Covalent–Organic Frameworks for Selective and Sensitive Detection of Antibiotics from Water
Abstract
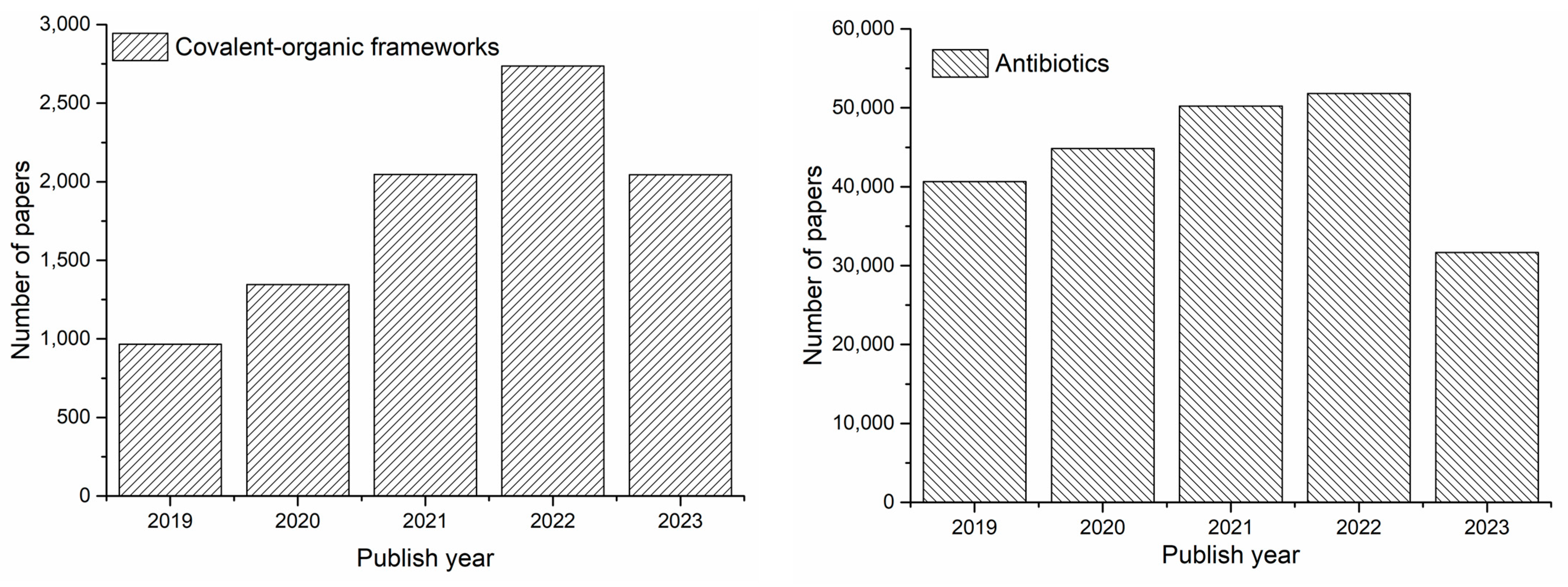
1. Introduction
2. Antibiotics
2.1. Classification and Synthetic Pathways of Antibiotics
2.2. Hazards of Antibiotics
3. COF Structure and Classification
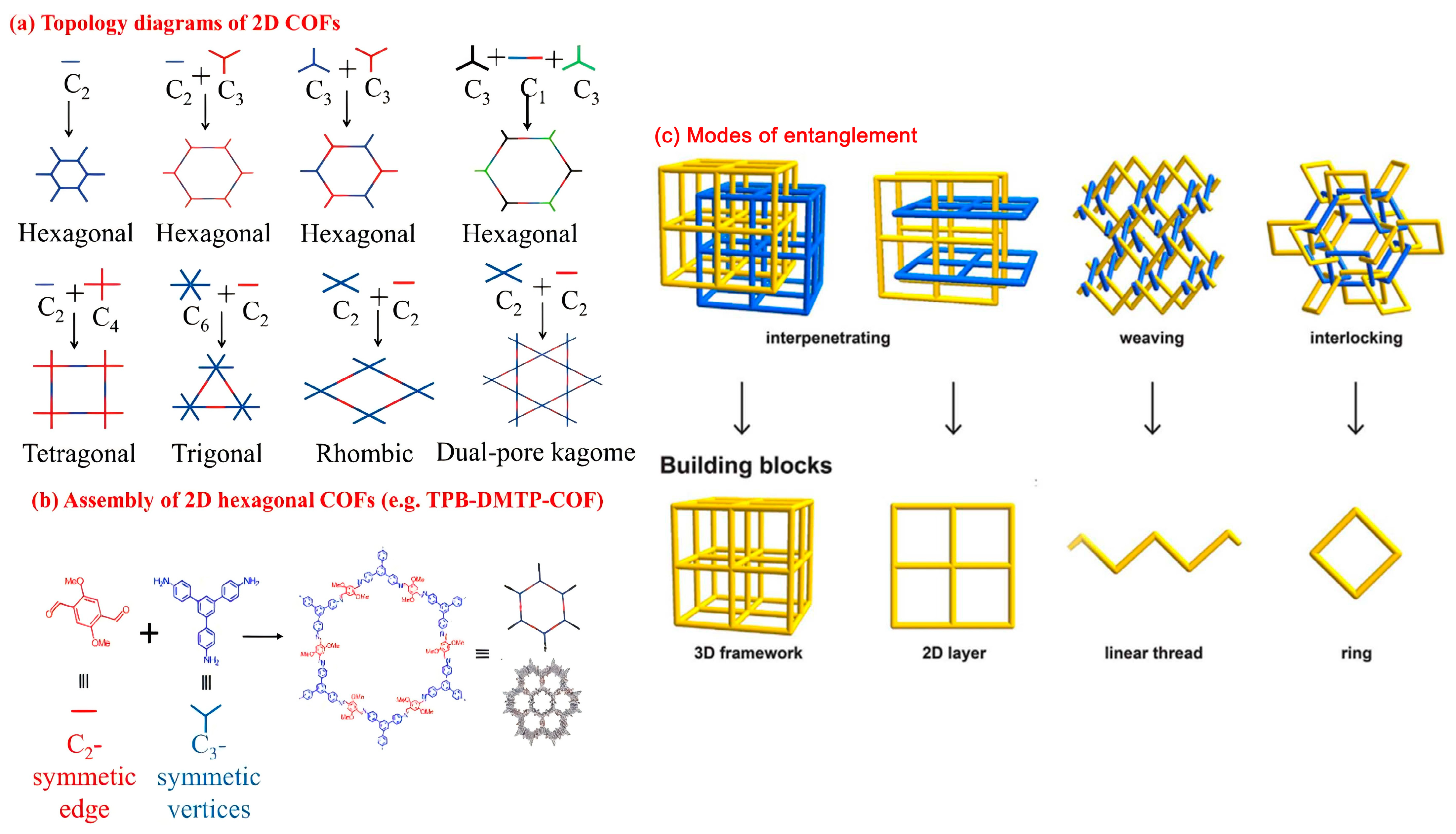
3.1. COF Structure
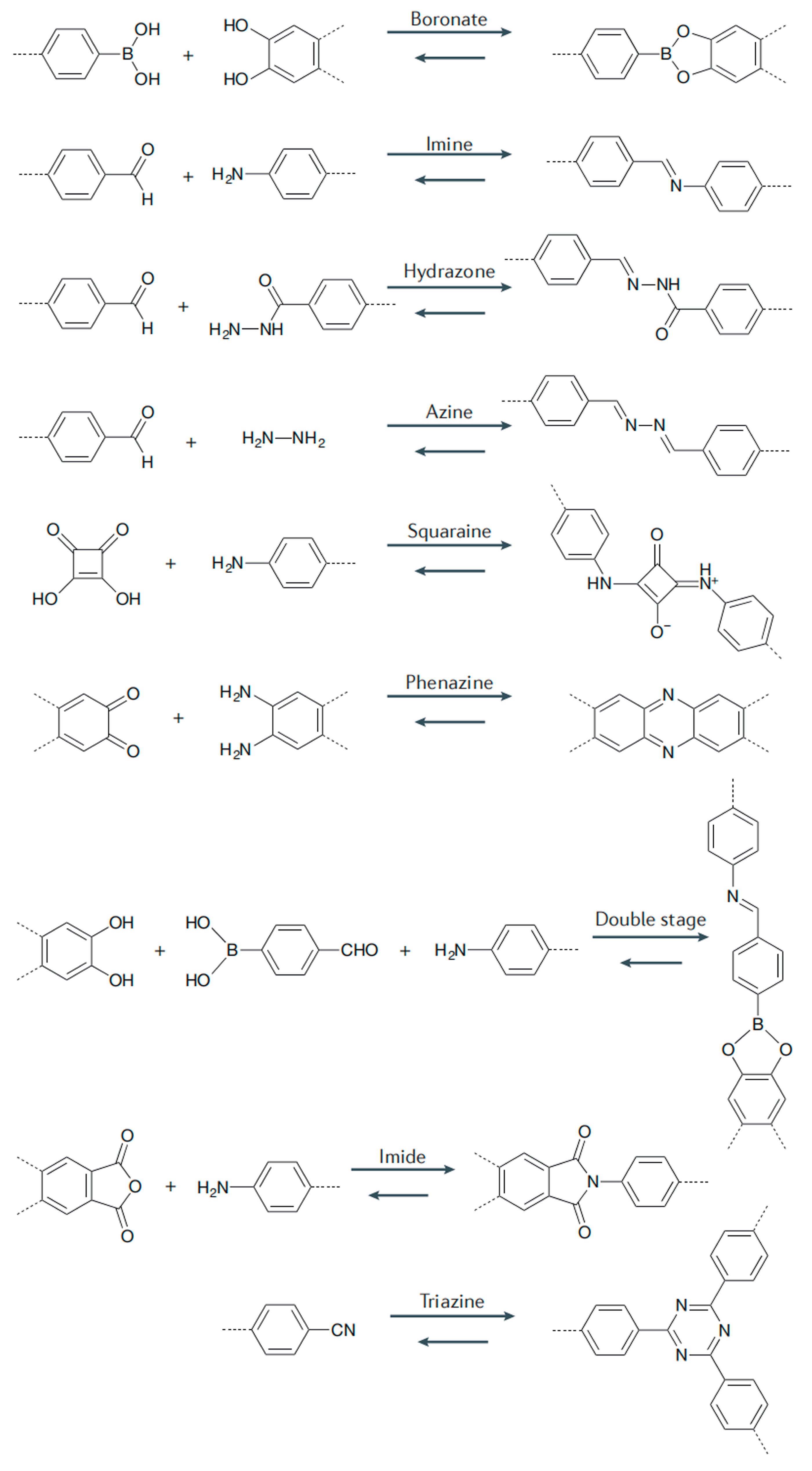
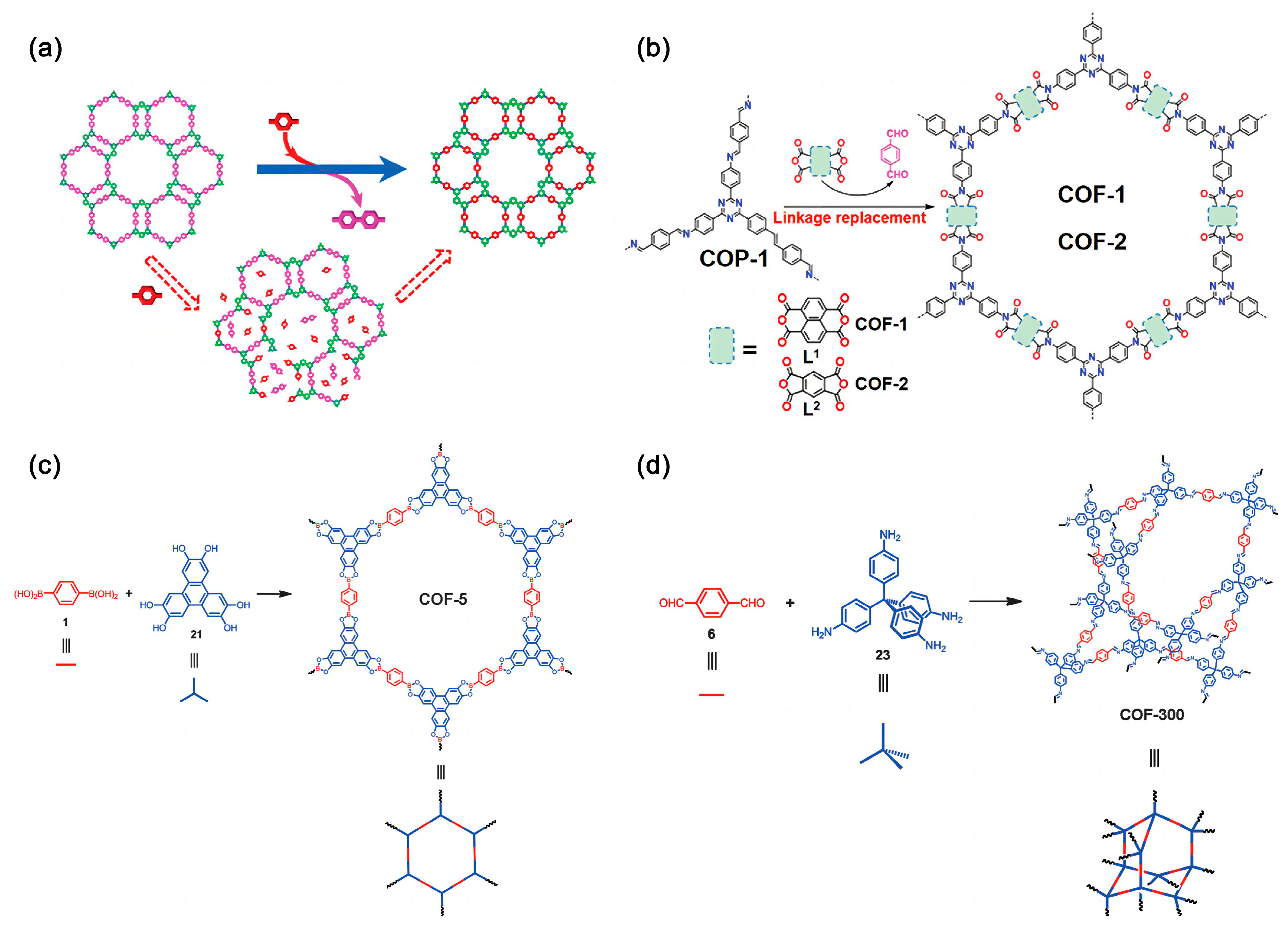
3.2. COF Classification
3.2.1. Boron-Based COFs
3.2.2. Triazine-Based COFs
3.2.3. Imine-Based COFs
3.2.4. Hydrazone-Based COFs
3.2.5. Azine-Based COFs
3.2.6. β-Ketoenamine-Based COFs
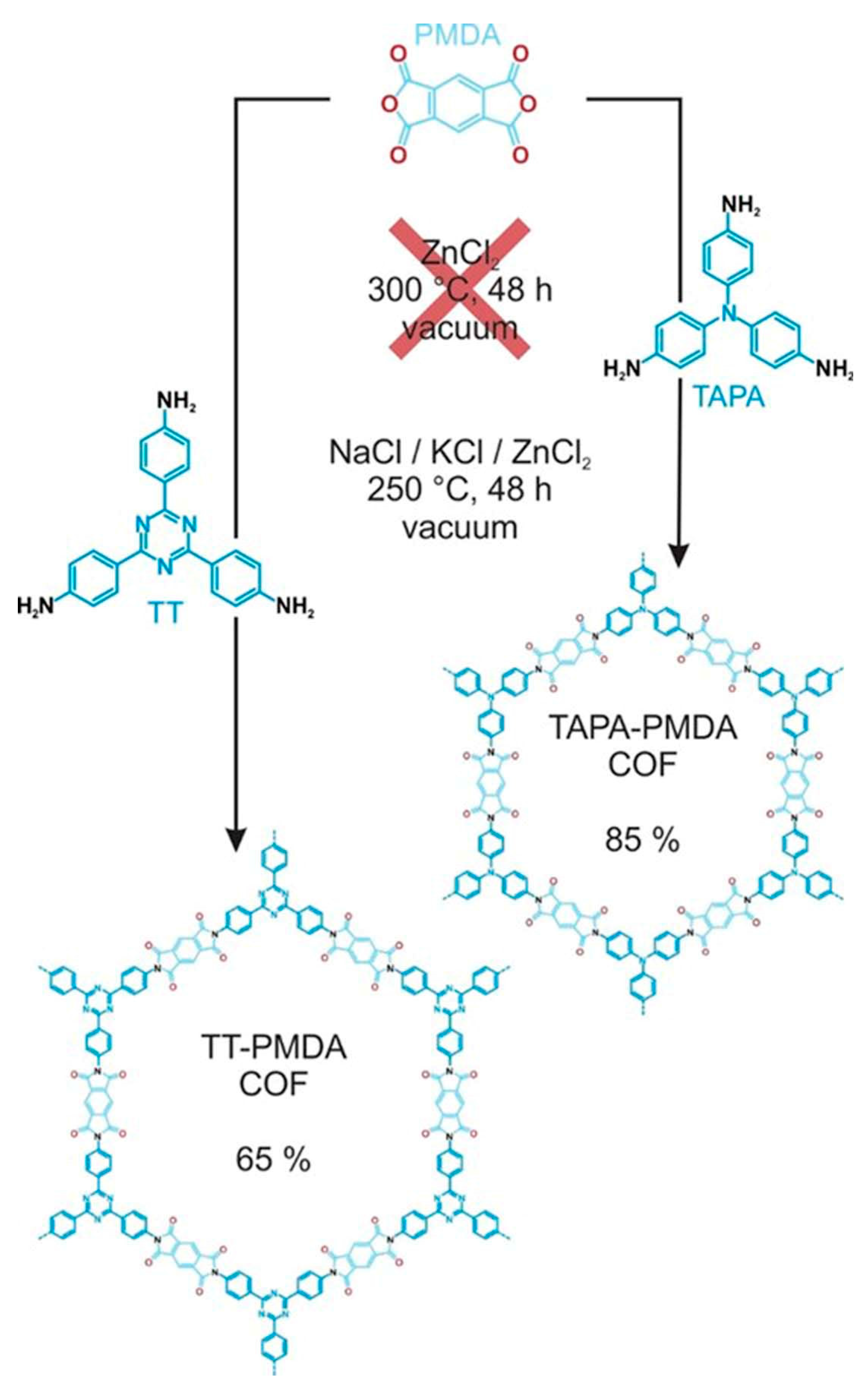
3.2.7. Imide-Based COFs
4. Synthetic Methods of COFs
4.1. Ionothermal Synthesis
4.2. Microwave-Assisted Synthesis
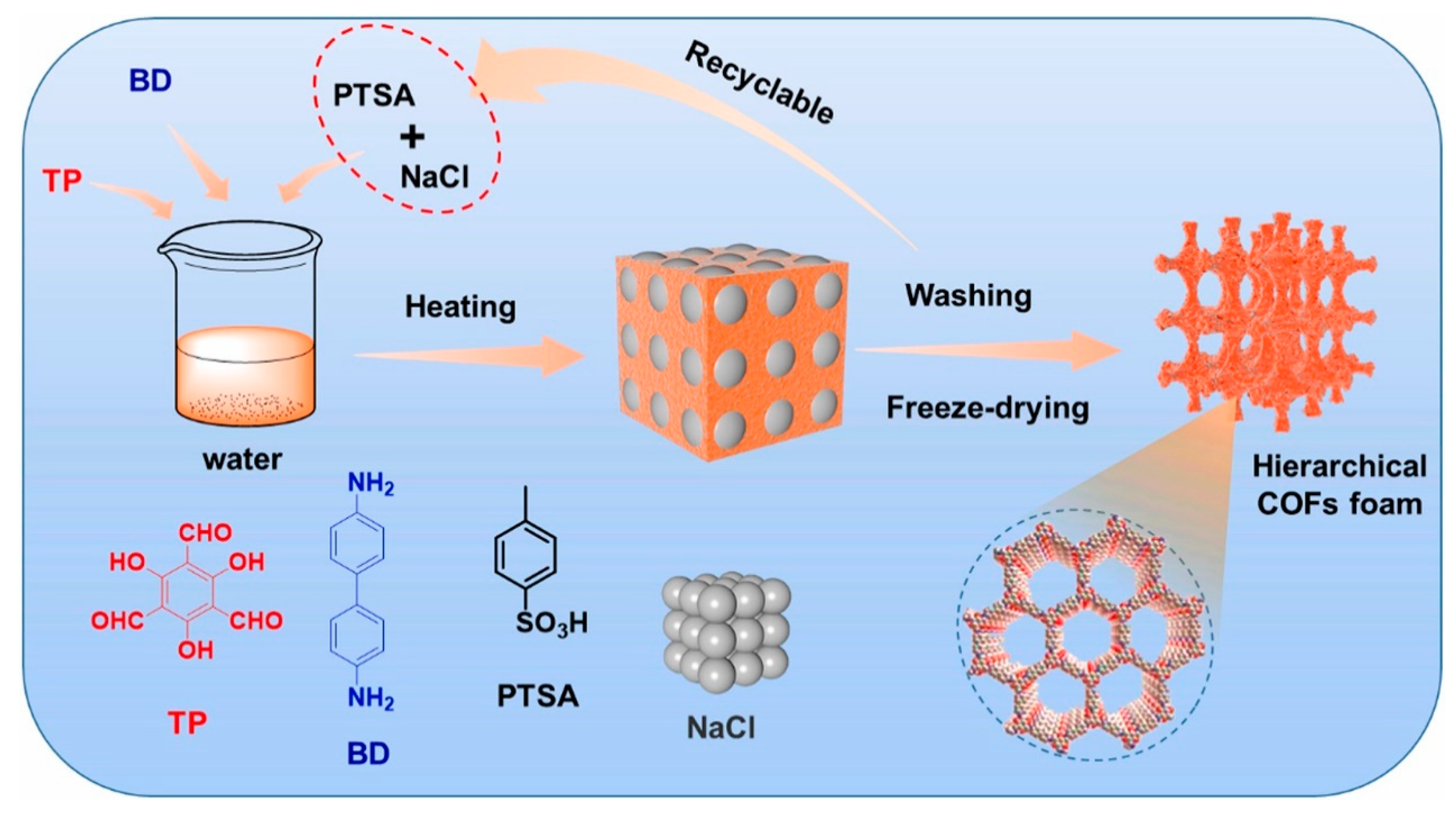
4.3. Room-Temperature Synthesis
4.4. Solvothermal Method
5. Physical and Chemical Properties of COFs
5.1. Chemical and Thermal Stability
5.2. High Porosity and Large Specific Surface Area
5.3. Rigid Topology and Good Modifiability
6. Application of COFs for Detection of Antibiotics
6.1. As Biosensors for Detection of Antibiotics
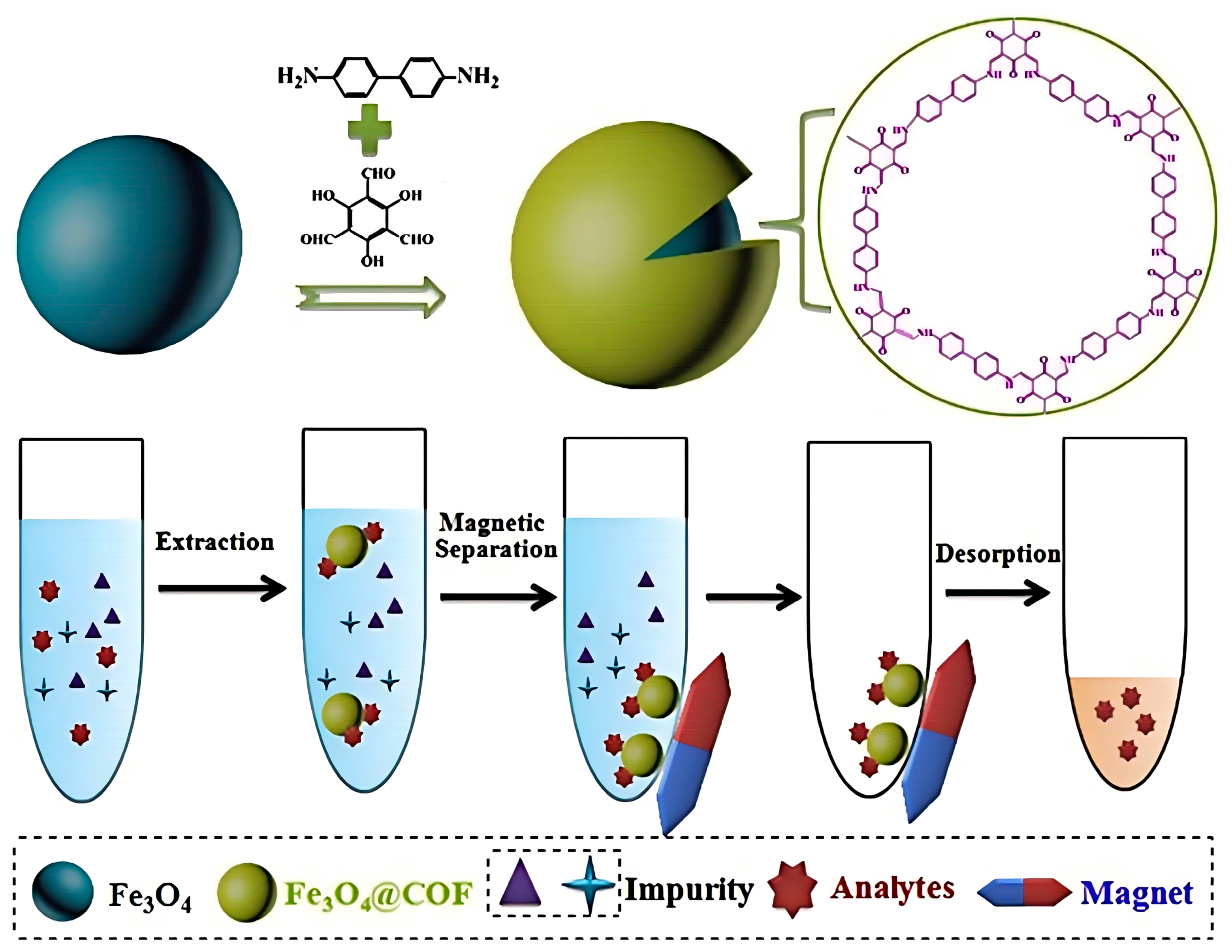
6.2. As the Adsorbent Materials for Pretreatment Technology
6.2.1. SPE
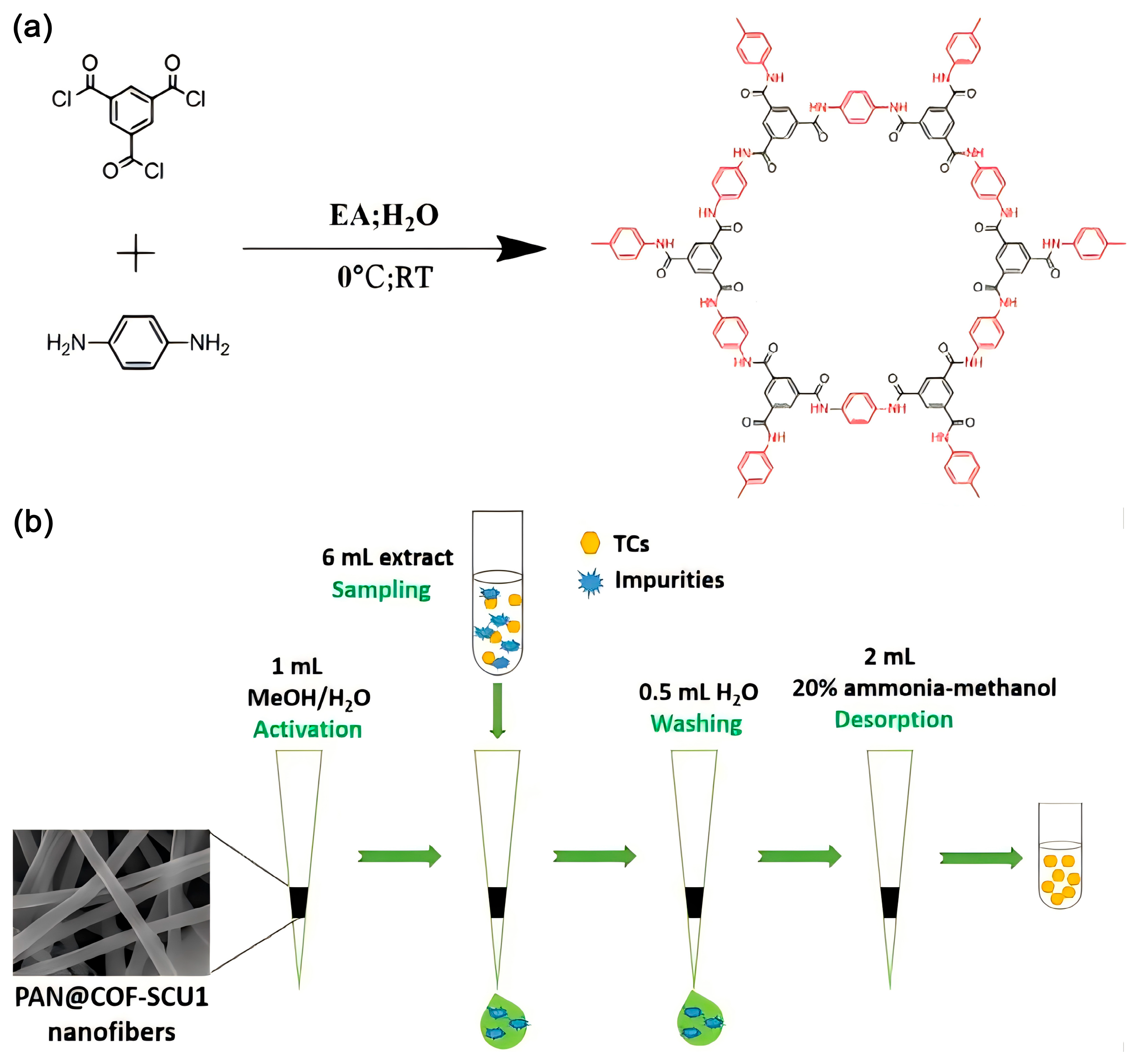
6.2.2. SPME
6.3. Comparison between COF-Based Materials and Other Materials for Detection of Antibiotics
7. Adsorption Mechanism of Antibiotics by COFs
7.1. Porosity
7.2. pH Value and Charge Interaction
7.3. π–π Interaction and Designability of Functional Group
8. Conclusions and Perspective
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
References
- Hutching, M.I.; Truman, A.W.; Wilkinson, B. Antibiotics: Past, present and future. Curr. Opin. Microbiol. 2019, 51, 72–80. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Lin, Y.; Hu, Y. Strategies of tuning catalysts for efficient photodegradation of antibiotics in water environments: A review. J. Mater. Chem. A 2021, 9, 2592–2611. [Google Scholar] [CrossRef]
- Chow, L.K.M.; Ghaly, T.M.; Gillings, M.R. A survey of sub-inhibitory concentrations of antibiotics in the environment. J. Environ. Sci. 2021, 99, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Zheng, D.; Yin, G.; Liu, M.; Chen, C.; Jiang, Y.; Hou, L.; Zheng, Y. A systematic review of antibiotics and antibiotic resistance genes in estuarine and coastal environments. Sci. Total Environ. 2021, 777, 146009–146026. [Google Scholar] [CrossRef]
- Kim, C.; Ryu, H.D.; Chung, E.G.; Kim, Y.; Lee, J. A review of analytical procedures for the simultaneous determination of medically important veterinary antibiotics in environmental water: Sample preparation, liquid chromatography, and mass spectrometry. J. Environ. Manag. 2018, 217, 629–645. [Google Scholar] [CrossRef]
- Bitew, Z.; Amare, M. Recent reports on electrochemical determination of selected antibiotics in pharmaceutical formulations: A mini review. Electrochem. Commun. 2020, 121, 106863–106876. [Google Scholar] [CrossRef]
- Zhang, C.; Deng, Y.; Zheng, J.; Zheng, J.; Zhang, Y.; Yang, L.; Liao, C.; Su, L.; Zhou, Y.; Gong, D.; et al. The application of the QuEChERS methodology in the determination of antibiotics in food: A review. Trends Anal. Chem. 2019, 118, 517–537. [Google Scholar] [CrossRef]
- Ahmed, S.; Ning, J.; Peng, D.; Chen, T.; Ahmad, I.; Ali, A.; Lei, Z.; Shabbir, M.A.; Cheng, G.; Yuan, Z. Current advances in immunoassays for the detection of antibiotics residues: A review. Food Agric. Immunol. 2020, 31, 268–290. [Google Scholar] [CrossRef]
- Du, C.; Zhang, Z.; Yu, G.; Wu, H.; Chen, H.; Zhou, L.; Zhang, Y.; Su, Y.; Tan, S.; Yang, L.; et al. A review of metal organic framework (MOFs)-based materials for antibiotics removal via adsorption and photocatalysis. Chemosphere 2021, 272, 129501. [Google Scholar] [CrossRef]
- Dong, J.; Han, X.; Liu, Y.; Li, H.; Cui, Y. Metal–covalent organic frameworks (MCOFS): A bridge between metal–organic frameworks and covalent organic frameworks. Angew. Chem. Int. Ed. 2020, 132, 13826–13837. [Google Scholar] [CrossRef]
- Zhang, P.; Wang, Z.; Cheng, P.; Chen, Y.; Zhang, Z. Design and application of ionic covalent organic frameworks. Coordin. Chem. Rev. 2021, 438, 213873. [Google Scholar] [CrossRef]
- Lin, S.; Diercks, C.S.; Zhang, Y.B.; Kornienko, N.; Nichols, E.M.; Zhao, Y.; Paris, A.R.; Kim, D.; Yang, P.; Yaghi, O.; et al. Covalent organic frameworks comprising cobalt porphyrins for catalytic CO2 reduction in water. Science 2015, 349, 1208–1213. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Liu, X.; Zhang, W.; Liu, Z.; Zhong, H.; Shao, B.; Liang, Q.; Liu, Y.; Zhang, W.; He, Q. Functionalization of covalent organic frameworks by metal modification: Construction, properties and applications. Chem. Eng. J. 2021, 404, 127136–127159. [Google Scholar] [CrossRef]
- Valenzuela, C.; Chen, C.; Sun, M.; Ye, Z.; Zhang, J. Strategies and applications of covalent organic frameworks as promising nanoplatforms in cancer therapy. J. Mater. Chem. B 2021, 9, 3450–3483. [Google Scholar] [CrossRef] [PubMed]
- Guan, X.; Chen, F.; Fang, Q.; Qiu, S. Design and applications of three dimensional covalent organic frameworks. Chem. Soc. Rev. 2020, 49, 1357–1384. [Google Scholar] [CrossRef]
- Liu, Y.; Zhou, W.; Teo, W.L.; Wang, K.; Zhang, L.; Zeng, Y.; Zhao, Y. Covalent-organic-framework-based composite materials. Chem 2020, 6, 3172–3202. [Google Scholar] [CrossRef]
- Keller, N.; Bein, T. Optoelectronic processes in covalent organic frameworks. Chem. Soc. Rev. 2021, 50, 1813–1845. [Google Scholar] [CrossRef]
