Halochromic Bacterial Cellulose/Anthocyanins Hybrid Polymer Film with Wound-Healing Potential
Abstract
1. Introduction
2. Materials and Methods
2.1. Microorganisms and Culturing
2.2. Selection of Plant Material and Preparation of Anthocyanin Extract (AE)
2.3. Anthocyanin Characterization
2.4. A Bacterial Cellulose Pellicle-Film Production and Purification
2.5. Analysis of Anthocyanin Load and Release Performance
2.6. Biological Activity Characterization of BC/AE
2.6.1. Antimicrobial Activity of Hydrogels with Elderberry Extract
2.6.2. Cytotoxicity Assay
2.6.3. Scratch Assay (Activation of Mouse Fibroblast Migration and Proliferation)
2.7. Physicochemical Characterization of BC/AE
2.7.1. Fourier Transform InfraRed Spectroscopy
2.7.2. Scanning Electron Microscopy (SEM)
2.8. Smartphone-Based Sensor Systems Based on BC Hydrogels and pH-Indicators
2.9. Animals
2.10. Statistical Analyses
3. Results
3.1. Black Elder Extracts, Appropriate Halochromic BC Hydrogels, and the Anthocyanin UV-VIS Spectra in the Range of pH of 4 to 12
3.2. Anthocyanins Upload and Release Performances
3.3. Smartphone-Based Sensor System
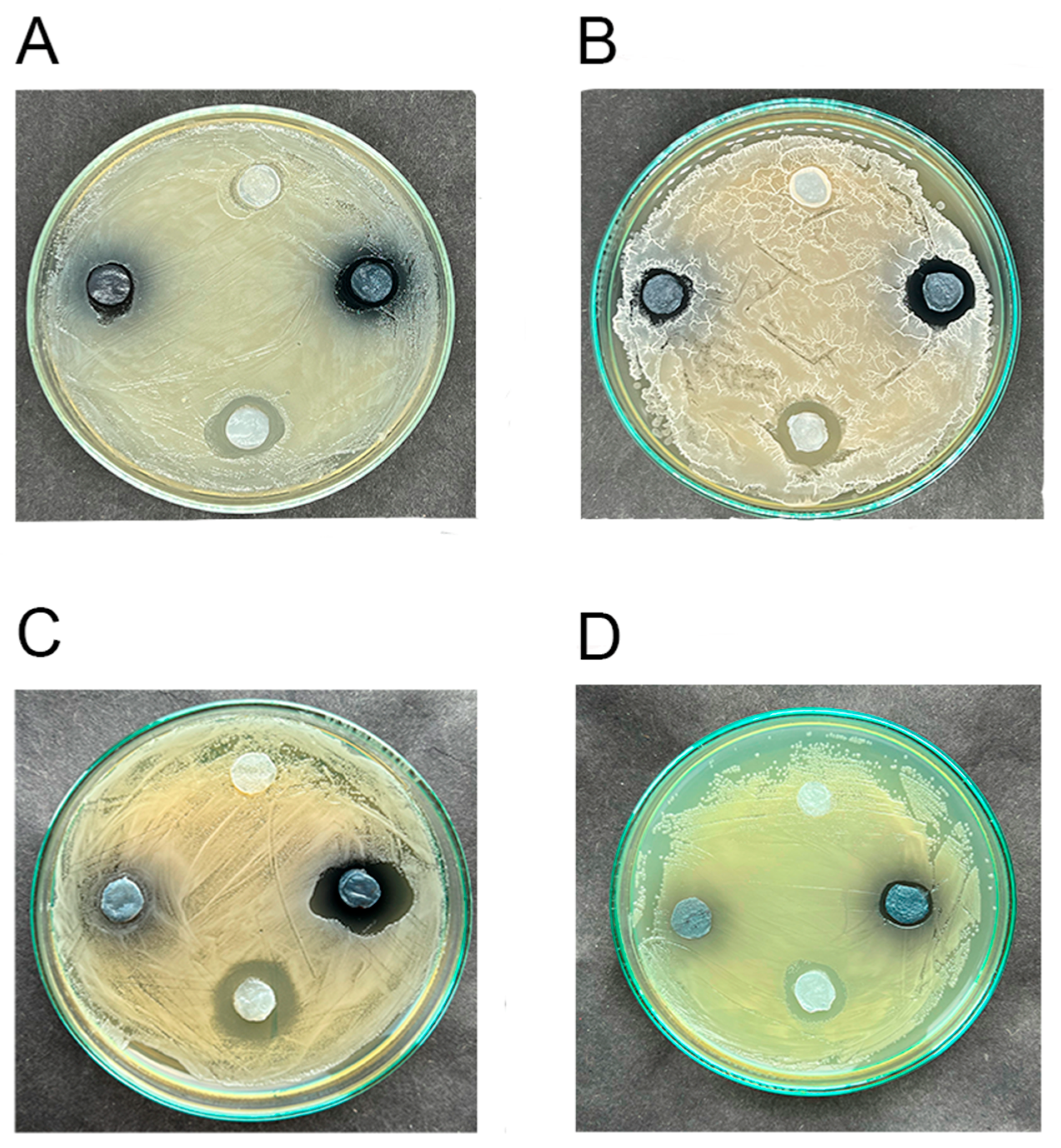
3.4. The Low Antimicrobial Activity of Hydrogels Impregnated with Elderberry Extract Is Shown
3.5. Hydrogels Loaded with the Elderberry Extract and Undecylenic Acid Exhibit More Potent Antimicrobial Activity than the Extract Alone
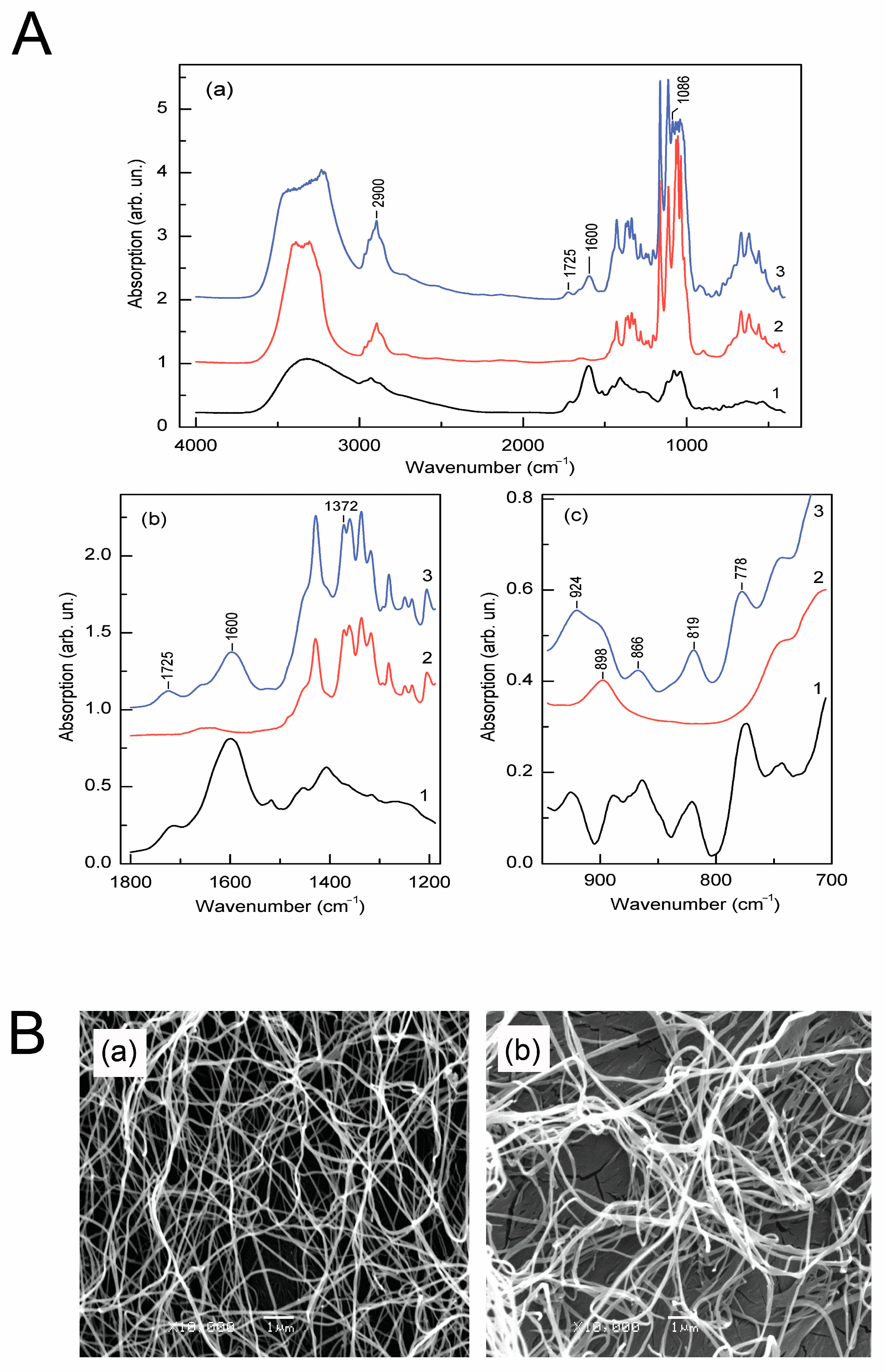
3.6. The Absorption Spectra of Biofilm Samples Proved the Presence of Flavonoid Rings
3.7. Scanning Electron Micrographs Show Thickened Cellulose Nanofibrils in Films Filled with Elderberry Extract
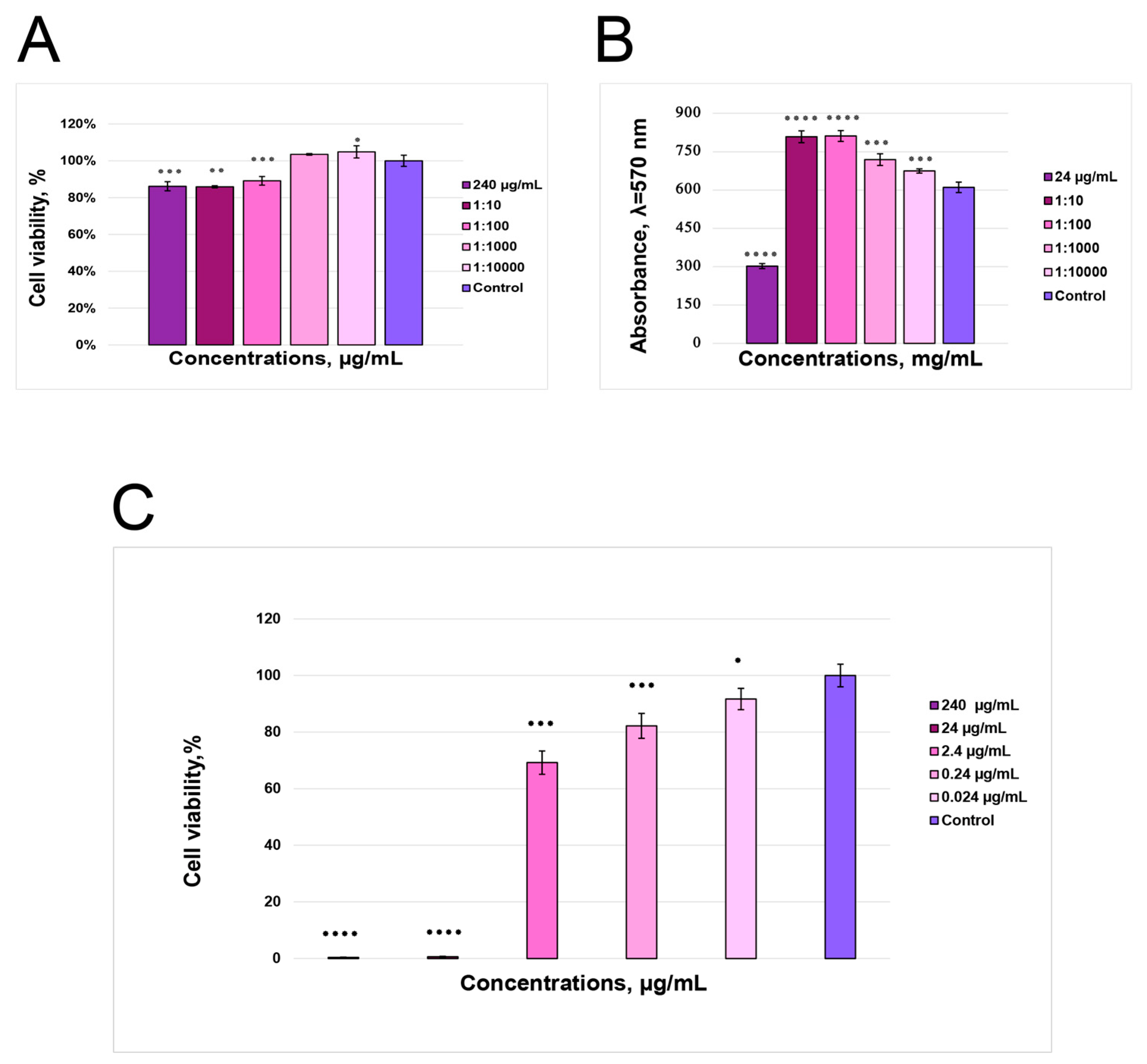
3.8. Cytotoxicity Assay Shows Working Anthocyanin Concentrations
3.9. In Vitro Fibroblast Migration Assay
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
Anthocyanin Content in the Sample Calculation
| Δ (A abs) | 0.012 |
| Δ (A rel) | 0.018 |
| Δ (MAPC rel) | 0.018 |
| Δ (MAPC abs) (mg/L) | 8.015 |
| Time, h | Reference Hydrogel | Hydrogel with the Elderberry Extract | ||
|---|---|---|---|---|
| Weight, g | Coefficient of Water Release, % | Weight, g | Coefficient of the Extract Release, % | |
| 0 | 4.467 ± 0.502 | 0 | 4.666 ± 0.408 | 0 |
| 1 | 3.926 ± 0.248 | 12.110 | 4.162 ± 0.315 | 10.802 |
| 2 | 3.686 ± 0.208 | 17.489 | 3.948 ± 0.513 | 15.377 |
| 3 | 3.521 ± 0.222 | 21.176 | 3.727 ± 0.547 | 20.116 |
| Time, h | Absorption, 517 nm | C-3-O-Glu Concentration, µg/mL |
|---|---|---|
| 0 | 1.1 ± 0.04 | 182.9 ± 6.9 |
| 3 | 0.812 ± 0.02 | 135.7 ± 2.4 |
