Building Chemical Interface Layers in Functionalized Graphene Oxide/Rubber Composites to Achieve Enhanced Mechanical Properties and Thermal Control Capability of Tires
Abstract
:1. Introduction
2. Experimental Methods
2.1. Materials
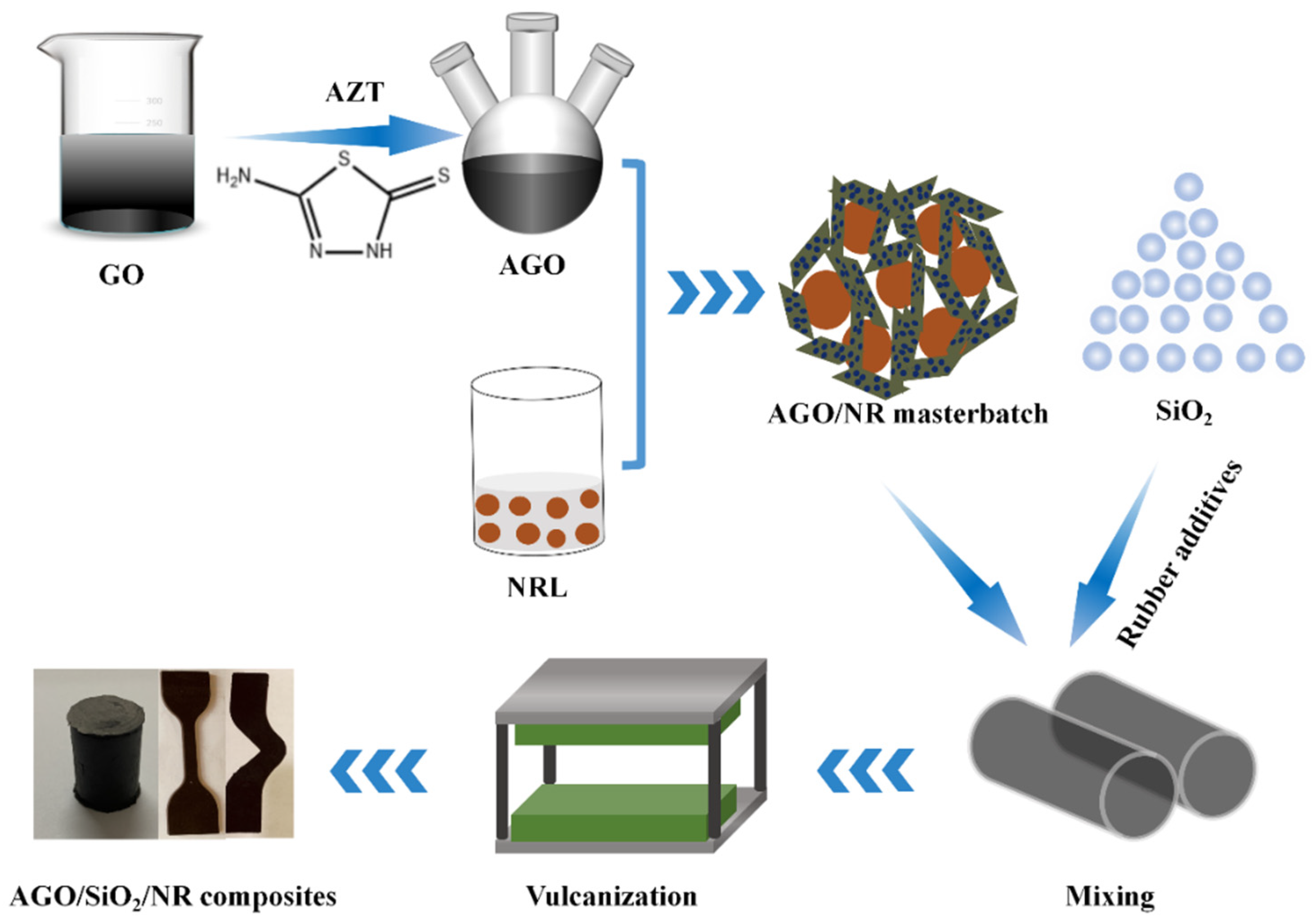
2.2. Preparation of AGO Fillers
2.3. Preparation of AGO/SiO2/NR Composites
2.4. Characterizations
3. Results and Discussion
3.1. Characterization of AGO
3.2. Vulcanization and Cross-Linking Properties of AGO/SiO2/NR Composites
3.3. Morphologies of NR/MGO Composites
3.4. Heat Build-Up and Thermal Conductivity Properties of NR Composites
3.5. Finite Element Simulation of Tire Temperature Field
3.6. Mechanical Properties of NR Composites
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Le, D.T.; Nguyen, D.T.; Le, N.D.; Nguyen, T.L. Traction Control Based on Wheel Slip Tracking of a Quarter-Vehicle Model with High-Gain Observers. Int. J. Dynam. Control. 2022, 10, 1130–1137. [Google Scholar] [CrossRef]
- Hakimi, N.M.F.; Lee, S.H.; Lum, W.C.; Mohamad, S.F.; Osman Al Edrus, S.S.; Park, B.-D.; Azmi, A. Surface Modified Nanocellulose and Its Reinforcement in Natural Rubber Matrix Nanocomposites: A Review. Polymer 2021, 13, 3241. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Yu, G.; Zhuang, T.; Pang, X. Reinforcing Effects of Carbon Nanotubes on Graphene/Trans-1,4-Polyisoprene/Natural Rubber Composites. J. Polym. Res. 2022, 29, 387. [Google Scholar] [CrossRef]
- Lima, M.A.; Loureiro, B.V.; Da Cunha Ribeiro, D.; Barbosa, J.P.; De Oliveira Dos Santos, M.; Morbach Dixini, P.V.; Siqueira, R.D.N. Optimized Sulfur Curing of Pulse-Damping Rubber Tubes. Mater. Chem. Phys. 2024, 319, 129369. [Google Scholar] [CrossRef]
