Exploring the Processing Potential of Polylactic Acid, Polyhydroxyalkanoate, and Poly(butylene succinate-co-adipate) Binary and Ternary Blends
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Blend Preparation
2.3. Sample Preparation
2.4. Testing Methods
3. Results
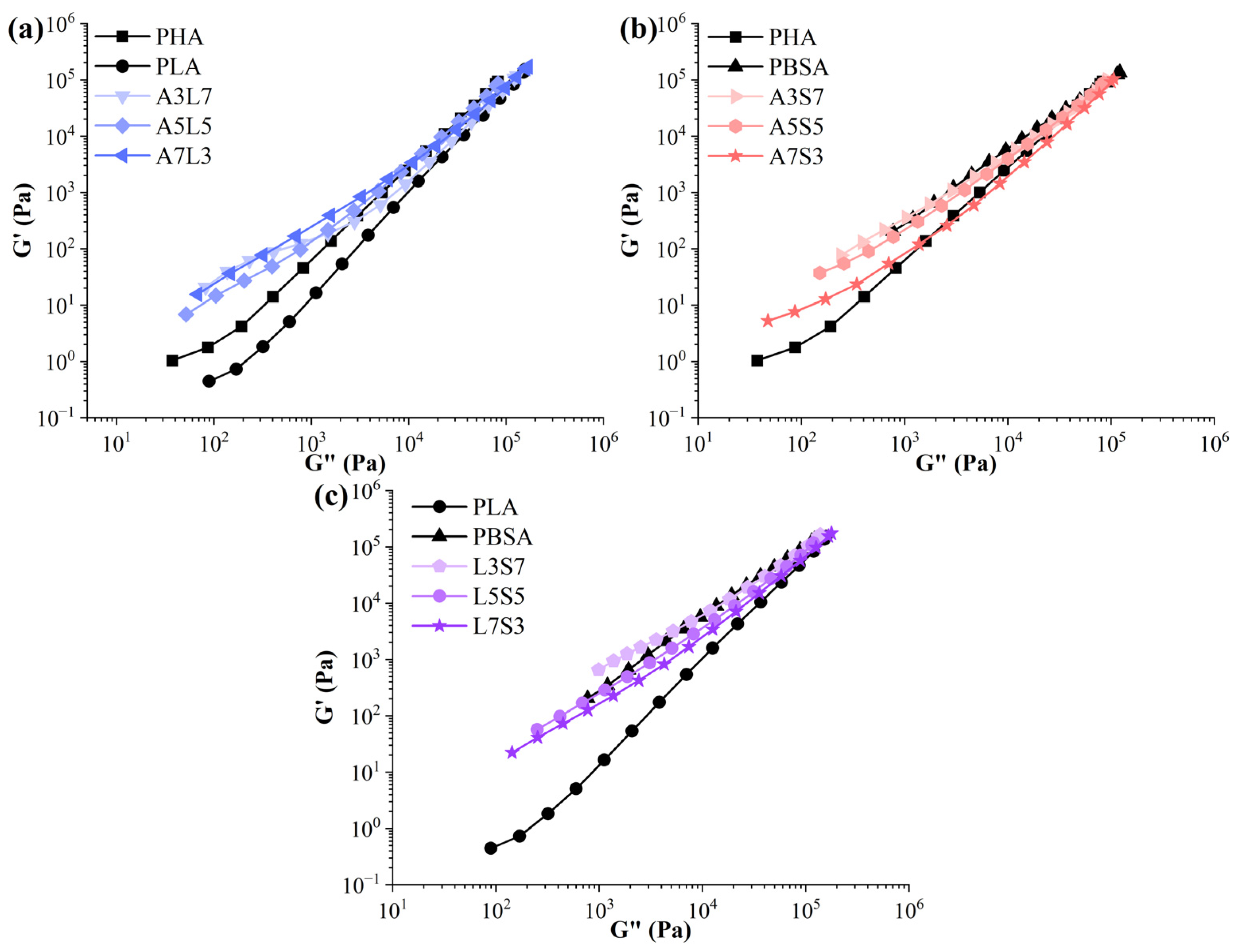
3.1. Rheology
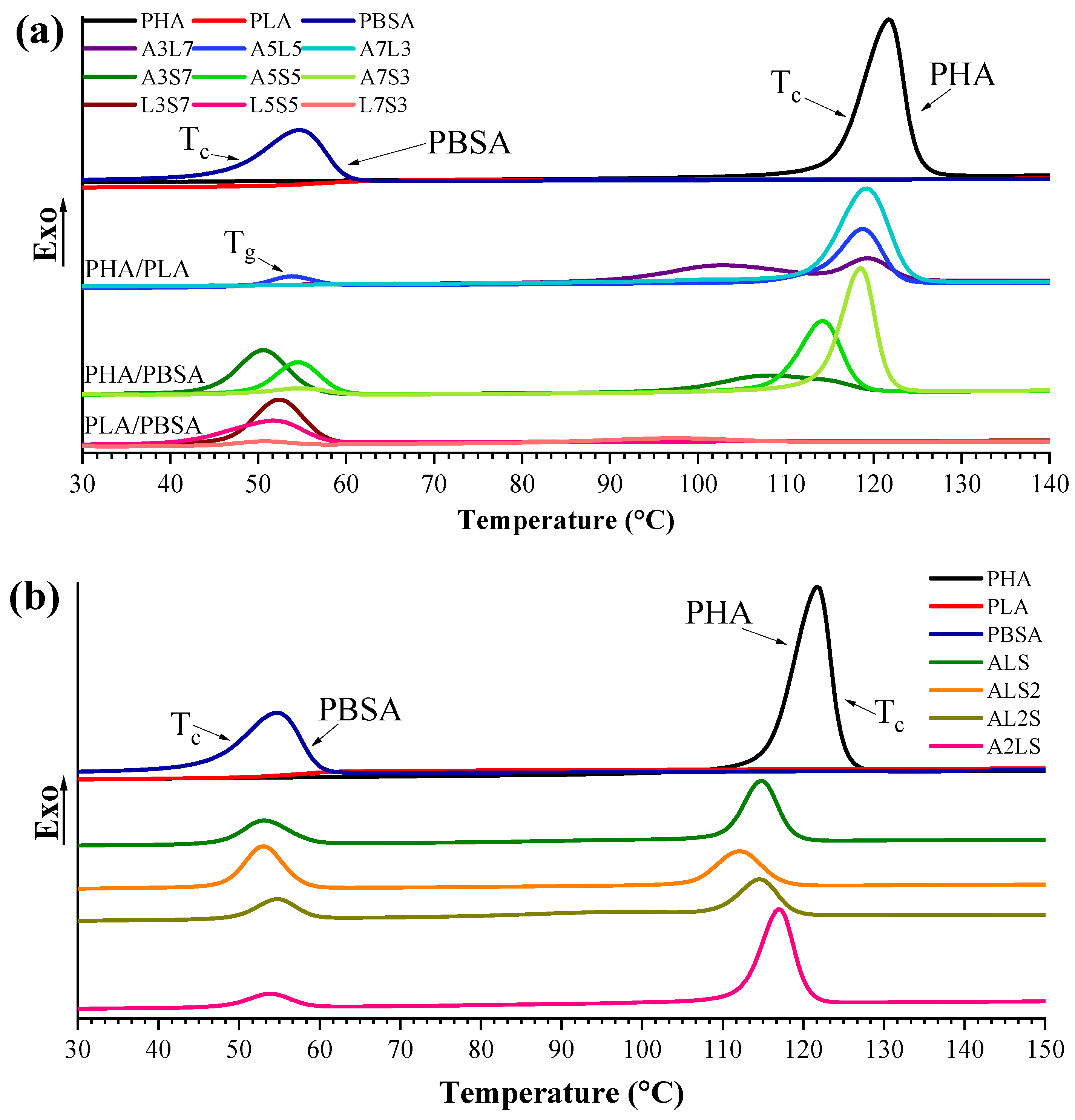
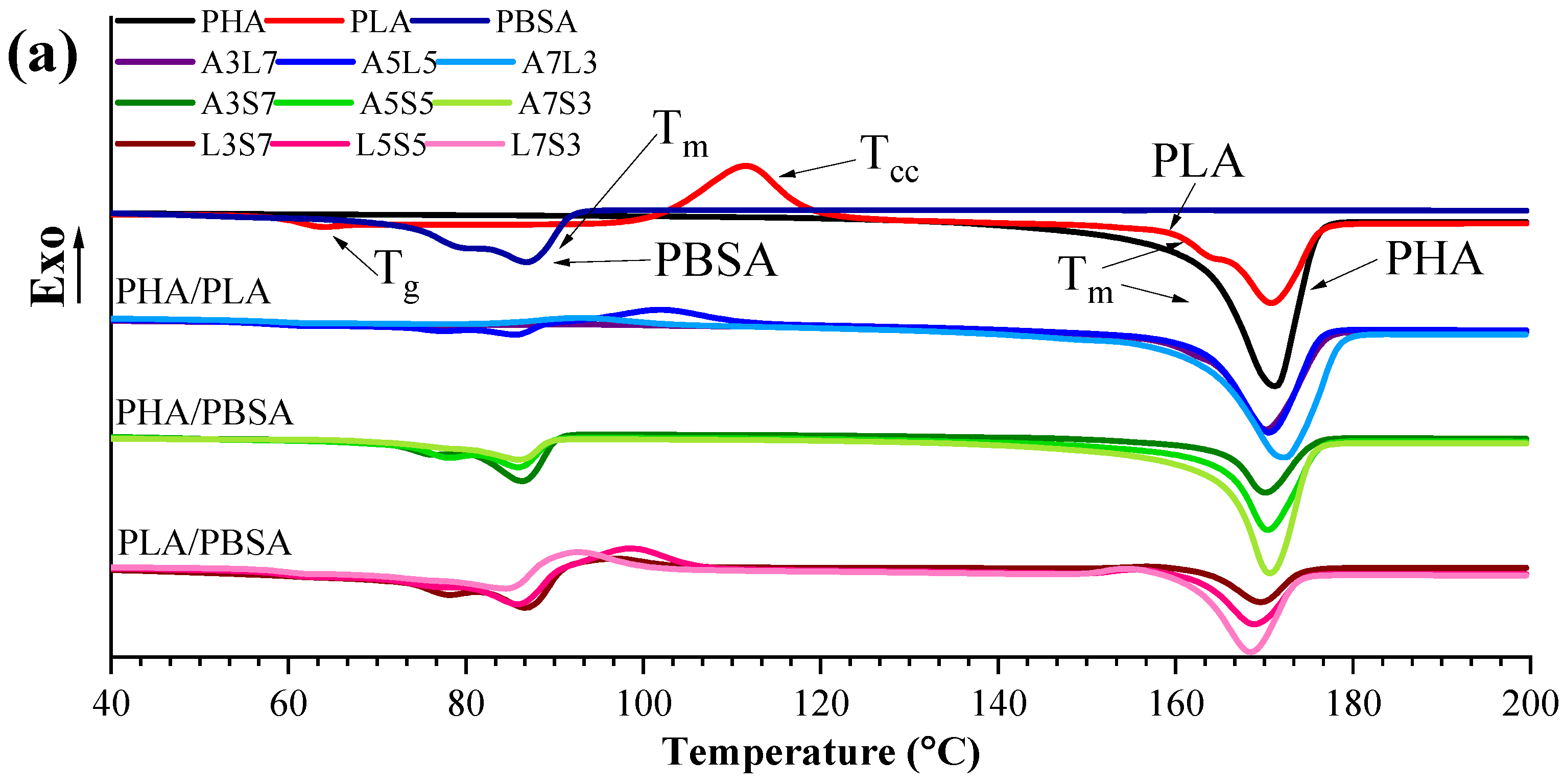
3.2. Calorimetric Properties
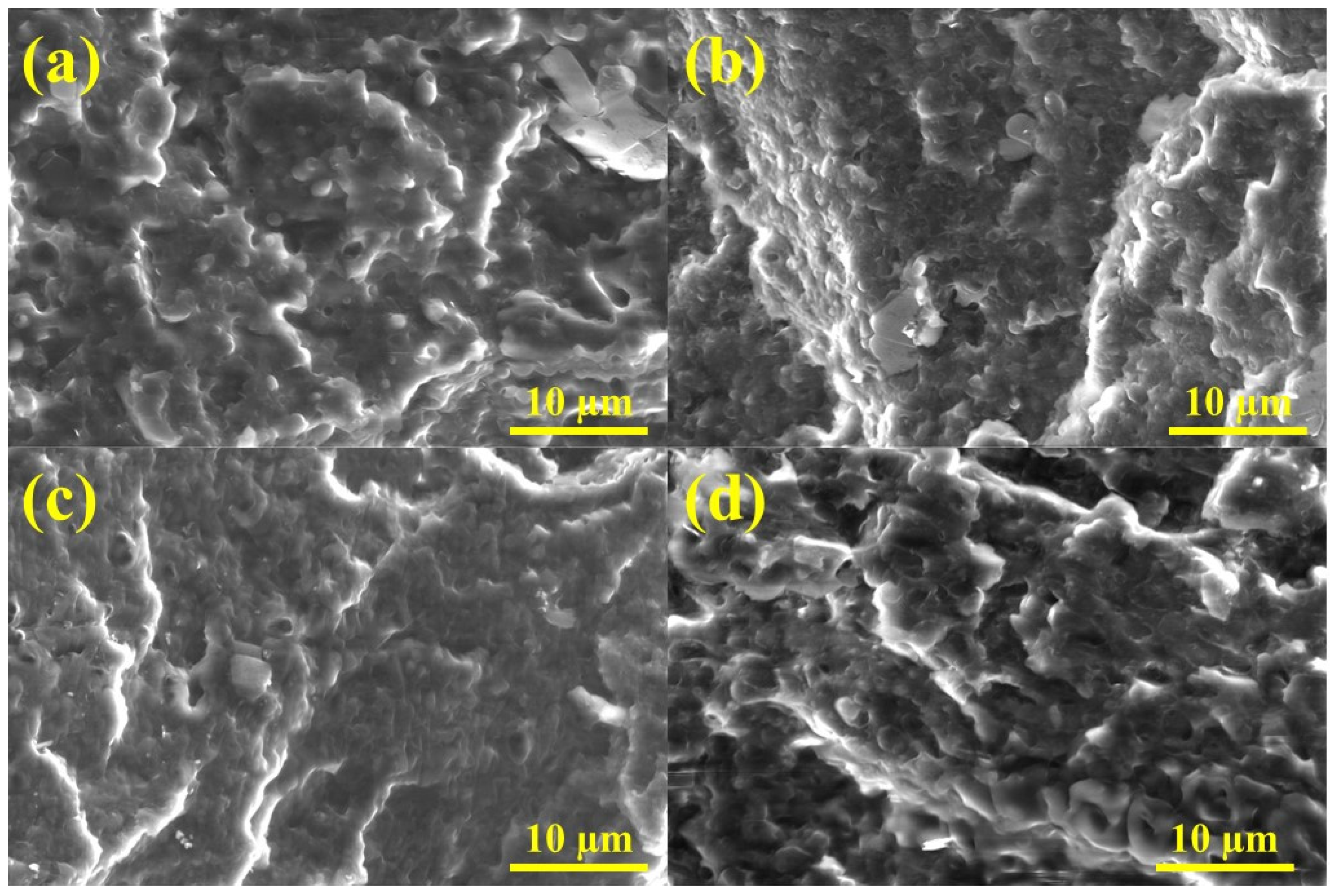
3.3. Ternary Blends’ Morphology and Compatibility
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Nofar, M.; Sacligil, D.; Carreau, P.J.; Kamal, M.R.; Heuzey, M.C. Poly (Lactic Acid) Blends: Processing, Properties and Applications. Int. J. Biol. Macromol. 2019, 125, 307–360. [Google Scholar] [CrossRef] [PubMed]
- Rosenboom, J.G.; Langer, R.; Traverso, G. Bioplastics for a Circular Economy. Nat. Rev. Mater. 2022, 7, 117–137. [Google Scholar] [CrossRef] [PubMed]
- Adams, B. Bio-Based Feedstock and Land Use; Hudson Publishing Company: Hudson, OH, USA, 2024; pp. 1–8. [Google Scholar]
- Walker, S.; Rothman, R. Life Cycle Assessment of Bio-Based and Fossil-Based Plastic: A Review. J. Clean. Prod. 2020, 261, 121158. [Google Scholar] [CrossRef]
- Platnieks, O.; Gaidukovs, S.; Kumar Thakur, V.; Barkane, A.; Beluns, S. Bio-Based Poly (Butylene Succinate): Recent Progress, Challenges and Future Opportunities. Eur. Polym. J. 2021, 161, 110855. [Google Scholar] [CrossRef]
- Ali, S.S.; Elsamahy, T.; Abdelkarim, E.A.; Al-Tohamy, R.; Kornaros, M.; Ruiz, H.A.; Zhao, T.; Li, F.; Sun, J. Biowastes for Biodegradable Bioplastics Production and End-of-Life Scenarios in Circular Bioeconomy and Biorefinery Concept. Bioresour. Technol. 2022, 363, 127869. [Google Scholar] [CrossRef] [PubMed]
- Platnieks, O.; Sereda, A.; Gaidukovs, S.; Thakur, V.K.; Barkane, A.; Gaidukova, G.; Filipova, I.; Ogurcovs, A.; Fridrihsone, V. Adding Value to Poly (Butylene Succinate) and Nanofibrillated Cellulose-Based Sustainable Nanocomposites by Applying Masterbatch Process. Ind. Crops Prod. 2021, 169, 113669. [Google Scholar] [CrossRef]
- Saharan, B.; Sharma, D. Bioplastics-For Sustainable Development: A Review Bioplastics—For Sustainable Development: A Review. Int. J. Microb. Resour. Technol. 2015, 1, 10–23. [Google Scholar]