- Nguyen, Q.X.T.; Manh Khong, H.; Duc La, D.; Dang, T.D. Self-Assembly of the porphyrin monomer on the surface of Fe/graphene material: A novel sensing material for the detection of chloramphenicol antibiotic in aqueous solution. ChemPhysChem 2024, e202400355. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, H.; Tian, D.; Phan, A.; Seididamyeh, M.; Alanazi, M.; Xu, Z.; Sultanbawa, Y.; Zhang, R. Luminescent sensors for residual antibiotics detection in food: Recent advances and perspectives. Coord. Chem. Rev. 2024, 498, 215455–215482. [Google Scholar] [CrossRef]
- Tan, G.; Wang, S.; Yu, J.; Chen, J.; Liao, D.; Liu, M.; Nezamzadeh-Ejhieh, A.; Pan, Y.; Liu, J. Detection mechanism and the outlook of metal-organic frameworks for the detection of hazardous substances in milk. Food Chem. 2024, 430, 136934. [Google Scholar] [CrossRef]
- Ji, M.; Li, J.; Liu, A.; Ma, D. Covalent organic frameworks-based materials for antibiotics fluorescence detection. Heliyon 2024, 10, e33118–e33132. [Google Scholar] [CrossRef] [PubMed]
- Pilli, P.; Kommalapati, H.S.; Golla, V.M.; Khemchandani, R.; Ramachandran, R.K.; Samanthula, G. Covalent organic frameworks: Spotlight on applications in the pharmaceutical arena. Bioanalysis 2024, 16, 279–305. [Google Scholar] [CrossRef] [PubMed]
- Fenneman, A.C.; Weidner, M.; Chen, L.A.; Nieuwdorp, M.; Blaser, M. Antibiotics in the pathogenesis of diabetes and inflammatory diseases of the gastrointestinal tract. Nat. Rev. Gastro. Hepatol. 2023, 20, 81–100. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Yang, L.; Yen, H.; Zhao, F.; Wang, X.; Zhou, T.; Feng, Q.; Chen, L. Occurrence, spatial distribution and ecological risks of antibiotics in soil in urban agglomeration. J. Environ. Sci. 2023, 125, 678–690. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Li, J.; Tang, L.; Meng, J.; Li, J. Antibiotics removal from piggery wastewater by a novel aerobic-microaerobic system: Efficiency and mechanism. Chem. Eng. J. 2023, 454, 140265. [Google Scholar] [CrossRef]
- Fu, Y.; Zhu, Y.; Dong, H.; Li, J.; Zhang, W.; Shao, Y.; Shao, Y. Effects of heavy metals and antibiotics on antibiotic resistance genes and microbial communities in soil. Process Saf. Environ. 2023, 169, 418–427. [Google Scholar] [CrossRef]
- Butler, J.; Handy, R.D.; Upton, M.; Besinis, A. Review of antimicrobial nanocoatings in medicine and dentistry: Mechanisms of action, biocompatibility performance, safety, and benefits compared to antibiotics. ACS Nano 2023, 17, 7064–7092. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.D.; Lee, T.; Van Tran, T.; Nguyen, V.; Nong, L.; Giang, L.; Vo, B. Multicomponent photocatalysts for synergic removal of antibiotics in aqueous media: A review. Environ. Chem. Lett. 2023, 21, 935–980. [Google Scholar] [CrossRef]
- Jumpertz, M.; Guilhaumou, R.; Million, M.; Parola, P.; Lagier, J.; Brouqui, P.; Cassir, N. Subcutaneously administered antibiotics: A review. J. Antimicrob. Chemother. 2023, 78, 1–7. [Google Scholar] [CrossRef]
- Le, V.V.; Tran, Q.G.; Ko, S.R.; Lee, S.; Oh, H.; Kim, H.; Ahn, C. How do freshwater microalgae and cyanobacteria respond to antibiotics? Crit. Rev. Biotechnol. 2023, 43, 191–211. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Vyas, R.K.; Vyas, S. A review on antibiotics pervasiveness in the environment and their removal from wastewater. Sep. Sci. Technol. 2023, 58, 326–344. [Google Scholar] [CrossRef]
- Wu, J.; Wang, J.; Li, Z.; Guo, S.; Li, K.; Xu, P.; Ok, Y.; Jones, D.; Zou, J. Antibiotics and antibiotic resistance genes in agricultural soils: A systematic analysis. Crit. Rev. Environ. Sci. Technol. 2023, 53, 847–864. [Google Scholar] [CrossRef]
- Mueller, U.; Schubert, M.; Teich, F.; Puetter, H.; Schierle-Arndt, K.; Pastré, J. Metal–organic frameworks—Prospective industrial applications. J. Mater. Chem. 2006, 16, 626–636. [Google Scholar] [CrossRef]
- Arqueros, C.; Zamora, F.; Montoro, C. A perspective on the application of covalent organic frameworks for detection and water treatment. Nanomaterials 2021, 11, 1651. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Yang, L.; Li, M.; Kuang, L.; Song, Y.; Wang, L. Covalent organic frameworks for fluorescent sensing: Recent developments and future challenges. Coord. Chem. Rev. 2021, 440, 213957. [Google Scholar] [CrossRef]
- Gong, Y.N.; Guan, X.; Jiang, H.L. Covalent organic frameworks for photocatalysis: Synthesis, structural features, fundamentals and performance. Coord. Chem. Rev. 2023, 475, 214889. [Google Scholar] [CrossRef]
- Wang, J.; Zhuang, S. Covalent organic frameworks (COFs) for environmental applications. Coord. Chem. Rev. 2019, 400, 213046. [Google Scholar] [CrossRef]
- Diercks, C.S.; Yaghi, O.M. The atom, the molecule, and the covalent organic framework. Science 2017, 355, eaal1585. [Google Scholar] [CrossRef]
- Lohse, M.S.; Bein, T. Covalent organic frameworks: Structures, synthesis, and applications. Adv. Funct. Mater. 2018, 28, 1705553–1705624. [Google Scholar] [CrossRef]
- Kandambeth, S.; Dey, K.; Banerjee, R. Covalent organic frameworks: Chemistry beyond the structure. J. Am. Chem. Soc. 2018, 141, 1807–1822. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Tu, B.; Zhang, G.; Liu, P.; Hu, K.; Wang, J.; Yang, Z.; Huang, Z.; Fang, M.; Hou, J.; et al. Advancing osmotic power generation by covalent organic framework monolayer. Nat. Nanotechnol. 2022, 176, 22–628. [Google Scholar] [CrossRef] [PubMed]