| Time, h | Absorption, 517 nm | C-3-O-Glu Concentration, µg/mL |
|---|---|---|
| 1 | 0.082 ± 0.01 | 13.9 ± 1.5 |
| 2 | 0.124 ± 0.01 | 20.7 ± 1.8 |
| 3 | 0.144 ± 0.01 | 24.0 ± 1.3 |
References
- James, G.A.; Swogger, E.; Wolcott, R.; Pulcini, E.D.; Secor, P.; Sestrich, J.; Costerton, J.W.; Stewart, P.S. Biofilms in Chronic Wounds. Wound Repair Regen. 2008, 16, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, A.R.; Bernstein, J.M. Chronic Wound Infection: Facts and Controversies. Clin. Dermatol. 2010, 28, 519–526. [Google Scholar] [CrossRef] [PubMed]
- Nagaraj, S.; Ramlal, S.; Kingston, J.; Batra, H.V. Development of IgY Based Sandwich ELISA for the Detection of Staphylococcal Enterotoxin G (SEG), an Egc Toxin. Int. J. Food Microbiol. 2016, 237, 136–141. [Google Scholar] [CrossRef] [PubMed]
- Dou, L.; Bai, Y.; Liu, M.; Shao, S.; Yang, H.; Yu, X.; Wen, K.; Wang, Z.; Shen, J.; Yu, W. ‘Three-To-One’ Multi-Functional Nanocomposite-Based Lateral Flow Immunoassay for Label-Free and Dual-Readout Detection of Pathogenic Bacteria. Biosens. Bioelectron. 2022, 204, 114093. [Google Scholar] [CrossRef]
- Mckillip, J.L.; Drake, M. Real-Time Nucleic Acid–Based Detection Methods for Pathogenic Bacteria in Food. J. Food Prot. 2004, 67, 823–832. [Google Scholar] [CrossRef] [PubMed]
- Saptalena, L.G.; Kuklya, A.; Telgheder, U. Gas Chromatography–Differential Mobility Spectrometry and Gas Chromatography–Mass Spectrometry for the Detection of Coliform Bacteria. Int. J. Mass Spectrom. 2015, 388, 17–25. [Google Scholar] [CrossRef]
- Aebisher, D.; Bartusik, D.; Tabarkiewicz, J. Laser Flow Cytometry as a Tool for the Advancement of Clinical Medicine. Biomed. Pharmacother. 2017, 85, 434–443. [Google Scholar] [CrossRef] [PubMed]
- Pahlow, S.; Meisel, S.; Cialla-May, D.; Weber, K.; Rösch, P.; Popp, J. Isolation and Identification of Bacteria by Means of Raman Spectroscopy. Adv. Drug Deliv. Rev. 2015, 89, 105–120. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.-H.; Sun, D.-W. Recent Applications of Spectroscopic and Hyperspectral Imaging Techniques with Chemometric Analysis for Rapid Inspection of Microbial Spoilage in Muscle Foods. Compr. Rev. Food Sci. Food Saf. 2015, 14, 478–490. [Google Scholar] [CrossRef]
- Altun, N.; Hervello, M.F.; Lombó, F.; González, P. Using Staining as Reference for Spectral Imaging: Its Application for the Development of an Analytical Method to Predict the Presence of Bacterial Biofilms. Talanta 2023, 261, 124655. [Google Scholar] [CrossRef] [PubMed]