- Naser, A.Z.; Deiab, I.; Defersha, F.; Yang, S. Expanding Poly(Lactic Acid) (PLA) and Polyhydroxyalkanoates (PHA’s) Applications: A Review on Modifications and Effects. Polymers 2021, 13, 4271. [Google Scholar] [CrossRef] [PubMed]
- Arrigo, R.; D’Anna, A.; Frache, A. Fully Bio-Based Ternary Polymer Blends: Structural Characterization and Mechanical Behavior. Mater. Today Sustain. 2023, 21, 100314. [Google Scholar] [CrossRef]
- Imre, B.; Pukánszky, B. Compatibilization in Bio-Based and Biodegradable Polymer Blends. Eur. Polym. J. 2013, 49, 1215–1233. [Google Scholar] [CrossRef]
- Sabalina, A.; Gaidukovs, S.; Jurinovs, M.; Grase, L.; Platnieks, O. Fabrication of Poly(Lactic Acid), Poly(Butylene Succinate), and Poly(Hydroxybutyrate) Bio-Based and Biodegradable Blends for Application in Fused Filament Fabrication-Based 3D Printing. J. Appl. Polym. Sci. 2023, 140, e54031. [Google Scholar] [CrossRef]
- Chrissafis, K.; Paraskevopoulos, K.M.; Bikiaris, D.N. Effect of Molecular Weight on Thermal Degradation Mechanism of the Biodegradable Polyester Poly(Ethylene Succinate). Thermochim. Acta 2006, 440, 166–175. [Google Scholar] [CrossRef]
- Kanabenja, W.; Passarapark, K.; Subchokpool, T.; Nawaaukkaratharnant, N.; Román, A.J.; Osswald, T.A.; Aumnate, C.; Potiyaraj, P. 3D Printing Filaments from Plasticized Polyhydroxybutyrate/Polylactic Acid Blends Reinforced with Hydroxyapatite. Addit. Manuf. 2022, 59, 103130. [Google Scholar] [CrossRef]
- Kervran, M.; Vagner, C.; Cochez, M.; Ponçot, M.; Saeb, M.R.; Vahabi, H. Thermal Degradation of Polylactic Acid (PLA)/Polyhydroxybutyrate (PHB) Blends: A Systematic Review. Polym. Degrad. Stab. 2022, 201, 109995. [Google Scholar] [CrossRef]
- Ou-Yang, Q.; Guo, B.; Xu, J. Preparation and Characterization of Poly(Butylene Succinate)/Polylactide Blends for Fused Deposition Modeling 3D Printing. ACS Omega 2018, 3, 14309–14317. [Google Scholar] [CrossRef] [PubMed]
- Murariu, M.; Dubois, P. PLA Composites: From Production to Properties. Adv. Drug Deliv. Rev. 2016, 107, 17–46. [Google Scholar] [CrossRef] [PubMed]
- D’Anna, A.; Arrigo, R.; Frache, A. PLA/PHB Blends: Biocompatibilizer Effects. Polymers 2019, 11, 1416. [Google Scholar] [CrossRef] [PubMed]
- Luoma, E.; Rokkonen, T.; Tribot, A.; Nättinen, K.; Lahtinen, J. Poly(Butylene Succinate-Co-Adipate)/Poly(Hydroxybutyrate) Blend Films and Their Thermal, Mechanical and Gas Barrier Properties. Polym. Renew. Resour. 2022, 13, 83–101. [Google Scholar] [CrossRef]