- Lu, M.; Zhang, M.; Liu, J.; Yu, T.; Chang, J.; Shang, L.; Li, S.; Lan, Y. Confining and highly dispersing single polyoxometalate clusters in covalent organic frameworks by covalent linkages for CO2 photoreduction. J. Am. Chem. Soc. 2022, 144, 1861–1871. [Google Scholar] [CrossRef] [PubMed]
- Huang, N.; Wang, P.; Jiang, D. Covalent organic frameworks: A materials platform for structural and functional designs. Nat. Rev. Mater. 2016, 1, 1–19. [Google Scholar] [CrossRef]
- Chen, L.; Furukawa, K.; Gao, J.; Nagai, A.; Nakamura, T.; Dong, Y.; Jiang, D. Photoelectric covalent organic frameworks: Converting open lattices into ordered donor–acceptor heterojunctions. J. Am. Chem. Soc. 2014, 136, 9806–9809. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Li, X.; Liu, M.; Yang, S.; Xu, Q.; Zeng, G. Quantitative construction of boronic-ester linkages in covalent organic frameworks for the carbon dioxide reduction. Angew. Chem. 2024, 136, e202317785. [Google Scholar] [CrossRef]
- Yue, L.; Wang, X.; Chen, L.; Shen, D.; Shao, Z.; Wu, H.; Xiao, S.; Liang, W.; Yu, Y.; Li, Y. In situ interface engineering of highly nitrogen-rich triazine-based covalent organic frameworks for an ultra-stable, dendrite-free lithium-metal anode. Energy Environ. Sci. 2024, 17, 1117–1131. [Google Scholar] [CrossRef]
- Tilford, R.W.; Gemmill, W.R.; zur Loye, H.C.; Lavigne, J.J. Facile synthesis of a highly crystalline, covalently linked porous boronate network. Chem. Mater. 2006, 18, 5296–5301. [Google Scholar] [CrossRef]
- Yaghi, O.M.; O’Keeffe, M.; Ockwig, N.W.; Chae, H.K.; Eddaoudi, M.; Kim, J. Reticular synthesis and the design of new materials. Nature 2003, 423, 705–714. [Google Scholar] [CrossRef]
- Cote, A.P.; Benin, A.I.; Ockwig, N.W.; O’Keeffe, M.; Matzger, A.J.; Yaghi, O.M. Porous, crystalline, covalent organic frameworks. Science 2005, 310, 1166–1170. [Google Scholar] [CrossRef]
- Liu, Y.; Feng, Y.; Huang, Z.; Zhang, X. Thermal conductivity of 3D boron-based covalent organic frameworks from molecular dynamics simulations. J. Phys. Chem. C 2016, 120, 17060–17068. [Google Scholar] [CrossRef]
- Li, H.; Li, H.; Dai, Q.; Li, H.; Bredas, J.L. Hydrolytic stability of boronate ester-linked covalent organic frameworks. Adv. Theory Simul. 2018, 1, 1700015–1700024. [Google Scholar] [CrossRef]
- Bao, T.; Tang, P.; Kong, D.; Mao, Z.; Chen, Z. Polydopamine-supported immobilization of covalent-organic framework-5 in capillary as stationary phase for electrochromatographic separation. J. Chromatogr. A 2016, 1445, 140–148. [Google Scholar] [CrossRef] [PubMed]
- Kuhn, P.; Antonietti, M.; Thomas, A. Porous, covalent triazine-based frameworks prepared by ionothermal synthesis. Angew. Chem. Int. Ed. 2008, 47, 3450–3453. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Byun, J.; Rörich, I.; Ramanan, C.; Blom, P.W.M.; Lu, H.; Wang, D.; da Silva, L.C.; Li, R.; Wang, L.; et al. Asymmetric covalent triazine framework for enhanced visible-light photoredox catalysis via energy transfer cascade. Angew. Chem. Int. Ed. 2018, 57, 8316–8320. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Magano, A.; Salaverri, N.; Marzo, L.; Mas-Ballest´e, R.; Aleman´, J. Synergistic combination of triazine and phenanthroline moieties in a covalent triazine framework tailored for heterogeneous photocatalytic metal-free C-Br and C-Cl activation. Appl. Catal. B Environ. 2022, 317, 121791–121801. [Google Scholar] [CrossRef]
- Li, L.; Lu, F.; Xue, R.; Ma, B.; Li, Q.; Wu, N.; Liu, H.; Yao, W.; Guo, H.; Yang, W. Ultrastable triazine-based covalent organic framework with an interlayer hydrogen bonding for supercapacitor applications. ACS Appl. Mater. Interfaces 2019, 11, 26355–26363. [Google Scholar] [CrossRef] [PubMed]
- Liao, H.; Ding, H.; Li, B.; Ai, X.; Wang, C. Covalent organic frameworks: Potential host materials for sulfur impregnation in lithium–sulfur batteries. J. Mater. Chem. A 2014, 2, 8854–8858. [Google Scholar] [CrossRef]
- Yang, C.; Liu, C.; Cao, Y.; Yan, X. Facile room-temperature solution-phase synthesis of a spherical covalent organic framework for high-resolution chromatographic separation. Chem. Commun. 2015, 51, 12254–12257. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Huang, J.; Yuan, C.; Liu, Y.; Cui, Y. Chiral 3D covalent organic frameworks for high performance liquid chromatographic enantioseparation. J. Am. Chem. Soc. 2018, 140, 892–895. [Google Scholar] [CrossRef]
- DeBlase, C.R.; Dichtel, W.R. Moving beyond boron: The emergence of new linkage chemistries in covalent organic frameworks. Macromolecules 2016, 49, 5297–5305. [Google Scholar] [CrossRef]
- Uribe-Romo, F.J.; Hunt, J.R.; Furukawa, H.; Klöck, C.; O’Keeffe, M.; Yaghi, O.M. A Crystalline imine-linked 3-D porous covalent organic framework. J. Am. Chem. Soc. 2009, 131, 4570–4571. [Google Scholar] [CrossRef]
- Fang, Q.; Zhuang, Z.; Gu, S.; Kaspar, R.B.; Zheng, J.; Wang, J.; Qiu, S.; Yan, Y. Designed synthesis of large-pore crystalline polyimide covalent organic frameworks. Nat. Commun. 2014, 5, 1–8. [Google Scholar] [CrossRef]
- Jin, S.; Sakurai, T.; Kowalczyk, T.; Dalapati, S.; Xu, F.; Wei, H.; Chen, X.; Gao, J.; Seki, S.; Irle, S.; et al. Two-dimensional tetrathiafulvalene covalent organic frameworks: Towards latticed conductive organic salts. Chem. Eur. J. 2014, 20, 14608–14613. [Google Scholar] [CrossRef] [PubMed]
