Flame-Retardant Glass Fiber-Reinforced Epoxy Resins with Phosphorus-Containing Bio-Based Benzoxazines and Graphene
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
3. Results and Discussion
3.1. Flammability Tests
3.2. Strength Characteristics
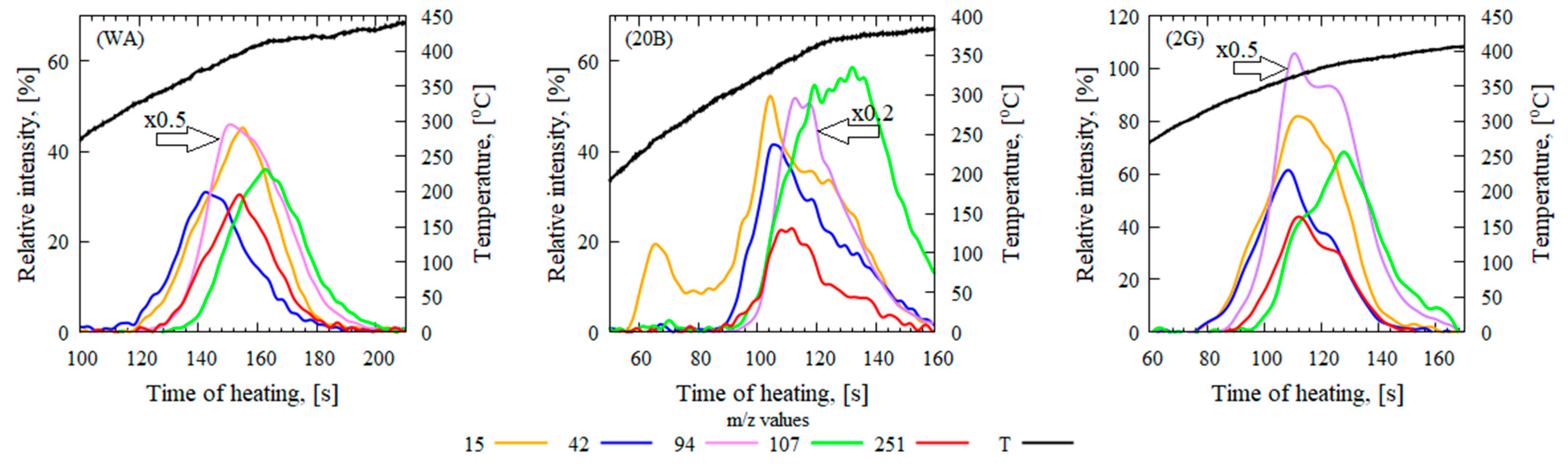
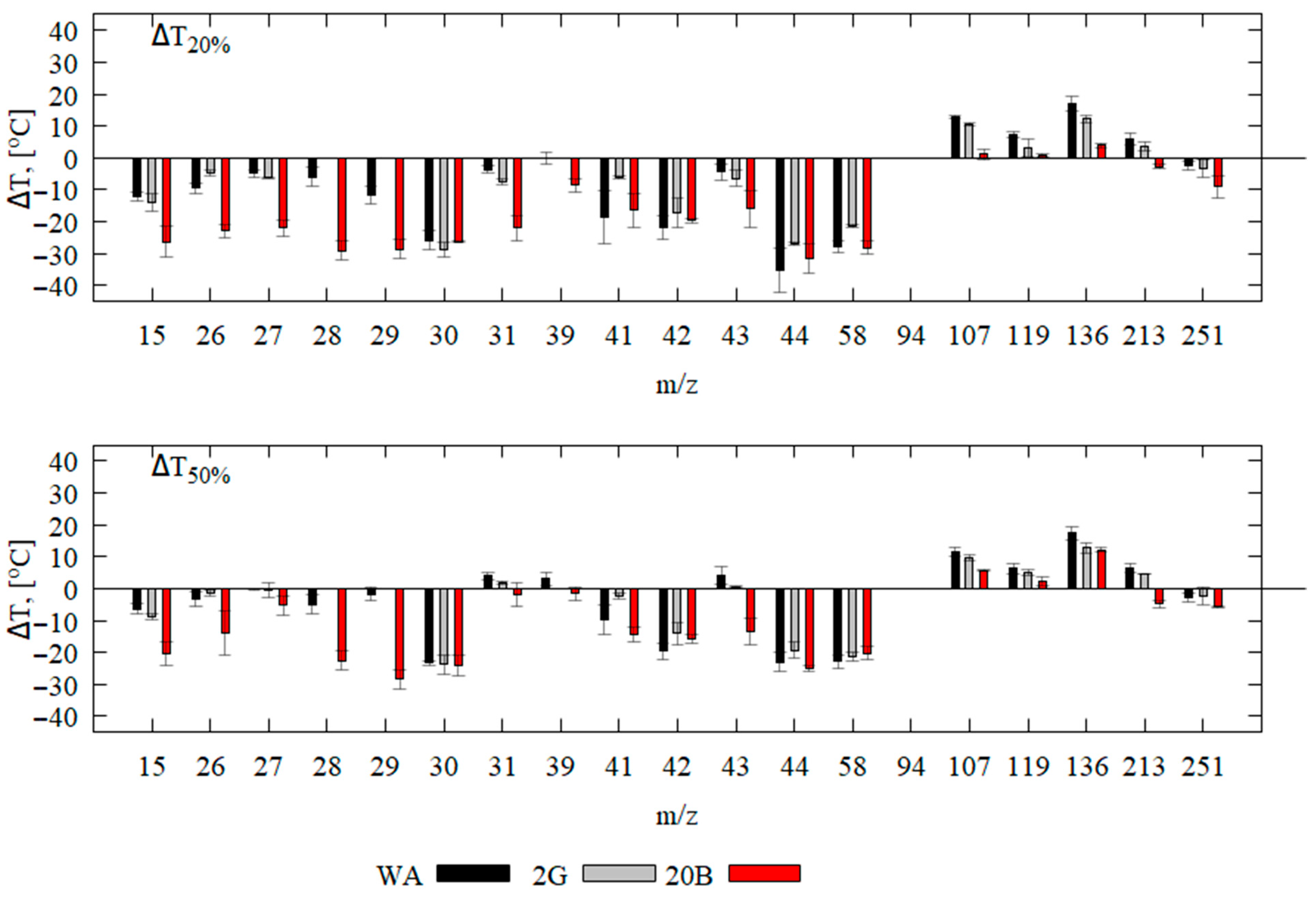
3.3. Analysis of Pyrolysis Products by Mass Spectrometry (In Situ) and GC-MS
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, Q.; Wang, D.; Li, Z.; Li, Z.; Peng, X.; Liu, C.; Zhang, Y.; Zheng, P. Recent Developments in the Flame-Retardant System of Epoxy Resin. Materials 2020, 13, 2145. [Google Scholar] [CrossRef] [PubMed]
- Zhi, M.; Yang, X.; Fan, R.; Yue, S.; Zheng, L.; Liu, Q.; He, Y. A Comprehensive Review of Reactive Flame-Retardant Epoxy Resin: Fundamentals, Recent Developments, and Perspectives. Polym. Degrad. Stab. 2022, 201, 109976. [Google Scholar] [CrossRef]
- Iqbal, M.A.; Iqbal, M.A.; Fedel, M. Fire Retardancy of Aluminum Hydroxide Reinforced Flame Retardant Modified Epoxy Resin Composite. Russ. J. Appl. Chem. 2018, 91, 680–686. [Google Scholar] [CrossRef]
- Tao, Z.; Yang, S.; Chen, J.; Fan, L. Synthesis and Characterization of Imide Ring and Siloxane-Containing Cycloaliphatic Epoxy Resins. Eur. Polym. J. 2007, 43, 1470–1479. [Google Scholar] [CrossRef]
- Yang, P.; Ren, M.; Chen, K.; Liang, Y.; Lü, Q.-F.; Zhang, T. Synthesis of a Novel Silicon-Containing Epoxy Resin and Its Effect on Flame Retardancy, Thermal, and Mechanical Properties of Thermosetting Resins. Mater. Today Commun. 2019, 19, 186–195. [Google Scholar] [CrossRef]