- Aliotta, L.; Seggiani, M.; Lazzeri, A.; Gigante, V.; Cinelli, P. A Brief Review of Poly (Butylene Succinate) (PBS) and Its Main Copolymers: Synthesis, Blends, Composites, Biodegradability, and Applications. Polymers 2022, 14, 844. [Google Scholar] [CrossRef] [PubMed]
- Hedrick, M.M.; Wu, F.; Mohanty, A.K.; Misra, M. Morphology and Performance Relationship Studies on Biodegradable Ternary Blends of Poly(3-Hydroxybutyrate-: Co -3-Hydroxyvalerate), Polylactic Acid, and Polypropylene Carbonate. RSC Adv. 2020, 10, 44624–44632. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Yu, C.; Wongwiwattana, P.; Thomas, N.L. Optimising Properties of Poly (Lactic Acid ) Blends through Co-Continuous Phase Morphology. J. Polym. Environ. 2018, 26, 1–254. [Google Scholar] [CrossRef]
- Zhou, S.; Hrymak, A.N.; Kamal, M.R. Properties of Microinjection-Molded Multi-Walled Carbon Nanotubes-Filled Poly(Lactic Acid)/Poly[(Butylene Succinate)-Co-Adipate] Blend Nanocomposites. J. Mater. Sci. 2018, 53, 9013–9025. [Google Scholar] [CrossRef]
- Boey, J.Y.; Mohamad, L.; Khok, Y.S.; Tay, G.S.; Baidurah, S. A Review of the Applications and Biodegradation of Polyhydroxyalkanoates and Poly(Lactic Acid) and Its Composites. Polymers 2021, 13, 1544. [Google Scholar] [CrossRef] [PubMed]
- Aliotta, L.; Vannozzi, A.; Canesi, I.; Cinelli, P.; Coltelli, M.B.; Lazzeri, A. Poly(Lactic Acid) (PLA)/Poly(Butylene Succinate-Co-Adipate) (PBSA) Compatibilized Binary Biobased Blends: Melt Fluidity, Morphological, Thermo-Mechanical and Micromechanical Analysis. Polymers 2021, 13, 218. [Google Scholar] [CrossRef] [PubMed]
- Righetti, M.C.; Cinelli, P.; Aliotta, L.; Bianchi, E.; Tricoli, F.; Seggiani, M.; Lazzeri, A. Immiscible PHB/PBS and PHB/PBSA Blends: Morphology, Phase Composition and Modelling of Elastic Modulus. Polym. Int. 2022, 71, 47–56. [Google Scholar] [CrossRef]
- Yang, X.; Xu, H.; Odelius, K.; Hakkarainen, M. Poly(Lactide)-g-Poly(Butylene Succinate-Co-Adipate) with High Crystallization Capacity and Migration Resistance. Materials 2016, 9, 313. [Google Scholar] [CrossRef] [PubMed]
- Olejnik, O.; Masek, A.; Zawadziłło, J. Processability and Mechanical Properties of Thermoplastic Polylactide/Polyhydroxybutyrate (PLA/PHB) Bioblends. Materials 2021, 14, 898. [Google Scholar] [CrossRef] [PubMed]
