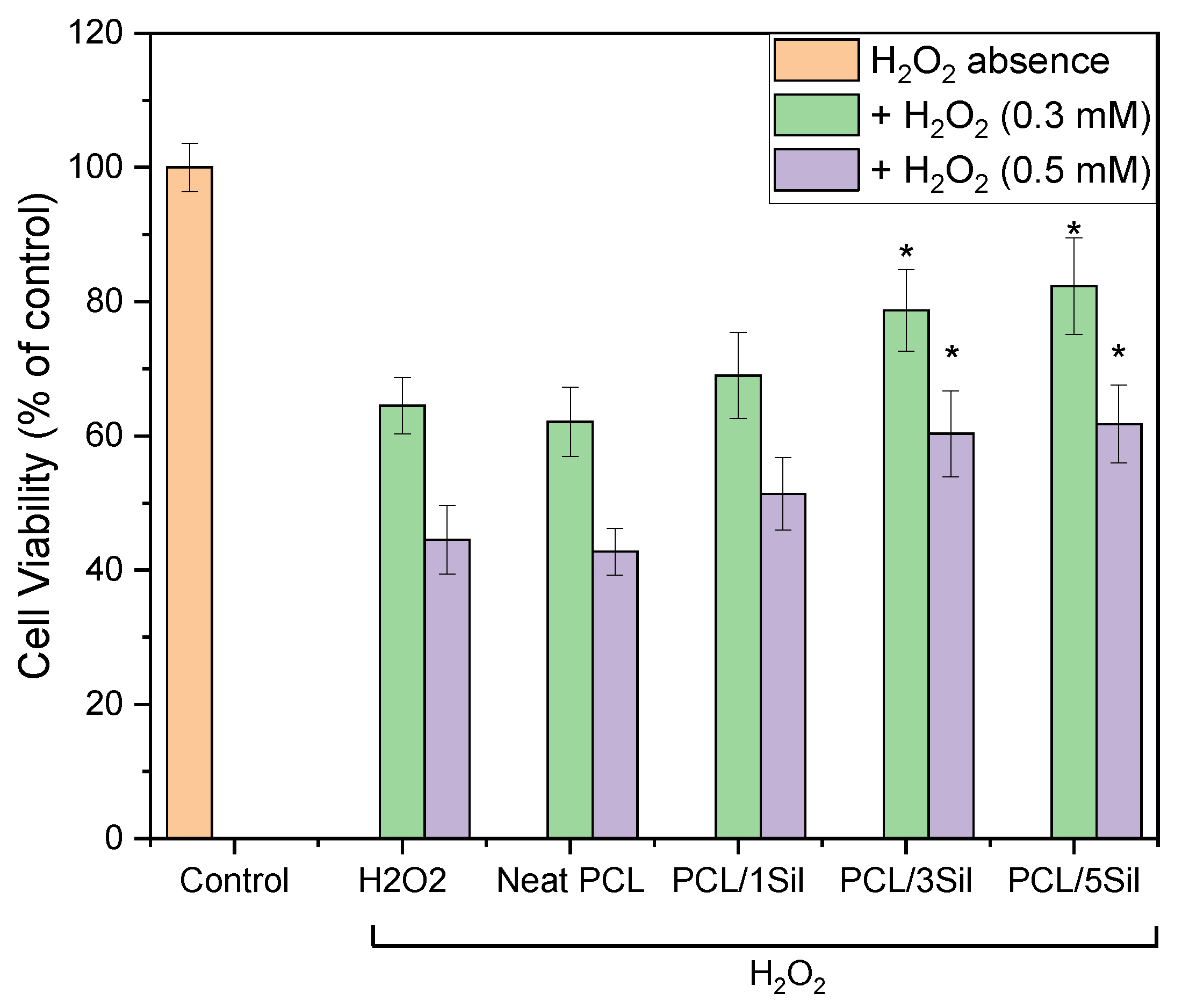
3.2.3. Antioxidant Activity of Fibrous Membranes in H2O2-Induced Oxidative Stress
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Abenavoli, L.; Izzo, A.A.; Milić, N.; Cicala, C.; Santini, A.; Capasso, R. Milk thistle (Silybum marianum): A concise overview on its chemistry, pharmacological, and nutraceutical uses in liver diseases. Phytother. Res. 2018, 32, 2202–2213. [Google Scholar] [CrossRef] [PubMed]
- Bijak, M.J.M. Silybin, a major bioactive component of milk thistle (Silybum marianum L. Gaernt.)—Chemistry, bioavailability, and metabolism. Molecules 2017, 22, 1942. [Google Scholar] [CrossRef] [PubMed]
- Esmaeil, N.; Anaraki, S.B.; Gharagozloo, M.; Moayedi, B. Silymarin impacts on immune system as an immunomodulator: One key for many locks. Int. Immunopharmacol. 2017, 50, 194–201. [Google Scholar] [CrossRef]
- Gazak, R.; Walterova, D.; Kren, V. Silybin and silymarin-new and emerging applications in medicine. Curr. Med. Chem. 2007, 14, 315–338. [Google Scholar] [CrossRef] [PubMed]
- Ullah, A.; Munir, S.; Badshah, S.L.; Khan, N.; Ghani, L.; Poulson, B.G.; Emwas, A.H.; Jaremko, M. Important Flavonoids and Their Role as a Therapeutic Agent. Molecules 2020, 25, 5243. [Google Scholar] [CrossRef] [PubMed]
- Zarenezhad, E.; Abdulabbas, H.T.; Kareem, A.S.; Kouhpayeh, S.A.; Barbaresi, S.; Najafipour, S.; Mazarzaei, A.; Sotoudeh, M.; Ghasemian, A. Protective role of flavonoids quercetin and silymarin in the viral-associated inflammatory bowel disease: An updated review. Arch. Microbiol. 2023, 205, 252. [Google Scholar] [CrossRef] [PubMed]
- Ahlenstiel, T.; Burkhardt, G.; Köhler, H.; Kuhlmann, M.K. Bioflavonoids attenuate renal proximal tubular cell injury during cold preservation in Euro-Collins and University of Wisconsin solutions. Kidney Int. 2003, 63, 554–563. [Google Scholar] [CrossRef] [PubMed]
- Federico, A.; Dallio, M.; Loguercio, C. Silymarin/Silybin and Chronic Liver Disease: A Marriage of Many Years. Molecules 2017, 22, 191. [Google Scholar] [CrossRef] [PubMed]
- Ferenci, P. Silymarin in the treatment of liver diseases: What is the clinical evidence? Clin. Liver Dis. 2016, 7, 8–10. [Google Scholar] [CrossRef]
- Saller, R.; Meier, R.; Brignoli, R.J.D. The use of silymarin in the treatment of liver diseases. Drugs 2001, 61, 2035–2063. [Google Scholar] [CrossRef] [PubMed]
- Boojar, M.M.A.; Boojar, M.M.A.; Golmohammad, S. Overview of Silibinin anti-tumor effects. J. Herb. Med. 2020, 23, 100375. [Google Scholar] [CrossRef]
- Nawaz, A.; Zaib, S.; Khan, I.; Ahmed, A.; Shahzadi, K.; Riaz, H. Silybum marianum: An overview of its phytochemistry and pharmacological activities with emphasis on potential anticancer properties. Anti-Cancer Agents Med. Chem. 2023, 23, 1519–1534. [Google Scholar] [CrossRef] [PubMed]
