Structural Characterization and Biological Properties Analysis of Exopolysaccharides Produced by Weisella cibaria HDL-4
Abstract
1. Introduction
2. Materials and Methods
2.1. Isolation and Identification of Strains
2.2. Isolation and Purification of HDL-4 EPS
2.3. Purity of HDL-4 EPS
2.4. Monosaccharide Composition of HDL-4 EPS
2.5. Molecular Weight of HDL-4 EPS
2.6. Fourier-Transform Infrared (FT-IR) Spectroscopy Analysis
2.7. X-ray Diffraction (XRD) Analysis
2.8. Nuclear Magnetic Resonance (NMR) Spectroscopy Analysis
2.9. Scanning Electron Microscopy (SEM) and Atomic Force Microscopy (AFM) Analysis
2.10. Water Contact Angle Measurement
2.11. Thermal Analysis
2.12. Heavy-Metal-Chelating Activity
2.13. Rheological Properties
2.14. Emulsifying Activity (EA) Assay
2.15. Scavenging Assay of DPPH and ABTS Radical
2.16. Probiotic Proliferation Test
2.17. Water Solubility Index (WSI) and Water Holding Capacity (WHC) Analysis
2.18. Statistical Analysis
3. Results and Discussion
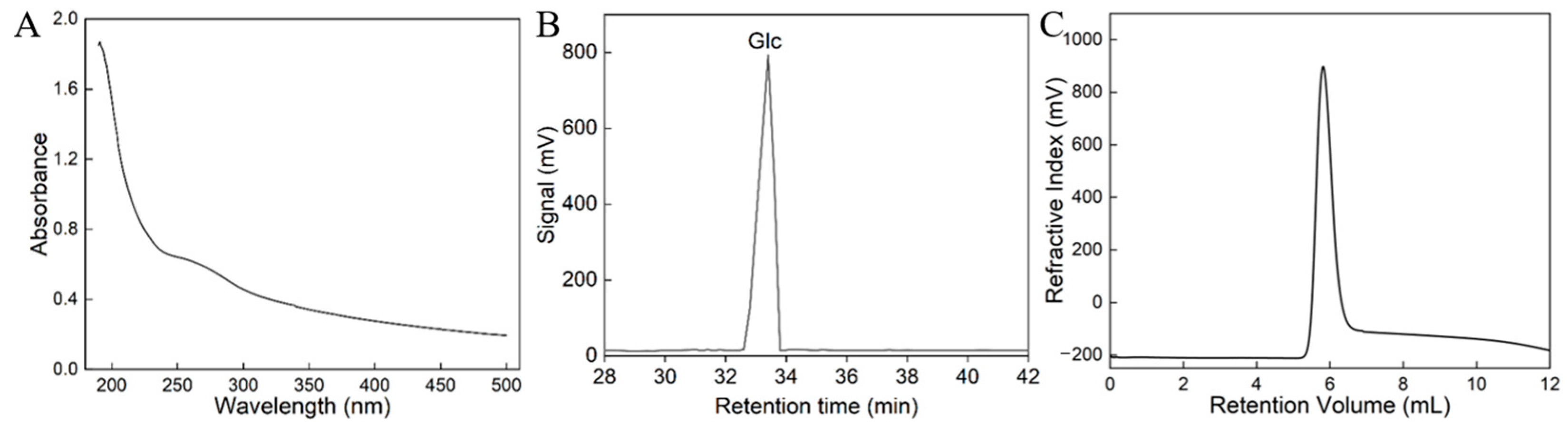
3.1. Strain Identiffcation, Extraction, and Quantification of HDL-4 EPS
3.2. Monosaccharide Composition and Mw Analysis
3.3. FT-IR Analysis
3.4. XRD Analysis
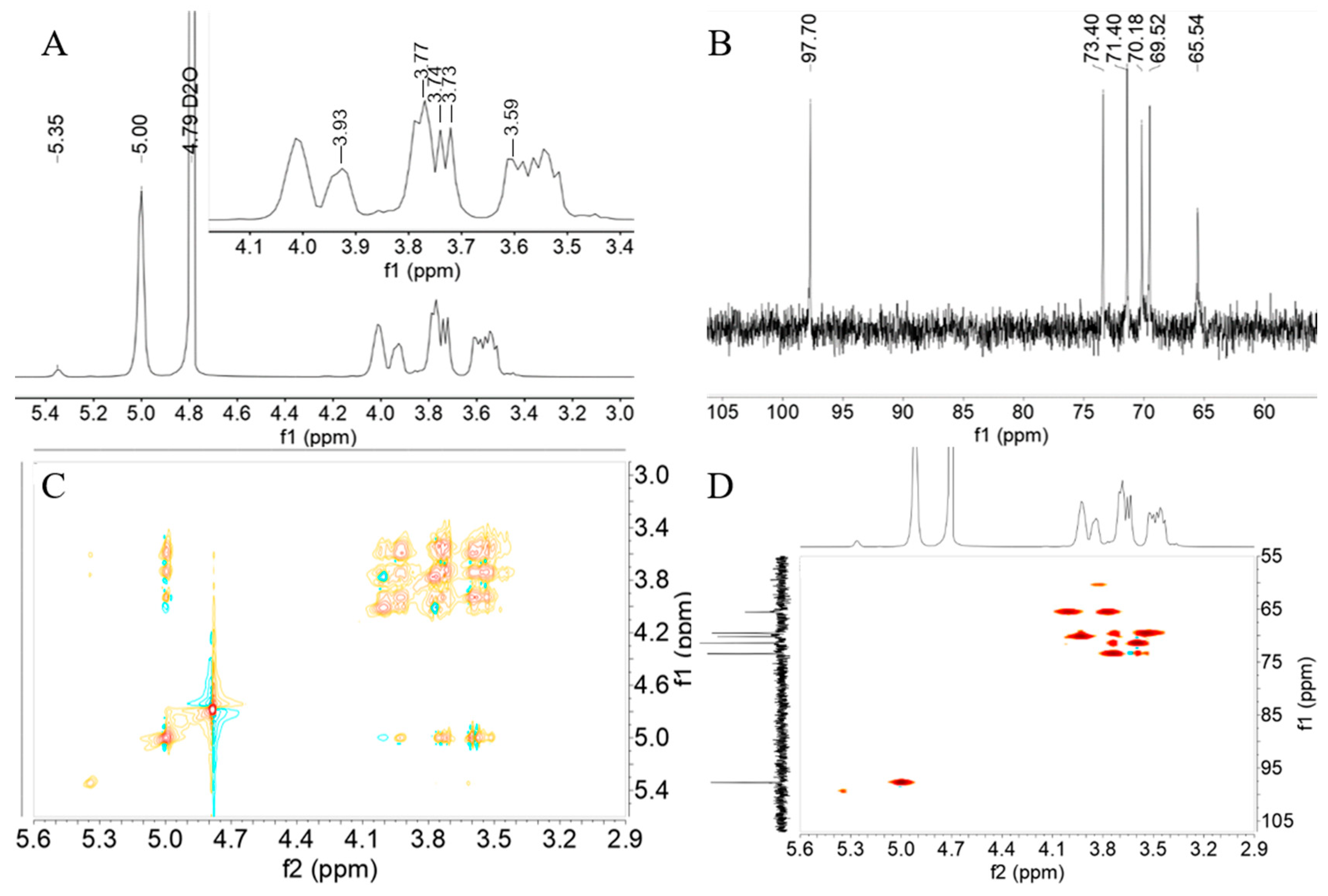
3.5. NMR Analysis
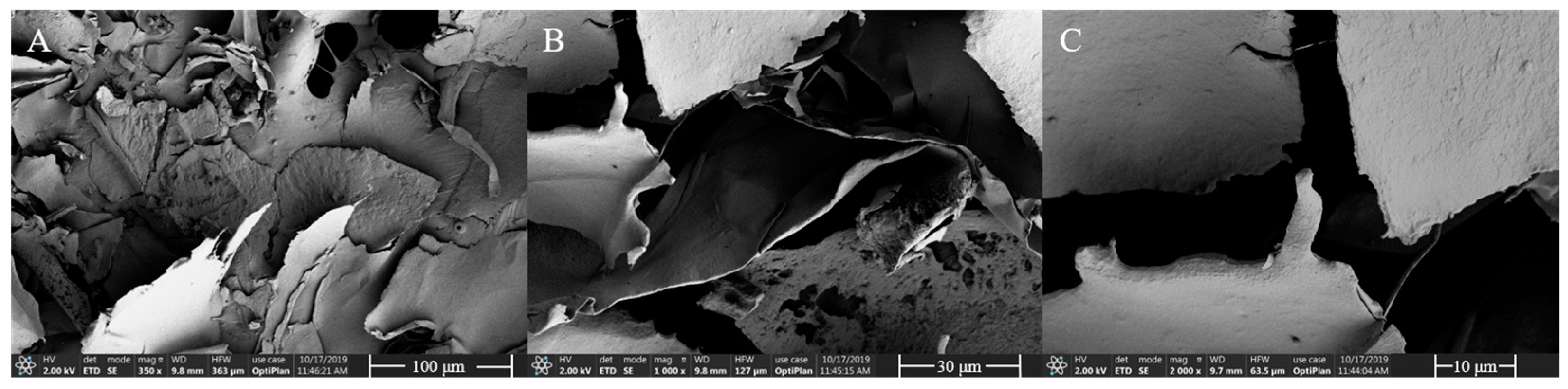
3.6. SEM Analysis
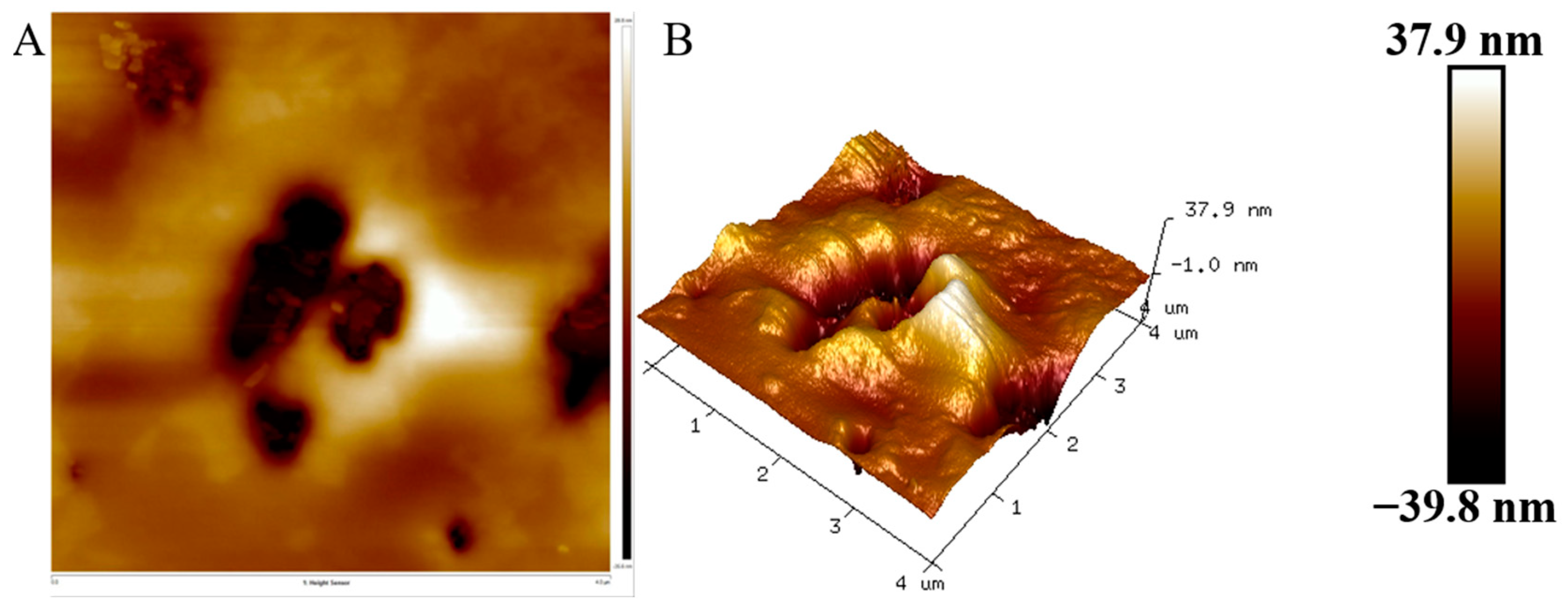
3.7. AFM Analysis
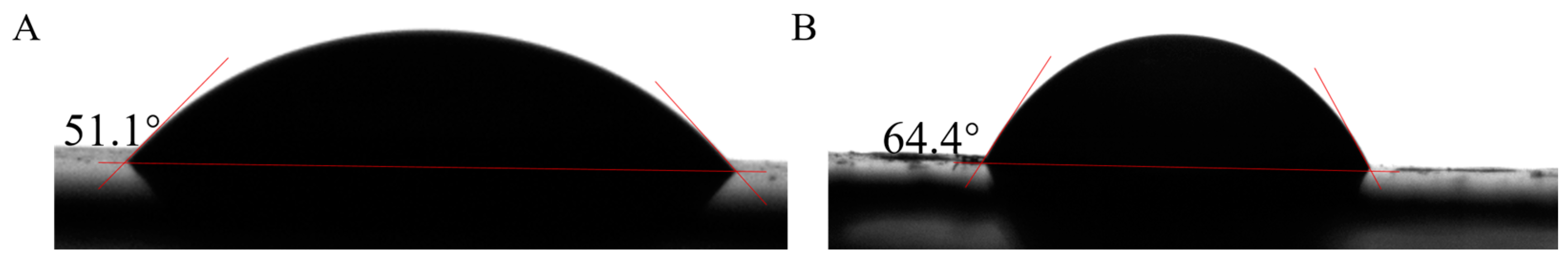
3.8. Water Contact Angle Analysis
3.9. Thermal Properties Analysis
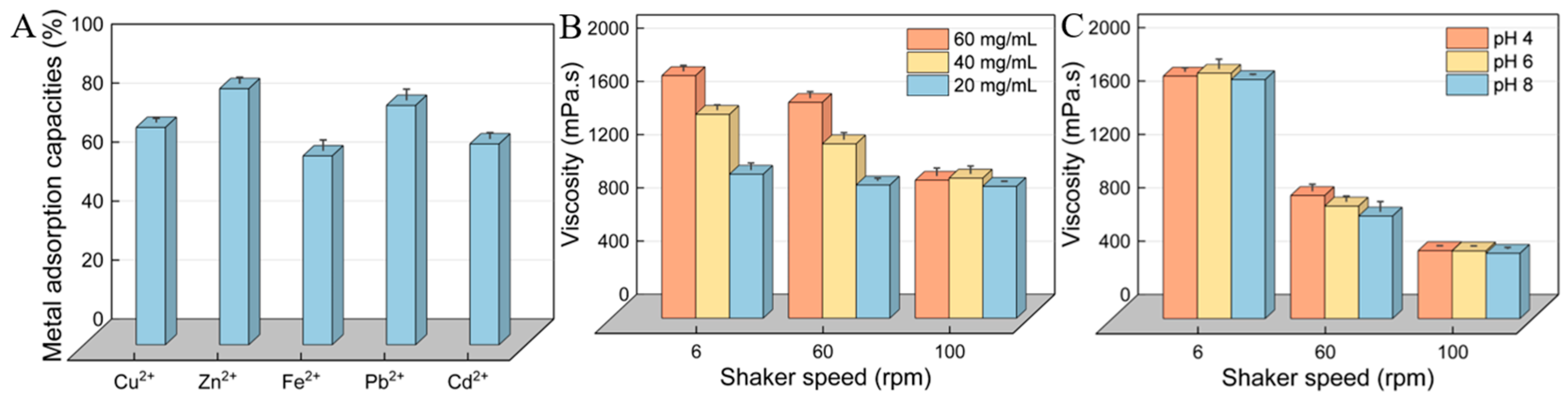
3.10. Heavy-Metal-Chelating Activity of HDL-4 EPS
3.11. Rheological Property of HDL-4 EPS
3.12. EA Activity of HDL-4 EPS
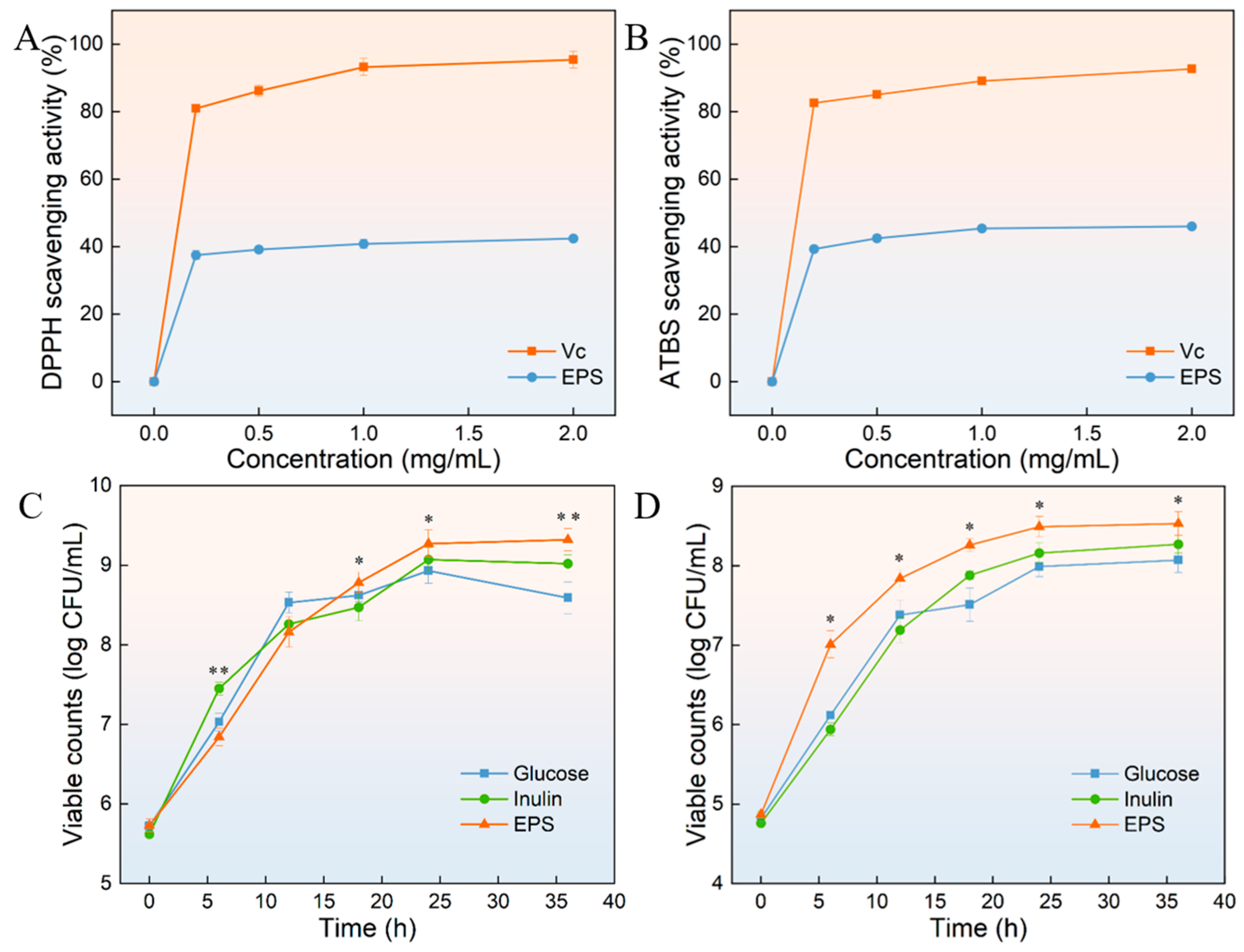
3.13. DPPH Radical and ABTS Radical Scavenging Activity of HDL-4 EPS
3.14. Probiotic Proliferation
3.15. WSI and WHC Analysis of HDL-4 EPS
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Nwodo, U.U.; Green, E.; Okoh, A.I. Bacterial Exopolysaccharides: Functionality and Prospects. Int. J. Mol. Sci. 2012, 13, 14002–14015. [Google Scholar] [CrossRef] [PubMed]
- Antczak-Chrobot, A.; Wojtczak, M. The impact of exopolysaccharides on the purification process—Filtration, sedimentation and particle size distribution. Sugar Ind. Int. 2024, 149, 116–121. [Google Scholar] [CrossRef]
- Di, W.; Zhang, Y.C.; Yi, H.X.; Han, X.; Wang, S.M.; Zhang, L.W. Research methods for structural analysis of lactic acid Bacteria induced exopolysaccharides, Chin. J. Anal. Chem. 2018, 46, 875–882. [Google Scholar] [CrossRef]
