Polymeric Mixed Micelle-Loaded Hydrogel for the Ocular Delivery of Fexofenadine for Treating Allergic Conjunctivitis
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Experimental Design
2.3. Preparation of FEX-PMM
2.4. Characterization of FEX-PMM
2.4.1. Drug Entrapment Efficiency
2.4.2. Particle Size and Zeta Potential
2.4.3. In Vitro Drug Release Study
2.5. Selection of the Optimized FEX-PMM Formula
2.6. Characterization of the Optimized FEX-PMM Formula
2.6.1. Morphology
2.6.2. Differential Scanning Calorimetry
2.6.3. Stability Study
2.7. Preparation of FEX-PMM Hydrogel
2.8. Evaluation of FEX-PMM Hydrogel
2.8.1. Determination of pH
2.8.2. Rheological Studies
2.8.3. Evaluation of Gel Spreadability
2.8.4. Ex Vivo Mucoadhesive Strength
2.8.5. In Vitro Drug Release Study
2.9. In Vivo Studies
2.9.1. Animals
2.9.2. Draize Test
2.9.3. Pharmacodynamics Study
2.10. Statistical Analysis of Data
3. Results and Discussion
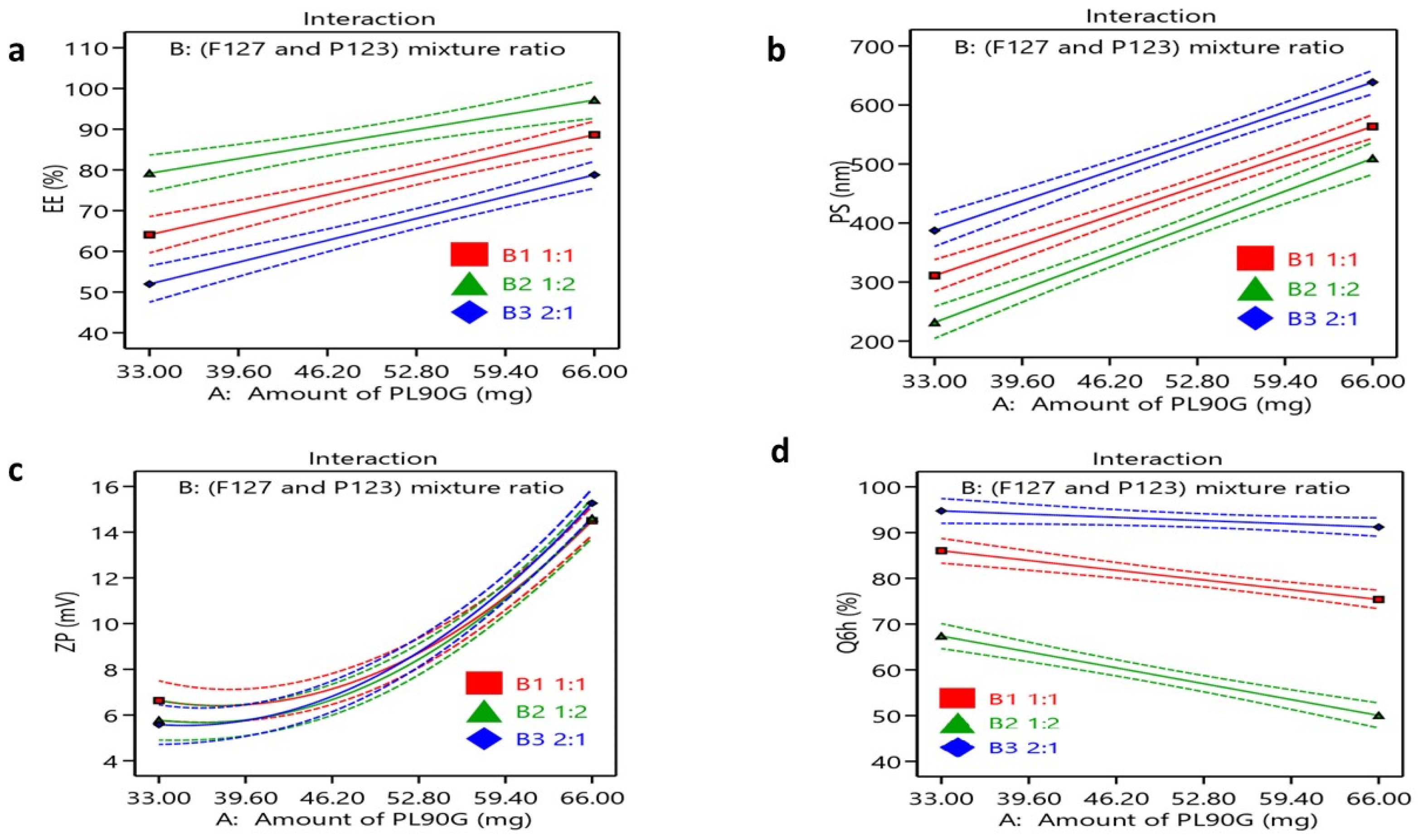
3.1. Factorial Design Optimization
3.2. Characterization of FEX—PMM
3.2.1. Drug Entrapment Efficiency
3.2.2. Particle Size
3.2.3. Zeta Potential
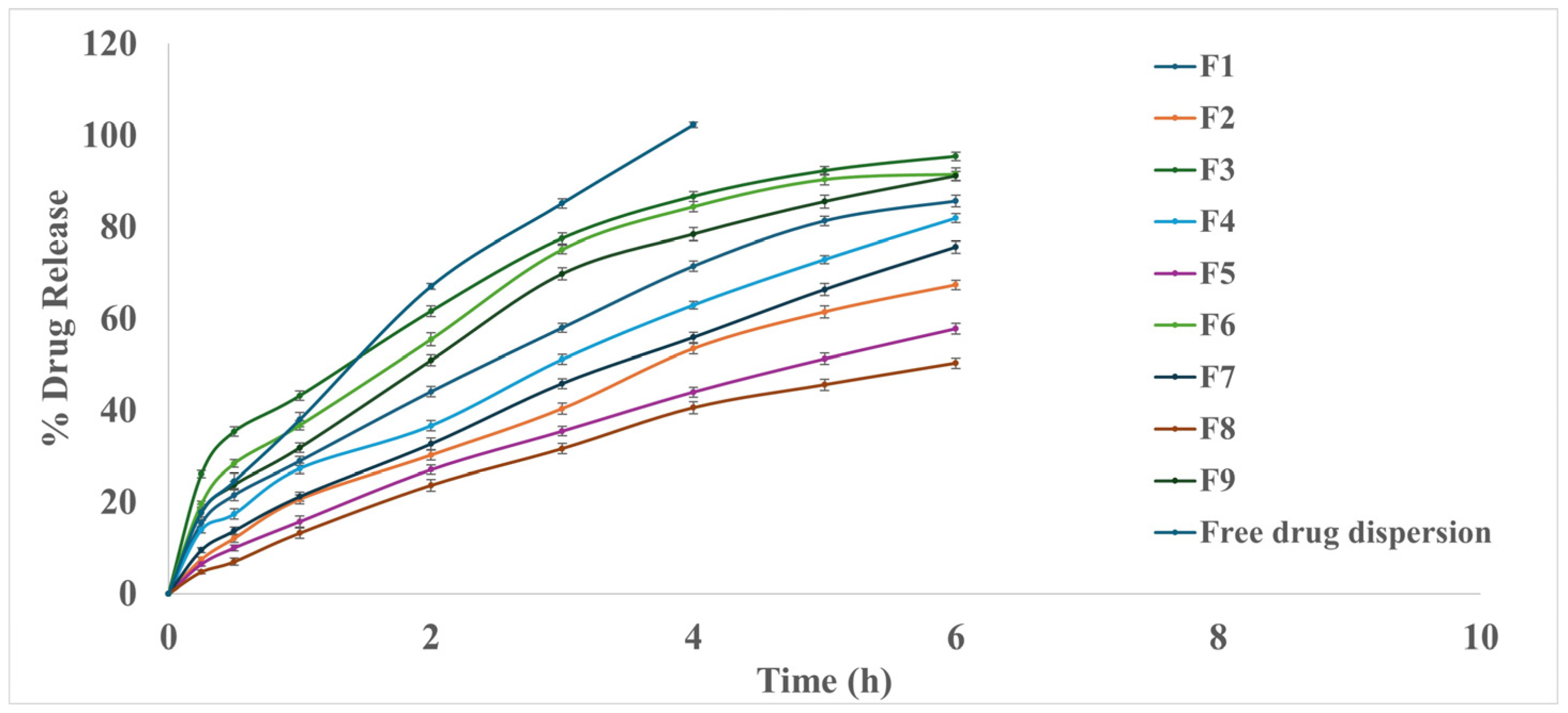
3.2.4. In Vitro Drug Release Study
3.3. Selection of the Optimized FX-PMM Formula
3.4. Characterization of the Optimized FEX-PMM Formula
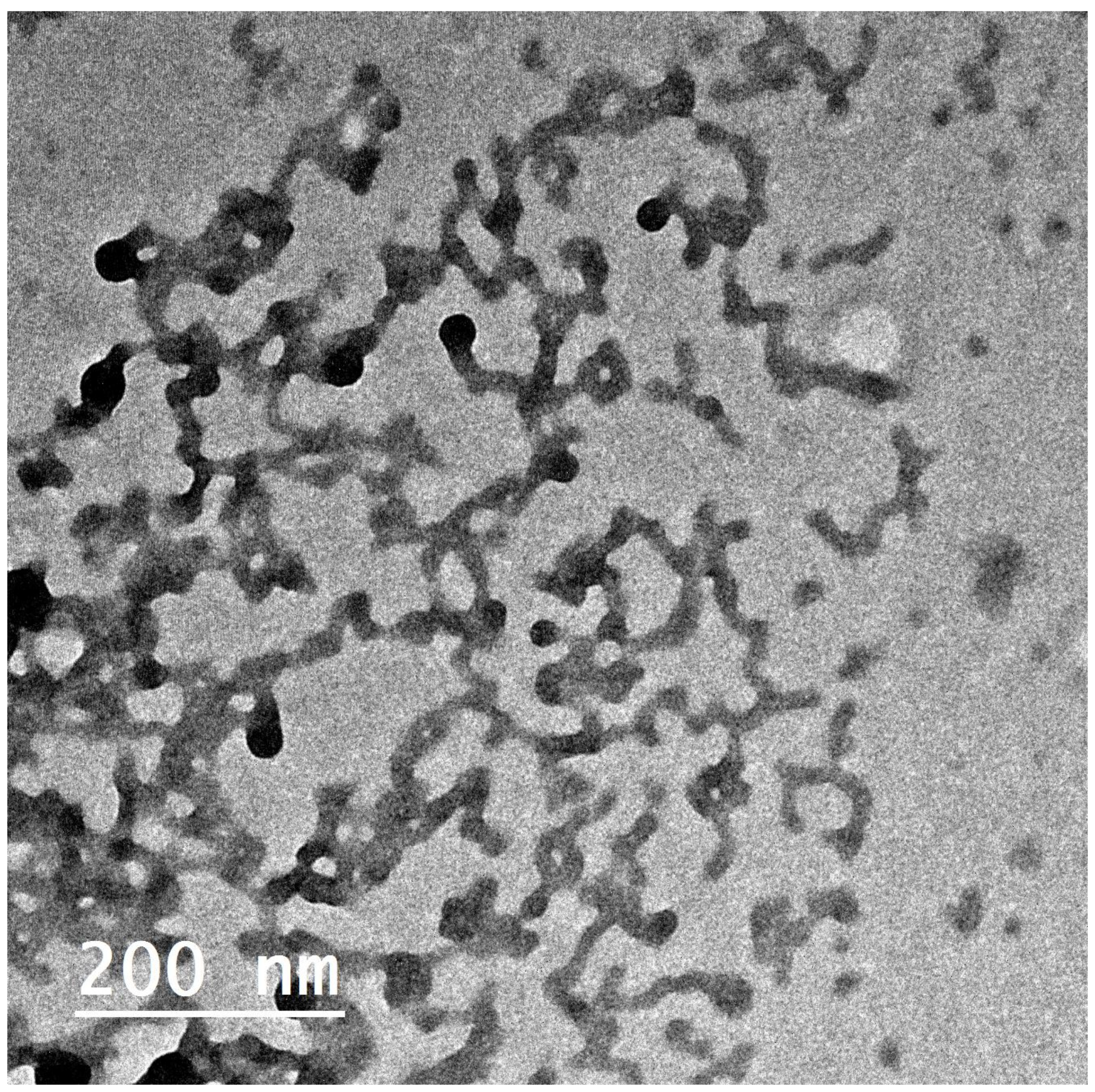
3.4.1. Morphology
3.4.2. Differential Scanning Calorimetry Studies
3.4.3. Stability Study
3.5. Evaluation of FEX-PMM Hydrogel
3.5.1. Determination of pH
3.5.2. Rheological Studies
3.5.3. Measurement of Gel Spreadability
3.5.4. Ex Vivo Muco-Adhesive Strength
3.5.5. In Vitro Drug Release Study
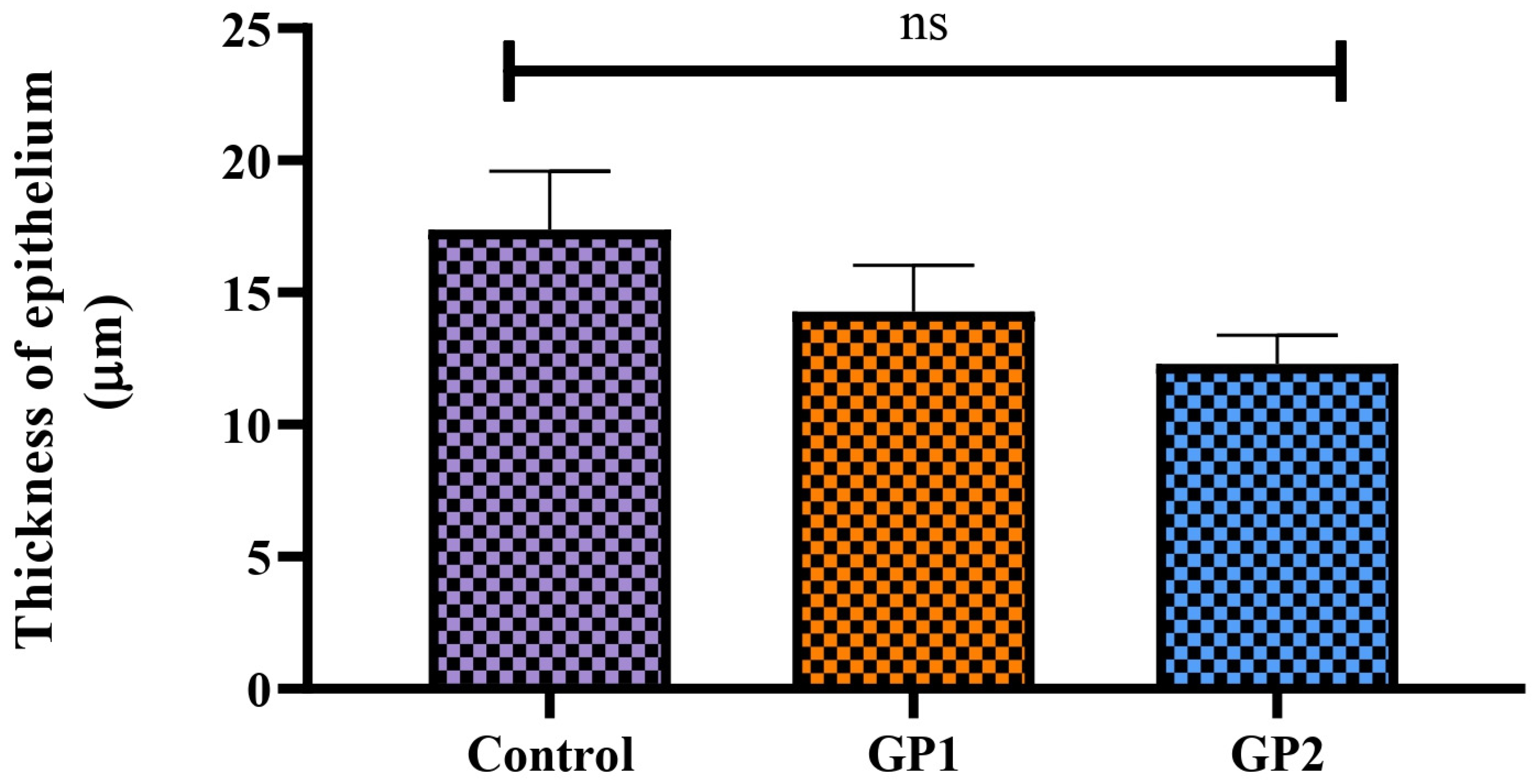
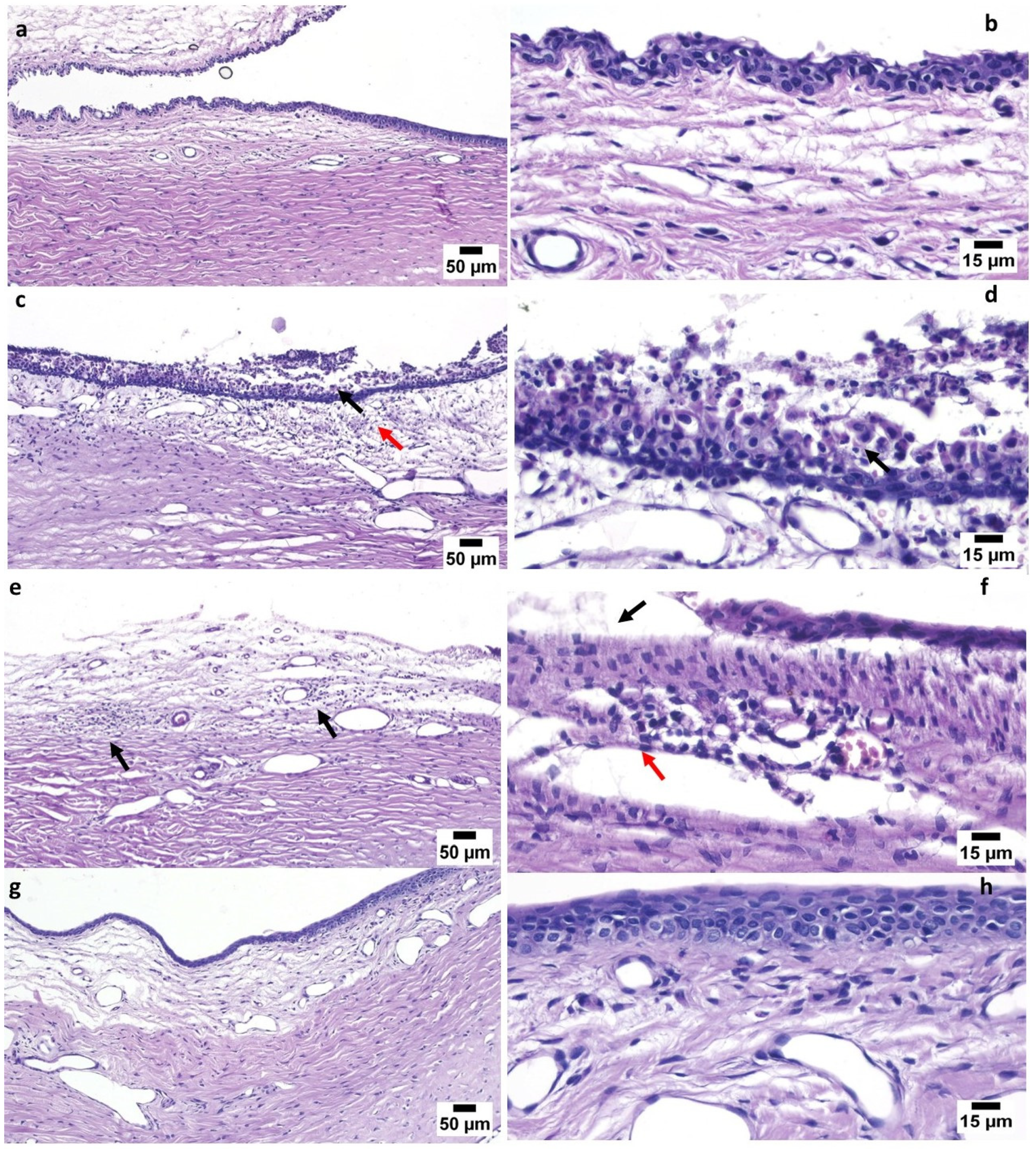
3.6. In Vivo Studies
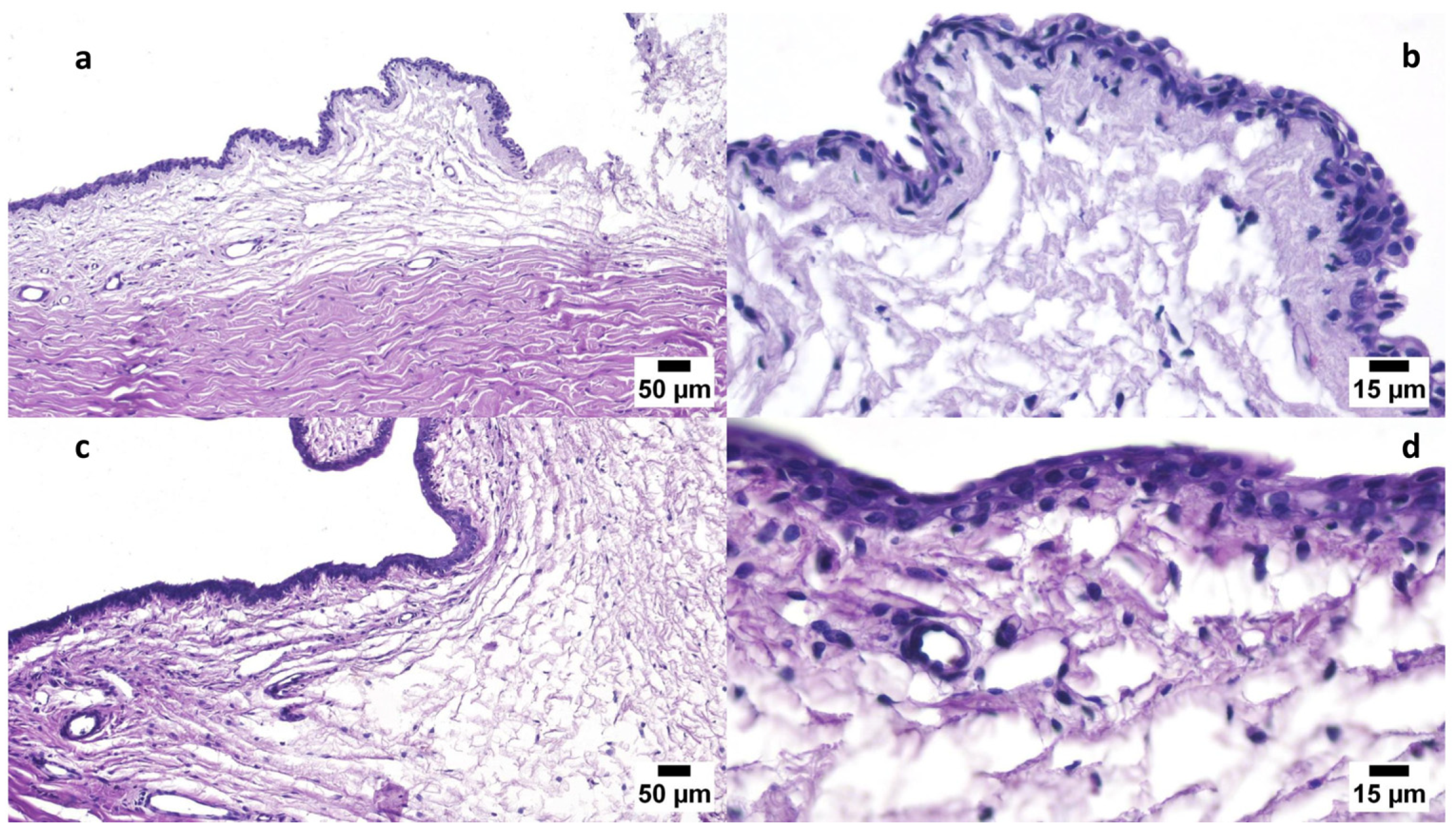
3.6.1. Draize Test
3.6.2. Pharmacodynamics Study
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Okur, N.U.; Yozgatli, V.; Okur, M.E. In vitro-in vivo evaluation of tetrahydrozoline-loaded ocular in situ gels on rabbits for allergic conjunctivitis management. Drug Dev. Res. 2020, 81, 716–727. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Bansal, R.; Sharma, A.; Kapil, A. Red Eyes—Conjunctivitis, Corneal Ulcers, Dry Eye Disease, and Acute Uveitis. In Ophthalmic Signs in Practice of Medicine; Gupta, A., Bansal, R., Sharma, A., Kapil, A., Eds.; Springer Nature: Singapore, 2023; pp. 493–542. [Google Scholar]
- Leonardi, A.; Castegnaro, A.; Valerio, A.L.G.; Lazzarini, D. Epidemiology of allergic conjunctivitis: Clinical appearance and treatment patterns in a population-based study. Curr. Opin. Allergy Clin. Immunol. 2015, 15, 482–488. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.C.; Lin, M.T.; Ng, A.H.C.; Wong, T.T.; Mehta, J.S. Nanotechnology for the Treatment of Allergic Conjunctival Diseases. Pharmaceuticals 2020, 13, 351. [Google Scholar] [CrossRef]
- Zi, Y.; Deng, Y.; Ji, M.; Qin, Y.; Nong, L.; Liu, Z.; Jin, M. The effectiveness of olopatadine hydrochloride eye drops for allergic conjunctivitis: Protocol for a systematic review. Medicine 2020, 99, e18618. [Google Scholar] [CrossRef] [PubMed]
- Labib, B.A.; Chigbu, D.I. Therapeutic Targets in Allergic Conjunctivitis. Pharmaceuticals 2022, 15, 547. [Google Scholar] [CrossRef] [PubMed]