- Koltai, T.; Fliegel, L. Role of silymarin in cancer treatment: Facts, hypotheses, and questions. J. Evid. Based Integr. Med. 2022, 27, 2515690X211068826. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Li, Y.J.; Ding, Y.; Zhang, H.N.; Sun, T.; Zhang, K.; Yang, L.; Guo, Y.Y.; Liu, S.B.; Zhao, M.G.; et al. Silibinin Prevents Autophagic Cell Death upon Oxidative Stress in Cortical Neurons and Cerebral Ischemia-Reperfusion Injury. Mol. Neurobiol. 2016, 53, 932–943. [Google Scholar] [CrossRef]
- Borah, A.; Paul, R.; Choudhury, S.; Choudhury, A.; Bhuyan, B.; Talukdar, A.D.; Choudhury, M.D.; Mohanakumar, K.P. Neuroprotective Potential of Silymarin against CNS Disorders: Insight into the Pathways and Molecular Mechanisms of Action. CNS Neurosci. Ther. 2013, 19, 847–853. [Google Scholar] [CrossRef] [PubMed]
- Seo, S.; Kim, G.-Y.; Kim, M.-H.; Lee, K.W.; Kim, M.-J.; Chaudhary, M.; Bikram, K.; Kim, T.; Choi, S.; Yang, H.; et al. Nanocrystal Formulation to Enhance Oral Absorption of Silybin: Preparation, In Vitro Evaluations, and Pharmacokinetic Evaluations in Rats and Healthy Human Subjects. Pharmaceutics 2024, 16, 1033. [Google Scholar] [CrossRef]
- Parveen, R.; Baboota, S.; Ali, J.; Ahuja, A.; Vasudev, S.S.; Ahmad, S. Effects of silymarin nanoemulsion against carbon tetrachloride-induced hepatic damage. Arch. Pharmacal Res. 2011, 34, 767–774. [Google Scholar] [CrossRef]
- Calligaris, S.; Comuzzo, P.; Bot, F.; Lippe, G.; Zironi, R.; Anese, M.; Nicoli, M.C. Nanoemulsions as delivery systems of hydrophobic silybin from silymarin extract: Effect of oil type on silybin solubility, in vitro bioaccessibility and stability. LWT Food Sci. Technol. 2015, 63, 77–84. [Google Scholar] [CrossRef]
- Nagi, A.; Iqbal, B.; Kumar, S.; Sharma, S.; Ali, J.; Baboota, S. Quality by design based silymarin nanoemulsion for enhancement of oral bioavailability. J. Drug Deliv. Sci. Technol. 2017, 40, 35–44. [Google Scholar] [CrossRef]
- Piazzini, V.; Rosseti, C.; Bigagli, E.; Luceri, C.; Bilia, A.R.; Bergonzi, M.C. Prediction of Permeation and Cellular Transport of Silybum marianum Extract Formulated in a Nanoemulsion by Using PAMPA and Caco-2 Cell Models. Planta Medica 2017, 83, 1184–1193. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Liu, X.; Liu, K.; Zhao, B.; Li, Q.; Xi, J.; Li, X. Preparation and characterisation of silybin nanoemulsions based on complex emulsifiers: Stability, in vitro release properties and antioxidant capacity. Int. J. Food Sci. Technol. 2024, 59, 3331–3339. [Google Scholar] [CrossRef]