- Sutherland, I. Biofilm exopolysaccharides: A strong and sticky framework. Microbiology 2001, 147, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Schmid, J.; Fariña, J.; Fiehm, B.; Sieber, V. Editorial: Microbial exopolysaccharides: From genes to applications. Front. Microbiol. 2016, 7, 308. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.S.; Mody, K.; Jha, B. Bacterial exopolysaccharides—A perception. J. Basic Microbiol. 2007, 47, 103–117. [Google Scholar] [CrossRef]
- Gao, X.; Zhao, J.; Zhang, H.; Chen, W.; Zhai, Q. Modulation of gut health using probiotics: The role of probiotic effector molecules. J. Future Foods 2022, 2, 1–12. [Google Scholar] [CrossRef]
- Lynch, K.M.; Zannini, E.; Coffey, A.; Arendt, E.K. Lactic Acid bacteria exopolysaccharides in foods and beverages: Isolation, properties, characterization, and health benefits. Annu. Rev. Food Sci. Technol. 2018, 9, 155–176. [Google Scholar] [CrossRef]
- Saadat, Y.R.; Khosroushahi, A.Y.; Gargari, B.P. A comprehensive review of anticancer, immunomodulatory and health beneficial effects of the lactic acid bacteria exopolysaccharides. Carbohydr. Polym. 2019, 217, 79–89. [Google Scholar] [CrossRef]
- Angelin, J.; Kavitha, M. Exopolysaccharides from probiotic bacteria and their health potential. Int. J. Biol. Macromol. 2020, 162, 853–865. [Google Scholar] [CrossRef]
- Nesic, A.; Cabrera-Barjas, G.; Dimitrijevic-Brankovic, S.; Davidovic, S.; Radovanovic, N.; Delattre, C. Prospect of polysaccharide-based materials as advanced food packaging. Molecules 2020, 25, 135. [Google Scholar] [CrossRef]
- Barcelos, M.C.S.; Vespermann, K.A.C.; Pelissari, F.M.; Molina, G. Current status of biotechnological production and applications of microbial exopolysaccharides. Crit. Rev. Food Sci. Nutr. 2020, 60, 1475–1495. [Google Scholar] [CrossRef] [PubMed]
- Brüls, M.; Foroutanparsa, S.; Maljaars, C.E.P.; Olsthoorn, M.; Tas, R.P.; Voets, I.K. Investigating the impact of exopolysaccharides on yogurt network mechanics and syneresis through quantitative microstructural analysis. Food Hydrocoll. 2024, 150, 109629. [Google Scholar] [CrossRef]
- Yang, S.; Xu, X.Q.; Peng, Q.; Ma, L.; Qiao, Y.; Shi, B. Exopolysaccharides from lactic acid bacteria, as an alternative to antibiotics, on regulation of intestinal health and the immune system. Anim. Nutr. 2023, 13, 78–89. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.S.; Ye, G.B.; Qi, X.T.; Yang, Y.; Zhou, B.S.; Zhang, Y.Y.; Du, R.P.; Ge, J.P.; Ping, W.X. Purification, characterization and probiotic proliferation effect of exopolysaccharides produced by Lactiplantibacillus plantarum HDC-01 isolated from sauerkraut. Front. Microbiol. 2023, 14, 1210302. [Google Scholar] [CrossRef] [PubMed]