- Nailwal, Y.; Wonanke, A.D.D.; Addicoat, M.A.; Pal, S.K. A dual-function highly crystalline covalent organic framework for HCl sensing and visible-light heterogeneous photocatalysis. Macromolecules 2021, 54, 6595–6604. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, Y.; Li, H.; Guan, X.; Xue, M.; Yan, Y.; Valtchev, V.; Qiu, S.; Fang, Q. Three-dimensional mesoporous covalent organic frameworks through steric hindrance engineering. J. Am. Chem. Soc. 2020, 142, 3736–3741. [Google Scholar] [CrossRef]
- Stegbauer, L.; Schwinghammer, K.; Lotsch, B.V. A hydrazone-based covalent organic framework for photocatalytic hydrogen production. Chem. Sci. 2014, 5, 2789–2793. [Google Scholar] [CrossRef]
- Zhang, K.; Cai, S.; Yan, Y.; He, Z.; Lin, H.; Huang, X.; Zheng, S.; Fan, J.; Zhang, W. Construction of a hydrazone-linked chiral covalent organic framework–silica composite as the stationary phase for high performance liquid chromatography. J. Chromatogr. A 2017, 1519, 100–109. [Google Scholar] [CrossRef]
- Li, Z.; Feng, X.; Zou, Y.; Zhang, Y.; Xia, H.; Liu, X.; Mu, Y. A 2D azine-linked covalent organic framework for gas storage applications. Chem. Commun. 2014, 50, 13825–13828. [Google Scholar] [CrossRef]
- Dalapati, S.; Jin, S.; Gao, J.; Xu, Y.; Nagai, A. An azine-linked covalent organic framework. J. Am. Chem. Soc. 2013, 135, 17310–17313. [Google Scholar] [CrossRef]
- Traxler, M.; Gisbertz, S.; Pachfule, P.; Schmidt, J.; Roeser, J.; Reischauer, S.; Rabeah, S.; Rabeah, J.; Pieber, B. Acridine-functionalized covalent organic frameworks (COFs) as photocatalysts for metallaphotocatalytic C-N cross-coupling. Angew. Chem. Int. Ed. 2022, 61, e202117738. [Google Scholar] [CrossRef]
- DeBlase, C.R.; Silberstein, K.E.; Truong, T.T.; Abruña, H.D.; Dichtel, W.R. β-Ketoenamine-linked covalent organic frameworks capable of pseudocapacitive energy storage. J. Am. Chem. Soc. 2013, 135, 16821–16824. [Google Scholar] [CrossRef]
- Liu, F.; Zhou, P.; Hou, Y.; Tan, H.; Liang, Y.; Liang, J.; Zhang, Q.; Guo, S.; Tong, M.; Ni, J. Covalent organic frameworks for direct photosynthesis of hydrogen peroxide from water, air and sunlight. Nat. Commun. 2013, 14, 4344–4354. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Zheng, Y.; An, H.; Aguila, B.; Yang, C.; Dong, Y.; Xie, W.; Cheng, P.; Zhang, Z.; Chen, Y.; et al. Covalent organic frameworks with chirality enriched by biomolecules for efficient chiral separation. Angew. Chem. Int. Ed. 2018, 57, 16754–16759. [Google Scholar] [CrossRef]
- Abuzeid, H.R.; EL-Mahdy, A.F.M.; Kuo, S.W. Covalent organic frameworks: Design principles, synthetic strategies, and diverse applications. Giant 2021, 6, 100054–100081. [Google Scholar] [CrossRef]
- Li, Y.; Chen, W.; Xing, G.; Jiang, D.; Chen, L. New synthetic strategies toward covalent organic frameworks. Chem. Soc. Rev. 2020, 49, 2852–2868. [Google Scholar] [CrossRef]
- Ding, S.Y.; Wang, W. Covalent organic frameworks (COFs): From design to applications. Chem. Soc. Rev. 2013, 42, 548–568. [Google Scholar] [CrossRef]
- Bisbey, R.P.; Dichtel, W.R. Covalent organic frameworks as a platform for multidimensional polymerization. ACS Cent. Sci. 2017, 3, 533–543. [Google Scholar] [CrossRef]
- Bhadra, M.; Kandambeth, S.; Sahoo, M.K.; Addicoat, M.; Balaraman, E.; Banerjee, R. Triazine functionalized porous covalent organic framework for photo-organocatalytic E–Z isomerization of olefins. J. Am. Chem. Soc. 2019, 141, 6152–6156. [Google Scholar] [CrossRef]
- Sick, T.; Rotter, J.M.; Reuter, S.; Kandambeth, S.; Bach, N.N.; Döblinger, M.; Merz, J.; Clark, T.; Marder, T.B.; Bein, T.; et al. Switching on and off interlayer correlations and porosity in 2D covalent organic frameworks. J. Am. Chem. Soc. 2019, 141, 12570–12581. [Google Scholar] [CrossRef]
- Maschita, J.; Banerjee, T.; Savasci, G.; Haase, F.; Ochsenfeld, C.; Lotsch, B.V. Ionothermal synthesis of imide-linked covalent organic frameworks. Angew. Chem. Int. Ed. 2020, 59, 15750–15758. [Google Scholar] [CrossRef] [PubMed]
- Guan, X.; Ma, Y.; Li, H.; Yusran, Y.; Xue, M.; Fang, Q.; Yan, Y.; Valtchev, V.; Qiu, S. Fast, ambient temperature and pressure ionothermal synthesis of three-dimensional covalent organic frameworks. J. Am. Chem. Soc. 2018, 140, 4494–4498. [Google Scholar] [CrossRef] [PubMed]
- Dong, B.; Wang, W.; Pan, W.; Kang, G. Ionic liquid as a green solvent for ionothermal synthesis of 2D keto-enamine-linked covalent organic frameworks. Mater. Chem. Phys. 2019, 226, 244–249. [Google Scholar] [CrossRef]
- Dąbrowska, S.; Chudoba, T.; Wojnarowicz, J.; Lojkowski, W. Current trends in the development of microwave reactors for the synthesis of nanomaterials in laboratories and industries: A review. Crystals 2018, 8, 379. [Google Scholar] [CrossRef]
- Kumar, S.; Kulkarni, V.V.; Jangir, R. Covalent-organic framework composites: A review report on synthesis methods. ChemistrySelect 2021, 6, 11201–11223. [Google Scholar] [CrossRef]
- Sharma, R.K.; Yadav, P.; Yadav, M.; Gupta, R.; Rana, P.; Srivastava, A.; Zbořil, R.; Varma, R.S.; Antonietti, M.; Gawande, M.B. Recent development of covalent organic frameworks (COFs): Synthesis and catalytic (organic-electro-photo) applications. Mater. Horiz. 2020, 7, 411–454. [Google Scholar] [CrossRef]
- Nain, S.; Singh, R.; Ravichandran, S. Importance of microwave heating in organic synthesis. Adv. J. Chem. A 2019, 2, 94–104. [Google Scholar] [CrossRef]
- Zhang, F.; Zhang, J.; Zhang, B.; Tan, X.; Shao, D.; Shi, J.; Tan, D.; Liu, L.; Feng, J.; Han, B.; et al. Room-temperature synthesis of covalent organic framework (COF-LZU1) nanobars in CO2/water solvent. ChemSusChem 2018, 11, 3576–3580. [Google Scholar] [CrossRef]