- Maheshwari, H.; Agarwal, R.; Patil, C.; Katare, O.P. Preparation and Pharmacological Evaluation of Silibinin Liposomes. Arzneimittelforschung 2003, 53, 420–427. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.; Rai, A.; Reddy, N.D.; Raj, P.V.; Jain, P.; Deshpande, P.; Mathew, G.; Kutty, N.G.; Udupa, N.; Rao, C.M. Silymarin liposomes improves oral bioavailability of silybin besides targeting hepatocytes, and immune cells. Pharmacol. Rep. 2014, 66, 788–798. [Google Scholar] [CrossRef] [PubMed]
- Elmowafy, M.; Viitala, T.; Ibrahim, H.M.; Abu-Elyazid, S.K.; Samy, A.; Kassem, A.; Yliperttula, M. Silymarin loaded liposomes for hepatic targeting: In vitro evaluation and HepG2 drug uptake. Eur. J. Pharm. Sci. 2013, 50, 161–171. [Google Scholar] [CrossRef] [PubMed]
- Mohseni, M.; Ashkezari, M.D.; Akhlaghi, M.; Ansari, K.; Haghirosadat, B.F. A new therapeutic approach for the treatment of breast cancer using synthesis of liposomes containing silybinin and their characterization. New Cell. Mol. Biotechnol. J. 2021, 11, 57–70. [Google Scholar]
- Wu, W.; Wang, Y.; Que, L. Enhanced bioavailability of silymarin by self-microemulsifying drug delivery system. Eur. J. Pharm. Biopharm. 2006, 63, 288–294. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Yuan, Q.; Huang, Y.; Zhou, Y.; Liu, Y. Development of silymarin self-microemulsifying drug delivery system with enhanced oral bioavailability. AAPS PharmSciTech 2010, 11, 672–678. [Google Scholar] [CrossRef] [PubMed]
- Woo, J.S.; Kim, T.-S.; Park, J.-H.; Chi, S.-C. Formulation and biopharmaceutical evaluation of silymarin using SMEDDS. Arch. Pharmacal Res. 2007, 30, 82–89. [Google Scholar] [CrossRef] [PubMed]
- Tung, N.-T.; Tran, C.-S.; Nguyen, H.-A.; Nguyen, T.-D.; Chi, S.-C.; Pham, D.-V.; Bui, Q.-D.; Ho, X.-H. Formulation and biopharmaceutical evaluation of supersaturatable self-nanoemulsifying drug delivery systems containing silymarin. Int. J. Pharm. 2019, 555, 63–76. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-H.; Chang, C.-C.; Shih, T.-H.; Aljuffali, I.A.; Yeh, T.-S.; Fang, J.-Y. Self-nanoemulsifying drug delivery systems ameliorate the oral delivery of silymarin in rats with Roux-en-Y gastric bypass surgery. Int. J. Nanomed. 2015, 10, 2403–2416. [Google Scholar]
- Jia, L.-J.; Zhang, D.-R.; Li, Z.-Y.; Feng, F.-F.; Wang, Y.-C.; Dai, W.-T.; Duan, C.-X.; Zhang, Q. Preparation and characterization of silybin-loaded nanostructured lipid carriers. Drug Deliv. 2010, 17, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, B.; Ali, J.; Ganguli, M.; Mishra, S.; Baboota, S. Silymarin-Loaded Nanostructured Lipid Carrier Gel for The Treatment of Skin Cancer. Nanomedicine 2019, 14, 1077–1093. [Google Scholar] [CrossRef] [PubMed]
- Hirlekar, R.; Patil, E.; Bhairy, S. Solid nanostructured lipid carriers loaded with silymarin for oral delivery: Formulation development and evaluation. Curr. Trends Pharm. Pharm. Chem. 2021, 3, 56–67. [Google Scholar] [CrossRef]