- Bielory, L.; Delgado, L.; Katelaris, C.H.; Leonardi, A.; Rosario, N.; Vichyanoud, P. ICON: Diagnosis and management of allergic conjunctivitis. Ann. Allergy Asthma Immunol. 2020, 124, 118–134. [Google Scholar] [CrossRef] [PubMed]
- Reich, S.; Lopez, M.; Leff, J.; Herman, J. DEXTENZA versus Topical Steroid or Antihistamine Therapy for Treatment of Allergic Conjunctivitis. Clin. Ophthalmol. 2024, 18, 473–480. [Google Scholar] [CrossRef]
- Eedara, B.B.; Nyavanandi, D.; Narala, S.; Veerareddy, P.R.; Bandari, S. Improved Dissolution Rate and Intestinal Absorption of Fexofenadine Hydrochloride by the Preparation of Solid Dispersions: In Vitro and In Situ Evaluation. Pharmaceutics 2021, 13, 310. [Google Scholar] [CrossRef]
- Abdelhameed, A.H.; Abdelhafez, W.A.; Saleh, K.; Mohamed, M.S. Formulation, optimization, and in-vivo evaluation of nanostructured lipid carriers loaded with Fexofenadine HCL for oral delivery. J. Drug Deliv. Sci. Technol. 2022, 74, 103607. [Google Scholar] [CrossRef]
- Sultan, A.A.; El Nashar, N.F.; Ashmawy, S.M.; El Maghraby, G.M. Cubosomes for Enhancing Intestinal Absorption of Fexofenadine Hydrochloride: In situ and in vivo Investigation. Int. J. Nanomed. 2022, 17, 3543–3560. [Google Scholar] [CrossRef]
- Nasr, A.M.; Qushawy, M.K.; Elkhoudary, M.M.; Gawish, A.Y.; Elhady, S.S.; Swidan, S.A. Quality by Design for the Development and Analysis of Enhanced In-Situ Forming Vesicles for the Improvement of the Bioavailability of Fexofenadine HCl In Vitro and In Vivo. Pharmaceutics 2020, 12, 409. [Google Scholar] [CrossRef] [PubMed]
- Abdelhameed, A.H.; Abdelhafez, W.A.; Saleh, K.I.; Hamad, A.A.; Mohamed, M.S. Formulation and optimization of oral fast dissolving films loaded with nanosuspension to enhance the oral bioavailability of Fexofenadine HCL. J. Drug Deliv. Sci. Technol. 2023, 85, 104578. [Google Scholar] [CrossRef]
- Piao, H.M.; Balakrishnan, P.; Cho, H.J.; Kim, H.; Kim, Y.S.; Chung, S.J.; Shim, C.K.; Kim, D.D. Preparation and evaluation of fexofenadine microemulsions for intranasal delivery. Int. J. Pharm. 2010, 395, 309–316. [Google Scholar] [CrossRef] [PubMed]
- El-Dakroury, W.A.; Zewail, M.B.; Asaad, G.F.; Abdallah, H.M.I.; Shabana, M.E.; Said, A.R.; Doghish, A.S.; Azab, H.A.; Amer, D.H.; Hassan, A.E.; et al. Fexofenadine-loaded chitosan coated solid lipid nanoparticles (SLNs): A potential oral therapy for ulcerative colitis. Eur. J. Pharm. Biopharm. 2024, 196, 114205. [Google Scholar] [CrossRef] [PubMed]
- Güven, U.M.; Başaran, E. In vitro-in vivo evaluation of olopatadine incorporated chitosan nanoparticles for the treatment of ocular allergy. J. Drug Deliv. Sci. Technol. 2021, 64, 102518. [Google Scholar] [CrossRef]
- Tyagi, R.; Bhalla, V.; Mondal, P. Novel approaches in ophthalmic drug delivery: A comprehensive review. J. Popul. Ther. Clin. Pharmacol. 2023, 30, 604–621. [Google Scholar]
- Su, S.; Kang, M.P. Recent Advances in Nanocarrier-Assisted Therapeutics Delivery Systems. Pharmaceutics 2020, 12, 837. [Google Scholar] [CrossRef]
- Datta, D.; Priyanka Bandi, S.; Colaco, V.; Dhas, N.; Siva Reddy, D.V.; Vora, L.K. Fostering the unleashing potential of nanocarriers-mediated delivery of ocular therapeutics. Int. J. Pharm. 2024, 658, 124192. [Google Scholar] [CrossRef]
- Pham, D.T.; Chokamonsirikun, A.; Phattaravorakarn, V.; Tiyaboonchai, W. Polymeric micelles for pulmonary drug delivery: A comprehensive review. J. Mater. Sci. 2021, 56, 2016–2036. [Google Scholar] [CrossRef]
