Color-Stable Formulations for 3D-Photoprintable Dental Materials
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Color Stability and Sample Preparation
2.2.1. UV-Vis
2.2.2. Cielab
3. Results
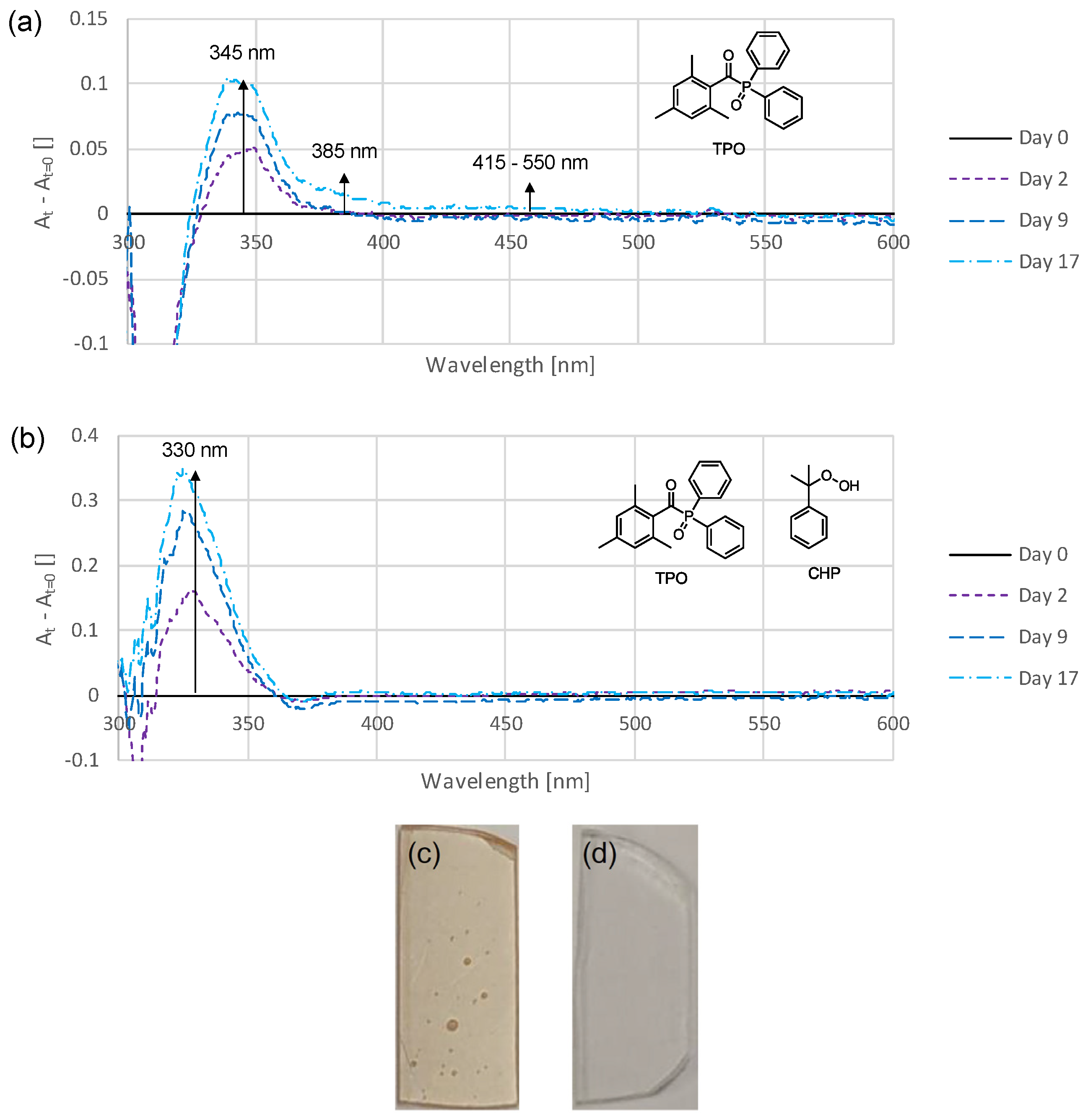
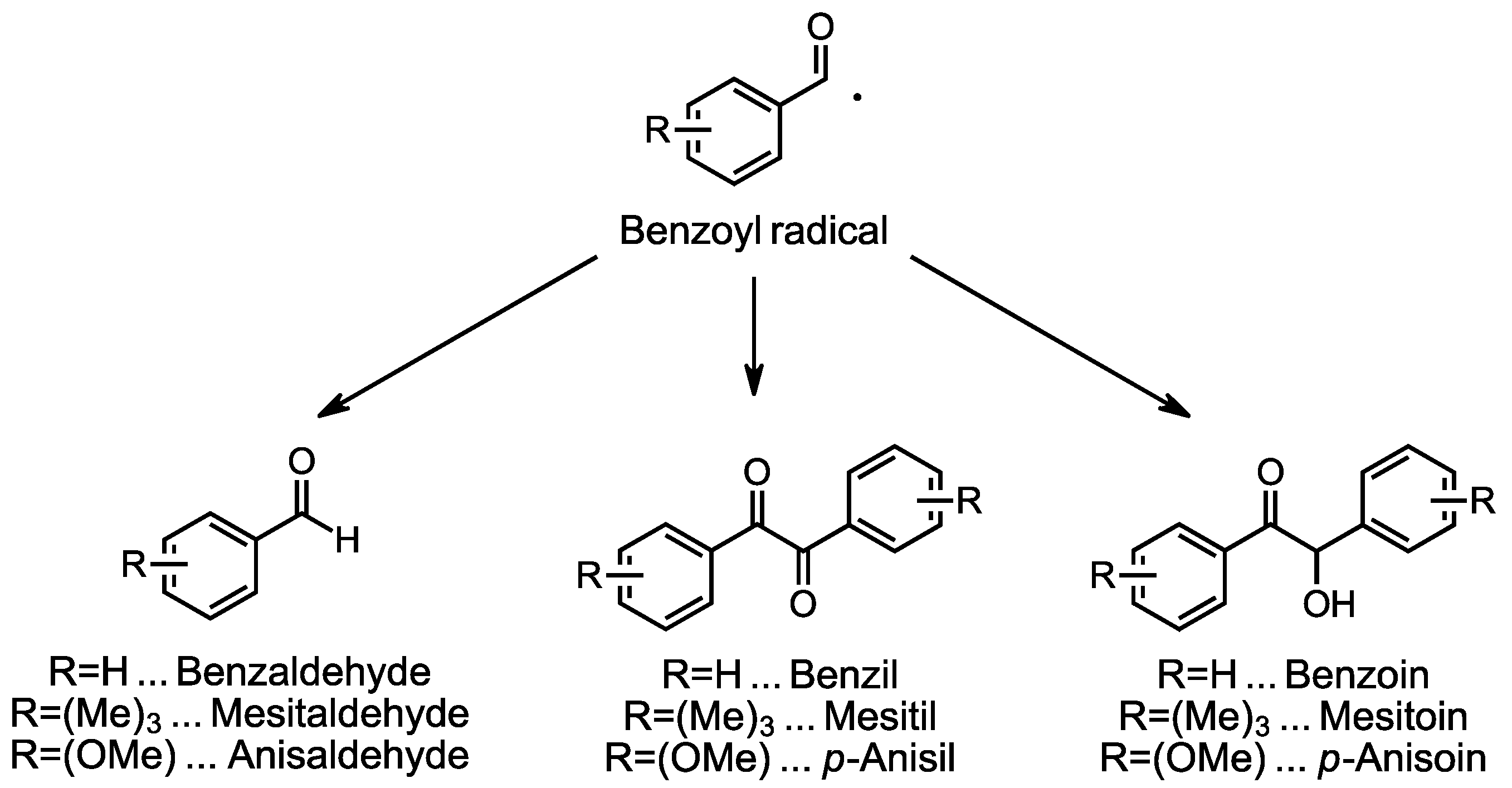
3.1. Time-Dependent Discoloration and Bleaching
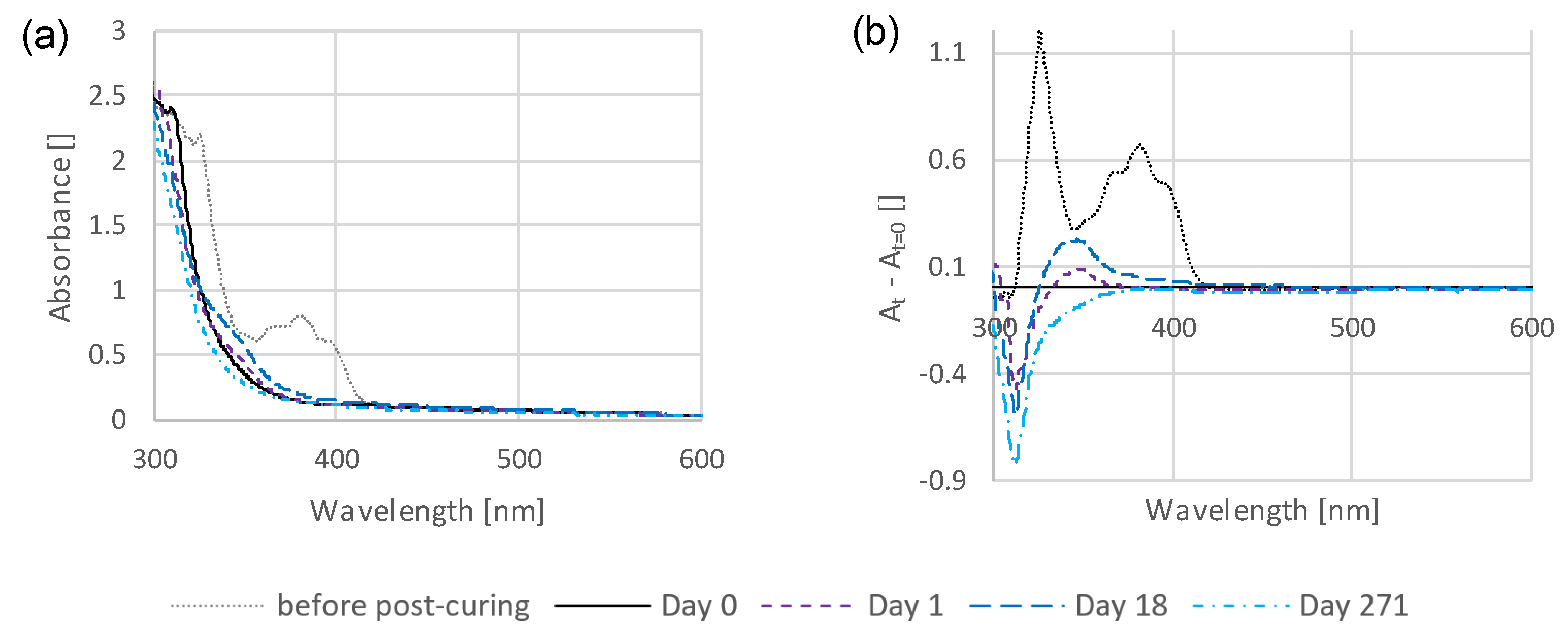
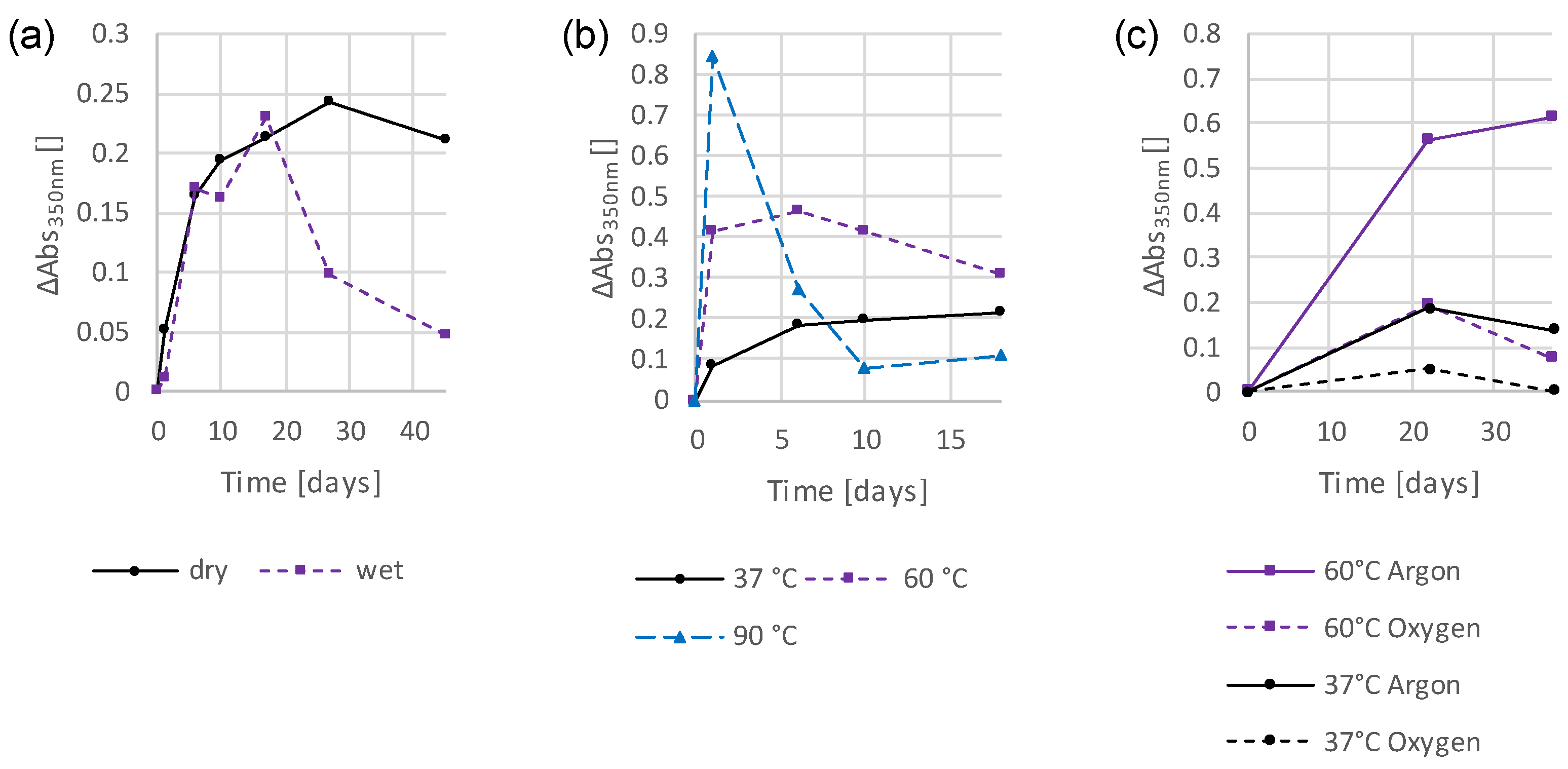
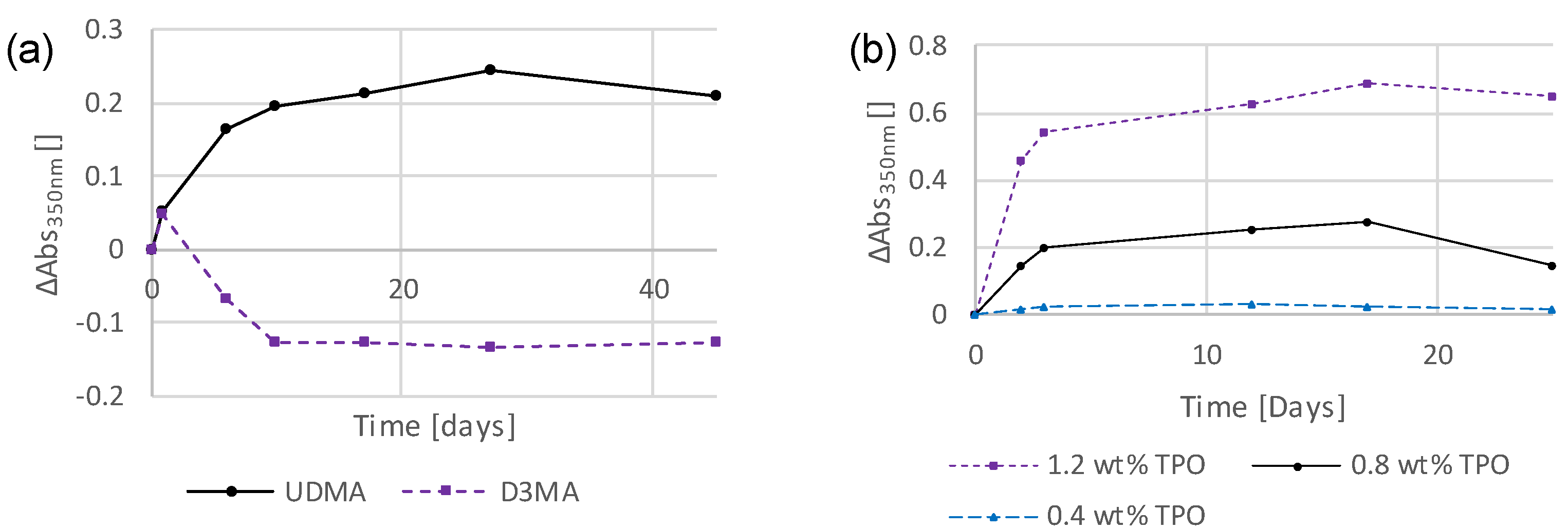
3.2. Preliminary Investigations into the Influence of Storage Conditions and Formulation Components on the Discoloration Behavior
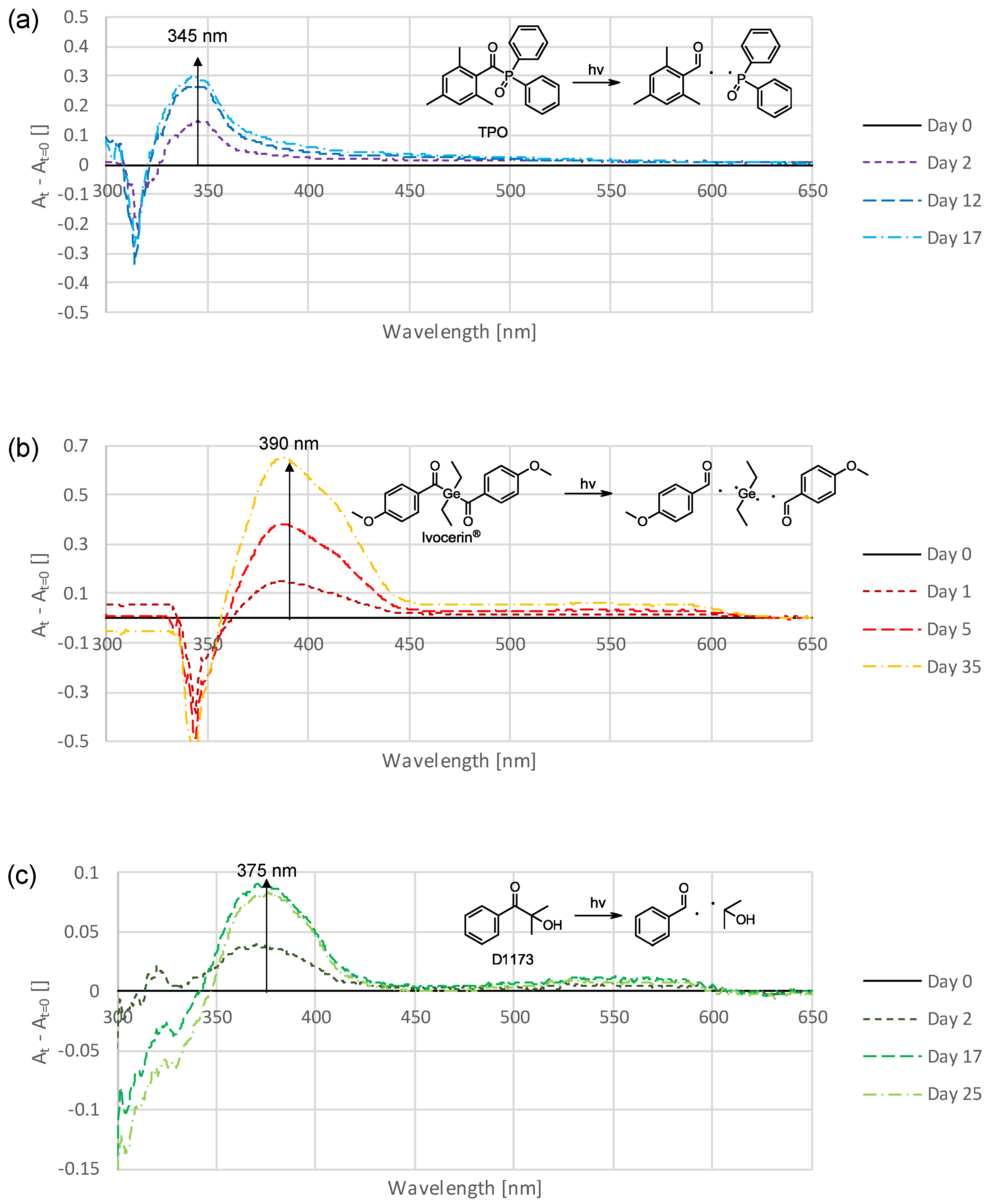
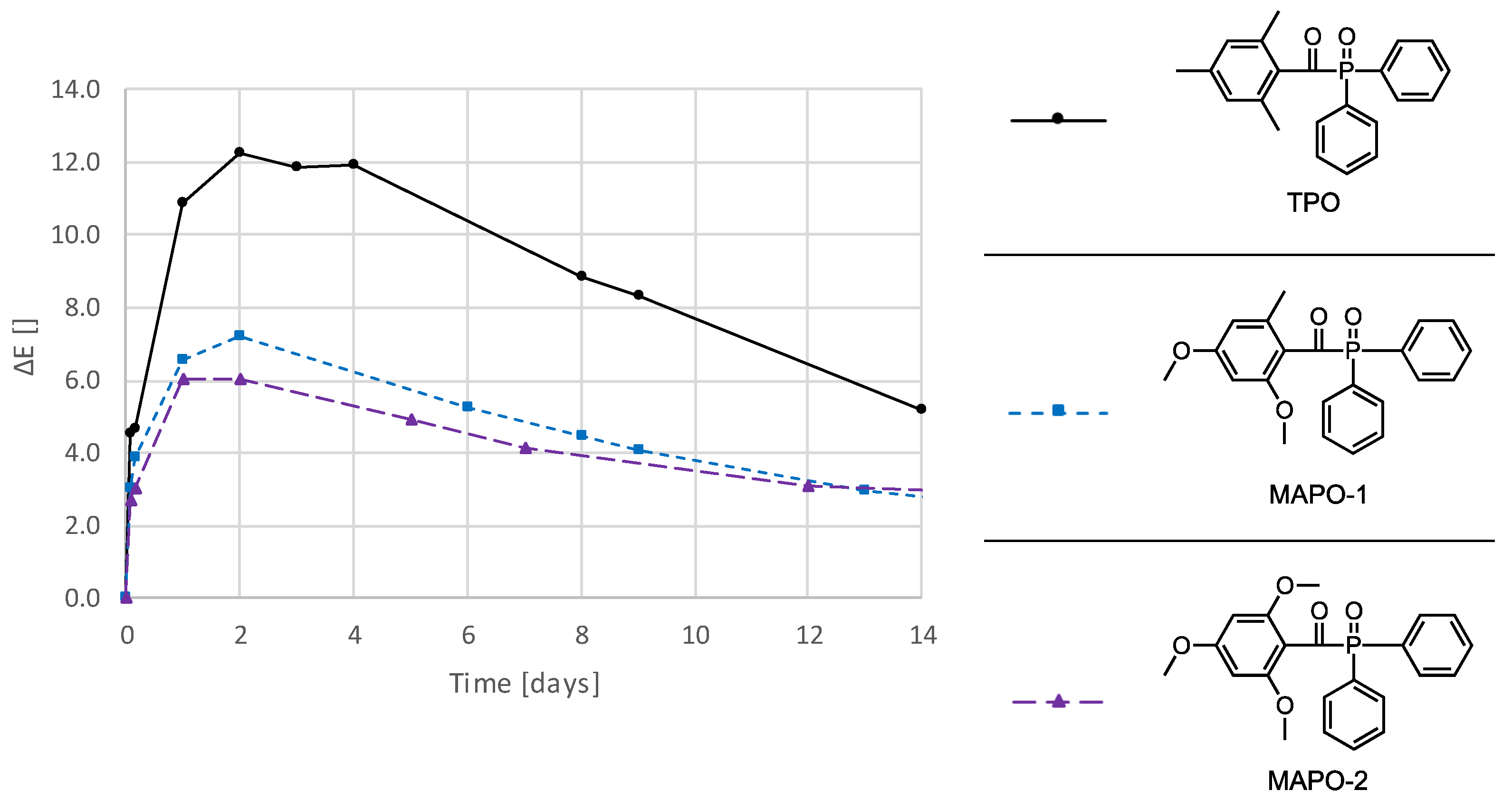
3.3. Photoinitiators
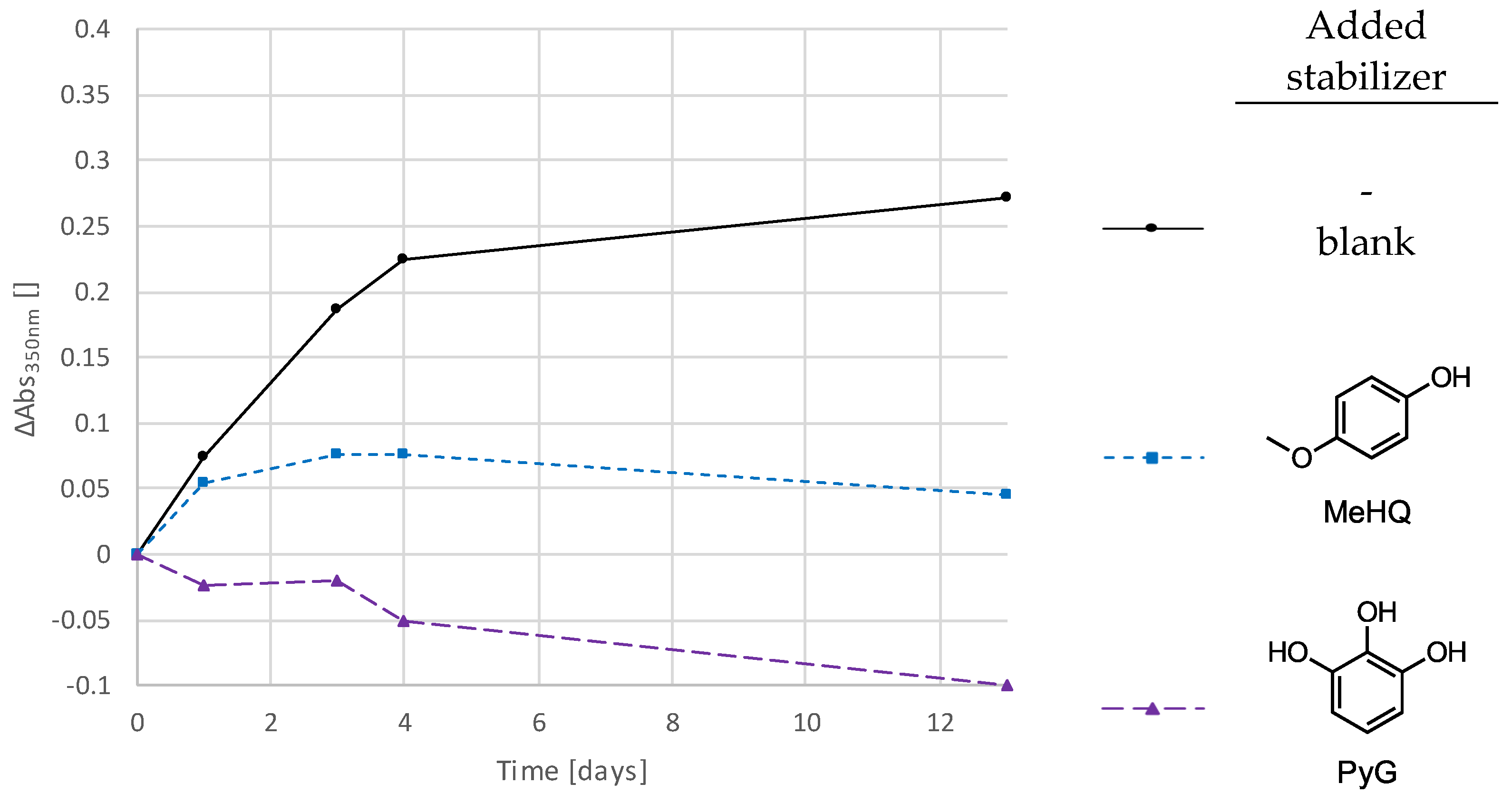
3.4. Addition of Stabilizers
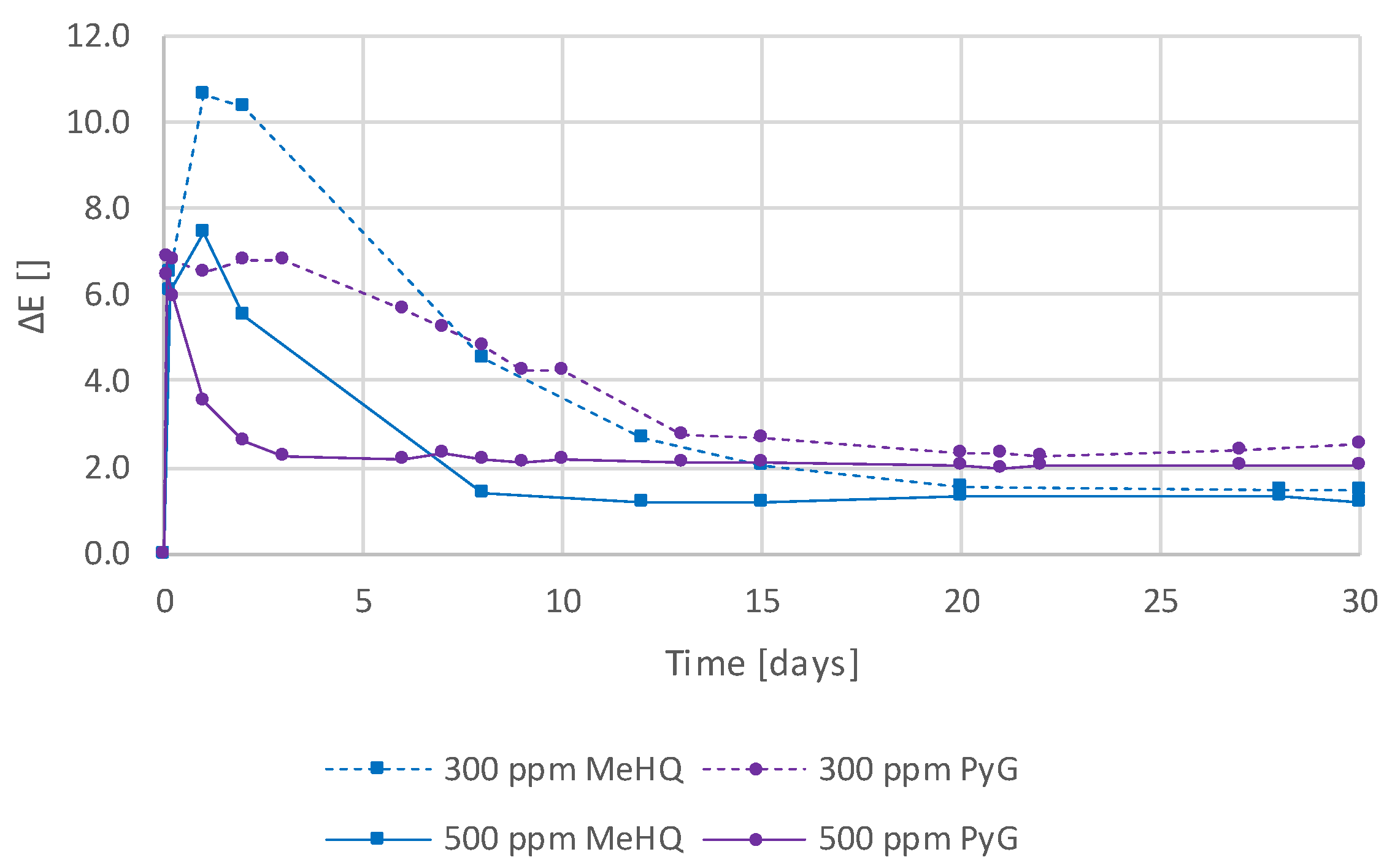
3.5. Addition of Hydroperoxide
4. Discussion
4.1. Photoinitiators for Improved Color Stability
4.2. Addition of Stabilizers for Improved Color Stability
4.3. Addition of Hydroperoxide for Improved Color Stability
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tahayeri, A.; Morgan, M.; Fugolin, A.P.; Bompolaki, D.; Athirasala, A.; Pfeifer, C.S.; Ferracane, J.L.; Bertassoni, L.E. 3D printed versus conventionally cured provisional crown and bridge dental materials. Dent. Mater. 2018, 34, 192–200. [Google Scholar] [CrossRef] [PubMed]
- Abdullah, A.O.; Tsitrou, E.A.; Pollington, S. Comparative in vitro evaluation of CAD/CAM vs conventional provisional crowns. J Appl. Oral Sci. 2016, 24, 258–263. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Xie, B.; Jin, J.; Chai, Y.; Chen, Y. 3D Printing Temporary Crown and Bridge by Temperature Controlled Mask Image Projection Stereolithography. Procedia Manufacturing 2018, 26, 1023–1033. [Google Scholar] [CrossRef]