- D’Anna, A.; Arrigo, R.; Frache, A. Rheology, Morphology and Thermal Properties of a PLA/PHB/Clay Blend Nanocomposite: The Influence of Process Parameters. J. Polym. Environ. 2022, 30, 102–113. [Google Scholar] [CrossRef]
- Abdelwahab, M.A.; Flynn, A.; Chiou, B.S.; Imam, S.; Orts, W.; Chiellini, E. Thermal, Mechanical and Morphological Characterization of Plasticized PLA-PHB Blends. Polym. Degrad. Stab. 2012, 97, 1822–1828. [Google Scholar] [CrossRef]
- Koyama, N.; Doi, Y. Miscibility of Binary Blends of Poly[(R)-3-Hydroxybutyric Acid] and Poly[(S)-Lactic Acid]. Polymer 1997, 38, 1589–1593. [Google Scholar] [CrossRef]
- Blümm, E.; Owen, A.J. Crystallization of Poly(3-Hydroxybutyrate)/Poly(L-Lactide) Blends. Polymer 1995, 36, 4077–4081. [Google Scholar] [CrossRef]
- Zhang, K.; Mohanty, A.K.; Misra, M. Fully Biodegradable and Biorenewable Ternary Blends from Polylactide, Poly(3-Hydroxybutyrate-Co-Hydroxyvalerate) and Poly(Butylene Succinate) with Balanced Properties. ACS Appl. Mater. Interfaces 2012, 4, 3091–3101. [Google Scholar] [CrossRef]
- Wang, Y.P.; Xiao, Y.J.; Duan, J.; Yang, J.H.; Wang, Y.; Zhang, C. liang Accelerated Hydrolytic Degradation of Poly(Lactic Acid) Achieved by Adding Poly(Butylene Succinate). Polym. Bull. 2016, 73, 1067–1083. [Google Scholar] [CrossRef]
- Huang, J.; Zou, W.; Luo, Y.; Wu, Q.B.; Lu, X.; Qu, J. Phase Morphology, Rheological Behavior, and Mechanical Properties of Poly (Lactic Acid)/Poly (Butylene Succinate)/Hexamethylene Diisocyanate Reactive Blends. ES Energy Environ. 2021, 12, 86–94. [Google Scholar] [CrossRef]
- Zhou, S.Y.; Huang, H.D.; Xu, L.; Yan, Z.; Zhong, G.J.; Hsiao, B.S.; Li, Z.M. In Situ Nanofibrillar Networks Composed of Densely Oriented Polylactide Crystals as Efficient Reinforcement and Promising Barrier Wall for Fully Biodegradable Poly(Butylene Succinate) Composite Films. ACS Sustain. Chem. Eng. 2016, 4, 2887–2897. [Google Scholar] [CrossRef]
- Ojijo, V.; Sinha Ray, S.; Sadiku, R. Role of Specific Interfacial Area in Controlling Properties of Immiscible Blends of Biodegradable Polylactide and Poly[(Butylene Succinate)-Co-Adipate]. ACS Appl. Mater. Interfaces 2012, 4, 6690–6701. [Google Scholar] [CrossRef] [PubMed]
- ASTM D6866-22; Standard Test Methods for Determining the Biobased Content of Solid, Liquid, and Gaseous Samples Using Radiocarbon Analysis. ASTM: West Conshohocken, PA, USA, 2024.