- Patel, H.S.; Shaikh, S.J.; Ray, D.; Aswal, V.K.; Vaidya, F.; Pathak, C.; Sharma, R.K. Formulation, Solubilization, and In Vitro Characterization of Quercetin-Incorporated Mixed Micelles of PEO-PPO-PEO Block Copolymers. Appl. Biochem. Biotechnol. 2022, 194, 445–463. [Google Scholar] [CrossRef]
- Mahajan, H.; Patel, H.S.; Ray, D.; Aswal, V.K.; Sharma, R.K.; Tandel, H. Mixed Pluronic/lecithin micelles formulation for oral bioavailability of candesartan cilexetil drug: In vitro characterization and in vivo pharmacokinetic investigations. Drug Dev. Ind. Pharm. 2024, 50, 23–35. [Google Scholar] [CrossRef] [PubMed]
- Signorini, S.; Delledonne, A.; Pescina, S.; Bianchera, A.; Sissa, C.; Vivero-Lopez, M.; Alvarez-Lorenzo, C.; Santi, P.; Padula, C.; Nicoli, S. A sterilizable platform based on crosslinked xanthan gum for controlled-release of polymeric micelles: Ocular application for the delivery of neuroprotective compounds to the posterior eye segment. Int. J. Pharm. 2024, 657, 124141. [Google Scholar] [CrossRef] [PubMed]
- Devi, S.; Bhatt, S.; Saini, V.; Kumar, M.; Deep, A. A Review on Polymeric Nano Micelles Based Delivery to the Posterior Segment of the Eye. Nanosci. Nanotechnol.-Asia 2020, 10, 591–601. [Google Scholar] [CrossRef]
- Safwat, M.A.; Mansour, H.F.; Hussein, A.K.; Abdelwahab, S.; Soliman, G.M. Polymeric micelles for the ocular delivery of triamcinolone acetonide: Preparation and in vivo evaluation in a rabbit ocular inflammatory model. Drug Deliv. 2020, 27, 1115–1124. [Google Scholar] [CrossRef] [PubMed]
- Naharros-Molinero, A.; Caballo-González, M.Á.; de la Mata, F.J.; García-Gallego, S. Direct and Reverse Pluronic Micelles: Design and Characterization of Promising Drug Delivery Nanosystems. Pharmaceutics 2022, 14, 2628. [Google Scholar] [CrossRef] [PubMed]
- Kadam, Y.; Yerramilli, U.; Bahadur, A. Solubilization of poorly water-soluble drug carbamezapine in Pluronic® micelles: Effect of molecular characteristics, temperature and added salt on the solubilizing capacity. Colloids Surf. B Biointerfaces 2009, 72, 141–147. [Google Scholar] [CrossRef] [PubMed]
- Kaur, J.; Singla, P.; Kaur, I. Binary Pluronics based mixed micellar systems: Effective solution for improved solubilization of Biochanin A. Spectrochim. Acta Part. A Mol. Biomol. Spectrosc. 2024, 304, 123279. [Google Scholar] [CrossRef]
- Basalious, E.B.; Shamma, R.N. Novel self-assembled nano-tubular mixed micelles of Pluronics P123, Pluronic F127 and phosphatidylcholine for oral delivery of nimodipine: In vitro characterization, ex vivo transport and in vivo pharmacokinetic studies. Int. J. Pharm. 2015, 493, 347–356. [Google Scholar] [CrossRef] [PubMed]
- Patel, H.S.; Kunjadiya, A.; Rahdar, A.; Sharma, R.K. Pluronic-phosphatidylcholine mixed polymeric nanomicellar formulation for curcumin drug bioavailability: Design, fabrication, characterization and in vitro bioinvestigations. J. Bioact. Compat. Polym. 2023, 38, 191–208. [Google Scholar] [CrossRef]
- Abdellatif, M.M.; Josef, M.; El-Nabarawi, M.A.; Teaima, M. Sertaconazole-Nitrate-Loaded Leciplex for Treating Keratomycosis: Optimization Using D-Optimal Design and In Vitro, Ex Vivo, and In Vivo Studies. Pharmaceutics 2022, 14, 2215. [Google Scholar] [CrossRef]
