Polymers Containing Phenothiazine, Either as a Dopant or as Part of Their Structure, for Dye-Sensitized and Bulk Heterojunction Solar Cells
Abstract
1. Introduction
2. Phenothiazine Polymers in Dye-Sensitized Solar Cells
- Substrate: Metal oxide—usually titanium dioxide (TiO2)—nanoparticles are typically employed as semiconductor materials in fabricating photoanodes for DSSCs. Metal oxide nanoparticles in the form of a paste are deposited on a transparent conductive oxide (TCO) surface, such as an indium tin oxide (ITO) or a fluorine-doped tin oxide (FTO) conductive substrate, which is usually made of glass [33,34]. To prevent charge recombination phenomena, a compact layer, also called a blocking layer, is applied before depositing a titanium dioxide layer, which helps to increase the current produced by the dye-sensitized solar cell (cf. Figure 3).
- Photosensitizer: Conjugated donor–acceptor organic or organometallic dye molecules capable of absorbing solar light and producing excited electrons are adsorbed onto the metal oxide surface to prepare the photoanode. Ruthenium-based complexes and organic dyes without metals have all been employed as dyes for DSSCs [35].
- Electrolyte: An electrolyte is injected between the photoanode and the counter electrode, which helps to regenerate the oxidized dye by transferring the electrons to the HOMO of the dye and thus helps in regulating the whole conversion process of the cell. The electrolyte frequently contains iodide/triiodide ions (I−/I3−) or other redox species and can be in liquid, gel or solid form.
- Counter Electrode: The counter electrode is typically made of a conductive glass substrate on which a metal, mostly platinum or a semiconducting polymer like poly(3,4-ethylenedioxythiophene) (PEDOT), is deposited as a catalyst. The catalytic ability of the counter electrode helps in the reduction of electrolyte species. Besides PEDOT, other semiconducting polymers like polypyrrole (PPy), poly(3-hexylthiophene) (P3HT) and polyaniline (PANI) have also been used as counter electrodes in DSSCs.
2.1. Phenothiazine-Based Polymers Applied in Electrolytes in Dye-Sensitized Solar Cells
| Composition of Polymer | VOC (mV) | JSC (mAcm−2) | FF (-) | PCE (%) | Ref. |
|---|---|---|---|---|---|
| a P1/TBAI/I2/PMII/EC:PC(3:1) | 600 | 12.84 | 0.56 | 4.38 | [61] |
| P2/TBAI/I2/PMII/EC:PC(3:1) | 600 | 14.56 | 0.55 | 4.84 | |
| P3/TBAI/I2/PMII/EC:PC(3:1) | 600 | 14.25 | 0.62 | 5.30 | |
| PAN/TBAI/I2/PMII/EC:PC(3:1) | 600 | 12.32 | 0.55 | 4.05 | |
| PMMA/PVDF/KI/I2 | 550 | 2.50 | 0.48 | 1.40 | [63] |
| PMMA/PVDF/KI/I2/0.004 g PTZ | 820 | 5.80 | 0.50 | 4.80 | |
| PMMA/PVDF/KI/I2/0.009 g PTZ | 740 | 3.30 | 0.48 | 2.30 | |
| PMMA/PVDF/KI/I2/0.014 g PTZ | 720 | 3.00 | 0.48 | 2.10 | |
| PMMA/PVDF/KI/I2/0.019 g PTZ | 740 | 3.50 | 0.44 | 2.30 | |
| PMMA/PVDF/KI/I2/0.024 g PTZ | 750 | 3.60 | 0.42 | 2.20 | |
| PMMA/PVDF/KI/I2/0.029 g PTZ | 650 | 2.80 | 0.49 | 1.80 | |
| PMMA/PVDF/KI/I2/0.034 g PTZ | 760 | 3.90 | 0.40 | 2.40 | |
| PVDF-PEO/KI/I2 | 650 | 7.50 | 0.50 | 3.50 | [65] |
| PVDF-PEO/KI/I2 DPA | 790 | 10.20 | 0.52 | 6.00 | |
| PVDF-PEO/KI/I2 PTZ | 850 | 13.20 | 0.53 | 8.50 | |
| PVDF/KI/I2 | 587 ± 5.8 | 3.43 ± 0.20 | 0.42 | 1.42 | [66] |
| PTZ-PVDF/KI/I2 | 616 ± 4.7 | 5.00 ± 0.17 | 0.57 | 2.92 |
2.2. Phenothiazine-Based Polymers as Photosensitizers in Dye-Sensitized Solar Cells
2.2.1. Phenothiazine in the Main Chain
2.2.2. Phenothiazine in the Side Chain
3. Phenothiazine Polymers in Bulk Heterojunction Solar Cells
4. Summary and Conclusions: Challenges, Solutions and Future Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bagher, A.M.; Vahid, M.M.A.; Mohsen, M. Types of solar cells and application. Am. J. Opt. Photonics 2015, 3, 94–113. [Google Scholar] [CrossRef]
- Becquerel, H. Comptes Rendus Acad. CR Acad. Sci. 1896, 122, 420. [Google Scholar]
- Chapin, D.M.; Fuller, C.S.; Pearson, G.L. A new silicon p-n junction photocell for converting solar radiation into electrical power. J. Appl. Phys. 1954, 25, 676. [Google Scholar] [CrossRef]
- Płaczek-Popko, E. Top PV market solar cells 2016. Opto-Electron. Rev. 2017, 25, 55–64. [Google Scholar] [CrossRef]
- Borchers, A.M.; Duke, J.M.; Parsons, G.R. Does willingness to pay for green energy differ by source? Energy Policy 2007, 35, 3327–3334. [Google Scholar] [CrossRef]