- ASTM D6400-21; Standard Specification for Labeling of Plastics Designed to be Aerobically Composted in Municipal or Industrial Facilities. ASTM: West Conshohocken, PA, USA, 2022.
- ASTM D1238-10; Standard Test Method for Melt Flow Rates of Thermoplastics by Extrusion Plastometer. ASTM: West Conshohocken, PA, USA, 2013.
- Baidurah, S. Methods of Analyses for Biodegradable Polymers: A Review. Polymers 2022, 14, 4928. [Google Scholar] [CrossRef] [PubMed]
- Colomines, G.; Ducruet, V.; Courgneau, C.; Guinault, A.; Barrier, S.D. Barrier Properties of Poly(Lactic Acid) and Its Mor-phological Changes Induced by Aroma Compound Sorption. Polym. Int. 2017, 59, 818–826. [Google Scholar] [CrossRef]
- Messin, T.; Follain, N.; Guinault, A.; Sollogoub, C.; Gaucher, V.; Delpouve, N.; Marais, S. Structure and Barrier Properties of Multinanolayered Biodegradable PLA/PBSA Films: Confinement Effect via Forced Assembly Coextrusion. ACS Appl. Mater. Interfaces 2017, 9, 29101–29112. [Google Scholar] [CrossRef] [PubMed]
- Renoux, J.D.; Dani, J.; Douchain, C.; Prashantha, K.; Lacrampe, M.F.; Krawczak, P. Simultaneous Plasticization and Blending of Isolate Soy Protein with Poly(Butylene Succinate–Co–Adipate). Appl. Polym. Sci. 2016, 135, 21–23. [Google Scholar]
- Mazur, K.E.; Jakubowska, P.; Gaweł, A.; Kuciel, S. Mechanical, Thermal and Hydrodegradation Behavior of Poly(3-Hydroxybutyrate-Co-3-Hydroxyvalerate) (PHBV) Composites with Agricultural Fibers as Reinforcing Fillers. Sustain. Mater. Technol. 2022, 31, e00390. [Google Scholar] [CrossRef]
- Zhang, D.; Jiang, N.; Chen, X.; He, B. Rheology of Crosslinked Entangled Polymers: Shear Stiffening in Oscillatory Shear. J. Appl. Polym. Sci. 2020, 137, 48421. [Google Scholar] [CrossRef]
- Saeidlou, S.; Huneault, M.A.; Li, H.; Park, C.B. Poly(Lactic Acid) Stereocomplex Formation: Application to PLA Rheological Property Modification. J. Appl. Polym. Sci. 2014, 131, 41073. [Google Scholar] [CrossRef]
- Barkoula, N.M.; Alcock, B.; Cabrera, N.O.; Peijs, T. Flame-Retardancy Properties of Intumescent Ammonium Poly(Phosphate) and Mineral Filler Magnesium Hydroxide in Combination with Graphene. Polym. Polym. Compos. 2008, 16, 101–113. [Google Scholar]
- Zhao, H.; Cui, Z.; Wang, X.; Turng, L.S.; Peng, X. Processing and Characterization of Solid and Microcellular Poly(Lactic Acid)/Polyhydroxybutyrate-Valerate (PLA/PHBV) Blends and PLA/PHBV/Clay Nanocomposites. Compos. Part B Eng. 2013, 51, 79–91. [Google Scholar] [CrossRef]
- Song, X.; Zhang, X.; Li, T.; Li, Z.; Chi, H. Mechanically Robust Hybrid POSS Thermoplastic Polyurethanes with Enhanced Surface Hydrophobicity. Polymers 2019, 11, 373. [Google Scholar] [CrossRef] [PubMed]
- Botchu, J.; Baek, S.W. Flow and Dynamic Rheological Characterization of Ethanolamine Gelled Propellant Flow and Dynamic Rheological Characterization of Ethanolamine Gel Propellant with Hybrid Gelling Agent. Stem 2015, 76, 62–67. [Google Scholar]
- Lyu, Y.; Chen, Y.; Lin, Z.; Zhang, J.; Shi, X. Manipulating Phase Structure of Biodegradable PLA/PBAT System: Effects on Dynamic Rheological Responses and 3D Printing. Compos. Sci. Technol. 2020, 200, 108399. [Google Scholar] [CrossRef]
- Bousmina, M. Effect of Interfacial Tension on Linear Viscoelastic Behavior of Immiscible Polymer Blends. Rheol. Acta 1999, 38, 251–254. [Google Scholar] [CrossRef]
- Wu, T.; Tong, Y.; Qiu, F.; Yuan, D.; Zhang, G.; Qu, J. Morphology, Rheology Property, and Crystallization Behavior of PLLA/OMMT Nanocomposites Prepared by an Innovative Eccentric Rotor Extruder. Polym. Adv. Technol. 2018, 29, 41–51. [Google Scholar] [CrossRef]
- Graebling, D.; Muller, R.; Palierne, J.F. Linear Viscoelastic Behavior of Some Incompatible Polymer Blends in the Melt. Interpretation of Data with a Model of Emulsion of Viscoelastic Liquids. Macromolecules 1993, 26, 320–329. [Google Scholar] [CrossRef]
- Hammani, S.; Moulai-Mostefa, N.; Samyn, P.; Bechelany, M.; Dufresne, A.; Barhoum, A. Morphology, Rheology and Crystallization in Relation to the Viscosity Ratio of Polystyrene/Polypropylene Polymer Blends. Materials 2020, 13, 926. [Google Scholar] [CrossRef] [PubMed]
- Cooke, M.E.; Rosenzweig, D.H. The Rheology of Direct and Suspended Extrusion Bioprinting. APL Bioeng. 2021, 5, 011502. [Google Scholar] [CrossRef] [PubMed]
- Ramli, H.; Zainal, N.F.A.; Hess, M.; Chan, C.H. Basic Principle and Good Practices of Rheology for Polymers for Teachers and Beginners. Chem. Teach. Int. 2022, 4, 307–326. [Google Scholar] [CrossRef]