- Alipour, M.; Bigdeli, M.R.; Aligholi, H.; Rasoulian, B.; Khaksarian, M. Sustained release of silibinin-loaded chitosan nanoparticle induced apoptosis in glioma cells. J. Biomed. Mater. Res. Part A 2020, 108, 458–469. [Google Scholar] [CrossRef]
- Pourgholi, A.; Dadashpour, M.; Mousapour, A.; Amandi, A.F.; Zarghami, N. Anticancer Potential of Silibinin Loaded Polymeric Nanoparticles against Breast Cancer Cells: Insight into the Apoptotic Genes Targets. Asian Pac. J. Cancer Prev. 2021, 22, 2587–2596. [Google Scholar] [CrossRef] [PubMed]
- Snima, K.S.; Arunkumar, P.; Jayakumar, R.; Lakshmanan, V.-K. Silymarin Encapsulated Poly(D, L-lactic-co-glycolic acid) Nanoparticles: A Prospective Candidate for Prostate Cancer Therapy. J. Biomed. Nanotechnol. 2014, 10, 559–570. [Google Scholar] [CrossRef] [PubMed]
- Tsai, Y.-H.; Yang, Y.-N.; Ho, Y.-C.; Tsai, M.-L.; Mi, F.-L. Drug release and antioxidant/antibacterial activities of silymarin-zein nanoparticle/bacterial cellulose nanofiber composite films. Carbohydr. Polym. 2018, 180, 286–296. [Google Scholar] [CrossRef] [PubMed]
- Bodbodak, S.; Nejatian, M.; Yazdi, A.P.G.; Rousta, L.K.; Rafiee, Z.; Jalali-Jivan, M.; Kharazmi, M.S.; Jafari, S.M. Improving the thermal stability of natural bioactive ingredients via encapsulation technology. Crit. Rev. Food Sci. Nutr. 2024, 64, 2824–2846. [Google Scholar] [CrossRef]
- Hajinezhad, M.R.; Roostaee, M.; Nikfarjam, Z.; Rastegar, S.; Sargazi, G.; Barani, M.; Sargazi, S. Exploring the potential of silymarin-loaded nanovesicles as an effective drug delivery system for cancer therapy: In vivo, in vitro, and in silico experiments. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2024. [Google Scholar] [CrossRef] [PubMed]
- Marras, S.I.; Kladi, K.P.; Tsivintzelis, I.; Zuburtikudis, I.; Panayiotou, C. Biodegradable polymer nanocomposites: The role of nanoclays on the thermomechanical characteristics and the electrospun fibrous structure. Acta Biomater. 2008, 4, 756–765. [Google Scholar] [CrossRef]
- Huang, Z.-M.; Zhang, Y.Z.; Kotaki, M.; Ramakrishna, S. A review on polymer nanofibers by electrospinning and their applications in nanocomposites. Compos. Sci. Technol. 2003, 63, 2223–2253. [Google Scholar] [CrossRef]
- Luraghi, A.; Peri, F.; Moroni, L. Electrospinning for drug delivery applications: A review. J. Control Release 2021, 334, 463–484. [Google Scholar] [CrossRef] [PubMed]
- Han, D.; Gouma, P.I. Electrospun bioscaffolds that mimic the topology of extracellular matrix, Nanomedicine: Nanotechnology. Biol. Med. 2006, 2, 37–41. [Google Scholar]
- Hinderer, S.; Layland, S.L.; Schenke-Layland, K. ECM and ECM-like materials—Biomaterials for applications in regenerative medicine and cancer therapy. Adv. Drug Deliv. Rev. 2016, 97, 260–269. [Google Scholar] [CrossRef] [PubMed]