- Sipos, B.; Katona, G.; Csóka, I. Risperidone-Loaded Nasal Thermosensitive Polymeric Micelles: Quality by Design-Based Formulation Study. Pharmaceutics 2024, 16, 703. [Google Scholar] [CrossRef] [PubMed]
- Shen, C.; Zhu, J.; Song, J.; Wang, J.; Shen, B.; Yuan, H.; Li, X. Formulation of pluronic F127/TPGS mixed micelles to improve the oral absorption of glycyrrhizic acid. Drug Dev. Ind. Pharm. 2020, 46, 1100–1107. [Google Scholar] [CrossRef] [PubMed]
- El-Dahmy, R.M.; Elsayed, I.; Elshafeey, A.H.; Gawad, N.A.A.E.; El-Gazayerly, O.N. Optimization of long circulating mixed polymeric micelles containing vinpocetine using simple lattice mixture design, in vitro and in vivo characterization. Int. J. Pharm. 2014, 477, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Abdellatif, M.M.; Ahmed, S.M.; El-Nabarawi, M.A.; Teaima, M. Oral Bioavailability Enhancement of Vancomycin Hydrochloride with Cationic Nanocarrier (Leciplex): Optimization, In Vitro, Ex Vivo, and In Vivo Studies. Sci. Pharm. 2023, 91, 1. [Google Scholar] [CrossRef]
- Kassem, A.; Refai, H.; El-Nabarawi, M.A.; Abdellatif, M.M. Formulation and Evaluation of Prednisolone Sodium Metazoate-Loaded Mucoadhesive Quatsomal Gel for Local Treatment of Recurrent Aphthous Ulcers: Optimization, In Vitro, Ex Vivo, and In Vivo Studies. Pharmaceutics 2023, 15, 1947. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, M.; Makky, A.; Abdellatif, M. Liposomal gels as carriers for safer topical delivery of tazarotene. J. Pharm. Res. Opin. 2013, 3, 82–90. [Google Scholar]
- Abdelbary, G.A.; Amin, M.M.; Zakaria, M.Y. Ocular ketoconazole-loaded proniosomal gels: Formulation, ex vivo corneal permeation and in vivo studies. Drug Deliv. 2017, 24, 309–319. [Google Scholar] [CrossRef] [PubMed]
- Yehia, S.A.; El-Ridi, M.S.; Tadros, M.I.; El-Sherif, N.G. Enhancement of the Oral Bioavailability of Fexofenadine Hydrochloride via Cremophor((R)) El-Based Liquisolid Tablets. Adv. Pharm. Bull. 2015, 5, 569–581. [Google Scholar] [CrossRef]
- Ghezzi, M.; Pescina, S.; Padula, C.; Santi, P.; Del Favero, E.; Cantù, L.; Nicoli, S. Polymeric micelles in drug delivery: An insight of the techniques for their characterization and assessment in biorelevant conditions. J. Control. Release 2021, 332, 312–336. [Google Scholar] [CrossRef]
- Suksiriworapong, J.; Rungvimolsin, T.; A-gomol, A.; Junyaprasert, V.B.; Chantasart, D. Development and Characterization of Lyophilized Diazepam-Loaded Polymeric Micelles. AAPS PharmSciTech 2014, 15, 52–64. [Google Scholar] [CrossRef]
- Toma, I.; Tefas, L.R.; Bogdan, C.; Tomuță, I. Development and characterization of Loratadine liposomal gel using QbD approach. Farmacia 2022, 70, 204–213. [Google Scholar] [CrossRef]
- Khurana, B.; Arora, D.; Narang, R.K. QbD based exploration of resveratrol loaded polymeric micelles based carbomer gel for topical treatment of plaque psoriasis: In vitro, ex vivo and in vivo studies. J. Drug Deliv. Sci. Technol. 2020, 59, 101901. [Google Scholar] [CrossRef]
- Ranch, K.M.; Maulvi, F.A.; Naik, M.J.; Koli, A.R.; Parikh, R.K.; Shah, D.O. Optimization of a novel in situ gel for sustained ocular drug delivery using Box-Behnken design: In vitro, ex vivo, in vivo and human studies. Int. J. Pharm. 2019, 554, 264–275. [Google Scholar] [CrossRef] [PubMed]