- Zhou, Y.; Feng, J.; Peng, H.; Qu, H.; Hao, J. Catalytic Pyrolysis and Flame Retardancy of Epoxy Resins with Solid Acid Boron Phosphate. Polym. Degrad. Stab. 2014, 110, 395–404. [Google Scholar] [CrossRef]
- Guo, Y.; Bao, C.; Song, L.; Yuan, B.; Hu, Y. In Situ Polymerization of Graphene, Graphite Oxide, and Functionalized Graphite Oxide into Epoxy Resin and Comparison Study of On-the-Flame Behavior. Ind. Eng. Chem. Res. 2011, 50, 7772–7783. [Google Scholar] [CrossRef]
- Im, J.S.; Lee, S.K.; In, S.J.; Lee, Y.-S. Improved Flame Retardant Properties of Epoxy Resin by Fluorinated MMT/MWCNT Additives. J. Anal. Appl. Pyrolysis 2010, 89, 225–232. [Google Scholar] [CrossRef]
- Zhu, Z.-M.; Wang, L.-X.; Lin, X.-B.; Dong, L.-P. Synthesis of a Novel Phosphorus-Nitrogen Flame Retardant and Its Application in Epoxy Resin. Polym. Degrad. Stab. 2019, 169, 108981. [Google Scholar] [CrossRef]
- Dai, X.; Li, P.; Sui, Y.; Zhang, C. Thermal and Flame-Retardant Properties of Intrinsic Flame-Retardant Epoxy Resin Containing Biphenyl Structures and Phosphorus. Eur. Polym. J. 2021, 147, 110319. [Google Scholar] [CrossRef]
- Liang, B.; Cao, J.; Hong, X.; Wang, C. Synthesis and Properties of a Novel Phosphorous-Containing Flame-Retardant Hardener for Epoxy Resin. J. Appl. Polym. Sci. 2013, 128, 2759–2765. [Google Scholar] [CrossRef]
- Ding, H.; Wang, X.; Song, L.; Hu, Y. Recent Advances in Flame Retardant Bio-Based Benzoxazine Resins. J. Renew. Mater. 2021, 10, 871–895. [Google Scholar] [CrossRef]
- Rao, B.S.; Palanisamy, A. Monofunctional Benzoxazine from Cardanol for Bio-Composite Applications. React. Funct. Polym. 2011, 71, 148–154. [Google Scholar] [CrossRef]