- Kessler, A.; Hickel, R.; Reymus, M. 3D Printing in Dentistry—State of the Art. Oper Dent 2020, 45, 30–40. [Google Scholar] [CrossRef] [PubMed]
- Berman, B. 3-D printing: The new industrial revolution. Business Horizons 2012, 55, 155–162. [Google Scholar] [CrossRef]
- Abduo, J.; Lyons, K.; Bennamoun, M. Trends in Computer-Aided Manufacturing in Prosthodontics: A Review of the Available Streams. Int. J. Dent. 2014, 2014, 783948. [Google Scholar] [CrossRef] [PubMed]
- van Noort, R. The future of dental devices is digital. Dent. Mater. 2012, 28, 3–12. [Google Scholar] [CrossRef]
- Dawood, A.; Marti, B.M.; Sauret-Jackson, V.; Darwood, A. 3D printing in dentistry. Br. Dent. J. 2015, 219, 521–529. [Google Scholar] [CrossRef] [PubMed]
- Nayar, S.; Bhuminathan, S.; Bhat, W.M. Rapid prototyping and stereolithography in dentistry. J. Pharm. BioAllied Sci. 2015, 7, S216–S219. [Google Scholar] [CrossRef]
- Lin, C.H.; Lin, Y.M.; Lai, Y.L.; Lee, S.Y. Mechanical properties, accuracy, and cytotoxicity of UV-polymerized 3D printing resins composed of Bis-EMA, UDMA, and TEGDMA. J. Prosthet. Dent. 2020, 123, 349–354. [Google Scholar] [CrossRef]
- Ling, L.; Taremi, N.; Malyala, R. A Novel Low-Shrinkage Resin for 3D Printing. J Dent 2022, 118, 103957. [Google Scholar] [CrossRef] [PubMed]