- Soliman, K.A.; Ullah, K.; Shah, A.; Jones, D.S.; Singh, T.R.R. Poloxamer-based in situ gelling thermoresponsive systems for ocular drug delivery applications. Drug Discov. Today 2019, 24, 1575–1586. [Google Scholar] [CrossRef] [PubMed]
- Talaei, S.; Mahboobian, M.M.; Mohammadi, M. Investigating the ocular toxicity potential and therapeutic efficiency of in situ gel nanoemulsion formulations of brinzolamide. Toxicol. Res. 2020, 9, 578–587. [Google Scholar] [CrossRef] [PubMed]
- Shokry, M.; Hathout, R.M.; Mansour, S. Exploring gelatin nanoparticles as novel nanocarriers for Timolol Maleate: Augmented in-vivo efficacy and safe histological profile. Int. J. Pharm. 2018, 545, 229–239. [Google Scholar] [CrossRef] [PubMed]
- Gumieniczek, A.; Hopkała, H.; Roliński, J.; Bojarska-Junak, A. Antioxidative and anti-inflammatory effects of repaglinide in plasma of diabetic animals. Pharmacol. Res. 2005, 52, 162–166. [Google Scholar] [CrossRef] [PubMed]
- El-Emam, G.A.; Girgis, G.N.S.; Hamed, M.F.; El-Azeem Soliman, O.A.; Abd El Gawad, A.E.G.H. Formulation and Pathohistological Study of Mizolastine–Solid Lipid Nanoparticles–Loaded Ocular Hydrogels. Int. J. Nanomed. 2021, 16, 7775–7799. [Google Scholar] [CrossRef] [PubMed]
- Sebbag, L.; Allbaugh, R.A.; Weaver, A.; Seo, Y.-J.; Mochel, J.P. Histamine-induced conjunctivitis and breakdown of blood–tear barrier in dogs: A model for ocular pharmacology and therapeutics. Front. Pharmacol. 2019, 10, 752. [Google Scholar] [CrossRef]
- Mandal, A.; Bisht, R.; Rupenthal, I.D.; Mitra, A.K. Polymeric micelles for ocular drug delivery: From structural frameworks to recent preclinical studies. J. Control. Release 2017, 248, 96–116. [Google Scholar] [CrossRef]
- Li, X.; Xia, X.; Zhang, J.; Adu-Frimpong, M.; Shen, X.; Yin, W.; He, Q.; Rong, W.; Shi, F.; Cao, X.; et al. Preparation, Physical Characterization, Pharmacokinetics and Anti-Hyperglycemic Activity of Esculetin-Loaded Mixed Micelles. J. Pharm. Sci. 2023, 112, 148–157. [Google Scholar] [CrossRef]
- Semenova, M.N.; Melik-Nubarov, N.S.; Semenov, V.V. Application of Pluronics for Enhancing Aqueous Solubility of Lipophilic Microtubule Destabilizing Compounds on the Sea Urchin Embryo Model. Int. J. Mol. Sci. 2023, 24, 14695. [Google Scholar] [CrossRef]
- Dutra, L.M.U.; Ribeiro, M.E.N.P.; Cavalcante, I.M.; Brito, D.H.A.D.; Semião, L.D.M.; Silva, R.F.D.; Fechine, P.B.A.; Yeates, S.G.; Ricardo, N.M.P.S. Binary mixture micellar systems of F127 and P123 for griseofulvin solubilisation. Polímeros 2015, 25, 433–439. [Google Scholar] [CrossRef]
- Fares, A.R.; ElMeshad, A.N.; Kassem, M.A.A. Enhancement of dissolution and oral bioavailability of lacidipine via pluronic P123/F127 mixed polymeric micelles: Formulation, optimization using central composite design and in vivo bioavailability study. Drug Deliv. 2018, 25, 132–142. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Qiu, L. Optimizing particle size of docetaxel-loaded micelles for enhanced treatment of oral epidermoid carcinoma. Nanomedicine 2016, 12, 1941–1949. [Google Scholar] [CrossRef]
- Nasr, M.; Hashem, F.; Teiama, M.; Tantawy, N.; Abdelmoniem, R. Folic acid grafted mixed polymeric micelles as a targeted delivery strategy for tamoxifen citrate in treatment of breast cancer. Drug Deliv. Transl. Res. 2024, 14, 945–958. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Liu, M.; Ke, L.; Wang, L.-J.; Wu, C.; Li, C.; Li, Z.; Wu, Y.-L. Flexible polymeric nanosized micelles for ophthalmic drug delivery: Research progress in the last three years. Nanoscale Adv. 2021, 3, 5240–5254. [Google Scholar] [CrossRef]