- Calò, E.; Maffezzoli, A.; Mele, G.; Martina, F.; Mazzetto, S.E.; Tarzia, A.; Stifani, C. Synthesis of a Novel Cardanol-Based Benzoxazine Monomer and Environmentally Sustainable Production of Polymers and Bio-Composites. Green Chem. 2007, 9, 754–759. [Google Scholar] [CrossRef]
- Rao, B.S.; Palanisamy, A. Synthesis of Bio Based Low Temperature Curable Liquid Epoxy, Benzoxazine Monomer System from Cardanol: Thermal and Viscoelastic Properties. Eur. Polym. J. 2013, 49, 2365–2376. [Google Scholar] [CrossRef]
- Mukherjee, S.; Amarnath, N.; Lochab, B. Oxazine Ring-Substituted 4th Generation Benzoxazine Monomers & Polymers: Stereoelectronic Effect of Phenyl Substituents on Thermal Properties. Macromolecules 2021, 54, 10001–10016. [Google Scholar] [CrossRef]
- Grishchuk, S.; Sorochynska, L.; Vorster, O.C.; Karger-Kocsis, J. Structure, Thermal, and Mechanical Properties of DDM-Hardened Epoxy/Benzoxazine Hybrids: Effects of Epoxy Resin Functionality and ETBN Toughening. J. Appl. Polym. Sci. 2013, 127, 5082–5093. [Google Scholar] [CrossRef]
- Chen, L.; Song, T.; Wang, J.; Shi, H.; Hao, J. Intrinsic Flame-Retardant Epoxy Resin Composites with Benzoxazine: Effect of a Catalyst and a Low Curing Temperature. J. Appl. Polym. Sci. 2019, 136, 47847. [Google Scholar] [CrossRef]
- Naiker, V.E.; Patil, D.A.; More, A.P.; Mhaske, S.T. Synthesis of High-Performance Bio-Based Benzoxazine for Flame Retardant Application. Polym. Adv. Technol. 2022, 33, 1481–1495. [Google Scholar] [CrossRef]
- Cao, J.; Duan, H.; Zou, J.; Zhang, J.; Wan, C.; Zhang, C.; Ma, H. Bio-Based Phosphorus-Containing Benzoxazine towards High Fire Safety, Heat Resistance and Mechanical Properties of Anhydride-Cured Epoxy Resin. Polym. Degrad. Stab. 2022, 198, 109878. [Google Scholar] [CrossRef]