- DuBois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Yang, Y.; Ye, G.B.; Qi, X.T.; Zhou, B.S.; Yu, L.S.; Song, G.; Du, R.P. Exploration of exopolysaccharide from Leuconostoc mesenteroides HDE-8: Unveiling structure, bioactivity, and food industry applications. Polymers 2024, 16, 954. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Jiang, J.; Liu, L.N.; Wang, S.; Ping, W.X.; Ge, J.P. Characterization of exopolysaccharides produced by Weissella confusa XG-3 and their potential biotechnological applications. Int. J. Biol. Macromol. 2021, 178, 306–315. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Guo, S.X.; Ping, W.X.; Zhao, D.; Ge, J.P. Optimization production of exopolysaccharide from Leuconostoc lactis L2 and its partial characterization. Int. J. Biol. Macromol. 2020, 159, 630–639. [Google Scholar] [CrossRef]
- Abid, Y.; Azabou, S.; Joulak, I.; Casillo, A.; Lanzetta, R.; Corsaro, M.M.; Gharsallaoui, A.; Attia, H. Potential biotechnological properties of an exopolysaccharide produced by newly isolated Bacillus tequilensis-GM from spontaneously fermented goat milk. LWT-Food Sci. Technol. 2019, 105, 135–141. [Google Scholar] [CrossRef]
- Liu, L.N.; Xu, J.J.; Du, R.P.; Ping, W.X.; Ge, J.P.; Zhao, D. The response surface optimization of exopolysaccharide produced by Saccharomyces cerevisiae Y3 and its partial characterization. Prep. Biochem. Biotech. 2022, 52, 566–577. [Google Scholar] [CrossRef] [PubMed]
- Lobo, R.E.; Orrillo, P.A.; Ribotta, S.B.; de Valdez, G.F.; García, M.S.; Cabello, J.C.R.; Torino, M.I. Structural characterization of a homopolysaccharide produced by Weissella cibaria FMy 2-21-1 and its potential application as a green corrosion inhibiting film. Int. J. Biol. Macromol. 2022, 212, 193–201. [Google Scholar] [CrossRef] [PubMed]
- Aburas, H.; Ispirli, H.; Taylan, O.; Yilmaz, M.T.; Dertli, E. Structural and physicochemical characterisation and antioxidant activity of an α-D-glucan produced by sourdough isolate Weissella cibaria MED17. Int. J. Biol. Macromol. 2020, 161, 648–655. [Google Scholar] [CrossRef]
- Liu, L.; Du, Y.R.; Du, Y.B.; Yan, W.L.; Li, Y.Z.; Cui, K.; Li, Z.; Yu, P.; Zhang, W.C.; Feng, J.G.; et al. Exopolysaccharide from Weissella confusa J4-1 inhibits colorectal cancer via induction of cell cycle arrest. Int. J. Biol. Macromol. 2023, 253, 127625. [Google Scholar] [CrossRef] [PubMed]