- Razavi, M.S.; Ebrahimnejad, P.; Fatahi, Y.; D’Emanuele, A.; Dinarvand, R. Recent Developments of Nanostructures for the Ocular Delivery of Natural Compounds. Front. Chem. 2022, 10, 850757. [Google Scholar] [CrossRef] [PubMed]
- Patel, D.; Patel, D.; Ray, D.; Kuperkar, K.; Aswal, V.K.; Bahadur, P. Single and mixed Pluronics® micelles with solubilized hydrophobic additives: Underscoring the aqueous solution demeanor and micellar transition. J. Mol. Liq. 2021, 343, 117625. [Google Scholar] [CrossRef]
- Vara, J.; Gualdesi, M.S.; Fernández, M.A.; Ortiz, C.S. Design of polymeric carriers to enhance antimicrobial photodynamic inactivation. J. Drug Deliv. Sci. Technol. 2024, 94, 105494. [Google Scholar] [CrossRef]
- Mingkwan, T.; Suksiriworapong, J.; Chantasart, D. Pluronic® P123/TPGS and pluronic® F127/TPGS mixed micelles for the entrapment of itraconazole. Chiang Mai J. Sci. 2015, 42, 946–956. [Google Scholar]
- Kassem, A.A.; Abd El-Alim, S.H.; Basha, M.; Salama, A. Phospholipid complex enriched micelles: A novel drug delivery approach for promoting the antidiabetic effect of repaglinide. Eur. J. Pharm. Sci. 2017, 99, 75–84. [Google Scholar] [CrossRef]
- Popovici, C.; Popa, M.; Sunel, V.; Atanase, L.I.; Ichim, D.L. Drug Delivery Systems Based on Pluronic Micelles with Antimicrobial Activity. Polymers 2022, 14, 3007. [Google Scholar] [CrossRef]
- Khatoon, K.; Rizwanullah, M.; Amin, S.; Mir, S.R.; Akhter, S. Cilnidipine loaded transfersomes for transdermal application: Formulation optimization, in-vitro and in-vivo study. J. Drug Deliv. Sci. Technol. 2019, 54, 101303. [Google Scholar] [CrossRef]
- Danaei, M.; Dehghankhold, M.; Ataei, S.; Hasanzadeh Davarani, F.; Javanmard, R.; Dokhani, A.; Khorasani, S.; Mozafari, M.R. Impact of Particle Size and Polydispersity Index on the Clinical Applications of Lipidic Nanocarrier Systems. Pharmaceutics 2018, 10, 57. [Google Scholar] [CrossRef]
- Gerardos, A.M.; Balafouti, A.; Pispas, S. Mixed Copolymer Micelles for Nanomedicine. Nanomanufacturing 2023, 3, 233–247. [Google Scholar] [CrossRef]
- Özsoy, Y.; Güngör, S.; Kahraman, E.; Durgun, M.E. Chapter 4—Polymeric micelles as a novel carrier for ocular drug delivery. In Nanoarchitectonics in Biomedicine; Grumezescu, A.M., Ed.; William Andrew Publishing: Norwich, NY, USA, 2019; pp. 85–117. [Google Scholar]
- Xiong, X.-B.; Falamarzian, A.; Garg, S.M.; Lavasanifar, A. Engineering of amphiphilic block copolymers for polymeric micellar drug and gene delivery. J. Control. Release 2011, 155, 248–261. [Google Scholar] [CrossRef] [PubMed]
- Terreni, E.; Chetoni, P.; Tampucci, S.; Burgalassi, S.; Al-kinani, A.A.; Alany, R.G.; Monti, D. Assembling Surfactants-Mucoadhesive Polymer Nanomicelles (ASMP-Nano) for Ocular Delivery of Cyclosporine-A. Pharmaceutics 2020, 12, 253. [Google Scholar] [CrossRef] [PubMed]
- Wei, Z.; Hao, J.; Yuan, S.; Li, Y.; Juan, W.; Sha, X.; Fang, X. Paclitaxel-loaded Pluronic P123/F127 mixed polymeric micelles: Formulation, optimization and in vitro characterization. Int. J. Pharm. 2009, 376, 176–185. [Google Scholar] [CrossRef]