- Luo, Q.; Sun, Y.; Yu, B.; Li, C.; Song, J.; Tan, D.; Zhao, J. Synthesis of a Novel DPPA-Containing Benzoxazine to Flame-Retard Epoxy Resin with Maintained Thermal Properties. Polym. Adv. Technol. 2019, 30, 1989–1995. [Google Scholar] [CrossRef]
- Guo, W.; Wang, X.; Gangireddy, C.S.R.; Wang, J.; Pan, Y.; Xing, W.; Song, L.; Hu, Y. Cardanol Derived Benzoxazine in Combination with Boron-Doped Graphene toward Simultaneously Improved Toughening and Flame Retardant Epoxy Composites. Compos. Part A Appl. Sci. Manuf. 2019, 116, 13–23. [Google Scholar] [CrossRef]
- Lee, L.-H. Mechanisms of Thermal Degradation of Phenolic Condensation Polymers. II. Thermal Stability and Degradation Schemes of Epoxy Resins. J. Polym. Sci. Part A Gen. Pap. 1965, 3, 859–882. [Google Scholar] [CrossRef]
- Vlastaras, A.S. Thermal Degradation of an Anhydride-Cured Epoxy Resin by Laser Heating. J. Phys. Chem. 1970, 74, 2496–2501. [Google Scholar] [CrossRef]
- Lukashenko, I.; Khmel’Nitskii, R.; Brodskii, Y.; Kalinkevich, G.; Kovaleva, N.; Batizat, V. Pyrolytic Mass Spectrometry as a Means of Investigating Liquid Epoxy Resins. Polym. Sci. USSR 1976, 18, 1302–1311. [Google Scholar] [CrossRef]
- Yefremov, V.; Kolesnikov, B.; Ksandopulo, G. Mass Spectroscopic Examination of the Diffusive Flame of Solidified Epoxy Resin. Polym. Sci. USSR 1978, 20, 2903–2908. [Google Scholar] [CrossRef]
- ISO 4589-2:2017; Plastics—Determination of Burning Behaviour by Oxygen Index—Part 2: Ambient-Temperature Test 2005. International Organization for Standardization: Geneva, Switzerland, 2017.
- UL 94; Standard for Tests for Flammability of Plastic Materials for Parts in Devices and Appliances. Underwriters Laboratories: Northbrook, IL, USA, 1996.