- Osman, R.B.; Alharbi, N.; Wismeijer, D. Build Angle: Does It Influence the Accuracy of 3D-Printed Dental Restorations Using Digital Light-Processing Technology? Int J Prosthodont 2017, 30, 182–188. [Google Scholar] [CrossRef] [PubMed]
- Rosentritt, M.; Huber, C.; Strasser, T.; Schmid, A. Investigating the mechanical and optical properties of novel Urethandimethacrylate (UDMA) and Urethanmethacrylate (UMA) based rapid prototyping materials. Dent. Mater. 2021, 37, 1584–1591. [Google Scholar] [CrossRef] [PubMed]
- Tzeng, J.-J.; Yang, T.-S.; Lee, W.-F.; Chen, H.; Chang, H.-M. Mechanical Properties and Biocompatibility of Urethane Acrylate-Based 3D-Printed Denture Base Resin. Polymers 2021, 13, 822. [Google Scholar] [CrossRef] [PubMed]
- Dickens, S.H.; Stansbury, J.W.; Choi, K.M.; Floyd, C.J.E. Photopolymerization kinetics of methacrylate dental resins. Macromolecules 2003, 36, 6043–6053. [Google Scholar] [CrossRef]
- Asmussen, E.; Peutzfeldt, A. Influence of UEDMA, BisGMA and TEGDMA on selected mechanical properties of experimental resin composites. Dent. Mater. 1998, 14, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Floyd, C.J.E.; Dickens, S.H. Network structure of Bis-GMA- and UDMA-based resin systems. Dent. Mater. 2006, 22, 1143–1149. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.-J.; Kim, H.; Lee, J.; Park, Y.; Kim, J.-H. Effect of airborne particle abrasion treatment of two types of 3D-printing resin materials for permanent restoration materials on flexural strength. Dent. Mater. 2023, 39, 648–658. [Google Scholar] [CrossRef]