- Patil, S.S.; Chougale, R.D.; Manjappa, A.S.; Disouza, J.I.; Hajare, A.A.; Patil, K.S. Statistically developed docetaxel-laden mixed micelles for improved therapy of breast cancer. OpenNano 2022, 8, 100079. [Google Scholar] [CrossRef]
- Aburahma, M.H.; Abdelbary, G.A. Novel diphenyl dimethyl bicarboxylate provesicular powders with enhanced hepatocurative activity: Preparation, optimization, in vitro/in vivo evaluation. Int. J. Pharm. 2012, 422, 139–150. [Google Scholar] [CrossRef] [PubMed]
- Gupta, B.; Mishra, V.; Gharat, S.; Momin, M.; Omri, A. Cellulosic Polymers for Enhancing Drug Bioavailability in Ocular Drug Delivery Systems. Pharmaceuticals 2021, 14, 1201. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Sha, X.; Jiang, X.; Chen, Y.; Ren, Q.; Fang, X. Pluronic P105/F127 mixed micelles for the delivery of docetaxel against Taxol-resistant non-small cell lung cancer: Optimization and in vitro, in vivo evaluation. Int. J. Nanomed. 2013, 8, 73–84. [Google Scholar] [CrossRef]
- Fouda, N.H.; Abdelrehim, R.T.; Hegazy, D.A.; Habib, B.A. Sustained ocular delivery of Dorzolamide-HCl via proniosomal gel formulation: In-vitro characterization, statistical optimization, and in-vivo pharmacodynamic evaluation in rabbits. Drug Deliv. 2018, 25, 1340–1349. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; He, Z.; Liang, R.; Ma, Y.; Huang, W.; Jiang, R.; Shi, S.; Chen, H.; Li, X. Fabrication of a Micellar Supramolecular Hydrogel for Ocular Drug Delivery. Biomacromolecules 2016, 17, 798–807. [Google Scholar] [CrossRef] [PubMed]
- Modi, D.; Mohammad; Warsi, M.H.; Garg, V.; Bhatia, M.; Kesharwani, P.; Jain, G.K. Formulation development, optimization, and in vitro assessment of thermoresponsive ophthalmic pluronic F127-chitosan in situ tacrolimus gel. J. Biomater. Sci. Polym. Ed. 2021, 32, 1678–1702. [Google Scholar] [CrossRef] [PubMed]
- Khare, A.; Grover, K.; Pawar, P.; Singh, I. Mucoadhesive polymers for enhancing retention in ocular drug delivery. In Progress in Adhesion and Adhesives; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2015; pp. 451–484. [Google Scholar]
- Bhatt, P.; Narvekar, P.; Lalani, R.; Chougule, M.B.; Pathak, Y.; Sutariya, V. An in vitro Assessment of Thermo-Reversible Gel Formulation Containing Sunitinib Nanoparticles for Neovascular Age-Related Macular Degeneration. AAPS PharmSciTech 2019, 20, 281. [Google Scholar] [CrossRef] [PubMed]
- Kompella, U.B.; Hartman, R.R.; Patil, M.A. Extraocular, periocular, and intraocular routes for sustained drug delivery for glaucoma. Prog. Retin. Eye Res. 2021, 82, 100901. [Google Scholar] [CrossRef] [PubMed]
- Binkhathlan, Z.; Ali, R.; Alomrani, A.H.; Abul Kalam, M.; Alshamsan, A.; Lavasanifar, A. Role of Polymeric Micelles in Ocular Drug Delivery: An Overview of Decades of Research. Mol. Pharm. 2023, 20, 5359–5382. [Google Scholar] [CrossRef]
- Pescina, S.; Grolli Lucca, L.; Govoni, P.; Padula, C.; Del Favero, E.; Cantù, L.; Santi, P.; Nicoli, S. Ex Vivo Conjunctival Retention and Transconjunctival Transport of Poorly Soluble Drugs Using Polymeric Micelles. Pharmaceutics 2019, 11, 476. [Google Scholar] [CrossRef]



| Factors | Levels | ||
|---|---|---|---|
| X1: PL90G amount (mg) | 33 | 49.5 | 66 |
| X2: Pluronic (F127 and P123) mixture ratio | 1:1 | 1:2 | 2:1 |