- Dietvorst, J.; Ferrer-Vilanova, A.; Iyengar, S.N.; Russom, A.; Vigués, N.; Mas, J.; Vilaplana, L.; Marco, M.-P.; Guirado, G.; Muñoz-Berbel, X. Bacteria Detection at a Single-Cell Level through a Cyanotype-Based Photochemical Reaction. Anal. Chem. 2022, 94, 787–792. [Google Scholar] [CrossRef] [PubMed]
- Bordbar, M.M.; Sheini, A.; Hashemi, P.; Hajian, A.; Bagheri, H. Disposable Paper-Based Biosensors for the Point-of-Care Detection of Hazardous Contaminations—A Review. Biosensors 2021, 11, 316. [Google Scholar] [CrossRef]
- Mazur, F.; Tjandra, A.D.; Zhou, Y.; Gao, Y.; Chandrawati, R. Paper-Based Sensors for Bacteria Detection. Nat. Rev. Bioeng. 2023, 1, 180–192. [Google Scholar] [CrossRef]
- Belushkin, A.; Yesilkoy, F.; González-López, J.J.; Ruiz-Rodríguez, J.C.; Ferrer, R.; Fàbrega, A.; Altug, H. Rapid and Digital Detection of Inflammatory Biomarkers Enabled by a Novel Portable Nanoplasmonic Imager. Small 2020, 16, 1906108. [Google Scholar] [CrossRef] [PubMed]
- Cesewski, E.; Johnson, B.N. Electrochemical Biosensors for Pathogen Detection. Biosens. Bioelectron. 2020, 159, 112214. [Google Scholar] [CrossRef] [PubMed]
- Charaya, H.; La, T.-G.; Rieger, J.; Chung, H.-J. Thermochromic and Piezocapacitive Flexible Sensor Array by Combining Composite Elastomer Dielectrics and Transparent Ionic Hydrogel Electrodes. Adv. Mater. Technol. 2019, 4, 1900327. [Google Scholar] [CrossRef]
- Rahimi, R.; Ochoa, M.; Parupudi, T.; Zhao, X.; Yazdi, I.K.; Dokmeci, M.R.; Tamayol, A.; Khademhosseini, A.; Ziaie, B. A Low-Cost Flexible pH Sensor Array for Wound Assessment. Sens. Actuators B Chem. 2016, 229, 609–617. [Google Scholar] [CrossRef]
- Jankowska, D.A.; Bannwarth, M.B.; Schulenburg, C.; Faccio, G.; Maniura-Weber, K.; Rossi, R.M.; Scherer, L.; Richter, M.; Boesel, L.F. Simultaneous Detection of pH Value and Glucose Concentrations for Wound Monitoring Applications. Biosens. Bioelectron. 2017, 87, 312–319. [Google Scholar] [CrossRef]
- Pan, N.; Qin, J.; Feng, P.; Li, Z.; Song, B. Color-Changing Smart Fibrous Materials for Naked Eye Real-Time Monitoring of Wound pH. J. Mater. Chem. B 2019, 7, 2626–2633. [Google Scholar] [CrossRef]
- Youssef, K.; Ullah, A.; Rezai, P.; Hasan, A.; Amirfazli, A. Recent Advances in Biosensors for Real Time Monitoring of pH, Temperature, and Oxygen in Chronic Wounds. Mater. Today Bio 2023, 22, 100764. [Google Scholar] [CrossRef]
- Behrouznejad, B.; Sadat, S.B.; Masaeli, E. The Orchestration of Sustained Drug Delivery by Bacterial Cellulose/Gelatin Nanocomposites Reinforced with Carboxylic Carbon Nanotubes. Carbohydr. Polym. 2024, 333, 121917. [Google Scholar] [CrossRef] [PubMed]