- Medina, D.D.; Rotter, J.M.; Hu, Y.; Dogru, M.; Werner, V.; Auras, F.; Markiewicz, J.T.; Knochel, P.; Bein, T. Room temperature synthesis of covalent–organic framework films through vapor-assisted conversion. J. Am. Chem. Soc. 2015, 137, 1016–1019. [Google Scholar] [CrossRef] [PubMed]
- Bagheri, A.R.; Aramesh, N. Towards the room-temperature synthesis of covalent organic frameworks: A mini-review. J. Mater. Sci. 2021, 56, 1116–1132. [Google Scholar] [CrossRef]
- Song, Y.; Sun, Q.; Aguila, B.; Ma, S. Opportunities of covalent organic frameworks for advanced applications. Adv. Sci. 2019, 6, 1801410. [Google Scholar] [CrossRef]
- Liu, C.; Yu, Y.; Zhang, W.; Zeng, Q.; Lei, S. Room-temperature synthesis of covalent organic frameworks with a boronic ester linkage at the liquid/solid interface. Chem. Eur. J. 2016, 22, 18412–18418. [Google Scholar] [CrossRef]
- Liu, R.; Yan, Q.; Tang, Y.; Liu, R.; Huang, L.; Shuai, Q. NaCl template-assisted synthesis of self-floating COFs foams for the efficient removal of sulfamerazine. J. Hazard. Mater. 2022, 421, 126702–126710. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Wong, W.K.; Hu, Z.; Cheng, Y.; Yuan, D.; Khan, S.A.; Zhao, D. Room temperature batch and continuous flow synthesis of water-stable covalent organic frameworks (COFs). Chem. Mater. 2016, 28, 5095–5101. [Google Scholar] [CrossRef]
- You, J.; Zhao, Y.; Wang, L.; Bao, W. Recent developments in the photocatalytic applications of covalent organic frameworks: A review. J. Clean. Prod. 2021, 291, 125822. [Google Scholar] [CrossRef]
- Li, X.; Yang, C.; Sun, B.; Cai, S.; Chen, Z.; Lv, Y.; Zhang, J.; Liu, Y. Expeditious synthesis of covalent organic frameworks: A review. J. Mater. Chem. A 2020, 8, 16045–16060. [Google Scholar] [CrossRef]
- Raptakis, A.; Dianat, A.; Croy, A.; Cuniberti, G. Predicting the bulk modulus of single-layer covalent organic frameworks with square-lattice topology from molecular building-block properties. Nanoscale 2021, 13, 1077–1085. [Google Scholar] [CrossRef]
- Chen, X.; Addicoat, M.; Jin, E.; Zhai, L.; Xu, H.; Huang, N.; Guo, Z.; Liu, L.; Irle, S.; Jiang, D. Locking covalent organic frameworks with hydrogen bonds: General and remarkable effects on crystalline structure, physical properties, and photochemical activity. J. Am. Chem. Soc. 2015, 137, 3241–3247. [Google Scholar] [CrossRef] [PubMed]
- Torabi, E.; Mirzaei, M.; Bazargan, M.; Amiri, A. A critical review of covalent organic frameworks-based sorbents in extraction methods. Anal. Chim. Acta 2022, 1224, 340207. [Google Scholar] [CrossRef] [PubMed]
- Xie, Z.; Wang, B.; Yang, Z.; Yang, X.; Yu, X.; Xing, G.; Zhang, Y.; Chen, L. Stable 2D heteroporous covalent organic frameworks for efficient ionic conduction. Angew. Chem. Int. Ed. 2019, 58, 15742–15746. [Google Scholar] [CrossRef]
- Chen, H.; Yuan, X.; Wang, H.; Yu, H.; Jiang, L. Nanostructured covalent organic frameworks with elevated crystallization for (electro) photocatalysis and energy storage devices. J. Mater. Sci. 2021, 56, 13875–13924. [Google Scholar] [CrossRef]
- He, G.; Zhang, R.; Jiang, Z. Engineering covalent organic framework membranes. Acc. Mater. Res. 2021, 2, 630–643. [Google Scholar] [CrossRef]
- Rodríguez-San-Miguel, D.; Zamora, F. Processing of covalent organic frameworks: An ingredient for a material to succeed. Chem. Soc. Rev. 2019, 48, 4375–4386. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Yang, J.; Gao, F.; Zhang, Q. Covalent organic frameworks: Recent progress in biomedical applications. ACS Nano 2023, 17, 1879–1905. [Google Scholar] [CrossRef] [PubMed]
- Shahzad, U.; Marwani, H.M.; Saeed, M.; Asiri, A.M.; Repon, M.R.; Althomali, R.H.; Rahman, M.M. Progress and Perspectives on promising covalent-organic frameworks (COFs) materials for energy storage capacity. Chem. Rec. 2024, 24, e202300285. [Google Scholar] [CrossRef]
- Liang, R.; Cui, F.; Qi, Q.; Zhao, X. A study on constitutional isomerism in covalent organic frameworks: Controllable synthesis, transformation, and distinct difference in properties. CCS Chem. 2020, 2, 139–145. [Google Scholar] [CrossRef]
- Li, Y.; Guo, X.; Li, X.; Zhang, M.; Jia, Z.; Deng, Y.; Tian, Y.; Li, S.; Ma, L. Redox-active two-dimensional covalent organic frameworks (COFs) for selective reductive separation of valence-variable, redox-sensitive and long-lived radionuclides. Angew. Chem. Int. Ed. 2020, 132, 4197–4204. [Google Scholar] [CrossRef]
- Sasmal, H.S.; Kumar Mahato, A.; Majumder, P.; Banerjee, R. Landscaping covalent organic framework nanomorphologies. J. Am. Chem. Soc. 2022, 144, 11482–11498. [Google Scholar] [CrossRef]
- Wang, G.; Li, Y.; Shi, W.; Zhang, B.; Hou, L.; Wang, Y. A robust cluster-based Eu-MOF as multi-functional fluorescence sensor for detection of antibiotics and pesticides in water. Sens. Actuators B Chem. 2021, 331, 129377–129386. [Google Scholar] [CrossRef]
- Xu, Q.; Tan, Z.; Liao, X.; Wang, C. Recent advances in nanoscale metal-organic frameworks biosensors for detection of biomarkers. Chin. Chem. Lett. 2021, 5, 52–63. [Google Scholar] [CrossRef]
- Zhang, X.; Li, G.; Wu, D.; Zhang, B.; Hu, N.; Wang, H.; Liu, J.; Wu, Y. Recent advances in the construction of functionalized covalent organic frameworks and their applications to sensing. Biosens. Bioelectron. 2019, 145, 111699–111718. [Google Scholar]
- Yao, D.; Li, C.; Wang, H.; Wen, G.; Liang, A.; Jiang, Z. A new dual-mode SERS and RRS aptasensor for detecting trace organic molecules based on gold nanocluster-doped covalent-organic framework catalyst. Sens. Actuators B Chem. 2020, 319, 128308–128319. [Google Scholar] [CrossRef]