- Korobeinichev, O.P.; Paletsky, A.A.; Gonchikzhapov, M.B.; Glaznev, R.K.; Gerasimov, I.E.; Naganovsky, Y.K.; Shundrina, I.K.; Snegirev, A.Y.; Vinu, R. Kinetics of Thermal Decomposition of PMMA at Different Heating Rates and in a Wide Temperature Range. Thermochim. Acta 2019, 671, 17–25. [Google Scholar] [CrossRef]
- Korobeinichev, O.P. Dynamic Flame Probe Mass Spectrometry and Condensed-System Decomposition. Combust. Explos. Shock Waves 1987, 23, 565–576. [Google Scholar] [CrossRef]
- Osipova, K.N.; Bolshova, T.A.; Korobeinichev, O.P.; Kuibida, L.V.; Shmakov, A.G. Effect of Addition of Methyl Hexanoate and Ethyl Pentanoate on the Structure of Premixed N-Heptane/Toluene/O2/Ar Flame. Energy Fuels 2019, 33, 4585–4597. [Google Scholar] [CrossRef]
- Korobeinichev, O.; Shaklein, A.; Trubachev, S.; Karpov, A.; Paletsky, A.; Chernov, A.; Sosnin, E.; Shmakov, A. The Influence of Flame Retardants on Combustion of Glass Fiber-Reinforced Epoxy Resin. Polymers 2022, 14, 3379. [Google Scholar] [CrossRef] [PubMed]
- Korobeinichev, O.P.; Sosnin, E.A.; Shaklein, A.A.; Karpov, A.I.; Sagitov, A.R.; Trubachev, S.A.; Shmakov, A.G.; Paletsky, A.A.; Kulikov, I.V. The Effect of Flame-Retardant Additives DDM-DOPO and Graphene on Flame Propagation over Glass-Fiber-Reinforced Epoxy Resin under the Influence of External Thermal Radiation. Molecules 2023, 28, 5162. [Google Scholar] [CrossRef] [PubMed]
- Alamusi; Xiao, T.; Wu, L.; Liu, C.; Lei, L.; Liu, Y.; Ning, H.; Hu, R.; Liu, H.; Gu, B.; et al. Influences of Preparation Process on Electrical Conductivity and Thermal Expansion Coefficient of Epoxy/Graphene Nanocomposites. Mater. Res. Express 2019, 6, 116318. [Google Scholar] [CrossRef]
- Erickson, K. Thermal Decomposition Mechanisms Common to Polyurethane, Epoxy, Poly(Diallyl Phthalate), Polycarbonate and Poly(Phenylene Sulfide). J. Therm. Anal. Calorim. 2007, 89, 427–440. [Google Scholar] [CrossRef]
- Bozi, J.; Czégény, Z.; Mészáros, E.; Blazsó, M. Thermal Decomposition of Flame Retarded Polycarbonates. J. Anal. Appl. Pyrolysis 2007, 79, 337–345. [Google Scholar] [CrossRef]
- Creasy, W.R. Epoxy Resin Analysis by Fourier Transform Mass Spectrometry: A Comparison of Pyrolysis and Laser Ablation. Polymer 1992, 33, 4486–4492. [Google Scholar] [CrossRef]
| Binder Content~35% | ||||||
|---|---|---|---|---|---|---|
| WA | 2G18B | 2G | 10B | 20B | 2G8B | |
| ED-20 Resin | 100 | 100 | 100 | 100 | 100 | 100 |
| UP-606/2 | 3 | 3 | 3 | 3 | 3 | 3 |
| Graphene | - | 2 | 2 | - | - | 2 |
| CBz | - | 18 | - | 10 | 20 | 8 |
| Phosphorus, wt.% | 0 | 0.41 | 0 | 0.25 | 0.46 | 0.2 |
| FR (Flame-Retardant) Content, % | GFRER Sample | Binder Content, wt.% | Limiting Oxygen Index, vol.% | UL-94 HB (Rate of Flame Spread, mm/s) | Thickness, mm | Density, kg/m3 |