- Zmejkoski, D.Z.; Marković, Z.M.; Budimir, M.D.; Zdravković, N.M.; Trišić, D.D.; Bugárová, N.; Danko, M.; Kozyrovska, N.O.; Špitalský, Z.; Kleinová, A.; et al. Photoactive and Antioxidant Nanochitosan Dots/Biocellulose Hydrogels for Wound Healing Treatment. Mater. Sci. Eng. C 2021, 122, 111925. [Google Scholar] [CrossRef]
- Li, Y.; Chen, S.; Zhang, M.; Ma, X.; Zhao, J.; Ji, Y. Novel Injectable, Self-Healing, Long-Effective Bacteriostatic, and Healed-Promoting Hydrogel Wound Dressing and Controlled Drug Delivery Mechanisms. ACS Appl. Mater. Interfaces 2024, 16, 2140–2153. [Google Scholar] [CrossRef]
- Bakadia, B.M.; Boni, B.O.O.; Ahmed, A.A.Q.; Zheng, R.; Shi, Z.; Ullah, M.W.; Lamboni, L.; Yang, G. In Situ Synthesized Porous Bacterial Cellulose/Poly(Vinyl Alcohol)-Based Silk Sericin and Azithromycin Release System for Treating Chronic Wound Biofilm. Macromol. Biosci. 2022, 22, 2200201. [Google Scholar] [CrossRef]
- Kukharenko, O.; Bardeau, J.-F.; Zaets, I.; Ovcharenko, L.; Tarasyuk, O.; Porhyn, S.; Mischenko, I.; Vovk, A.; Rogalsky, S.; Kozyrovska, N. Promising Low Cost Antimicrobial Composite Material Based on Bacterial Cellulose and Polyhexamethylene Guanidine Hydrochloride. Eur. Polym. J. 2014, 60, 247–254. [Google Scholar] [CrossRef]
- Zmejkoski, D.Z.; Marković, Z.M.; Mitić, D.D.; Zdravković, N.M.; Kozyrovska, N.O.; Bugárová, N.; Todorović Marković, B.M. Antibacterial Composite Hydrogels of Graphene Quantum Dots and Bacterial Cellulose Accelerate Wound Healing. J. Biomed. Mater. Res. Part B Appl. Biomater. 2022, 110, 1796–1805. [Google Scholar] [CrossRef]
- Yang, C.; Zhu, Y.; Tian, Z.; Zhang, C.; Han, X.; Jiang, S.; Liu, K.; Duan, G. Preparation of Nanocellulose and Its Applications in Wound Dressing: A Review. Int. J. Biol. Macromol. 2024, 254, 127997. [Google Scholar] [CrossRef]
- Halib, N.; Ahmad, I.; Grassi, M.; Grassi, G. The Remarkable Three-Dimensional Network Structure of Bacterial Cellulose for Tissue Engineering Applications. Int. J. Pharm. 2019, 566, 631–640. [Google Scholar] [CrossRef] [PubMed]
- Hou, S.; Xia, Z.; Pan, J.; Wang, N.; Gao, H.; Ren, J.; Xia, X. Bacterial Cellulose Applied in Wound Dressing Materials: Production and Functional Modification—A Review. Macromol. Biosci. 2024, 24, 2300333. [Google Scholar] [CrossRef] [PubMed]
- De Assis, S.C.; Morgado, D.L.; Scheidt, D.T.; De Souza, S.S.; Cavallari, M.R.; Ando Junior, O.H.; Carrilho, E. Review of Bacterial Nanocellulose-Based Electrochemical Biosensors: Functionalization, Challenges, and Future Perspectives. Biosensors 2023, 13, 142. [Google Scholar] [CrossRef]