- Wang, M.; Hu, M.; Liu, J.; Guo, C.; Peng, D.; Jia, Q.; He, L.; Zhang, Z.; Du, M. Covalent organic framework-based electrochemical aptasensors for the ultrasensitive detection of antibiotics. Biosens. Bioelectron. 2019, 132, 8–16. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Waterhouse, G.I.N.; Xu, L.; Qiao, X.; Xu, Z. Three-dimensional electrochemical sensor with covalent organic framework decorated carbon nanotubes signal amplification for the detection of furazolidone. Sens. Actuators B Chem. 2020, 321, 128501–128535. [Google Scholar] [CrossRef]
- Yang, Y.; Li, G.; Wang, P.; Fan, L.; Shi, Y. Highly sensitive multiplex detection of foodborne pathogens using a SERS immunosensor combined with novel covalent organic frameworks based biologic interference-free Raman tags. Talanta 2022, 243, 123369. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Chi, K.; Li, D.; Deng, Y.; Ma, Y.; Xu, Q.; Hu, R.; Yang, Y. 2D-porphrinic covalent organic framework-based aptasensor with enhanced photoelectrochemical response for the detection of C-reactive protein. Biosens. Bioelectron. 2019, 129, 64–71. [Google Scholar] [CrossRef] [PubMed]
- Ramos, L. Critical overview of selected contemporary sample preparation techniques. J. Chromatogr. A 2012, 1221, 84–98. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Jin, P.; Zhu, F.; Nie, L.; Qiu, H. A review on the use of ionic liquids in preparation of molecularly imprinted polymers for applications in solid-phase extraction. Trends Anal. Chem. 2021, 134, 116132–116186. [Google Scholar] [CrossRef]
- Haniffa, M.A.C.M.; Ching, Y.C.; Illias, H.A.; Munawar, K.; Ibrahim, S.; Nguyen, D.H.; Chuah, C.H. Cellulose supported promising magnetic sorbents for magnetic solid-phase extraction: A review. Carbohyd. Polym. 2021, 253, 117245. [Google Scholar] [CrossRef]
- Fenton, J.L.; Burke, D.W.; Qian, D.; de la Cruz, M.O.; Dichtel, W.R. Polycrystalline covalent organic framework films act as adsorbents, not membranes. J. Am. Chem. Soc. 2021, 143, 1466–1473. [Google Scholar] [CrossRef]
- Li, N.; Du, J.; Wu, D.; Liu, J.; Li, N.; Sun, Z.; Li, G.; Wu, Y. Recent advances in facile synthesis and applications of covalent organic framework materials as superior adsorbents in sample pretreatment. Trends Anal. Chem. 2018, 108, 154–166. [Google Scholar] [CrossRef]
- Majd, M.; Yazdanpanah, M.; Bayatloo, M.R.; Nojavan, S. Recent advances and applications of cyclodextrins in magnetic solid phase extraction. Talanta 2021, 229, 122296. [Google Scholar] [CrossRef] [PubMed]
- Sajid, M.; Nazal, M.K.; Ihsanullah, I. Novel materials for dispersive (micro) solid-phase extraction of polycyclic aromatic hydrocarbons in environmental water samples: A review. Anal. Chim. Acta 2021, 1141, 246–262. [Google Scholar] [CrossRef] [PubMed]
- Han, S.; Leng, Q.; Teng, F.; Ding, Y.; Yao, A. Preparation of mesh covalent organic framework Tppa-2-based adsorption enhanced magnetic molecularly imprinted composite for selective extraction of tetracycline residues from animal-derived foods. Food Chem. 2022, 384, 132601. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Zhou, T.; Lei, Y. Exploration of a novel triazine-based covalent organic framework for solid-phase extraction of antibiotics. RSC Adv. 2020, 10, 11557–11564. [Google Scholar] [CrossRef]
- Wang, R.; Li, C.; Li, Q.; Zhang, S.; Lv, F.; Yan, Z. Electrospinning fabrication of covalent organic framework composite nanofibers for pipette tip solid phase extraction of tetracycline antibiotics in grass carp and duck. J. Chromatogr. A 2020, 1622, 461098–461106. [Google Scholar] [CrossRef] [PubMed]
- Wen, L.; Liu, L.; Wang, X.; Wang, M.; Lin, J.; Zhao, R. Spherical mesoporous covalent organic framework as a solid-phase extraction adsorbent for the ultrasensitive determination of sulfonamides in food and water samples by liquid chromatography-tandem mass spectrometry. J. Chromatogr. A 2020, 1625, 461275–461305. [Google Scholar] [CrossRef]
- Wen, A.; Li, G.; Wu, D.; Yu, Y.; Yang, Y.; Hu, N.; Wang, H.; Chen, J.; Wu, Y. Sulphonate functionalized covalent organic framework-based magnetic sorbent for effective solid phase extraction and determination of fluoroquinolones. J. Chromatogr. A 2020, 1612, 460651–460685. [Google Scholar] [CrossRef]
- Belardi, R.; Pawliszyn, J. Application of chemically modified fused silica fibers in the extraction of organics from water matrix samples and their rapid transfer to capillary columns. Water Pollut. Res. J. Can. 1989, 24, 179–191. [Google Scholar] [CrossRef]
- Zheng, J.; Yang, Q.; Ni, C.; Xie, X.; Shi, Y.; Sun, J.; Zhu, F.; Ouyang, G. Fabrications of novel solid phase microextraction fiber coatings based on new materials for high enrichment capability. Trends Anal. Chem. 2018, 108, 135–153. [Google Scholar] [CrossRef]
- Feng, J.; Feng, J.; Ji, X.; Li, C.; Han, S.; Sun, H.; Sun, M. Recent advances of covalent organic frameworks for solid-phase microextraction. Trends Anal. Chem. 2021, 137, 116208–116214. [Google Scholar] [CrossRef]
- Zhang, Y.; Ning, Y.; Liao, W.; Wang, W.; Wang, A. Covalent organic framework-LZU1@ PEI@ Fe3O4-based magnetic dispersive micro-solid phase extraction of tetracyclines from environmental water prior to HPLC analysis. Anal. Methods 2021, 13, 4320–4327. [Google Scholar] [CrossRef]
- Yue, Q.; Huang, Y.; Shen, X.; Yang, C.; Pang, Y. In situ growth of covalent organic framework on titanium fiber for headspace solid-phase microextraction of 11 phthalate esters in vegetables. Food Chem. 2020, 318, 126507. [Google Scholar] [CrossRef]