- Gonçalves, F.; Kawano, Y.; Pfeifer, C.; Stansbury, J.W.; Braga, R.R. Influence of BisGMA, TEGDMA, and BisEMA contents on viscosity, conversion, and flexural strength of experimental resins and composites. Eur. J. Oral Sci. 2009, 117, 442–446. [Google Scholar] [CrossRef] [PubMed]
- Amini, A.; Guijt, R.M.; Themelis, T.; De Vos, J.; Eeltink, S. Recent developments in digital light processing 3D-printing techniques for microfluidic analytical devices. J. Chromatogr. A 2023, 1692. [Google Scholar] [CrossRef]
- Bagheri, A.; Jin, J. Photopolymerization in 3D Printing. ACS Appl. Polym. Mater. 2019, 1, 593–611. [Google Scholar] [CrossRef]
- de Castro, E.F.; Nima, G.; Rueggeberg, F.A.; Araújo-Neto, V.G.; Faraoni, J.J.; Palma-Dibb, R.G.; Giannini, M. Effect of build orientation in gloss, roughness and color of 3D-printed resins for provisional indirect restorations. Dent. Mater. 2023, 39, e1–e11. [Google Scholar] [CrossRef]
- Scotti, C.K.; Velo, M.M.d.A.C.; Rizzante, F.A.P.; Nascimento, T.R.d.L.; Mondelli, R.F.L.; Bombonatti, J.F.S. Physical and surface properties of a 3D-printed composite resin for a digital workflow. J. Prosthet. Dent. 2020, 124, 614.e611–614.e615. [Google Scholar] [CrossRef]
- Shin, J.-W.; Kim, J.-E.; Choi, Y.-J.; Shin, S.-H.; Nam, N.-E.; Shim, J.-S.; Lee, K.-W. Evaluation of the Color Stability of 3D-Printed Crown and Bridge Materials against Various Sources of Discoloration: An In Vitro Study. Materials 2020, 13, 5359. [Google Scholar] [CrossRef]
- Gruber, S.; Kamnoedboon, P.; Özcan, M.; Srinivasan, M. CAD/CAM Complete Denture Resins: An In Vitro Evaluation of Color Stability. J. Prosthodontics 2021, 30, 430–439. [Google Scholar] [CrossRef] [PubMed]