|---|---|---|---|---|---|---|
| 0% | WA | 31 ± 2 | 22.7 | HB (0.29 ± 0.03) | 1.9 ± 0.1 | 1550 |
| 2% | 2G | 35 ± 2 | 24.8 (+2.1) | HB (0.30 ± 0.03) | 1.8 ± 0.1 | 1650 |
| 10% | 2G8B | 34 ± 2 | 25.2 (+2.5) | HB (0.24 ± 0.02) | 2.1 ± 0.1 | 1460 |
| 10B | 37.5 ± 2 | 25.7 (+3.0) | HB * | 1.9 ± 0.1 | 1650 | |
| 20% | 2G18B | 32.5 ± 3 | 26.1 (+3.4) | HB * | 2.2 ± 0.1 | 1500 |
| 20B | 28 ± 3 | 28.5 (+5.4) | HB * | 1.7 ± 0.1 | 1500 |
| FR Concentration, % | Sample | Peak Load, kN | Displacement, mm |
|---|---|---|---|
| 0 | WA | 11.7 ± 0.4 | 1.8 ± 0.1 |
| 2 | 2G | 13.8 ± 0.3 (+2.1) | 2.4 ± 0.1 |
| 10 | 2G8B | 13.8 ± 0.3 (+2.1) | 2.52 ± 0.03 |
| 10B | 13.8 ± 0.2 (+2.1) | 2.52 ± 0.02 | |
| 20 | 2G18B | 15.5 ± 0.3 (+3.8) | 4 ± 0.3 |
| 20B | 14.1 ± 0.1 (+2.4) | 2.6 ± 0.1 |
| Proposed Compounds | Detected Mass-to-Charge Ratio (m/z) | WA | 20B | 2G |
|---|---|---|---|---|
| Imax, % | Imax, % | Imax, % | ||
| 2,2′,4,4′,6,6′-Hexamethylbenzophenone | 251 | 34 ± 3 | 25 ± 2 | 47 ± 5 |
| Bisphenol-A | 213 | 55 ± 6 | 29 ± 3 | 56 ± 6 |
| Isopropylphenol | 136 | 20 ± 2 | 29 ± 3 | 32 ± 3 |
| p-Isopropenylphenol | 119 | 64 ± 7 | 82 ± 8 | 67 ± 7 |
| Cresol | 107 | 48 ± 6 | 61 ± 6 | 59 ± 5 |
| Phenol | 94 | 121 ± 22 | 286 ± 14 | 180 ± 16 |
| Acetone | 58 | 35 ± 2 | 19 ± 2 | 35 ± 2 |
| CH3+ | 15 | 59 ± 9 | 58 ± 3 | 70 ± 6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trubachev, S.; Paletsky, A.; Sosnin, E.; Tuzhikov, O.; Buravov, B.; Shmakov, A.; Chernov, A.; Kulikov, I.; Sagitov, A.; Hu, Y.; et al. Flame-Retardant Glass Fiber-Reinforced Epoxy Resins with Phosphorus-Containing Bio-Based Benzoxazines and Graphene. Polymers 2024, 16, 2333. https://doi.org/10.3390/polym16162333
Trubachev S, Paletsky A, Sosnin E, Tuzhikov O, Buravov B, Shmakov A, Chernov A, Kulikov I, Sagitov A, Hu Y, et al. Flame-Retardant Glass Fiber-Reinforced Epoxy Resins with Phosphorus-Containing Bio-Based Benzoxazines and Graphene. Polymers. 2024; 16(16):2333. https://doi.org/10.3390/polym16162333
Chicago/Turabian StyleTrubachev, Stanislav, Alexander Paletsky, Egor Sosnin, Oleg Tuzhikov, Boris Buravov, Andrey Shmakov, Anatoliy Chernov, Ilya Kulikov, Albert Sagitov, Yuan Hu, and et al. 2024. "Flame-Retardant Glass Fiber-Reinforced Epoxy Resins with Phosphorus-Containing Bio-Based Benzoxazines and Graphene" Polymers 16, no. 16: 2333. https://doi.org/10.3390/polym16162333
APA StyleTrubachev, S., Paletsky, A., Sosnin, E., Tuzhikov, O., Buravov, B., Shmakov, A., Chernov, A., Kulikov, I., Sagitov, A., Hu, Y., & Wang, X. (2024). Flame-Retardant Glass Fiber-Reinforced Epoxy Resins with Phosphorus-Containing Bio-Based Benzoxazines and Graphene. Polymers, 16(16), 2333. https://doi.org/10.3390/polym16162333