- Farooq, U.; Ullah, M.W.; Yang, Q.; Aziz, A.; Xu, J.; Zhou, L.; Wang, S. High-Density Phage Particles Immobilization in Surface-Modified Bacterial Cellulose for Ultra-Sensitive and Selective Electrochemical Detection of Staphylococcus Aureus. Biosens. Bioelectron. 2020, 157, 112163. [Google Scholar] [CrossRef] [PubMed]
- Bueno, J.M.; Sáez-Plaza, P.; Ramos-Escudero, F.; Jiménez, A.M.; Fett, R.; Asuero, A.G. Analysis and Antioxidant Capacity of Anthocyanin Pigments. Part II: Chemical Structure, Color, and Intake of Anthocyanins. Crit. Rev. Anal. Chem. 2012, 42, 126–151. [Google Scholar] [CrossRef]
- Iacobucci, G.A.; Sweeny, J.G. The Chemistry of Anthocyanins, Anthocyanidins and Related Flavylium Salts. Tetrahedron 1983, 39, 3005–3038. [Google Scholar] [CrossRef]
- Haș, I.M.; Teleky, B.-E.; Szabo, K.; Simon, E.; Ranga, F.; Diaconeasa, Z.M.; Purza, A.L.; Vodnar, D.-C.; Tit, D.M.; Nițescu, M. Bioactive Potential of Elderberry (Sambucus nigra L.): Antioxidant, Antimicrobial Activity, Bioaccessibility and Prebiotic Potential. Molecules 2023, 28, 3099. [Google Scholar] [CrossRef]
- Osman, A.G.; Avula, B.; Katragunta, K.; Ali, Z.; Chittiboyina, A.G.; Khan, I.A. Elderberry Extracts: Characterization of the Polyphenolic Chemical Composition, Quality Consistency, Safety, Adulteration, and Attenuation of Oxidative Stress- and Inflammation-Induced Health Disorders. Molecules 2023, 28, 3148. [Google Scholar] [CrossRef] [PubMed]
- Studzińska-Sroka, E.; Paczkowska-Walendowska, M.; Woźna, Z.; Plech, T.; Szulc, P.; Cielecka-Piontek, J. Elderberry Leaves with Antioxidant and Anti-Inflammatory Properties as a Valuable Plant Material for Wound Healing. Pharmaceuticals 2024, 17, 618. [Google Scholar] [CrossRef] [PubMed]
- Vendrame, S.; Alaba, T.; Marchi, N.; Tsakiroglou, P.; Klimis-Zacas, D. In Vitro and In Vivo Evaluation of Bioactive Compounds from Berries for Wound Healing. Curr. Dev. Nutr. 2024, 8, 102078. [Google Scholar] [CrossRef] [PubMed]
- Rosales-Murillo, S.S.; Sánchez-Bodón, J.; Hernández Olmos, S.L.; Ibarra-Vázquez, M.F.; Guerrero-Ramírez, L.G.; Pérez-Álvarez, L.; Vilas-Vilela, J.L. Anthocyanin-Loaded Polymers as Promising Nature-Based, Responsive, and Bioactive Materials. Polymers 2024, 16, 163. [Google Scholar] [CrossRef]
- Pourjavaher, S.; Almasi, H.; Meshkini, S.; Pirsa, S.; Parandi, E. Development of a Colorimetric pH Indicator Based on Bacterial Cellulose Nanofibers and Red Cabbage (Brassica oleraceae) Extract. Carbohydr. Polym. 2017, 156, 193–201. [Google Scholar] [CrossRef]