- Azzopardi, N.; Moharamzadeh, K.; Wood, D.J.; Martin, N.; van Noort, R. Effect of resin matrix composition on the translucency of experimental dental composite resins. Dent. Mater. 2009, 25, 1564–1568. [Google Scholar] [CrossRef]
- Ferracane, J.L.; Moser, J.B.; Greener, E.H. Ultraviolet light-induced yellowing of dental restorative resins. J. Prosthet. Dent. 1985, 54, 483–487. [Google Scholar] [CrossRef] [PubMed]
- Fonseca, A.S.Q.S.; Labruna Moreira, A.D.; de Albuquerque, P.P.A.C.; de Menezes, L.R.; Pfeifer, C.S.; Schneider, L.F.J. Effect of monomer type on the CC degree of conversion, water sorption and solubility, and color stability of model dental composites. Dent. Mater. 2017, 33, 394–401. [Google Scholar] [CrossRef]
- Manojlovic, D.; Dramićanin, M.D.; Lezaja, M.; Pongprueksa, P.; Van Meerbeek, B.; Miletic, V. Effect of resin and photoinitiator on color, translucency and color stability of conventional and low-shrinkage model composites. Dent. Mater. 2016, 32, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Albuquerque, P.P.A.C.; Moreira, A.D.L.; Moraes, R.R.; Cavalcante, L.M.; Schneider, L.F.J. Color stability, conversion, water sorption and solubility of dental composites formulated with different photoinitiator systems. J Dent 2013, 41, e67–e72. [Google Scholar] [CrossRef]
- Arikawa, H.; Takahashi, H.; Kanie, T.; Ban, S. Effect of various visible light photoinitiators on the polymerization and color of light-activated resins. Dent. Mater. J. 2009, 28, 454–460. [Google Scholar] [CrossRef]