- Calvin, M. Electrochemistry of excited molecules: Photo-electrochemical reactions of chlorophylls. Photochem. Photobiol. 1970, 14, 95–112. [Google Scholar]
- Sariciftci, N.S.; Smilowitz, L.; Heeger, A.J.; Wudl, F. Photoinduced electron transfer from a conducting polymer to buckminsterfullerene. Science 1992, 258, 1474–1476. [Google Scholar] [CrossRef]
- Ladomenou, K.; Nikolaou, V.; Charalambidis, G.; Coutsolelos, A.G. Artificial hemes for DSSC and/or BHJ applications. Dalton Trans. 2016, 45, 1111–1126. [Google Scholar] [CrossRef]
- Kishore, R.S.; Kel, O.; Banerji, N.; Emery, D.; Bollot, G.; Mareda, J.; Gomez-Casado, A.; Jonkheijm, P.; Huskens, J.; Maroni, P. Ordered and oriented supramolecular n/p-heterojunction surface architectures: Completion of the primary color collection. J. Am. Chem. Soc. 2009, 131, 11106–11116. [Google Scholar] [CrossRef] [PubMed]
- Koeppe, R.; Hoeglinger, D.; Troshin, P.A.; Lyubovskaya, R.N.; Razumov, V.F.; Sariciftci, N.S. Organic solar cells with semitransparent metal back contacts for power window applications. ChemSusChem Chem. Sustain. Energy Mater. 2009, 2, 309–313. [Google Scholar] [CrossRef]
- Kim, Y.-G.; Walker, J.; Samuelson, L.A.; Kumar, J. Efficient light harvesting polymers for nanocrystalline TiO2 photovoltaic cells. Nano Lett. 2003, 3, 523–525. [Google Scholar] [CrossRef]
- Huang, X.-M.; Chen, N.; Ye, D.-N.; Zhong, A.-G.; Liu, H.; Li, Z.; Liu, S.-Y. Structurally complementary star-shaped unfused ring electron acceptors with simultaneously enhanced device parameters for ternary organic solar cells. Sol. RRL 2023, 7, 2300143. [Google Scholar] [CrossRef]
- Ye, D.; Liu, L.; Peng, Q.; Qiu, J.; Gong, H.; Zhong, A.; Liu, S. Effect of controlling thiophene rings on DA polymer photocatalysts accessed via direct arylation for hydrogen production. Molecules 2023, 28, 4507. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Chen, S.; Zhong, A.; Sun, R.; Jin, J.; Yang, J.; Liu, D.; Niu, J.; Lu, S. Tuning photophysical properties via positional isomerization of the pyridine ring in donor–acceptor-structured aggregation-induced emission luminogens based on phenylmethylene pyridineacetonitrile derivatives. Molecules 2023, 28, 3282. [Google Scholar] [CrossRef]
- Yun, S.; Freitas, J.N.; Nogueira, A.F.; Wang, Y.; Ahmad, S.; Wang, Z.-S. Dye-sensitized solar cells employing polymers. Prog. Polym. Sci. 2016, 59, 1–40. [Google Scholar] [CrossRef]
- Liang, Y.; Yu, L. A new class of semiconducting polymers for bulk heterojunction solar cells with exceptionally high performance. Acc. Chem. Res. 2010, 43, 1227–1236. [Google Scholar] [CrossRef]
- Kang, H.; Lee, W.; Oh, J.; Kim, T.; Lee, C.; Kim, B.J. From fullerene–polymer to all-polymer solar cells: The importance of molecular packing, orientation, and morphology control. Acc. Chem. Res. 2016, 49, 2424–2434. [Google Scholar] [CrossRef]
- Blouin, N.; Michaud, A.; Gendron, D.; Wakim, S.; Blair, E.; Neagu-Plesu, R.; Belletete, M.; Durocher, G.; Tao, Y.; Leclerc, M. Toward a rational design of poly (2, 7-carbazole) derivatives for solar cells. J. Am. Chem. Soc. 2008, 130, 732–742. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Yang, L.; Price, S.C.; Knight, K.J.; You, W. Enhanced photovoltaic performance of low-bandgap polymers with deep LUMO levels. Angew. Chem. Int. Ed. 2010, 43, 7992–7995. [Google Scholar] [CrossRef]
- Ganesan, S.; Muthuraaman, B.; Mathew, V.; Vadivel, M.K.; Maruthamuthu, P.; Ashokkumar, M.; Suthanthiraraj, S.A. Influence of 2, 6 (N-pyrazolyl) isonicotinic acid on the photovoltaic properties of a dye-sensitized solar cell fabricated using poly (vinylidene fluoride) blended with poly (ethylene oxide) polymer electrolyte. Electrochim. Acta 2011, 56, 8811–8817. [Google Scholar] [CrossRef]
- Kusama, H.; Kurashige, M.; Arakawa, H. Influence of nitrogen-containing heterocyclic additives in I−/I3− redox electrolytic solution on the performance of Ru-dye-sensitized nanocrystalline TiO2 solar cell. J. Photochem. Photobiol. A Chem. 2005, 169, 169–176. [Google Scholar] [CrossRef]
- Ganesan, S.; Muthuraaman, B.; Madhavan, J.; Mathew, V.; Maruthamuthu, P.; Suthanthiraraj, S.A. The use of 2, 6-bis (N-pyrazolyl) pyridine as an efficient dopant in conjugation with poly (ethylene oxide) for nanocrystalline dye-sensitized solar cells. Electrochim. Acta 2008, 53, 7903–7907. [Google Scholar] [CrossRef]