- Guo, J.; Qian, H.; Zhao, X.; Yang, C.; Yan, X. In situ room-temperature fabrication of a covalent organic framework and its bonded fiber for solid-phase microextraction of polychlorinated biphenyls in aquatic products. J. Mater. Chem. A 2019, 7, 13249–13255. [Google Scholar] [CrossRef]
- Ji, W.; Guo, Y.; Xie, H.; Wang, X.; Jiang, X.; Guo, D. Rapid microwave synthesis of dioxin-linked covalent organic framework for efficient micro-extraction of perfluorinated alkyl substances from water. J. Hazard. Mater. 2020, 397, 122793–122801. [Google Scholar] [CrossRef] [PubMed]
- Szultka-Mlynska, M.; Pomastowski, P.; Buszewski, B. Application of solid phase microextraction followed by liquid chromatography-mass spectrometry in the determination of antibiotic drugs and their metabolites in human whole blood and tissue samples. J. Chromatogr. B 2018, 1086, 153–165. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.; Fan, R.; Zhang, J.; Qin, M.; Chen, W.; Jiang, X.; Zhu, K.; Ji, C.; Hao, S.; Yang, Y. Stimuli-responsive metal–organic framework on a metal–organic framework heterostructure for efficient antibiotic detection and anticounterfeiting. ACS Appl. Mater. Interfaces 2021, 13, 35689–35699. [Google Scholar] [CrossRef] [PubMed]
- Lu, D.; Liu, C.; Qin, M.; Deng, J.; Shi, G.; Zhou, T. Functionalized ionic liquids-supported metal organic frameworks for dispersive solid phase extraction of sulfonamide antibiotics in water samples. Anal. Chim. Acta 2020, 1133, 88–98. [Google Scholar] [CrossRef] [PubMed]
- Ayankojo, A.G.; Reut, J.; Ciocan, V.; Öpik, A.; Syritski, V. Molecularly imprinted polymer-based sensor for electrochemical detection of erythromycin. Talanta 2020, 209, 120502–120511. [Google Scholar] [CrossRef]
- Zhou, T.; Halder, A.; Sun, Y. Fluorescent nanosensor based on molecularly imprinted polymers coated on graphene quantum dots for fast detection of antibiotics. Biosensors 2018, 8, 82. [Google Scholar] [CrossRef]
- Jamieson, O.; Soares, T.C.C.; de Faria, B.A.; Husaon, A.; Mecozzi, F.; Rowley-Neale, S.J.; Banks, C.E.; Gruber, J.; Novakovic, K.; Peeters, M.; et al. Screen printed electrode based detection systems for the antibiotic amoxicillin in aqueous samples utilising molecularly imprinted polymers as synthetic receptors. Chemosensors 2019, 8, 5. [Google Scholar] [CrossRef]
- Yin, J.; Meng, Z.; Du, M.; Liu, C.; Song, M.; Wang, H. Pseudo-template molecularly imprinted polymer for selective screening of trace β-lactam antibiotics in river and tap water. J. Chromatogr. A 2010, 1217, 5420–5426. [Google Scholar] [CrossRef]
- Tan, B.; Zhao, H.; Du, L.; Gan, X.; Quan, X. A versatile fluorescent biosensor based on target-responsive graphene oxide hydrogel for antibiotic detection. Biosens. Bioelectron. 2016, 83, 267–273. [Google Scholar] [CrossRef]
- Yue, X.; Li, Z.; Zhao, S. A new electrochemical sensor for simultaneous detection of sulfamethoxazole and trimethoprim antibiotics based on graphene and ZnO nanorods modified glassy carbon electrode. Microchem. J. 2020, 159, 105440. [Google Scholar] [CrossRef]
- Wong, A.; Santos, A.M.; Cincotto, F.H.; Moraes, F.C.; Fatibello-Filho, O.; Sotomayor, M.D.P.T. A new electrochemical platform based on low cost nanomaterials for sensitive detection of the amoxicillin antibiotic in different matrices. Talanta 2020, 206, 120252–120259. [Google Scholar] [CrossRef]
- Anh, N.T.; Dinh, N.X.; Tuan, H.V.; Doan, M.Q.; Anh, N.H.; Khi, N.T.; Trang, V.T.; Tri, D.Q.; Le, A.T. Eco-friendly copper nanomaterials-based dual-mode optical nanosensors for ultrasensitive trace determination of amoxicillin antibiotics residue in tap water samples. Mater. Res. Bull. 2022, 147, 111649. [Google Scholar] [CrossRef]
- Zhang, L.; Chen, L. Fluorescence probe based on hybrid mesoporous silica/quantum dot/molecularly imprinted polymer for detection of tetracycline. ACS Appl. Mater. Interfaces 2016, 8, 16248–16256. [Google Scholar] [CrossRef]
- Taranova, N.A.; Berlina, A.N.; Zherdev, A.V.; Dzantiev, B.B. ‘Traffic light’ immunochromatographic test based on multicolor quantum dots for the simultaneous detection of several antibiotics in milk. Biosens. Bioelectron. 2015, 63, 255–261. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.; Wang, S.; Wu, P.; Wang, D.; Yi, J.; Li, L.; Ding, P.; Pan, H. A composite prepared from covalent organic framework and gold nanoparticles for the electrochemical determination of enrofloxacin. Adv. Powder Technol. 2021, 32, 2106–2115. [Google Scholar] [CrossRef]
- Hu, J.; Su, X.; Yuan, L.; Zheng, K.; Zou, X.; Sun, Z.; Xu, X.; Zhang, W. Competitive immunoassay using enzyme-regulated Fe3O4@COF/Fe3+ fluorescence probe for natural chloramphenicol detection. Anal. Chim. Acta 2023, 1277, 341680. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wang, D.; Tao, Y.; Wang, J.; Tian, M.; Liu, Q.; Yang, Y.; Zou, Y.; Ke, F.; Guo, X. Phenylboronic acid functionalized high-crystallinity fluorescent covalent organic framework act as a sensing platform with dual performance for rifamycin antibiotics and water and adsorbent for rifamycin antibiotics. Mater. Today Chem. 2023, 32, 10147. [Google Scholar] [CrossRef]
- Li, Q.; Zhu, S.; Wu, F.; Chen, F.; Guo, C. Slice-layer COFs-aerogel: A regenerative dispersive solid-phase extraction adsorbent for determination of ultra-trace quinolone antibiotics. Microchim. Acta 2023, 190, 369–381. [Google Scholar] [CrossRef]
- Han, X.; Yuan, C.; Hou, B.; Liu, L.; Li, H.; Liu, Y.; Cui, Y. Chiral covalent organic frameworks: Design, synthesis and property. Chem. Soc. Rev. 2020, 49, 6248–6272. [Google Scholar] [CrossRef]