- Rosca, I.; Petrovici, A.R.; Peptanariu, D.; Nicolescu, A.; Dodi, G.; Avadanei, M.; Ivanov, I.C.; Bostanaru, A.C.; Mares, M.; Ciolacu, D. Biosynthesis of dextran by Weissella confusa and its In vitro functional characteristics. Int. J. Biol. Macromol. 2018, 107, 1765–1772. [Google Scholar] [CrossRef]
- Zhang, J.; Xiao, Y.; Wang, H.C.; Zhang, H.; Chen, W.; Lu, W.W. Lactic acid bacteria-derived exopolysaccharide: Formation, immunomodulatory ability, health effects, and structure-function relationship. Microbiol. Res. 2023, 274, 127432. [Google Scholar] [CrossRef]
- Lakra, A.K.; Domdi, L.; Tilwani, Y.M.; Arul, V. Physicochemical and functional characterization of mannan exopolysaccharide from Weissella confusa MD1 with bioactivities. Int. J. Biol. Macromol. 2020, 143, 797–805. [Google Scholar] [CrossRef]
- Ozpinar, F.B.; Ispirli, H.; Kayacan, S.; Korkmaz, K.; Dere, S.; Sagdic, O.; Alkay, Z.; Tuneil, Y.E.; Ayyash, M.; Dertli, E. Physicochemical and structural characterisation of a branched dextran type exopolysaccharide (EPS) from Weissella confusa S6 isolated from fermented sausage (Sucuk). Int. J. Biol. Macromol. 2024, 264, 130507. [Google Scholar] [CrossRef]
- Li, W.; Tang, W.Z.; Ji, J.; Xia, X.D.; Rui, X.; Chen, X.H.; Jiang, M.; Zhou, J.Z.; Dong, M.S. Characterization of a novel polysaccharide with anti-colon cancer activity from Lactobacillus helveticus MB2-1. Carbohydr. Res. 2015, 411, 6–14. [Google Scholar] [CrossRef]
- Vasanthakumari, D.S.; Harikumar, S.; Beena, D.J.; Pandey, A.; Nampoothiri, K.M. Physicochemical characterization of an exopolysaccharide produced by a newly isolated Weissella cibaria. Appl. Biochem. Biotechnol. 2015, 176, 440–453. [Google Scholar] [CrossRef]
- Yilmaz, M.T.; Ispirli, H.; Taylan, O.; Alamoudi, M.; Dertli, E. Bioactive and technological properties of an α-D-glucan synthesized by Weissella cibaria PDER21. Carbohydr. Polym. 2022, 285, 119227. [Google Scholar] [CrossRef] [PubMed]
- Ning, E.J.; Sun, C.W.; Wang, X.F.; Chen, L.; Li, F.F.; Zhang, L.X.; Wang, L.P.; Ma, Y.N.; Zhu, J.; Li, X.; et al. Artemisia argyi polysaccharide alleviates intestinal inflammation and intestinal flora dysbiosis in lipopolysaccharide-treated mice. Food Med. Homol. 2024, 1, 9420008. [Google Scholar] [CrossRef]
- Kavitake, D.; Devi, P.B.; Singh, S.P.; Shetty, P.H. Characterization of a novel galactan produced by Weissella confusa KR780676 from an acidic fermented food. Int. J. Biol. Macromol. 2016, 86, 681–689. [Google Scholar] [CrossRef] [PubMed]