- Naik, P.; Planchat, A.; Pellegrin, Y.; Odobel, F.; Adhikari, A.V. Exploring the application of new carbazole based dyes as effective p-type photosensitizers in dye-sensitized solar cells. Sol. Energy 2017, 157, 1064–1073. [Google Scholar] [CrossRef]
- Mozaffari, S.; Nateghi, M.R. Effects of multi anchoring groups of catecholamine polymer dyes on the electrical characteristics of metal free dye-sensitized solar cells: A comparison study. Sol. Energy 2014, 106, 63–71. [Google Scholar] [CrossRef]
- Jung, I.H.; Zhao, D.; Jang, J.; Chen, W.; Landry, E.S.; Lu, L.; Talapin, D.V.; Yu, L. Development and structure/property relationship of new electron accepting polymers based on thieno [2′, 3′: 4, 5] pyrido [2, 3-g] thieno [3, 2-c] quinoline-4, 10-dione for all-polymer solar cells. Chem. Mater. 2015, 27, 5941–5948. [Google Scholar] [CrossRef]
- Maglione, C.; Carella, A.; Centore, R.; Chávez, P.; Lévêque, P.; Fall, S.; Leclerc, N. Novel low bandgap phenothiazine functionalized DPP derivatives prepared by direct heteroarylation: Application in bulk heterojunction organic solar cells. Dye. Pigment. 2017, 141, 169–178. [Google Scholar] [CrossRef]
- Revoju, S.; Matuhina, A.; Canil, L.; Salonen, H.; Hiltunen, A.; Abate, A.; Vivo, P. Structure-induced optoelectronic properties of phenothiazine-based materials. J. Mater. Chem. C 2020, 8, 15486–15506. [Google Scholar] [CrossRef]
- Kim, G.; Yeom, H.R.; Cho, S.; Seo, J.H.; Kim, J.Y.; Yang, C. Easily attainable phenothiazine-based polymers for polymer solar cells: Advantage of insertion of S, S-dioxides into its polymer for inverted structure solar cells. Macromolecules 2012, 45, 1847–1857. [Google Scholar] [CrossRef]
- Buene, A.F.; Almenningen, D.M. Phenothiazine and phenoxazine sensitizers for dye-sensitized solar cells–an investigative review of two complete dye classes. J. Mater. Chem. C 2021, 9, 11974–11994. [Google Scholar] [CrossRef]
- Revoju, S.; Biswas, S.; Eliasson, B.; Sharma, G.D. Phenothiazine-based small molecules for bulk heterojunction organic solar cells; variation of side-chain polarity and length of conjugated system. Org. Electron. 2019, 65, 232–242. [Google Scholar] [CrossRef]
- Yang, L.; Li, L.; Wang, C.; Zhu, F.; Lu, Y.; Janietz, S.; Wedel, A.; Hua, Y.; Yin, S. Luminescence properties of PPV-based conjugated polymers containing phenothiazine and phenothiazine-5-oxide units. J. Lumin. 2007, 122, 714–716. [Google Scholar] [CrossRef]
- Meyer, G.J. The 2010 millennium technology grand prize: Dye-sensitized solar cells. ACS Nano 2010, 4, 4337–4343. [Google Scholar] [CrossRef] [PubMed]
- Pervez, A.; Javed, K.; Iqbal, Z.; Shahzad, M.; Khan, U.; Latif, H.; Shah, S.A.; Ahmad, N. Fabrication and comparison of dye-sensitized solar cells by using TiO2 and ZnO as photo electrode. Optik 2019, 182, 175–180. [Google Scholar] [CrossRef]
- Yu, Z.; Perera, I.R.; Daeneke, T.; Makuta, S.; Tachibana, Y.; Jasieniak, J.J.; Mishra, A.; Bäuerle, P.; Spiccia, L.; Bach, U. Indium tin oxide as a semiconductor material in efficient p-type dye-sensitized solar cells. NPG Asia Mater. 2016, 8, e305. [Google Scholar] [CrossRef]
- Wu, C.G. Ruthenium-based complex dyes for dye-sensitized solar cells. J. Chin. Chem. Soc. 2022, 69, 1242–1252. [Google Scholar] [CrossRef]
- Xu, Z.; Li, T.; Liu, Q.; Zhang, F.; Hong, X.; Xie, S.; Lin, C.; Liu, X.; Guo, W. Controllable and large-scale fabrication of rectangular CuS network films for indium tin oxide-and Pt-free flexible dye-sensitized solar cells. Sol. Energy Mater. Sol. Cells 2018, 179, 297–304. [Google Scholar] [CrossRef]
- Munusami, V.; Arutselvan, K.; Vadivel, S. Dye-sensitized solar cells (DSSCs) as a potential photovoltaic technology based on La2MoO6/bio-carbon hybrid composite photoanodes with~12.5% efficiency. Surf. Interfaces 2021, 22, 100844. [Google Scholar] [CrossRef]
- Kakiage, K.; Aoyama, Y.; Yano, T.; Oya, K.; Fujisawa, J.-i.; Hanaya, M. Highly-efficient dye-sensitized solar cells with collaborative sensitization by silyl-anchor and carboxy-anchor dyes. Chem. Commun. 2015, 51, 15894–15897. [Google Scholar] [CrossRef] [PubMed]
- Venkatesan, S.; My, N.H.T.; Teng, H.; Lee, Y.-L. Thin films of solid-state polymer electrolytes for dye-sensitized solar cells. J. Power Sources 2023, 564, 232896. [Google Scholar] [CrossRef]
- Kurokawa, Y.; Kato, T.; Pandey, S.S. Controlling the electrocatalytic activities of conducting polymer thin films toward suitability as cost-effective counter electrodes of dye-sensitized solar cells. Synth. Met. 2023, 296, 117362. [Google Scholar] [CrossRef]