| Response | Desirability constraints | ||
| Y1: EE (%) | Maximize | ||
| Y2: PS (nm) | Minimize | ||
| Y3: ZP (mV) | In the measured range of formulae | ||
| Y4: Q6h (%) | Maximize | ||
| Formula Code | PL90G Amount (mg) (X1) | Pluronic (F127 and P123) Mixture Ratio (X2) | EE % (Y1) | PS (nm) (Y2) | PDI | ZP (mV) (Y3) | Q6h (%) (Y4) |
|---|---|---|---|---|---|---|---|
| F1 | 33 | 2:1 | 51.20 ± 2.75 | 385.95 ± 4.23 | 0.33 ± 0.008 | −5.52 ± 0.12 | 85.60 ± 1.26 |
| F2 | 33 | 1:1 | 62.70 ± 1.41 | 302.20 ± 9.03 | 0.45 ± 0.002 | −6.67 ± 0.36 | 67.33 ± 1.03 |
| F3 | 33 | 1:2 | 79.35 ± 2.61 | 236.35 ± 6.43 | 0.49 ± 0.005 | −5.84 ± 0.12 | 95.38 ± 0.92 |
| F4 | 49.5 | 2:1 | 66.99 ± 2.19 | 515.95 ± 3.60 | 0.48 ± 0.025 | −7.76 ± 0.05 | 81.84 ± 0.98 |
| F5 | 49.5 | 1:1 | 79.10 ± 1.62 | 455.45 ± 5.41 | 0.26 ± 0.005 | −7.82 ± 0.48 | 57.74 ± 1.17 |
| F6 | 49.5 | 1:2 | 87.87 ± 3.53 | 360.95 ± 5.16 | 0.41 ± 0.043 | −7.32 ± 0.28 | 91.46 ± 1.38 |
| F7 | 66 | 2:1 | 77.80 ± 0.92 | 633.50 ± 2.44 | 0.57 ± 0.010 | −15.47 ± 0.33 | 75.57 ± 1.29 |
| F8 | 66 | 1:1 | 89.48 ± 1.90 | 559.05 ± 1.62 | 0.52 ± 0.020 | −14.12 ± 0.53 | 50.27 ± 1.11 |
| F9 | 66 | 1:2 | 97.25 ± 1.48 | 514.00 ± 6.26 | 0.47 ± 0.018 | −14.67 ± 0.23 | 91.07 ± 1.06 |
| Source | EE (%) | PS (nm) | ZP (mV) | Q6h (%) |
|---|---|---|---|---|
| p Value | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
| Model | Linear | Linear | Quadratic | 2F1 |
| X1 = A; Amount of PL90G | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
| X2 = B; Pluronic (F127 and P123) mixture ratio | <0.0001 | <0.0001 | 0.4646 | <0.0001 |
| Adequate precision | 31.96 | 31.96 | 36.44 | 52.00 |
| R2 | 0.9895 | 0.9895 | 0.9977 | 0.9969 |
| Adjusted R2 | 0.9791 | 0.9791 | 0.9939 | 0.9939 |
| Predicted R2 | 0.9345 | 0.9345 | 0.9865 | 0.9654 |
| Significant factors | X1, X2 | X1, X2 | X1 | X1, X2 |
| HPMC Percent (%) | pH | Spreadability (g.cm/s) | MS (dyne/cm2) | Viscosity Cp | Qh8 (%) |
|---|---|---|---|---|---|
| 2% | 7.4 ± 0.15 | 9.79 ± 0.23 | 16,190 ± 62.09 | 698.5 ± 20.50 | 75.15 ± 1.3 |
| 4% | 7.3 ± 0.2 | 7.26 ± 0.08 | 18,147 ± 49.12 | 2397 ± 20.75 | 60.97 ± 1.20 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
El-Shahed, S.A.; Hassan, D.H.; El-Nabarawi, M.A.; El-Setouhy, D.A.; Abdellatif, M.M. Polymeric Mixed Micelle-Loaded Hydrogel for the Ocular Delivery of Fexofenadine for Treating Allergic Conjunctivitis. Polymers 2024, 16, 2240. https://doi.org/10.3390/polym16162240
El-Shahed SA, Hassan DH, El-Nabarawi MA, El-Setouhy DA, Abdellatif MM. Polymeric Mixed Micelle-Loaded Hydrogel for the Ocular Delivery of Fexofenadine for Treating Allergic Conjunctivitis. Polymers. 2024; 16(16):2240. https://doi.org/10.3390/polym16162240
Chicago/Turabian StyleEl-Shahed, Sherouk A., Doaa H. Hassan, Mohamed A. El-Nabarawi, Doaa Ahmed El-Setouhy, and Menna M. Abdellatif. 2024. "Polymeric Mixed Micelle-Loaded Hydrogel for the Ocular Delivery of Fexofenadine for Treating Allergic Conjunctivitis" Polymers 16, no. 16: 2240. https://doi.org/10.3390/polym16162240
APA StyleEl-Shahed, S. A., Hassan, D. H., El-Nabarawi, M. A., El-Setouhy, D. A., & Abdellatif, M. M. (2024). Polymeric Mixed Micelle-Loaded Hydrogel for the Ocular Delivery of Fexofenadine for Treating Allergic Conjunctivitis. Polymers, 16(16), 2240. https://doi.org/10.3390/polym16162240