- Ispirli, H.; Sagdic, O.; Yilmaz, M.T.; Dertli, E. Physicochemical characterisation of an α-glucan from Lactobacillus reuteri E81 as a potential exopolysaccharide suitable for food applications. Process Biochem. 2019, 79, 91–96. [Google Scholar] [CrossRef]
- Kačuráková, M.; Capek, P.; Sasinková, V.; Wellner, N.; Ebringerová, A. FT-IR study of plant cell wall model compounds: Pectic polysaccharides and hemicelluloses. Carbohydr. Polym. 2000, 43, 195–203. [Google Scholar] [CrossRef]
- Shingel, K.I. Determination of structural peculiarities of dexran, pullulan and γ-irradiated pullulan by Fourier-transform IR spectroscopy. Carbohydr. Res. 2002, 337, 1445–1451. [Google Scholar] [CrossRef]
- Das, D.; Goyal, A. Characterization and biocompatibility of glucan: A safe food additive from probiotic Lactobacillus plantarum DM5. J. Sci. Food Agric. 2014, 94, 683–690. [Google Scholar] [CrossRef] [PubMed]
- Heperkan, Z.D.; Bolluk, M.; Bülbül, S. Structural analysis and properties of dextran produced by Weissella confusa and the effect of different cereals on its rheological characteristics. Int. J. Biol. Macromol. 2020, 143, 305–313. [Google Scholar] [CrossRef]
- Mathivanan, K.; Chandirika, J.U.; Mathimani, T.; Vinothkanna, A.; Rajaram, R.; Annadurai, G. Optimization, compositional analysis, and characterization of exopolysaccharides produced by multi-metal resistant Bacillus cereus KMS3-1. Carbohydr. Polym. 2020, 227, 115369. [Google Scholar] [CrossRef]
- Yilmaz, M.T.; Ispirli, H.; Taylan, O.; Bilgrami, A.L.; Dertli, E. Structural and bioactive characteristics of a dextran produced by Lactobacillus kunkeei AK1. Int. J. Biol. Macromol. 2022, 200, 293–302. [Google Scholar] [CrossRef]
- Ahmed, R.Z.; Siddiqui, K.; Arman, M.; Ahmed, N. Characterization of high molecular weight dextran produced by Weissella cibaria CMGDEX3. Carbohydr. Polym. 2012, 90, 441–446. [Google Scholar] [CrossRef] [PubMed]
- Naessens, M.; Cerdobbel, A.; Soetaert, W.K.; Vandamme, E.J. Leuconostoc dextransucrase and dextran: Production, properties and applications. J. Chem. Technol. Biotechnol. 2005, 80, 845–860. [Google Scholar] [CrossRef]