- Kwon, J.; Ganapathy, V.; Kim, Y.H.; Song, K.-D.; Park, H.-G.; Jun, Y.; Yoo, P.J.; Park, J.H. Nanopatterned conductive polymer films as a Pt, TCO-free counter electrode for low-cost dye-sensitized solar cells. Nanoscale 2013, 5, 7838–7843. [Google Scholar] [CrossRef]
- Thokala, S.; Singh, S.P. Phenothiazine-based hole transport materials for perovskite solar cells. ACS Omega 2020, 5, 5608–5619. [Google Scholar] [CrossRef]
- Olivier, Y.; Niedzialek, D.; Lemaur, V.; Pisula, W.; Müllen, K.; Koldemir, U.; Reynolds, J.R.; Lazzaroni, R.; Cornil, J.; Beljonne, D. 25th anniversary article: High-mobility hole and electron transport conjugated polymers: How structure defines function. Adv. Mater. 2014, 26, 2119–2136. [Google Scholar] [CrossRef]
- Andreani, L.C.; Bozzola, A.; Kowalczewski, P.; Liscidini, M.; Redorici, L. Silicon solar cells: Toward the efficiency limits. Adv. Phys. X 2019, 4, 1548305. [Google Scholar] [CrossRef]
- Tan, H.; Pan, C.; Wang, G.; Wu, Y.; Zhang, Y.; Chen, X.; Zou, Y.; Yu, G.; Zhang, M. Synthesis and characterization of conjugated polymers with main-chain donors and pendent acceptors for dye-sensitized solar cells. RSC Adv. 2013, 3, 16612–16618. [Google Scholar] [CrossRef]
- Amasawa, E.; Sasagawa, N.; Kimura, M.; Taya, M. Design of a new energy-harvesting electrochromic window based on an organic polymeric dye, a cobalt couple, and PProDOT-Me2. Adv. Energy Mater. 2014, 4, 1400379. [Google Scholar] [CrossRef]
- Wang, G.; Wu, Y.; Ding, W.; Yu, G.; Hu, Z.; Wang, H.; Liu, S.; Zou, Y.; Pan, C. Photovoltaic performance of long-chain poly (triphenylamine-phenothiazine) dyes with a tunable π-bridge for dye-sensitized solar cells. J. Mater. Chem. A 2015, 3, 14217–14227. [Google Scholar] [CrossRef]
- Wang, H.; Ding, W.; Wang, G.; Pan, C.; Duan, M.; Yu, G. Tunable molecular weights of poly (triphenylamine-2, 2′-bithiophene) and their effects on photovoltaic performance as sensitizers for dye-sensitized solar cells. J. Appl. Polym. Sci. 2016, 47, 133. [Google Scholar] [CrossRef]
- Ramasamy, S.; Boopathy, M.; Johnsanthoshkumar, S.; Subramanian, K. Structural engineering of poly-(methacrylate) bearing push-pull type pendants oxindole-phenothiazine with tetrazole anchoring acceptor for efficient organic photovoltaic cells. Polymer 2017, 115, 128–136. [Google Scholar] [CrossRef]
- Xiong, S.; Wang, Y.; Lin, J.; Yu, X.; Tao, J.; Wu, Y.; Yu, G.; Pan, C.; Yamauchi, Y. D-π-A conjugated polymer dyes-covered TiO2 compact layers for enhancing photovoltaic performance of dye-sensitized solar cells. Synth. Met. 2018, 244, 73–79. [Google Scholar] [CrossRef]
- Wang, G.; Liu, Z.; Wang, X.; Liu, J.; Chen, Y.; Liu, B. Electrochemical Polymerization-Fabricated Several Triphenylamine–Carbazolyl-Based Polymers with Improved Short-Circuit Current and High Adsorption Stability in Dye-Sensitized Solar Cells. ACS Omega 2019, 4, 15215–15225. [Google Scholar] [CrossRef] [PubMed]
- Giri, D.; Raut, S.K.; Patra, S.K. Diketopyrrolopyrrole/perylene-diimide and thiophene based D-π-A low bandgap polymer sensitizers for application in dye sensitized solar cells. Dye. Pigment. 2020, 174, 108032. [Google Scholar] [CrossRef]
- Chinnasamy, S.; Shanmugam, M.; Ramanathan, S. Novel Metal-Organic Polymer [Ruthenium Bis (II)(2, 2′–Bipyridyl 4, 4′− Dicarboxylic Acid)(N-Methyl morpholine)] n (BF 4) 2n for Dye-Sensitized Solar Cell Application. In Advances in Materials Research: Select Proceedings of ICAMR 2019; Springer: Berlin/Heidelberg, Germany, 2021; pp. 1223–1231. [Google Scholar]
- Tian, Y.; Wang, K.; Zhang, H.; Wu, X.; Zhong, C. Novel polymeric metal complexes of salicylaldehyde schiff base derivative being used for dye sensitizer. Tetrahedron 2022, 113, 132756. [Google Scholar] [CrossRef]
- Parwati, K.; Tiwari, R.; Verma, D.K.; Kumar, D.; Yadav, S.; Rai, R.; Singh, S.; Srivastava, P.; Krishnamoorthi, S. Ionic Liquid-Supported Vat Dye (Congo Red)-Based Donor–Acceptor Type Conjugated Polymeric Material as Photosensitizer for Dye-Sensitized Solar Cells. Energy Technol. 2024, 12, 2300598. [Google Scholar] [CrossRef]
- Boschloo, G.; Hagfeldt, A. Characteristics of the iodide/triiodide redox mediator in dye-sensitized solar cells. Acc. Chem. Res. 2009, 42, 1819–1826. [Google Scholar] [CrossRef] [PubMed]