- Keirouz, A.; Wang, Z.; Reddy, V.S.; Nagy, Z.K.; Vass, P.; Buzgo, M.; Ramakrishna, S.; Radacsi, N. The History of Electrospinning: Past, Present, and Future Developments. Adv. Mater. Technol. 2023, 8, 2201723. [Google Scholar] [CrossRef]
- Kapadnis, P.D.; Shrotriya, S.N. Electrospun silybin enriched scaffolds of polyethylene oxide as wound dressings: Enhanced wound closure, reepithelization in rat excisional wound model. Indian J. Pharm. Educ. Res. 2019, 53, 301–309. [Google Scholar] [CrossRef]
- Wu, Q.; Xu, S.; Xu, W.; Meng, H. In vitro anticancer efficacy of silibinin-loaded PLGA nanofibers against A549 non-small-cell lung cancer cells. ScienceAsia 2023, 49, 403–410. [Google Scholar] [CrossRef]
- Dadashpour, M.; Kalavi, S.; Gorgzadeh, A.; Nosrati, R.; Amandi, A.F.; Mohammadikhah, M.; Sara, M.R.S.; Alizadeh, E. Preparation and in vitro evaluation of cell adhesion and long-term proliferation of stem cells cultured on silibinin co-embedded PLGA/Collagen electrospun composite nanofibers. Exp. Cell Res. 2024, 435, 113926. [Google Scholar] [CrossRef] [PubMed]
- Najafi, R.; Asadi, A.; Zahri, S.; Abdolmaleki, A.J.G. Cell, Tissue, Comparison of Biocompatibility and Morphology of PC12 Cell Line on a Polycaprolactane/Silymarin Scaffold and a Polycaprolactane/Tragacanth Scaffold. Gene Cell Tissue 2023, 10, e131955. [Google Scholar] [CrossRef]
- Xie, Y.; Yi, Y.; Hu, X.; Shangguan, M.; Wang, L.; Lu, Y.; Qi, J.; Wu, W. Synchronous microencapsulation of multiple components in silymarin into PLGA nanoparticles by an emulsification/solvent evaporation method. Pharm. Dev. Technol. 2016, 21, 672–679. [Google Scholar] [CrossRef] [PubMed]
- Kurowiak, J.; Klekiel, T.; Będziński, R. Biodegradable polymers in biomedical applications: A review—Developments. Perspect. Future Chall. 2023, 24, 16952. [Google Scholar]
- Dash, T.K.; Konkimalla, V.B. Polymeric modification and its implication in drug delivery: Poly-ε-caprolactone (PCL) as a model polymer. Mol. Pharm. 2012, 9, 2365–2379. [Google Scholar] [CrossRef] [PubMed]
- Kayan, G.Ö.; Kayan, A. Polycaprolactone Composites/Blends and Their Applications Especially in Water Treatment. ChemEngineering 2023, 7, 104. [Google Scholar] [CrossRef]
- Arampatzis, A.S.; Giannakoula, K.; Kontogiannopoulos, K.N.; Theodoridis, K.; Aggelidou, E.; Rat, A.; Kampasakali, E.; Willems, A.; Christofilos, D.; Kritis, A.; et al. Novel electrospun poly-hydroxybutyrate scaffolds as carriers for the wound healing agents alkannins and shikonins. Regen. Biomater. 2021, 8, rbab011. [Google Scholar] [CrossRef] [PubMed]
- Tsioptsias, C.; Spartali, C.; Marras, S.I.; Ntampou, X.; Tsivintzelis, I.; Panayiotou, C. Thermochemical Transition in Low Molecular Weight Substances: The Example of the Silybin Flavonoid. Molecules 2022, 27, 6345. [Google Scholar] [CrossRef] [PubMed]