- de Oliveira, D.C.R.S.; Rocha, M.G.; Gatti, A.; Correr, A.B.; Ferracane, J.L.; Sinhoret, M.A.C. Effect of different photoinitiators and reducing agents on cure efficiency and color stability of resin-based composites using different LED wavelengths. J Dent 2015, 43, 1565–1572. [Google Scholar] [CrossRef]
- Hadis, M.A.; Shortall, A.C.; Palin, W.M. Competitive light absorbers in photoactive dental resin-based materials. Dent. Mater. 2012, 28, 831–841. [Google Scholar] [CrossRef] [PubMed]
- Schneider, L.F.J.; Pfeifer, C.S.C.; Consani, S.; Prahl, S.A.; Ferracane, J.L. Influence of photoinitiator type on the rate of polymerization, degree of conversion, hardness and yellowing of dental resin composites. Dent. Mater. 2008, 24, 1169–1177. [Google Scholar] [CrossRef]
- Shin, D.-H.; Rawls, H. Degree of conversion and color stability of the light curing resin with new photoinitiator systems. Dent. Mater. 2009, 25, 1030–1038. [Google Scholar] [CrossRef]
- Segurola, J.; Allen, N.S.; Edge, M.; McMahon, A.; Wilson, S. Photoyellowing and discolouration of UV cured acrylated clear coatings systems: Influence of photoinitiator type. Polym. Degrad. Stab. 1999, 64, 39–48. [Google Scholar] [CrossRef]
- Kirchmayr, R.; Berner, G.; Huesler, R.; Rist, G. Nonyellowing photoinitiators. Farbe Lack 1982, 88, 910–916. [Google Scholar]
- Griesser, M.; Neshchadin, D.; Dietliker, K.; Moszner, N.; Liska, R.; Gescheidt, G. Decisive Reaction Steps at Initial Stages of Photoinitiated Radical Polymerizations. Angew. Chem., Int. Ed. 2009, 48, 9359–9361. [Google Scholar] [CrossRef]