- Farhana, N.K.; Saidi, N.M.; Bashir, S.; Ramesh, S.; Ramesh, K. Review on the revolution of polymer electrolytes for dye-sensitized solar cells. Energy Fuels 2021, 35, 19320–19350. [Google Scholar] [CrossRef]
- Cui, Y.; Zhang, X.; Feng, J.; Zhang, J.; Zhu, Y. Enhanced photovoltaic performance of quasi-solid-state dye-sensitized solar cells by incorporating a quaternized ammonium salt into poly (ethylene oxide)/poly (vinylidene fluoride-hexafluoropropylene) composite polymer electrolyte. Electrochim. Acta 2013, 108, 757–762. [Google Scholar] [CrossRef]
- Shanmukaraj, D.; Wang, G.; Murugan, R.; Liu, H.-K. Ionic conductivity and electrochemical stability of poly (methylmethacrylate)–poly (ethylene oxide) blend-ceramic fillers composites. J. Phys. Chem. Solids 2008, 69, 243–248. [Google Scholar] [CrossRef]
- Li, Y.; Li, X.; Guo, H.; Wang, Z.; Li, T. Effect on properties of PVDF-HFP based composite polymer electrolyte doped with nano-SiO2. Iran. Polym. J. 2014, 23, 487–494. [Google Scholar] [CrossRef]
- Song, M.; Park, J.S.; Kim, Y.H.; Karim, M.A.; Jin, S.-H.; Ree, R.S.; Cho, Y.R.; Gal, Y.-S.; Lee, J.W. Synthesis and characterization of polymer electrolytes containing phenothiazine-based click polymers for dye-sensitized solar cell applications. Macromol. Res. 2011, 19, 654–659. [Google Scholar] [CrossRef]
- Kusama, H.; Arakawa, H. Influence of alkylaminopyridine additives in electrolytes on dye-sensitized solar cell performance. Sol. Energy Mater. Sol. Cells 2004, 81, 87–99. [Google Scholar] [CrossRef]
- Amudha, S.; SUTHANTHIRARAJ, S.A.; Maruthamuthu, P. A Novel Solid State Dye-Sensitized Solar Cell Containing PMMA/PVDF-Type Blended Polymer Electrolyte. Chem. Sci. Trans. 2013, 2, 955–963. [Google Scholar]
- Rajendran, S.; Mahendran, O.; Mahalingam, T. Thermal and ionic conductivity studies of plasticized PMMA/PVdF blend polymer electrolytes. Eur. Polym. J. 2002, 38, 49–55. [Google Scholar] [CrossRef]
- Ganesan, S.; Mathew, V.; Paul, B.J.; Maruthamuthu, P.; Suthanthiraraj, S.A. Influence of organic nitrogenous compounds phenothiazine and diphenyl amine in poly (vinylidene fluoride) blended with poly (ethylene oxide) polymer electrolyte in dye-sensitized solar cells. Electrochim. Acta 2013, 102, 219–224. [Google Scholar] [CrossRef]
- Senthil, R.; Theerthagiri, J.; Madhavan, J. Organic dopant added polyvinylidene fluoride based solid polymer electrolytes for dye-sensitized solar cells. J. Phys. Chem. Solids 2016, 89, 78–83. [Google Scholar] [CrossRef]
- Giri, H.; Ma, G.; Almtiri, M.; Gu, X.; Scott, C.N. Side Chain Effects on the Conductivity of Phenothiazine-Derived Polyaniline. Chem. Mater. 2024, 36, 2279–2288. [Google Scholar] [CrossRef]
- Fang, Z.; Eshbaugh, A.A.; Schanze, K.S. Low-bandgap donor− acceptor conjugated polymer sensitizers for dye-sensitized solar cells. J. Am. Chem. Soc. 2011, 133, 3063–3069. [Google Scholar] [CrossRef] [PubMed]
- Xie, Q.; Zhou, J.; Hu, J.; Peng, D.; Liu, Y.; Liao, Y.; Zhu, C.; Zhong, C. Synthesis and Photovoltaic properties of branched chain polymeric metal complexes containing Phenothiazine and Thiophene derivative for dye-sensitized solar cells. J. Chem. Sci. 2015, 127, 395–403. [Google Scholar] [CrossRef]
- Chen, X.; Liu, Y.; Xie, Q.; Zhou, J.; Liao, Y.; Zhu, C.; Chen, T.; Zhong, C. Synthesis and characterization of stable main-chain polymeric metal complex dyes based on phenothiazine or carbazole units for dye-sensitized solar cells. Polym. J. 2016, 48, 813–819. [Google Scholar] [CrossRef]
- Arslan, B.S.; Ülüş, S.N.; Gezgin, M.; Arkan, B.; Güzel, E.; Avcı, D.; Nebioğlu, M.; Şişman, İ. Insight into the effects of the donors and pi-spacers on the photovoltaic performance of quinoline and pyridocarbazole based DSSCs. Opt. Mater. 2020, 106, 109974. [Google Scholar] [CrossRef]
- Prakash, G.; Subramanian, K. Interaction of pyridine π-bridge-based poly (methacrylate) dyes for the fabrication of dye-sensitized solar cells with the influence of different strength phenothiazine, fluorene and anthracene sensitizers as donor units with new anchoring mode. New J. Chem. 2018, 42, 17939–17949. [Google Scholar] [CrossRef]
- Meng, L.; Kim, D.; Yang, E.; Suh, H.; Park, S.H. Automatic formation of electron transport layer in BHJ solar cells using phenothiazine-based conjugated small molecular electrolytes. J. Mater. Chem. C 2022, 10, 15137–15144. [Google Scholar] [CrossRef]