- Gendy, E.A.; Oyekunle, D.T.; Ifthikar, J.; Jawad, A.; Chen, Z. A review on the adsorption mechanism of different organic contaminants by covalent organic framework (COF) from the aquatic environment. Environ. Sci. Pollut. R. 2022, 29, 32566–32593. [Google Scholar] [CrossRef]
- Jiang, W.; Cui, W.; Liang, R.; Qiu, J. Difunctional covalent organic framework hybrid material for synergistic adsorption and selective removal of fluoroquinolone antibiotics. J. Hazard. Mater. 2021, 413, 125302–125311. [Google Scholar] [CrossRef]
- Gendy, E.A.; Ifthikar, J.; Ali, J.; Oyekunle, D.T.; Elkhlifia, Z.; Shahib, I.I.; Khodair, A.I.; Chen, Z. Removal of heavy metals by covalent organic frameworks (COFs): A review on its mechanism and adsorption properties. J. Environ. Chem. Eng. 2021, 9, 105687–105700. [Google Scholar] [CrossRef]
- Haug, W.K.; Moscarello, E.M.; Wolfson, E.R.; McGrier, P.L. The luminescent and photophysical properties of covalent organic frameworks. Chem. Soc. Rev. 2020, 49, 839–864. [Google Scholar] [CrossRef]
- Zhang, N.; Ishag, A.; Li, Y.; Wang, H.; Guo, H.; Mei, P.; Meng, Q.; Sun, Y. Recent investigations and progress in environmental remediation by using covalent organic framework-based adsorption method: A review. J. Clean. Prod. 2020, 277, 123360. [Google Scholar] [CrossRef]
- Xu, G.; Dong, X.; Hou, L.; Wang, X.; Liu, L. Room-temperature synthesis of flower-shaped covalent organic frameworks for solid-phase extraction of quinolone antibiotics. Anal. Chim. Acta 2020, 1126, 82–90. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Wu, H.; Yildirim, T. Structural stability and elastic properties of prototypical covalent organic frameworks. Chem. Phys. Lett. 2010, 499, 103–107. [Google Scholar] [CrossRef]
- Tchinsa, A.; Hossain, M.F.; Wang, T.; Zhou, Y. Removal of organic pollutants from aqueous solution using metal organic frameworks (MOFs)-based adsorbents: A review. Chemosphere 2021, 284, 131393–131406. [Google Scholar] [CrossRef]
- Guan, S.; Wu, H.; Yang, L.; Wang, Z.; Wu, J. Use of a magnetic covalent organic framework material with a large specific surface area as an effective adsorbent for the extraction and determination of six fluoroquinolone antibiotics by HPLC in milk sample. J. Sep. Sci. 2020, 43, 3775–3784. [Google Scholar] [CrossRef] [PubMed]


| Linkages Species | COFs | SBET (m2 g–1) | Pore Volume (cm3 g–1) | Pore Size (nm) | Ref. |
|---|---|---|---|---|---|
| Boronate ester | COF-5 | - | - | 2.7 | [52] |
| Imine | TpBD | 885 | - | 2.3 | [58] |
| Imine | CCOF-5 | 655 | 0.51 | 0.62 | [59] |
| Amide | COF-6 | 613 | 0.42 | 0.59 | [59] |
| Imine | Tx-COF-2 | 1137 | - | 1.51 | [64] |
| Triazine | CTF-1 | 791 | 1.2 | [53] | |
| Triazine | CTF-Th | 78 | - | - | [54] |
| Triazine | Phen-CTF | 358 | [55] | ||
| Hydrazone | TFPT-COF | 1.185 | 3.8 | [66] | |
| Hydrazone | BtaMth COF | 723 | 0.46 | 1.41 | [67] |
| Amide | COF-1 | 714 | - | 3.7 | [73] |
| Azine | ACOF-1 | 1.176 | - | 1.1 | [68] |
| Azine | Py-Azine COF | 1.210 | - | 1.9 | [69] |
| β-ketoenamines | Acridine COF | 654 | - | - | [70] |
| Materials | Detection Methods | Antibiotics Targets | Limits of Detection | Recovery (%) | Relative Standard Deviation (%) | Ref. |
|---|---|---|---|---|---|---|
| Zn(II)-MOF | Photoluminescence sensing | Aminoglycosides | 37.6 ng L−1 | 97 | [136] | |
| Zr-MOF | Solid-phase extraction | Sulfonamides antibiotics | 0.20 μg·mL−1 | 91–109.4 | 1.0–8.6 | [137] |
| MIP | Sensor | Erythromycin | 0.10 μg·mL−1 | 91–102 | [138] | |
| Graphene Quantum Dots | Fluorescent emission | Tetracycline | 1.0 μg·L−1 | 85.3–103.3 | [139] | |
| MIP | Sensor | Amoxicillin | 1.89 ng mL−1 | [140] | ||
| MIP | Solid-phase extraction | β-lactam | 9.56 μg·L−1 | 60–90 | [141] | |
| Graphene oxide hydrogel | Fluorescent biosensor | Oxytetracycline | 25 μg·L−1 | [142] | ||
| ZnO nanorods | Sensor | Trimethoprim | 0.3 μg·mL−1 | 93.2–108 | [143] | |
| Nanomaterial | Sensor | Amoxicillin | 50 ng L−1 | 97 | 3.3 | [144] |
| Copper nanomaterials | Sensor | Amoxicillin | 1.71 μg·mL−1 | 95 | 5 | [145] |
| Quantum dot | Fluorescence sensor | Tetracycline | 50 ng L−1 | 90.2–97.2 | 2.2–5.7 | [146] |
| Quantum dots | Fluorescence sensor | Ofloxacin | 0.3 ng mL−1 | 92–101 | 8 | [147] |
| COFs | Enrofloxacin | 0.05 ng mL−1 | 96.7–102.2 | 0.9–6.4 | [148] | |
| Fe3O4@COFs | Fluorescence sensor | Tetracycline | 0.092 ng mL−1 | 96.4–103.7 | [149] | |
| Ba@COF | Fluorescence sensor | Rifampicin | 0.03 μg·mL−1 | 75.20–123.46 | [150] | |
| COFs | Solid-phase extraction | Quinolone | 0.02–0.06 ng∙L−1 | 68.2–104 | <10% | [151] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hao, Y.; Xia, Y.; Huang, J.; Zhong, C.; Li, G. Covalent–Organic Frameworks for Selective and Sensitive Detection of Antibiotics from Water. Polymers 2024, 16, 2319. https://doi.org/10.3390/polym16162319
Hao Y, Xia Y, Huang J, Zhong C, Li G. Covalent–Organic Frameworks for Selective and Sensitive Detection of Antibiotics from Water. Polymers. 2024; 16(16):2319. https://doi.org/10.3390/polym16162319
Chicago/Turabian StyleHao, Ying, Yanjie Xia, Jingjing Huang, Chenglin Zhong, and Guizhen Li. 2024. "Covalent–Organic Frameworks for Selective and Sensitive Detection of Antibiotics from Water" Polymers 16, no. 16: 2319. https://doi.org/10.3390/polym16162319
APA StyleHao, Y., Xia, Y., Huang, J., Zhong, C., & Li, G. (2024). Covalent–Organic Frameworks for Selective and Sensitive Detection of Antibiotics from Water. Polymers, 16(16), 2319. https://doi.org/10.3390/polym16162319