- Zhou, S.; Li, N.; Peng, H.; Yang, X.; Lin, D. The Development of Highly pH-Sensitive Bacterial Cellulose Nanofibers/Gelatin-Based Intelligent Films Loaded with Anthocyanin/Curcumin for the Fresh-Keeping and Freshness Detection of Fresh Pork. Foods 2023, 12, 3719. [Google Scholar] [CrossRef]
- Festa, J.; Singh, H.; Hussain, A.; Da Boit, M. Elderberries as a Potential Supplement to Improve Vascular Function in a SARS-CoV-2 Environment. J. Food Biochem. 2022, 46, e14091. [Google Scholar] [CrossRef] [PubMed]
- Arghavani, S.; Mohseni-Shahri, F.S.; Moeinpour, F. Anthocyanin-loaded Bacterial Cellulose Nanofiber as a Green Sensor for Monitoring the Selective Naked Eye and Visual Detection of Al(III) Ions. Anal. Sci. Adv. 2023, 4, 324–334. [Google Scholar] [CrossRef]
- Parizadeh, P.; Moeinpour, F.; Mohseni-Shahri, F.S. Anthocyanin-Induced Color Changes in Bacterial Cellulose Nanofibers for the Accurate and Selective Detection of Cu(II) in Water Samples. Chemosphere 2023, 326, 138459. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Durst, R.W.; Wrolstad, R.E.; Eisele, T.; Giusti, M.M.; Hach, J.; Hofsommer, H.; Koswig, S.; Krueger, D.A.; Martin, S.K.; et al. Determination of Total Monomeric Anthocyanin Pigment Content of Fruit Juices, Beverages, Natural Colorants, and Wines by the pH Differential Method: Collaborative Study. J. AOAC Int. 2005, 88, 1269–1278. [Google Scholar] [CrossRef]
- Startseva, Y.D.; Hodyna, D.M.; Semenyuta, I.V.; Tarasyuk, O.P.; Rogalsky, S.P.; Metelytsia, L.O. Undecylenic Acid and N,N-Dibutylundecenamide as Effective Antibacterials against Antibiotic-Resistant Strains. Ukr. Biochem. J. 2023, 95, 55–63. [Google Scholar] [CrossRef]
- Präbst, K.; Engelhardt, H.; Ringgeler, S.; Hübner, H. Basic Colorimetric Proliferation Assays: MTT, WST, and Resazurin. In Cell Viability Assays; Gilbert, D.F., Friedrich, O., Eds.; Methods in Molecular Biology; Springer: New York, NY, USA, 2017; Volume 1601, pp. 1–17. ISBN 978-1-4939-6959-3. [Google Scholar]
- Salem, K.S.; Kasera, N.K.; Rahman, M.A.; Jameel, H.; Habibi, Y.; Eichhorn, S.J.; French, A.D.; Pal, L.; Lucia, L.A. Comparison and Assessment of Methods for Cellulose Crystallinity Determination. Chem. Soc. Rev. 2023, 52, 6417–6446. [Google Scholar] [CrossRef]
- Strugała, P.; Loi, S.; Bażanów, B.; Kuropka, P.; Kucharska, A.Z.; Włoch, A.; Gabrielska, J. A Comprehensive Study on the Biological Activity of Elderberry Extract and Cyanidin 3-O-Glucoside and Their Interactions with Membranes and Human Serum Albumin. Molecules 2018, 23, 2566. [Google Scholar] [CrossRef]