- Badgurjar, D.; Duvva, N.; Bagui, A.; Pooja; Gahlot, S.; Pawar, R.; Singh, S.P.; Garg, A.; Giribabu, L.; Chitta, R. Phenothiazine functionalized fulleropyrrolidines: Synthesis, charge transport and applications to organic solar cells. Photochem. Photobiol. Sci. 2023, 22, 379–393. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; Kim, S.H.; Lee, H.H.; Lee, K.; Ma, W.; Gong, X.; Heeger, A.J. New architecture for high-efficiency polymer photovoltaic cells using solution-based titanium oxide as an optical spacer. Adv. Mater. 2006, 18, 572–576. [Google Scholar] [CrossRef]
- Kitamura, C.; Tanaka, S.; Yamashita, Y. Design of narrow-bandgap polymers. Syntheses and properties of monomers and polymers containing aromatic-donor and o-quinoid-acceptor units. Chem. Mater. 1996, 8, 570–578. [Google Scholar] [CrossRef]
- Zhu, Z.; Waller, D.; Gaudiana, R.; Morana, M.; Mühlbacher, D.; Scharber, M.; Brabec, C. Panchromatic conjugated polymers containing alternating donor/acceptor units for photovoltaic applications. Macromolecules 2007, 40, 1981–1986. [Google Scholar] [CrossRef]
- Zhu, Y.; Champion, R.D.; Jenekhe, S.A. Conjugated donor− acceptor copolymer semiconductors with large intramolecular charge transfer: Synthesis, optical properties, electrochemistry, and field effect carrier mobility of thienopyrazine-based copolymers. Macromolecules 2006, 39, 8712–8719. [Google Scholar] [CrossRef]
- Turkarslan, O.; Ak, M.; Tanyeli, C.; Akhmedov, I.M.; Toppare, L. Enhancing electrochromic properties of conducting polymers via copolymerization: Copolymer of 1-(4-fluorophenyl)-2, 5-di (thiophen-2-yl)-1H-pyrrole with 3, 4-ethylene dioxythiophene. J. Polym. Sci. Part A Polym. Chem. 2007, 45, 4496–4503. [Google Scholar] [CrossRef]
- Shi, C.; Yao, Y.; Yang; Pei, Q. Regioregular copolymers of 3-alkoxythiophene and their photovoltaic application. J. Am. Chem. Soc. 2006, 128, 8980–8986. [Google Scholar] [CrossRef]
- Wong, M.; Wong, K. Investigation of the factors affecting the power conversion efficiency of all-solution-processed ‘bilayer’P3HT: PCBM solar cells. Synth. Met. 2013, 170, 1–6. [Google Scholar] [CrossRef]
- Liu, F.; Chen, D.; Wang, C.; Luo, K.; Gu, W.; Briseno, A.L.; Hsu, J.W.; Russell, T.P. Molecular weight dependence of the morphology in P3HT: PCBM solar cells. ACS Appl. Mater. Interfaces 2014, 6, 19876–19887. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Pröller, S.; Niedermeier, M.A.; Körstgens, V.; Philipp, M.; Su, B.; Moseguí González, D.; Yu, S.; Roth, S.V.; Müller-Buschbaum, P. Development of the morphology during functional stack build-up of P3HT: PCBM bulk heterojunction solar cells with inverted geometry. ACS Appl. Mater. Interfaces 2015, 7, 602–610. [Google Scholar] [CrossRef]
- Kadem, B.; Hassan, A.; Cranton, W. Efficient P3HT: PCBM bulk heterojunction organic solar cells; effect of post deposition thermal treatment. J. Mater. Sci. Mater. Electron. 2016, 27, 7038–7048. [Google Scholar] [CrossRef]
- Xiao, B.; Tang, A.; Zhang, J.; Mahmood, A.; Wei, Z.; Zhou, E. Achievement of high Voc of 1.02 V for P3HT-based organic solar cell using a benzotriazole-containing non-fullerene acceptor. Adv. Energy Mater. 2017, 7, 1602269. [Google Scholar] [CrossRef]
- Sartorio, C.; Campisciano, V.; Chiappara, C.; Cataldo, S.; Scopelliti, M.; Gruttadauria, M.; Giacalone, F.; Pignataro, B. Enhanced power-conversion efficiency in organic solar cells incorporating copolymeric phase-separation modulators. J. Mater. Chem. A 2018, 6, 3884–3894. [Google Scholar] [CrossRef]
- Ghosekar, I.C.; Patil, G.C. Thermal stability analysis of buffered layer P3HT/P3HT: PCBM organic solar cells. IET Optoelectron. 2019, 13, 240–246. [Google Scholar] [CrossRef]
- Uddin, S.I.; Tahir, M.; Aziz, F.; Sarker, M.R.; Muhammad, F.; Nawaz Khan, D.; Hamid Md Ali, S. Thickness optimization and photovoltaic properties of bulk heterojunction solar cells based on PFB–PCBM layer. Energies 2020, 13, 5915. [Google Scholar] [CrossRef]
- Sen, S.; Islam, R. Effect of different layers on the performance of P3HT: PCBM-based organic solar cell. Braz. J. Phys. 2021, 51, 1661–1669. [Google Scholar] [CrossRef]
- Li, K.C.; Hsu, Y.C.; Lin, J.T.s.; Yang, C.C.; Wei, K.H.; Lin, H.C. Novel narrow-band-gap conjugated copolymers containing phenothiazine-arylcyanovinyl units for organic photovoltaic cell applications. J. Polym. Sci. Part A Polym. Chem. 2008, 46, 4285–4304. [Google Scholar] [CrossRef]