- Arampatzis, A.S.; Kontogiannopoulos, K.N.; Theodoridis, K.; Aggelidou, E.; Rat, A.; Willems, A.; Tsivintzelis, I.; Papageorgiou, V.P.; Kritis, A.; Assimopoulou, A.N. Electrospun wound dressings containing bioactive natural products: Physico-chemical characterization and biological assessment. Biomater. Res. 2021, 25, 23. [Google Scholar] [CrossRef] [PubMed]
- Chou, S.F.; Carson, D.; Woodrow, K.A. Current strategies for sustaining drug release from electrospun nanofibers. J. Control. Release 2015, 220, 584–591. [Google Scholar] [CrossRef] [PubMed]
- Zeng, J.; Yang, L.; Liang, Q.; Zhang, X.; Guan, H.; Xu, X.; Chen, X.; Jing, X. Influence of the drug compatibility with polymer solution on the release kinetics of electrospun fiber formulation. J. Control Release 2005, 105, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Varghese, L.; Agarwal, C.; Tyagi, A.; Singh, R.P.; Agarwal, R. Silibinin efficacy against human hepatocellular carcinoma. Clin. Cancer Res. 2005, 11, 8441–8448. [Google Scholar] [CrossRef] [PubMed]
- Dan, W.; Fan, Y.; Hou, T.; Wei, Y.; Liu, B.; Que, T.; Yu, B.; Zeng, J.; Li, L. Silibinin inhibits the migration, invasion and epithelial-mesenchymal transition of prostate cancer by activating the autophagic degradation of YAP. J. Cancer 2022, 13, 3415–3426. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.P.; Agarwal, R. Mechanisms and preclinical efficacy of silibinin in preventing skin cancer. Eur. J. Cancer 2005, 41, 1969–1979. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, C.; Singh, R.P.; Dhanalakshmi, S.; Tyagi, A.K.; Tecklenburg, M.; Sclafani, R.A.; Agarwal, R. Silibinin upregulates the expression of cyclin-dependent kinase inhibitors and causes cell cycle arrest and apoptosis in human colon carcinoma HT-29 cells. Oncogene 2003, 22, 8271–8282. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.O.; Jeong, Y.J.; Im, H.G.; Kim, C.H.; Chang, Y.C.; Lee, I.S. Silibinin suppresses PMA-induced MMP-9 expression by blocking the AP-1 activation via MAPK signaling pathways in MCF-7 human breast carcinoma cells. Biochem. Biophys. Res. Commun. 2007, 354, 165–171. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Ge, Y.; Ping, X.; Yu, M.; Lou, D.; Shi, W. Synergistic apoptotic effects of silibinin in enhancing paclitaxel toxicity in human gastric cancer cell lines. Mol. Med. Rep. 2018, 18, 1835–1841. [Google Scholar] [CrossRef] [PubMed]
- Jafari, S.; Bakhshaei, A.; Eskandani, M.; Molavi, O. Silibinin-Loaded Nanostructured Lipid Carriers for Growth Inhibition of Cisplatin-Resistant Ovarian Cancer Cells. Assay Drug Dev. Technol. 2022, 20, 339–348. [Google Scholar] [CrossRef] [PubMed]