- Kovalcik, A. Recent Advances in 3D Printing of Polyhydroxyalkanoates: A Review. EuroBiotech J. 2021, 5, 48–55. [Google Scholar] [CrossRef]
- Qahtani, M.; Wu, F.; Misra, M.; Gregori, S.; Mielewski, D.F.; Mohanty, A.K. Experimental Design of Sustainable 3D-Printed Poly(Lactic Acid)/Biobased Poly(Butylene Succinate) Blends via Fused Deposition Modeling. ACS Sustain. Chem. Eng. 2019, 7, 14460–14470. [Google Scholar] [CrossRef]
- Weinmann, S.; Bonten, C. Thermal and Rheological Properties of Modified Polyhydroxybutyrate (PHB). Polym. Eng. Sci. 2019, 59, 1057–1064. [Google Scholar] [CrossRef]
- Conrad, J.D.; Harrison, G.M. The Rheology and Processing of Renewable Resource Polymers. AIP Conf. Proc. 2008, 1027, 114–116. [Google Scholar]
- Gerard, T.; Budtova, T. Morphology and Molten-State Rheology of Polylactide and Polyhydroxyalkanoate Blends. Eur. Polym. J. 2012, 48, 1110–1117. [Google Scholar] [CrossRef]
- Choi, I.S.; Kim, Y.K.; Hong, S.H.; Seo, H.J.; Hwang, S.H.; Kim, J.; Lim, S.K. Effects of Polybutylene Succinate Content on the Rheological Properties of Polylactic Acid/Polybutylene Succinate Blends and the Characteristics of Their Fibers. Materials 2024, 17, 662. [Google Scholar] [CrossRef] [PubMed]
- Yi, X.; Xu, L.; Wang, Y.L.; Zhong, G.J.; Ji, X.; Li, Z.M. Morphology and Properties of Isotactic Polypropylene/Poly(Ethylene Terephthalate) in Situ Microfibrillar Reinforced Blends: Influence of Viscosity Ratio. Eur. Polym. J. 2010, 46, 719–730. [Google Scholar] [CrossRef]
- Omonov, T.S.; Harrats, C.; Moldenaers, P.; Groeninckx, G. Phase Continuity Detection and Phase Inversion Phenomena in Immiscible Polypropylene/Polystyrene Blends with Different Viscosity Ratios. Polymer 2007, 48, 5917–5927. [Google Scholar] [CrossRef]
- Dae Han, C.; Woo Kim, Y. The Effect of Mixing on the Modes of Dispersion and Rheological Properties of Two-Phase Polymer Blends in Extrusion. J. Appl. Polym. Sci. 1975, 19, 2831–2843. [Google Scholar] [CrossRef]
- Utracki, L.A.; Wilkie, C.A. Polymer Blends Handbook; Springer: Berlin/Heidelberg, Germany, 2014; pp. 517–675. [Google Scholar]
- Sanchez, L.C.; Beatrice, C.A.G.; Lotti, C.; Marini, J.; Bettini, S.H.P.; Costa, L.C. Rheological Approach for an Additive Manufacturing Printer Based on Material Extrusion. Int. J. Adv. Manuf. Technol. 2019, 105, 2403–2414. [Google Scholar] [CrossRef]
- Maia, J.; Covas, J.; de Cindio, B.; Gabriele, D. Rheology in materials engineering. In Rheology; Universidad de Huelva: Huelva, Spain, 2010. [Google Scholar]
- Xu, H.; Yu, Y.; Li, Y. Crystallization, Rheological and Mechanical Properties of Poly(Butylene Succinate)/Poly(Propylene Carbonate)/Poly(Vinyl Acetate) Ternary Blends. Colloid Polym. Sci. 2021, 299, 1447–1458. [Google Scholar] [CrossRef]
- Wu, F.; Misra, M.; Mohanty, A.K. Super Toughened Poly(Lactic Acid)-Based Ternary Blends via Enhancing Interfacial Compatibility. ACS Omega 2019, 4, 1955–1968. [Google Scholar] [CrossRef] [PubMed]
- Vigil Fuentes, M.A.; Thakur, S.; Wu, F.; Misra, M.; Gregori, S.; Mohanty, A.K. Study on the 3D Printability of Poly(3-Hydroxybutyrate-Co-3-Hydroxyvalerate)/Poly(Lactic Acid) Blends with Chain Extender Using Fused Filament Fabrication. Sci. Rep. 2020, 10, 11804. [Google Scholar] [CrossRef]
- Qiao, H.; Maazouz, A.; Lamnawar, K. Study of Morphology, Rheology, and Dynamic Properties toward Unveiling the Partial Miscibility in Poly(Lactic Acid)—Poly(Hydroxybutyrate-Co-Hydroxyvalerate) Blends. Polymers 2022, 14, 5359. [Google Scholar] [CrossRef] [PubMed]
- Patti, A.; Acierno, S.; Cicala, G.; Acierno, D. Predicting the Printability of Poly(Lactide) Acid Filaments in Fused Deposition Modeling (FDM) Technology: Rheological Measurements and Experimental Evidence. ChemEngineering 2023, 7, 1. [Google Scholar] [CrossRef]
- Wang, L.; Jing, X.; Cheng, H.; Hu, X.; Yang, L.; Huang, Y. Blends of Linear and Long-Chain Branched Poly(l-Lactide)s with High Melt Strength and Fast Crystallization Rate. Ind. Eng. Chem. Res. 2012, 51, 10088–10099. [Google Scholar] [CrossRef]
- Ansari, S.; Rashid, M.A.I.; Waghmare, P.R.; Nobes, D.S. Measurement of the Flow Behavior Index of Newtonian and Shear-Thinning Fluids via Analysis of the Flow Velocity Characteristics in a Mini-Channel. SN Appl. Sci. 2020, 2, 1787. [Google Scholar] [CrossRef]
- Diani, J.; Gall, K. Finite Strain 3D Thermoviscoelastic Constitutive Model. Society 2006, 51, 486–492. [Google Scholar]