- Cho, N.S.; Park, J.-H.; Lee, S.-K.; Lee, J.; Shim, H.-K.; Park, M.-J.; Hwang, D.-H.; Jung, B.-J. Saturated and efficient red light-emitting fluorene-based alternating polymers containing phenothiazine derivatives. Macromolecules 2006, 39, 177–183. [Google Scholar] [CrossRef]
- Padhy, H.; Huang, J.H.; Sahu, D.; Patra, D.; Kekuda, D.; Chu, C.W.; Lin, H.C. Synthesis and applications of low-bandgap conjugated polymers containing phenothiazine donor and various benzodiazole acceptors for polymer solar cells. J. Polym. Sci. Part A: Polym. Chem. 2010, 48, 4823–4834. [Google Scholar] [CrossRef]
- Lyu, F.; Park, H.; Lee, S.-H.; Lee, Y.-S. Synthesis and characterization of phenothiazine-isoindigo copolymers for photovoltaic applications. Bull. Korean Chem. Soc. 2014, 35, 1875–1878. [Google Scholar] [CrossRef]
- Sang, G.; Zou, Y.; Li, Y. Two polythiophene derivatives containing phenothiazine units: Synthesis and photovoltaic properties. J. Phys. Chem. C 2008, 112, 12058–12064. [Google Scholar] [CrossRef]
- Sang, G.; Zou, Y.; Huang, Y.; Zhao, G.; Yang, Y.; Li, Y. All-polymer solar cells based on a blend of poly [3-(10-n-octyl-3-phenothiazine-vinylene) thiophene-co-2, 5-thiophene] and poly [1, 4-dioctyloxyl-p-2, 5-dicyanophenylenevinylene]. Appl. Phys. Lett. 2009, 94, 193302. [Google Scholar] [CrossRef]
- Wong, W.-Y.; Chow, W.-C.; Cheung, K.-Y.; Fung, M.-K.; Djurišić, A.B.; Chan, W.-K. Harvesting solar energy using conjugated metallopolyyne donors containing electron-rich phenothiazine–oligothiophene moieties. J. Organomet. Chem. 2009, 694, 2717–2726. [Google Scholar] [CrossRef]
- Jo, M.Y.; Do, T.T.; Ha, Y.E.; Won, Y.S.; Kim, J.H. Enhanced efficiency in polymer solar cells by incorporation of phenothiazine-based conjugated polymer electrolytes. Org. Electron. 2015, 16, 18–25. [Google Scholar] [CrossRef]
- Wessling, R.; Delgado Andrés, R.; Morhenn, I.; Acker, P.; Maftuhin, W.; Walter, M.; Würfel, U.; Esser, B. Phenothiazine-Based Donor–Acceptor Polymers as Multifunctional Materials for Charge Storage and Solar Energy Conversion. Macromol. Rapid Commun. 2022, 45, 2200699. [Google Scholar] [CrossRef] [PubMed]
- Harvey, P.D.; Fortin, D. Photoproperties of the polymeric {[M (dmb) 2] Y]} n materials: Photoinduced intrachain energy and intermolecular electron transfers, and design of photovoltaic cells. Coord. Chem. Rev. 1998, 171, 351–354. [Google Scholar] [CrossRef]
- Wong, W.Y. Metallopolyyne polymers as new functional materials for photovoltaic and solar cell applications. Macromol. Chem. Phys. 2008, 209, 14–24. [Google Scholar] [CrossRef]
- Gnida, P.; Amin, M.F.; Pająk, A.K.; Jarząbek, B. Polymers in High-Efficiency Solar Cells: The Latest Reports. Polymers 2022, 14, 1946. [Google Scholar] [CrossRef]
- Hau, S.K.; Yip, H.-L.; Leong, K.; Jen, A.K.-Y. Spraycoating of silver nanoparticle electrodes for inverted polymer solar cells. Org. Electron. 2009, 10, 719–723. [Google Scholar] [CrossRef]
- Al-Busaidi, I.J.; Haque, A.; Al Rasbi, N.K.; Khan, M.S. Phenothiazine-based derivatives for optoelectronic applications: A review. Synth. Met. 2019, 257, 116189. [Google Scholar] [CrossRef]
- Seo, Y.H.; Lee, W.H.; Park, J.H.; Bae, C.; Hong, Y.; Park, J.W.; Kang, I.N. Side-chain effects on phenothiazine-based donor–acceptor copolymer properties in organic photovoltaic devices. J. Polym. Sci. Part A Polym. Chem. 2012, 50, 649–658. [Google Scholar] [CrossRef]





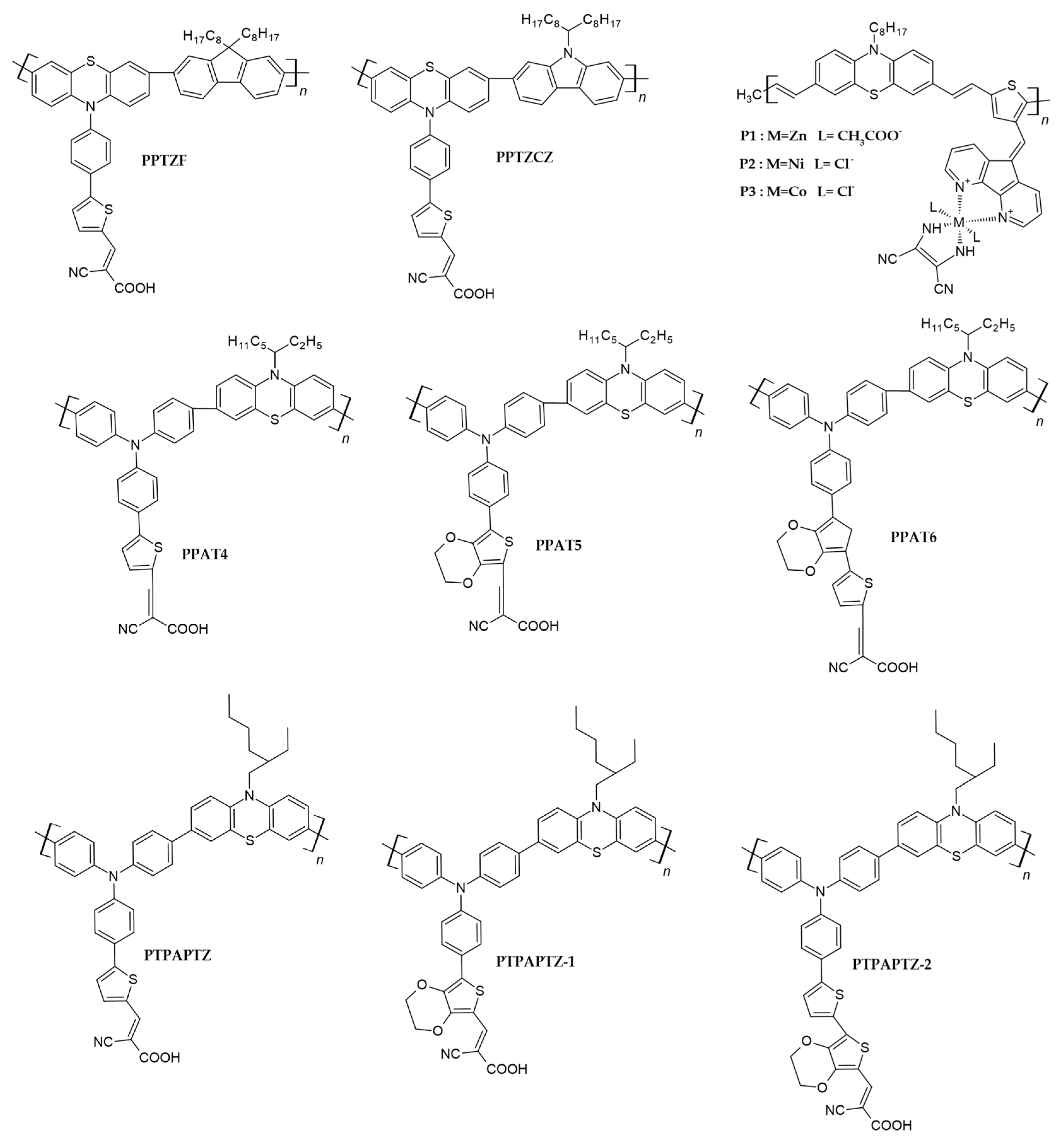







| Polymer | VOC (mV) | JSC (mAcm−2) | FF (-) | PCE (%) | Ref. |
|---|---|---|---|---|---|
| a PPTZF | 742 | 5.30 | 0.77 | 3.0 | [45] |
| PPTZCZ | 775 | 6.06 | 0.75 | 3.5 | |
| PTPACZ | 769 | 8.12 | 0.71 | 4.4 | |
| b P1 | 620 | 4.12 | 0.61 | 1.57 | [69] |
| P2 | 570 | 3.64 | 0.63 | 1.31 | |
| P3 | 540 | 3.77 | 0.60 | 1.22 | |
| b P1 | 640 | 4.30 | 0.68 | 1.88 | [70] |
| P2 | 610 | 4.21 | 0.66 | 1.70 | |
| a PAT | 660 | 6.91 | 0.66 | 3.0 | [47] |
| PPAT4 | 720 | 10.87 | 0.60 | 4.7 | |
| PPAT5 | 700 | 8.05 | 0.65 | 3.7 | |
| PPAT6 | 650 | 9.80 | 0.64 | 4.1 | |
| a PTPAPTZ | 650 | 9.80 | 0.75 | 4.08 | [50] |
| PTPAPTZ-1 | 700 | 8.05 | 0.82 | 3.66 | |
| PTPAPTZ-2 | 720 | 10.87 | 0.78 | 4.71 |
| Polymer | VOC [mV] | JSC [mAcm−2] | FF | PCE [%] | Ref. |
|---|---|---|---|---|---|
| a POTZP1 | 740 ± 0.03 | 5.90 ± 0.04 | 0.68 ± 0.02 | 3.97 ± 0.03 | [49] |