- van Leeuwen, S.S.; Kralj, S.; van Geel-Schutten, I.H.; Gerwig, G.J.; Dijkhuizen, L.; Kamerling, J.P. Structural analysis of the α-D-glucan (EPS35-5) produced by the Lactobacillus reuteri strain 35-5 glucansucrase GTFA enzyme. Carbohydr. Res. 2008, 343, 1251–1265. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kralj, S.; Stripling, E.; Sanders, P.; van Geel-Schutten, G.H.; Dijkhuizen, L. Highly hydrolytic reuteransucrase from probiotic Lactobacillus reuteri strain ATCC 55730. Appl. Environ. Microbiol. 2005, 71, 3942–3950. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wang, W.; Ju, Y.; Liu, N.; Shi, S.; Hao, L. Structural characteristics of microbial exopolysaccharides in association with their biological activities: A review. Chem. Biol. Technol. Agric. 2023, 10, 137. [Google Scholar] [CrossRef]
- Zhao, W.; Zhang, J.; Jiang, Y.Y.; Zhao, X.; Hao, X.N.; Li, L.; Yang, Z.N. Characterization and antioxidant activity of the exopolysaccharide produced by Bacillus amyloliquefaciens GSBa-1. J. Microbiol. Biotechnol. 2018, 28, 1282–1292. [Google Scholar] [CrossRef] [PubMed]
- Ye, G.B.; Li, G.L.; Wang, C.L.; Ling, B.; Yang, R.R.; Huang, S.Y. Extraction and characterization of dextran from Leuconostoc pseudomesenteroides YB-2 isolated from mango juice. Carbohydr. Polym. 2019, 207, 218–223. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Li, H.X.; Wang, S.; Zhao, F.; Fang, F.; Guo, J.S.; Long, M.; Shen, Y. Differences in exopolysaccharides of three microbial aggregates. Environ. Technol. 2022, 43, 2909–2921. [Google Scholar] [CrossRef]
- Wang, Y.P.; Li, C.; Liu, P.; Ahmed, Z.; Xiao, P.; Bai, X.J. Physical characterization of exopolysaccharide produced by Lactobacillus plantarum KF5 isolated from Tibet Kefir. Carbohydr. Polym. 2010, 82, 895–903. [Google Scholar] [CrossRef]
- Netsopa, S.; Niamsanit, S.; Sakloetsakun, D.; Milintawisamai, N. Characterization and rheological behavior of dextran from Weissella confusa R003. Int. J. Polym. Sci. 2018, 2018, 5790526. [Google Scholar] [CrossRef]
- Zhou, Q.Q.; Feng, F.; Yang, Y.F.; Zhao, F.K.; Du, R.P.; Zhou, Z.J.; Han, Y. Characterization of a dextran produced by Leuconostoc pseudomesenteroides XG5 from homemade wine. Int. J. Biol. Macromol. 2018, 107, 2234–2241. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, Z.; Wang, Y.P.; Anjum, N.; Ahmad, A.; Khan, S.T. Characterization of exopolysaccharide produced by Lactobacillus kefiranofaciens ZW3 isolated from Tibet kefir—Part II. Food Hydrocoll. 2013, 30, 343–350. [Google Scholar] [CrossRef]
- Wang, B.B.; Song, Q.Z.; Zhao, F.K.; Han, Y.; Zhou, Z.J. Production optimization, partial characterization and properties of an exopolysaccharide from Lactobacillus sakei L3. Int. J. Biol. Macromol. 2019, 141, 21–28. [Google Scholar] [CrossRef]