- Bourg, V.; Valette, R.; Moigne, N.L.; Ienny, P.; Guillard, V.; Bergeret, A. Shear and Extensional Rheology of Linear and Branched Polybutylene Succinate Blends. Polymers 2021, 13, 652. [Google Scholar] [CrossRef] [PubMed]
- Zhiguo, Q.; Jinnan, C.; Baohua, G.; Zunhao, L. Rheology Properties of Poly(Butylene Succinate-Cobutylene Adipate)/Attapulgite Nanocomposites. Adv. Intell. Syst. Res. 2012, 27, 578–580. [Google Scholar]
- 7Arsad, A.; Rahmat, A.R.; Hassan, A.; Mokhtar, M.; Dali, S.N.M. Flow Characteristics and Dynamic Behavior of Polyamide 6/Acrylonitile Butadiene Styrene (PA6/ABS) Blends. Int. J. Polym. Mater. Polym. Biomater. 2013, 62, 209–214. [Google Scholar] [CrossRef]
- Nagy, D.; Weltsch, Z. Crystallinity and Oscillatory Shear Rheology of Polyethylene Blends. Materials 2023, 16, 6402. [Google Scholar] [CrossRef] [PubMed]
- Khonakdar, H.A.; Jafari, S.H.; Hesabi, M.N. Miscibility Analysis, Viscoelastic Properties and Morphology of Cyclic Olefin Copolymer/Polyolefin Elastomer (COC/POE) Blends. Compos. Part B Eng. 2015, 69, 111–119. [Google Scholar] [CrossRef]
- Singh, M.K.; Hu, M.; Cang, Y.; Hsu, H.P.; Therien-Aubin, H.; Koynov, K.; Fytas, G.; Landfester, K.; Kremer, K. Glass Transition of Disentangled and Entangled Polymer Melts: Single-Chain-Nanoparticles Approach. Macromolecules 2020, 53, 7312–7321. [Google Scholar] [CrossRef] [PubMed]
- Walha, F.; Lamnawar, K.; Maazouz, A.; Jaziri, M. Rheological, Morphological and Mechanical Studies of Sustainably Sourced Polymer Blends Based on Poly(Lactic Acid) and Polyamide 11. Polymers 2016, 8, 61. [Google Scholar] [CrossRef] [PubMed]
- Dae Han, C.; Kim, J. Rheological Technique for Determining the Order-Disorder Transition of Block Copolymers. J. Polym. Sci. Part B Polym. Phys. 1987, 25, 1741–1764. [Google Scholar] [CrossRef]
- Cuiffo, M.A.; Snyder, J.; Elliott, A.M.; Romero, N.; Kannan, S.; Halada, G.P. Impact of the Fused Deposition (FDM) Printing Process on Polylactic Acid (PLA) Chemistry and Structure. Appl. Sci. 2017, 7, 579. [Google Scholar] [CrossRef]
- Su, S.; Kopitzky, R.; Tolga, S.; Kabasci, S. Polylactide (PLA) and Its Blends with Poly(Butylene Succinate) (PBS): A Brief Review. Polymers 2019, 11, 1193. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.Q.; Huang, H.X. Foaming of Poly(Lactic Acid) Using Supercritical Carbon Dioxide as Foaming Agent: Influence of Crystallinity and Spherulite Size on Cell Structure and Expansion Ratio. Ind. Eng. Chem. Res. 2014, 53, 2277–2286. [Google Scholar] [CrossRef]
- Tsuji, H.; Sawada, M.; Bouapao, L. Biodegradable Polyesters as Crystallization-Accelerating Agents of Poly(l-lactide). ACS Appl. Mater. Interfaces 2009, 1, 1719–1730. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Wolcott, M.P.; Zhang, J. Study of Biodegradable Polylactide/Poly(Butylene Adipate-Co-Terephthalate) Blends. Biomacromolecules 2006, 7, 199–207. [Google Scholar] [CrossRef] [PubMed]
- Lascano, D.; Quiles-Carrillo, L.; Balart, R.; Boronat, T.; Montanes, N. Toughened Poly (Lactic Acid)-PLA Formulations by Binary Blends with Poly(Butylene Succinate-Co-Adipate)-PBSA and Their Shape Memory Behaviour. Materials 2019, 12, 622. [Google Scholar] [CrossRef] [PubMed]
- Szuman, K.; Krucińska, I.; Boguń, M.; Draczyński, Z. PLA/PHA- Biodegradable Blends for Pneumothermic Fabrication of Nonwovens. Autex Res. J. 2016, 16, 119–127. [Google Scholar] [CrossRef]
- Pan, P.; Zhu, B.; Kai, W.; Dong, T.; Inoue, Y. Polymorphic Transition in Disordered Poly(L-Lactide) Crystals Induced by Annealing at Elevated Temperatures. Macromolecules 2008, 41, 4296–4304. [Google Scholar] [CrossRef]
- Ma, P.; Hristova-Bogaerds, D.G.; Lemstra, P.J.; Zhang, Y.; Wang, S. Toughening of PHBV/PBS and PHB/PBS Blends via in Situ Compatibilization Using Dicumyl Peroxide as a Free-Radical Grafting Initiator. Macromol. Mater. Eng. 2012, 297, 402–410. [Google Scholar] [CrossRef]
- Zhang, K.; Nagarajan, V.; Misra, M.; Mohanty, A.K. Supertoughened Renewable PLA Reactive Multiphase Blends System: Phase Morphology and Performance. ACS Appl. Mater. Interfaces 2014, 6, 12436–12448. [Google Scholar] [CrossRef] [PubMed]
- Zembouai, I.; Bruzaud, S.; Kaci, M.; Benhamida, A.; Corre, Y.; Grohens, Y.; Lopez-Cuesta, J.-M.; Corre, Y.-M. Poly(3-Hydroxybutyrate-Co-3-Hydroxyvalerate)/Polylactide Blends: Thermal Stability, Flammability and Thermo-Mechanical Behavior. J. Polym. Environ. 2014, 22, 131–139. [Google Scholar] [CrossRef]