| POTZP2 | 780 ± 0.01 | 8.83 ± 0.02 | 0.61 ± 0.00 | 4.42 ± 0.01 | |
| POTZP3 | 850 ± 0.01 | 11.45 ±0.01 | 0.74 ± 0.02 | 5.91 ± 0.01 | |
| b PPNPP | 710 | 4.35 | 0.68 | 2.18 | [72] |
| * PPNPP | 760 | 7.23 | 0.69 | 4.12 |
| Device Architecture | VOC (mV) | JSC (mAcm−2) | FF (-) | PCE (%) | Ref. |
|---|---|---|---|---|---|
| ITO/PEDOT-PSS/PF−PZB50(1):PCBM(3)/LiF/Al | 780 | 2.38 | 0.29 | 0.53 | [91] |
| ITO/PEDOT:PSS/P2(1):PCBM(4)/LiF/Al | 430 | 1.86 | 0.27 | 0.22 | [90] |
| ITO/PEDOT:PSS/P6(1):PCBM(4)/LiF/Al | 520 | 1.46 | 0.22 | 0.17 | |
| ITO/PEDOT:PSS/P8(1):PCBM(4)/LiF/Al | 270 | 2.21 | 0.27 | 0.16 | |
| ITO/PEDOT:PSS/P10(1):PCBM(4)/LiF/Al | 530 | 1.30 | 0.26 | 0.18 | |
| ITO/PEDOT:PSS/P12(1):PCBM(1)/LiF/Al | 550 | 2.10 | 0.25 | 0.29 | |
| ITO/PEDOT:PSS/P12(1)PCBM(2)/LiF/Al | 560 | 2.30 | 0.28 | 0.36 | |
| ITO/PEDOT:PSS/P12(1):PCBM(4)/LiF/Al | 640 | 2.70 | 0.29 | 0.51 | |
| ITO/PEDOT:PSS/PP6DHTBT(1):PC61BM(1)/Ca/Al | 670 | 1.92 | 0.32 | 0.41 | [92] |
| ITO/PEDOT:PSS/PP6DHTBSe(1):PC61BM(1)/Ca/Al | 650 | 1.43 | 0.30 | 0.28 | |
| ITO/PEDOT:PSS/PP6DHTBX(1):PC61BM(1)/Ca/Al | 690 | 1.24 | 0.29 | 0.25 | |
| ITO/PEDOT:PSS/PP6DHTBT(1):PC71BM(1)/Ca/Al | 730 | 2.95 | 0.34 | 0.74 | |
| ITO/PEDOT:PSS/PP6DHTBT(1):PC71BM(3)/Ca/Al | 730 | 3.80 | 0.33 | 0.88 | |
| ITO/PEDOT:PSS/PP6DHTBT(1):PC71BM(4)/Ca/Al | 750 | 4.60 | 0.35 | 1.20 | |
| ITO/PEDOT:PSS/PPTDTBT(1):PC71BM(2)/Al | 770 | 5.75 | 0.38 | 1.69 | [28] |
| ITO/PEDOT:PSS/PPTDTBT-SS(1):PC71BM(1.5)/Al | 810 | 4.03 | 0.30 | 0.97 | |
| ITO/PEDOT:PSS/PPT-II(1):PC71BM(1)/LiF/Al | 790 | 0.47 | 0.24 | 0.09 | [93] |
| ITO/PEDOT:PSS/PPT-II(1):PC71BM(2)/LiF/Al | 650 | 1.9 | 0.26 | 0.33 | |
| ITO/PEDOT:PSS/PPT-II(1):PC71BM(3)/LiF/Al | 650 | 2.4 | 0.26 | 0.40 | |
| ITO/PEDOT:PSS/PPT-II(1):PC71BM(4)/LiF/Al | 640 | 2.5 | 0.27 | 0.50 | |
| a ITO/PEDOT:PSS/PPT-II(1):PC71BM(4)/LiF/Al | 660 | 3.3 | 0.26 | 0.58 | |
| b ITO/PEDOT:PSS/PPT-II(1):PC71BM(4)/LiF/Al | 680 | 3.6 | 0.26 | 0.64 | |
| c ITO/PEDOT:PSS/PPT-II(1):PC71BM(4)/LiF/Al | 700 | 3.9 | 0.28 | 0.74 | |
| ITO/PEDOT:PSS/POPTZ-PT(1):PCBM(1)/Au | 700 | 0.38 | 0.32 | 0.09 | [94] |
| ITO/PEDOT:PSS/PTZV-PT(1):PCBM(1)/Au | 730 | 4.03 | 0.34 | 1.0 | |
| ITO/PEDOT:PSS/PTZV-PT(1):DOCN-PPV(1)/LiF/Al | 850 | 3.14 | 0.28 | 0.80 | [95] |
| ITO/PEDOT:PSS/P1(1):PCBM(4)/Al | 630 | 2.73 | 0.32 | 0.55 | [96] |
| ITO/PEDOT:PSS/P1(1):PCBM(5)/Al | 780 | 3.71 | 0.37 | 1.06 | |
| ITO/PEDOT:PSS/P2(1):PCBM(4)/Al | 730 | 3.95 | 0.30 | 0.86 | |
| ITO/PEDOT:PSS/P2(1):PCBM(5)/Al | 790 | 4.06 | 0.41 | 1.29 | |
| ITO/PEDOT/P3HT:PCBM/PHPT/Al | 610 | 7.41 | 0.59 | 2.69 | [97] |
| ITO/PEDOT/P3HT:PCBM/PcoPT/Al | 610 | 8.02 | 0.60 | 2.96 |
| Device Architecture | VOC (mV) | JSC (mAcm−2) | FF (-) | PCE (%) | Ref. |
|---|---|---|---|---|---|
| ITO/PEDOT:PSS/PPTDTBT(1):PC71BM(2)/Al | 780 | 4.80 | 0.39 | 1.47 | [28] |
| ITO/PEDOT:PSS/PPTDTBT-SS(1):PC71BM(1.5)/Al | 920 | 4.11 | 0.32 | 1.22 | |
| ITO/ZnO/P(PT-T2)(1)/PC61BM(1.5)/MoOx/Ag | 788 | 0.54 | 0.32 | 0.14 | [98] |
| ITO/ZnO/P(PT-DPP)b(1)/PC61BM(1.5)MoOx/Ag | 677 | 1.73 | 0.35 | 0.40 | |
| ITO/ZnO/P(PT-DPP)c(1)/PC61BM(1)MoOx/Ag | 497 | 4.57 | 0.39 | 0.89 | |
| ITO/ZnO/P(PT-DPP)d(1)/PC61BM(3)MoOx/Ag | 757 | 6.12 | 0.40 | 1.87 | |
| ITO/ZnO/P(PT-BTZ)(1)/PC61BM(1)MoOx/Ag | 675 | 1.80 | 0.29 | 0.36 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amin, M.F.; Anwar, A.; Gnida, P.; Jarząbek, B. Polymers Containing Phenothiazine, Either as a Dopant or as Part of Their Structure, for Dye-Sensitized and Bulk Heterojunction Solar Cells. Polymers 2024, 16, 2309. https://doi.org/10.3390/polym16162309
Amin MF, Anwar A, Gnida P, Jarząbek B. Polymers Containing Phenothiazine, Either as a Dopant or as Part of Their Structure, for Dye-Sensitized and Bulk Heterojunction Solar Cells. Polymers. 2024; 16(16):2309. https://doi.org/10.3390/polym16162309
Chicago/Turabian StyleAmin, Muhammad Faisal, Amna Anwar, Paweł Gnida, and Bożena Jarząbek. 2024. "Polymers Containing Phenothiazine, Either as a Dopant or as Part of Their Structure, for Dye-Sensitized and Bulk Heterojunction Solar Cells" Polymers 16, no. 16: 2309. https://doi.org/10.3390/polym16162309
APA StyleAmin, M. F., Anwar, A., Gnida, P., & Jarząbek, B. (2024). Polymers Containing Phenothiazine, Either as a Dopant or as Part of Their Structure, for Dye-Sensitized and Bulk Heterojunction Solar Cells. Polymers, 16(16), 2309. https://doi.org/10.3390/polym16162309