- Kim, G.-T.; Go, H.-B.; Yu, J.-H.; Yang, S.-Y.; Kim, K.-M.; Choi, S.-H.; Kwon, J.-S. Cytotoxicity, Colour Stability and Dimensional Accuracy of 3D Printing Resin with Three Different Photoinitiators. Polymers 2022, 14, 979. [Google Scholar] [CrossRef]
- Lu, Y.; Wang, L.; Dal Piva, A.M.d.O.; Tribst, J.P.M.; Čokić, S.M.; Zhang, F.; Werner, A.; Kleverlaan, C.J.; Feilzer, A.J. Effect of printing layer orientation and polishing on the fatigue strength of 3D-printed dental zirconia. Dent. Mater. 2023. [Google Scholar] [CrossRef]
- Sa, L.; Kaiwu, L.; Shenggui, C.; Junzhong, Y.; Yongguang, J.; Lin, W.; Li, R. 3D printing dental composite resins with sustaining antibacterial ability. J. Mater. Sci. 2019, 54, 3309–3318. [Google Scholar] [CrossRef]
- Wuersching, S.N.; Hickel, R.; Edelhoff, D.; Kollmuss, M. Initial biocompatibility of novel resins for 3D printed fixed dental prostheses. Dent. Mater. 2022, 38, 1587–1597. [Google Scholar] [CrossRef]
- Dietlin, C.; Trinh, T.T.; Schweizer, S.; Graff, B.; Morlet-Savary, F.; Noirot, P.-A.; Lalevée, J. Rational Design of Acyldiphenylphosphine Oxides as Photoinitiators of Radical Polymerization. Macromolecules 2019, 52, 7886–7893. [Google Scholar] [CrossRef]
- Gorsche, C.; Harikrishna, R.; Baudis, S.; Knaack, P.; Husar, B.; Laeuger, J.; Hoffmann, H.; Liska, R. Real Time-NIR/MIR-Photorheology: A Versatile Tool for the in Situ Characterization of Photopolymerization Reactions. Anal. Chem. 2017, 89, 4958–4968. [Google Scholar] [CrossRef] [PubMed]