- Chu, S.C.; Chiou, H.L.; Chen, P.H.; Yang, S.F.; Hsieh, Y.S. Silibinin inhibits the invasion of human lung cancer cells via decreased productions of urokinase-plasminogen activator and matrix metalloproteinase-2. Mol. Carcinog. 2004, 40, 143–149. [Google Scholar] [CrossRef] [PubMed]
- Cormick, B.P.M.; Langle, Y.; Belgorosky, D.; Vanzulli, S.; Balarino, N.; Sandes, E.; Eiján, A.M. Flavonoid silybin improves the response to radiotherapy in invasive bladder cancer. J. Cell. Biochem. 2018, 119, 5402–5412. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Sun, Y.; Xu, F.; Liu, W.; Hayashi, T.; Mizuno, K.; Hattori, S.; Fujisaki, H.; Ikejima, T. Autophagy and glycolysis independently attenuate silibinin-induced apoptosis in human hepatocarcinoma HepG2 and Hep3B cells. Hum. Exp. Toxicol. 2021, 40, 2048–2062. [Google Scholar] [CrossRef] [PubMed]
- Boojar, M.M.A.; Hassanipour, M.; Mehr, S.E.; Boojar, M.M.A.; Dehpour, A.R. New Aspects of Silibinin Stereoisomers and their 3-O-galloyl Derivatives on Cytotoxicity and Ceramide Metabolism in Hep G2 hepatocarcinoma Cell Line. Iran. J. Pharm. Res. 2016, 15, 421–433. [Google Scholar]
- Zappavigna, S.; Vanacore, D.; Lama, S.; Potenza, N.; Russo, A.; Ferranti, P.; Dallio, M.; Federico, A.; Loguercio, C.; Sperlongano, P.; et al. Silybin-induced apoptosis occurs in parallel to the increase of ceramides synthesis and mirnas secretion in human hepatocarcinoma cells. Int. J. Mol. Sci. 2019, 20, 2190. [Google Scholar] [CrossRef] [PubMed]
- Hepel, M.; Andreescu, S. Oxidative Stress and Human Health. In Oxidative Stress: Diagnostics, Prevention, and Therapy; ACS Symposium Series; American Chemical Society: Washington, DC, USA, 2015; Volume 2. [Google Scholar]
- Imlay, J.A.; Chin, S.M.; Linn, S. Toxic DNA damage by hydrogen peroxide through the fenton reaction in vivo and in vitro. Science 1988, 240, 640–642. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Zhang, T.; Chen, Z.; Wang, Y.; Ma, S.; Liu, J.; Liu, J. The beneficial effect of ginsenosides extracted by pulsed electric field against hydrogen peroxide-induced oxidative stress in HEK-293 cells. J. Ginseng Res. 2017, 41, 169–179. [Google Scholar] [CrossRef]
- Tsialtas, I.; Georgantopoulos, A.; Karipidou, M.E.; Kalousi, F.D.; Karra, A.G.; Leonidas, D.D.; Psarra, A.M.G. Anti-apoptotic and antioxidant activities of the mitochondrial estrogen receptor beta in n2a neuroblastoma cells. Int. J. Mol. Sci. 2021, 22, 7620. [Google Scholar] [CrossRef]
- Surai, P.F. Silymarin as a natural antioxidant: An overview of the current evidence and perspectives. Antioxidants 2015, 4, 204–247. [Google Scholar] [CrossRef]
- Anthony, K.P.; Saleh, M.A. Free Radical Scavenging and Antioxidant Activities of Silymarin Components. Antioxidants 2013, 2, 398–407. [Google Scholar] [CrossRef]