- Vasavi, H.S.; Arun, A.B.; Rekha, P.-D. Anti-Quorum Sensing Activity of Psidium Guajava L. Flavonoids against Chromobacterium violaceum and Pseudomonas aeruginosa PAO1. Microbiol. Immunol. 2014, 58, 286–293. [Google Scholar] [CrossRef]
- Paczkowski, J.E.; Mukherjee, S.; McCready, A.R.; Cong, J.-P.; Aquino, C.J.; Kim, H.; Henke, B.R.; Smith, C.D.; Bassler, B.L. Flavonoids Suppress Pseudomonas aeruginosa Virulence through Allosteric Inhibition of Quorum-Sensing Receptors. J. Biol. Chem. 2017, 292, 4064–4076. [Google Scholar] [CrossRef]
- Li, Y.; Kong, D.; Wu, H. Comprehensive Chemical Analysis of the Flower Buds of Five Lonicera Species by ATR-FTIR, HPLC-DAD, and Chemometric Methods. Rev. Bras. Farmacogn. 2018, 28, 533–541. [Google Scholar] [CrossRef]
- Eskilson, O.; Zattarin, E.; Berglund, L.; Oksman, K.; Hanna, K.; Rakar, J.; Sivlér, P.; Skog, M.; Rinklake, I.; Shamasha, R.; et al. Nanocellulose Composite Wound Dressings for Real-Time pH Wound Monitoring. Mater. Today Bio 2023, 19, 100574. [Google Scholar] [CrossRef] [PubMed]
- Tamayol, A.; Akbari, M.; Zilberman, Y.; Comotto, M.; Lesha, E.; Serex, L.; Bagherifard, S.; Chen, Y.; Fu, G.; Ameri, S.K.; et al. Flexible pH-Sensing Hydrogel Fibers for Epidermal Applications. Adv. Healthc. Mater. 2016, 5, 711–719. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Li, X.; Nagao, M.; Elias, A.L.; Narain, R.; Chung, H.-J. A pH-Indicating Colorimetric Tough Hydrogel Patch towards Applications in a Substrate for Smart Wound Dressings. Polymers 2017, 9, 558. [Google Scholar] [CrossRef] [PubMed]
- Gamerith, C.; Luschnig, D.; Ortner, A.; Pietrzik, N.; Guse, J.-H.; Burnet, M.; Haalboom, M.; van der Palen, J.; Heinzle, A.; Sigl, E.; et al. pH-Responsive Materials for Optical Monitoring of Wound Status. Sens. Actuators B Chem. 2019, 301, 126966. [Google Scholar] [CrossRef]
- Orlovska, I.; Podolich, O.; Kukharenko, O.; Zaets, I.; Reva, O.; Khirunenko, L.; Zmejkoski, D.; Rogalsky, S.; Barh, D.; Tiwari, S.; et al. Bacterial Cellulose Retains Robustness but Its Synthesis Declines after Exposure to a Mars-like Environment Simulated Outside the International Space Station. Astrobiology 2021, 21, 706–717. [Google Scholar] [CrossRef] [PubMed]
- Le Bourvellec, C.; Renard, C.M.G.C. Interactions between Polyphenols and Macromolecules: Quantification Methods and Mechanisms. Crit. Rev. Food Sci. Nutr. 2012, 52, 213–248. [Google Scholar] [CrossRef] [PubMed]
- Phan, A.D.T.; Netzel, G.; Wang, D.; Flanagan, B.M.; D’Arcy, B.R.; Gidley, M.J. Binding of Dietary Polyphenols to Cellulose: Structural and Nutritional Aspects. Food Chem. 2015, 171, 388–396. [Google Scholar] [CrossRef] [PubMed]