- Pospíšil, J. Mechanistic action of phenolic antioxidants in polymers—A review. Polym. Degrad. Stab. 1988, 20, 181–202. [Google Scholar] [CrossRef]
- Pospıšil, J.; Habicher, W.D.; Pilař, J.; Nešpůrek, S.; Kuthan, J.; Piringer, G.O.; Zweifel, H. Discoloration of polymers by phenolic antioxidants. Polym. Degrad. Stab. 2002, 77, 531–538. [Google Scholar] [CrossRef]
- Wolf, T.J.A.; Voll, D.; Barner-Kowollik, C.; Unterreiner, A.-N. Elucidating the Early Steps in Photoinitiated Radical Polymerization via Femtosecond Pump–Probe Experiments and DFT Calculations. Macromolecules 2012, 45, 2257–2266. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bassenheim, D.; Rist, K.; Moszner, N.; Catel, Y.; Liska, R.; Knaack, P. Color-Stable Formulations for 3D-Photoprintable Dental Materials. Polymers 2024, 16, 2323. https://doi.org/10.3390/polym16162323
Bassenheim D, Rist K, Moszner N, Catel Y, Liska R, Knaack P. Color-Stable Formulations for 3D-Photoprintable Dental Materials. Polymers. 2024; 16(16):2323. https://doi.org/10.3390/polym16162323
Chicago/Turabian StyleBassenheim, David, Kai Rist, Norbert Moszner, Yohann Catel, Robert Liska, and Patrick Knaack. 2024. "Color-Stable Formulations for 3D-Photoprintable Dental Materials" Polymers 16, no. 16: 2323. https://doi.org/10.3390/polym16162323
APA StyleBassenheim, D., Rist, K., Moszner, N., Catel, Y., Liska, R., & Knaack, P. (2024). Color-Stable Formulations for 3D-Photoprintable Dental Materials. Polymers, 16(16), 2323. https://doi.org/10.3390/polym16162323