- Yilmaz, M.T.; Ispirli, H.; Taylan, O.; Tasdemir, V.; Sagdic, O.; Dertli, E. Characterisation and functional roles of a highly branched dextran produced by a bee pollen isolate Leuconostoc mesenteroides BI-20. Food Biosci. 2022, 45, 101330. [Google Scholar] [CrossRef]
- Xing, H.W.; Du, R.P.; Zhao, F.K.; Han, Y.; Xiao, H.Z.; Zhou, Z.J. Optimization, chain conformation and characterization of exopolysaccharide isolated from Leuconostoc mesenteroides DRP105. Int. J. Biol. Macromol. 2018, 112, 1208–1216. [Google Scholar] [CrossRef]
- Yuan, Y.V.; Carrington, M.F.; Walsh, N.A. Extracts from dulse (Palmaria palmata) are effective antioxidants and inhibitors of cell proliferation in vitro. Food Chem. Toxicol. 2005, 43, 1073–1081. [Google Scholar] [CrossRef]
- Raj K., K.; Sardar, U.R.; Bhargavi, E.; Devi, I.; Bhunia, B.; Tiwari, O.N. Advances in exopolysaccharides based bioremediation of heavy metals in soil and water: A critical review. Carbohydr. Polym. 2018, 199, 353–364. [Google Scholar] [CrossRef]
- Nowak, K.; Wiater, A.; Choma, A.; Wiącek, D.; Bieganowski, A.; Siwulski, M.; Waśko, A. Fungal (1 → 3)-α-D-glucans as a new kind of biosorbent for heavy metals. Int. J. Biol. Macromol. 2019, 137, 960–965. [Google Scholar] [CrossRef]
- de Oliveira, J.M.; Amaral, S.A.; Burkert, C.A.V. Rheological, textural and emulsifying properties of an exopolysaccharide produced by Mesorhizobium loti grown on a crude glycerol-based medium. Int. J. Biol. Macromol. 2018, 120, 2180–2187. [Google Scholar] [CrossRef]
- Wang, Y.; Du, R.; Qiao, X.; Zhao, B.; Zhou, Z.; Han, Y. Optimization and characterization of exopolysaccharides with a highly branched structure extracted from Leuconostoc citreum B-2. Int. J. Biol. Macromol. 2020, 142, 73–84. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.N.; Xu, J.J.; Na, R.Y.; Du, R.P.; Ping, W.X.; Ge, J.P.; Zhao, D. Purification, characterization and partial biological activities of exopolysaccharide produced by Saccharomyces cerevisiae Y3. Int. J. Biol. Macromol. 2022, 206, 777–787. [Google Scholar] [CrossRef]
- Elnahas, M.O.; Amin, M.A.; Hussein, M.M.D.; Shanbhag, V.C.; Ali, A.E.; Wall, J.D. Isolation, characterization and bioactivities of an extracellular polysaccharide produced from Streptomyces sp. MOE6. Molecules 2017, 22, 1396. [Google Scholar] [CrossRef] [PubMed]
- Pei, F.Y.; Ma, Y.S.; Chen, X.; Liu, H. Purification and structural characterization and antioxidant activity of levan from Bacillus megaterium PFY-147. Int. J. Biol. Macromol. 2020, 161, 1181–1188. [Google Scholar] [CrossRef] [PubMed]