- Taleb, A.; Ahmad, K.A.; Ihsan, A.U.; Qu, J.; Lin, N.; Hezam, K.; Koju, N.; Hui, L.; Qilong, D. Antioxidant effects and mechanism of silymarin in oxidative stress induced cardiovascular diseases. Biomed. Pharmacother. 2018, 102, 689–698. [Google Scholar] [CrossRef]
- Asghar, Z.; Masood, Z. Evaluation of antioxidant properties of silymarin and its potential to inhibit peroxyl radicals in vitro. Pak. J. Pharm. Sci. 2008, 21, 249–254. [Google Scholar] [PubMed]
- Rolo, A.P.; Oliveira, P.J.; Moreno, A.J.; Palmeira, C.M. Protection against post-ischemic mitochondrial injury in rat liver by silymarin or TUDC. Hepatol. Res. Off. J. Jpn. Soc. Hepatol. 2003, 26, 217–224. [Google Scholar] [CrossRef]
- Muthumani, M.; Prabu, S.M. Silibinin potentially protects arsenic-induced oxidative hepatic dysfunction in rats. Toxicol. Mech. Methods 2012, 22, 277–288. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.Y.; Wang, H.J.; Wang, J.; Tashiro, S.; Onodera, S.; Ikejima, T. The protective effect of silibinin against mitomycin C-induced intrinsic apoptosis in human melanoma A375-S2 cells. J. Pharmacol. Sci. 2009, 111, 137–146. [Google Scholar] [CrossRef]



| Samples | Encapsulation Efficiency 1 (%) | Loading 1 (wt%) |
|---|---|---|
| PCL/1Sil | 93 ± 3 | 0.9 ± 0.1 |
| PCL/3Sil | 90 ± 2 | 2.7 ± 0.1 |
| PCL/5Sil | 89 ± 3 | 4.5 ± 0.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Spartali, C.; Psarra, A.-M.G.; Marras, S.I.; Tsioptsias, C.; Georgantopoulos, A.; Kalousi, F.D.; Tsakalof, A.; Tsivintzelis, I. Silybin-Functionalized PCL Electrospun Fibrous Membranes for Potential Pharmaceutical and Biomedical Applications. Polymers 2024, 16, 2346. https://doi.org/10.3390/polym16162346
Spartali C, Psarra A-MG, Marras SI, Tsioptsias C, Georgantopoulos A, Kalousi FD, Tsakalof A, Tsivintzelis I. Silybin-Functionalized PCL Electrospun Fibrous Membranes for Potential Pharmaceutical and Biomedical Applications. Polymers. 2024; 16(16):2346. https://doi.org/10.3390/polym16162346
Chicago/Turabian StyleSpartali, Christina, Anna-Maria G. Psarra, Sotirios I. Marras, Costas Tsioptsias, Achilleas Georgantopoulos, Foteini D. Kalousi, Andreas Tsakalof, and Ioannis Tsivintzelis. 2024. "Silybin-Functionalized PCL Electrospun Fibrous Membranes for Potential Pharmaceutical and Biomedical Applications" Polymers 16, no. 16: 2346. https://doi.org/10.3390/polym16162346
APA StyleSpartali, C., Psarra, A.-M. G., Marras, S. I., Tsioptsias, C., Georgantopoulos, A., Kalousi, F. D., Tsakalof, A., & Tsivintzelis, I. (2024). Silybin-Functionalized PCL Electrospun Fibrous Membranes for Potential Pharmaceutical and Biomedical Applications. Polymers, 16(16), 2346. https://doi.org/10.3390/polym16162346