- Siemińska-Kuczer, A.; Szymańska-Chargot, M.; Zdunek, A. Recent Advances in Interactions between Polyphenols and Plant Cell Wall Polysaccharides as Studied Using an Adsorption Technique. Food Chem. 2022, 373, 131487. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Zhang, S.; Ye, Y.; Liu, X.; He, J.; Wei, L.; Zhang, D.; Zhou, J.; Cai, J. Robust Electrospinning-Constructed Cellulose Acetate@Anthocyanin Ultrafine Fibers: Synthesis, Characterization, and Controlled Release Properties. Polymers 2022, 14, 4036. [Google Scholar] [CrossRef]
- Land, K.J.; Boeras, D.I.; Chen, X.-S.; Ramsay, A.R.; Peeling, R.W. REASSURED Diagnostics to Inform Disease Control Strategies, Strengthen Health Systems and Improve Patient Outcomes. Nat. Microbiol. 2018, 4, 46–54. [Google Scholar] [CrossRef]
- Stępień, A.E.; Trojniak, J.; Tabarkiewicz, J. Health-Promoting Properties: Anti-Inflammatory and Anticancer Properties of Sambucus nigra L. Flowers and Fruits. Molecules 2023, 28, 6235. [Google Scholar] [CrossRef] [PubMed]
- Horta-Velázquez, A.; Mota-Morales, J.D.; Morales-Narváez, E. Next-Generation of Smart Dressings: Integrating Multiplexed Sensors and Theranostic Functions. Int. J. Biol. Macromol. 2024, 254, 127737. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zubova, G.; Melnyk, H.; Zaets, I.; Sergeyeva, T.; Havryliuk, O.; Rogalsky, S.; Khirunenko, L.; Zaika, L.; Ruban, T.; Antonenko, S.; et al. Halochromic Bacterial Cellulose/Anthocyanins Hybrid Polymer Film with Wound-Healing Potential. Polymers 2024, 16, 2327. https://doi.org/10.3390/polym16162327
Zubova G, Melnyk H, Zaets I, Sergeyeva T, Havryliuk O, Rogalsky S, Khirunenko L, Zaika L, Ruban T, Antonenko S, et al. Halochromic Bacterial Cellulose/Anthocyanins Hybrid Polymer Film with Wound-Healing Potential. Polymers. 2024; 16(16):2327. https://doi.org/10.3390/polym16162327
Chicago/Turabian StyleZubova, Ganna, Hanna Melnyk, Iryna Zaets, Tetyana Sergeyeva, Olesia Havryliuk, Sergiy Rogalsky, Lyudmila Khirunenko, Leonid Zaika, Tetiana Ruban, Svitlana Antonenko, and et al. 2024. "Halochromic Bacterial Cellulose/Anthocyanins Hybrid Polymer Film with Wound-Healing Potential" Polymers 16, no. 16: 2327. https://doi.org/10.3390/polym16162327
APA StyleZubova, G., Melnyk, H., Zaets, I., Sergeyeva, T., Havryliuk, O., Rogalsky, S., Khirunenko, L., Zaika, L., Ruban, T., Antonenko, S., & Kozyrovska, N. (2024). Halochromic Bacterial Cellulose/Anthocyanins Hybrid Polymer Film with Wound-Healing Potential. Polymers, 16(16), 2327. https://doi.org/10.3390/polym16162327