- Du, R.P.; Xing, H.W.; Yang, Y.P.; Jiang, H.J.; Zhou, Z.J.; Han, Y. Optimization, purification and structural characterization of a dextran produced by L-mesenteroides isolated from Chinese sauerkraut. Carbohydr. Polym. 2017, 174, 409–416. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.M.; Cui, Y.L.; Wang, X.; Yue, F.F.; Shan, Y.Y.; Liu, B.F.; Zhou, Y.; Yi, Y.L.; Lu, X. Purification, characterization and bioactivity of exopolysaccharides produced by Lactobacillus plantarum KX041. Int. J. Biol. Macromol. 2019, 128, 480–492. [Google Scholar] [CrossRef]
- Du, R.P.; Yu, L.S.; Sun, M.; Ye, G.B.; Yang, Y.; Zhou, B.S.; Qian, Z.G.; Ling, H.Z.; Ge, J.P. Characterization of dextran biosynthesized by glucansucrase from Leuconostoc pseudomesenteroides and their potential biotechnological applications. Antioxidants 2023, 12, 275. [Google Scholar] [CrossRef] [PubMed]
- Nehal, F.; Sahnoun, M.; Smaoui, S.; Jaouadi, B.; Bejar, S.; Mohammed, S. Characterization, high production and antimicrobial activity of exopolysaccharides from Lactococcus lactis F-mou. Microb. Pathogen. 2019, 132, 10–19. [Google Scholar] [CrossRef]



| Organic Agents | E24 (%) | E48 (%) |
|---|---|---|
| Hexane | 5.57 ± 0.28 | 6.18 ± 0.20 |
| Benzene | 12.30 ± 0.91 | 17.3 ± 0.57 |
| Petroleum ether | 15.65 ± 0.81 | 23.73 ± 1.18 |
| Gasoline | 15.97 ± 0.65 | 20.70 ± 0.86 |
| Soybean oil | 26.80 ± 0.78 | 36.84 ± 0.98 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, B.; Wang, C.; Yang, Y.; Yu, W.; Bin, X.; Song, G.; Du, R. Structural Characterization and Biological Properties Analysis of Exopolysaccharides Produced by Weisella cibaria HDL-4. Polymers 2024, 16, 2314. https://doi.org/10.3390/polym16162314
Zhou B, Wang C, Yang Y, Yu W, Bin X, Song G, Du R. Structural Characterization and Biological Properties Analysis of Exopolysaccharides Produced by Weisella cibaria HDL-4. Polymers. 2024; 16(16):2314. https://doi.org/10.3390/polym16162314
Chicago/Turabian StyleZhou, Bosen, Changli Wang, Yi Yang, Wenna Yu, Xiaoyun Bin, Gang Song, and Renpeng Du. 2024. "Structural Characterization and Biological Properties Analysis of Exopolysaccharides Produced by Weisella cibaria HDL-4" Polymers 16, no. 16: 2314. https://doi.org/10.3390/polym16162314
APA StyleZhou, B., Wang, C., Yang, Y., Yu, W., Bin, X., Song, G., & Du, R. (2024). Structural Characterization and Biological Properties Analysis of Exopolysaccharides Produced by Weisella cibaria HDL-4. Polymers, 16(16), 2314. https://doi.org/10.3390/polym16162314