| Sample | PHA (A) (wt.%) | PLA (L) (wt.%) | PBSA (S) (wt.%) |
|---|---|---|---|
| PHA | 100 | 0 | 0 |
| PLA | 0 | 100 | 0 |
| PBSA | 0 | 0 | 100 |
| A3L7 | 30.0 | 70.0 | 0 |
| A5L5 | 50.0 | 50.0 | 0 |
| A7L3 | 70.0 | 30.0 | 0 |
| A3S7 | 30.0 | 0 | 70.0 |
| A5S5 | 50.0 | 0 | 50.0 |
| A7S3 | 70.0 | 0 | 30.0 |
| L3S7 | 0 | 30.0 | 70.0 |
| L5S5 | 0 | 50.0 | 50.0 |
| L7S3 | 0 | 70.0 | 30.0 |
| ALS | 33.3 | 33.3 | 33.3 |
| ALS2 | 25.0 | 25.0 | 50.0 |
| AL2S | 25.0 | 50.0 | 25.0 |
| A2LS | 50.0 | 25.0 | 25.0 |
| Samples | ) | ||
|---|---|---|---|
| PHA | 0.597 | 3.440 | 753 |
| PLA | 0.625 | 3.606 | 966 |
| PBSA | 0.591 | 3.659 | 5954 |
| A3L7 | 0.651 | 3.432 | 702 |
| A5L5 | 0.615 | 3.374 | 699 |
| A7L3 | 0.568 | 3.808 | 1610 |
| A3S7 | 0.540 | 3.633 | 2537 |
| A5S5 | 0.559 | 3.532 | 1554 |
| A7S3 | 0.675 | 3.297 | 648 |
| L3S7 | 0.553 | 3.791 | 9051 |
| L5S5 | 0.600 | 3.563 | 2570 |
| L7S3 | 0.661 | 3.555 | 1193 |
| ALS | 0.660 | 3.265 | 747 |
| ALS2 | 0.588 | 3.707 | 1671 |
| AL2S | 0.624 | 3.495 | 1036 |
| A2LS | 0.595 | 3.682 | 1371 |
| Samples | Component | Tc (°C) | ΔHc (J/g) | χc (%) | Tm (°C) | ΔHm (J/g) | Tc (°C) | ΔHc (J/g) |
|---|---|---|---|---|---|---|---|---|
| PHA | 121.7 | 84.0 | 58 | 171.2 | 92.9 | |||
| PLA | – | – | – | 170.7 | 41.9 | 111.6 | 42.1 | |
| PBSA | 54.6 | 44.0 | 40 | 86.8 | 27.4 | |||
| A3L7 | PHA | 119.3 ** | 11.3 | 26 | 170.1 | 50.9 | ||
| PLA | 102.9 ** | 22.6 | 35 | 170.1 * | 50.9 * | 91.7 | 0.4 | |
| A5L5 | PHA | 118.8 | 31.8 | 44 | 170.6 | 51.9 | ||
| PLA | – | – | – | 170.6 * | 51.9 * | 101.78 | 10.8 | |
| A7L3 | PHA | 119.1 | 60.8 | 60 | 173.2 | 69.2 | ||
| PLA | – | – | – | 173.2 * | 69.2 * | 92.9 | 3.8 | |
| A3S7 | PHA | 108.1 | 20.8 | 48 | 170.2 | 22.0 | ||
| PBSA | 50.4 | 29.3 | 38 | 86.3 | 20.89 | |||
| A5S5 | PHA | 114.2 | 38.8 | 53 | 170.5 | 40.8 | ||
| PBSA | 54.6 | 19.2 | 35 | 85.8 | 13.6 | |||
| A7S3 | PHA | 118.5 | 56.3 | 55 | 170.6 | 62.8 | ||
| PBSA | 54.9 | 4.6 | 14 | 86.0 | 10.9 | |||
| L3S7 | PLA | – | – | – | 169.6 | 12.5 | 96.8 | 4.2 |
| PBSA | 52.4 | 26.4 | 34 | 86.6 | 36.8 | |||
| L5S5 | PLA | – | – | – | 169.0 | 19.7 | 98.6 | 18.1 |
| PBSA | 51.9 | 18.1 | 33 | 85.8 | 23.4 | |||
| L7S3 | PLA | 97.5 | 5.1 | 8 | 168.4 | 29.6 | 95.8 | 4.2 |
| PBSA | 50.8 | 1.8 | 5 | 84.4 | 14.0 | |||
| Samples | Component | Tc (°C) | ΔHc (J/g) | χc (%) | Tm (°C) | ΔHm (J/g) | Tc (°C) | ΔHc (J/g) |
|---|---|---|---|---|---|---|---|---|
| ALS | PHA | 114.7 | 23.4 | 49 | 169.1 | 35.3 | ||
| PLA | – | – | – | 169.1 * | 35.3 * | 98.1 | 6.8 | |
| PBSA | 53.2 | 11.7 | 32 | 76.2 | 0.3 | |||
| ALS2 | PHA | 112.1 | 18.4 | 51 | 169.9 | 31.9 | ||
| PLA | – | – | – | 169.9 * | 31.9 * | 101.1 | 8.1 | |
| PBSA | 53.1 | 19.5 | 36 | 85.6 | 17.3 | |||
| AL2S | PHA | 114.6 | 14.0 | 38 | 170.1 | 38.6 | ||
| PLA | – | – | – | 170.1 * | 38.6 * | 96.8 | 15.0 | |
| PBSA | 54.8 | 8.2 | 30 | 68.7 | 8.2 | |||
| A2LS | PHA | 117.1 | 38. | 52 | 170.2 | 43.5 | ||
| PLA | – | – | – | 170.2 * | 43.5 * | 95.8 | 4.2 | |
| PBSA | 53.8 | 5.9 | 22 | 85.2 | 0.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sabalina, A.; Gaidukovs, S.; Aunins, A.; Gromova, A.; Gaidukova, G.; Orlova, L.; Platnieks, O. Exploring the Processing Potential of Polylactic Acid, Polyhydroxyalkanoate, and Poly(butylene succinate-co-adipate) Binary and Ternary Blends. Polymers 2024, 16, 2288. https://doi.org/10.3390/polym16162288
Sabalina A, Gaidukovs S, Aunins A, Gromova A, Gaidukova G, Orlova L, Platnieks O. Exploring the Processing Potential of Polylactic Acid, Polyhydroxyalkanoate, and Poly(butylene succinate-co-adipate) Binary and Ternary Blends. Polymers. 2024; 16(16):2288. https://doi.org/10.3390/polym16162288
Chicago/Turabian StyleSabalina, Alisa, Sergejs Gaidukovs, Arturs Aunins, Anda Gromova, Gerda Gaidukova, Liga Orlova, and Oskars Platnieks. 2024. "Exploring the Processing Potential of Polylactic Acid, Polyhydroxyalkanoate, and Poly(butylene succinate-co-adipate) Binary and Ternary Blends" Polymers 16, no. 16: 2288. https://doi.org/10.3390/polym16162288
APA StyleSabalina, A., Gaidukovs, S., Aunins, A., Gromova, A., Gaidukova, G., Orlova, L., & Platnieks, O. (2024). Exploring the Processing Potential of Polylactic Acid, Polyhydroxyalkanoate, and Poly(butylene succinate-co-adipate) Binary and Ternary Blends. Polymers, 16(16), 2288. https://doi.org/10.3390/polym16162288