- Hosseinmardi, A.; Amiralian, N.; Martin, D.J.; Annamalai, P.K. Achieving Ultra-Tear Resistant High-Performance Natural Rubber Nanocomposite via Bio-Inspired Lignocellulosic Compatibilization. Ind. Crop. Prod. 2024, 207, 117729. [Google Scholar] [CrossRef]
- Shi, X.; Sun, S.; Zhao, A.; Zhang, H.; Zuo, M.; Song, Y.; Zheng, Q. Influence of Carbon Black on the Payne Effect of Filled Natural Rubber Compounds. Compos. Sci. Technol. 2021, 203, 108586. [Google Scholar] [CrossRef]
- Wang, X.; Luo, Z.; Liang, J.; Zhong, J. Hybrid Enhancement of Silica and Aramid Pulp on Improving Performance and Reducing Dynamic Heat Generation of Natural Rubber Composites. Polym. Compos. 2023, 44, 5579–5588. [Google Scholar] [CrossRef]
- Ruamcharoen, J.; Munlee, R.; Ruamcharoen, P. Eco-friendly Bio-based Composites of Cassava Starch and Natural Rubber Compatibilized with Nanoclays. Polym. Compos. 2023, 44, 1071–1082. [Google Scholar] [CrossRef]
- Sethulekshmi, A.S.; Saritha, A.; Joseph, K. A Comprehensive Review on the Recent Advancements in Natural Rubber Nanocomposites. Int. J. Biol. Macromol. 2022, 194, 819–842. [Google Scholar] [CrossRef]
- Yang, G.; Zhang, X.; Wang, R.; Liu, X.; Zhang, J.; Zong, L.; Yang, H. Ultra-Stretchable Graphene Aerogels at Ultralow Temperatures. Mater. Horiz. 2023, 10, 1865–1874. [Google Scholar] [CrossRef]
- Fu, Q.; Yang, Z.; Jia, H.; Wen, Y.; Luo, Y.; Ding, L. Integration of Experimental Methods and Molecular Dynamics Simulations for a Comprehensive Understanding of Enhancement Mechanisms in Graphene Oxide (GO)/Rubber Composites. J. Polym. Res. 2023, 30, 277. [Google Scholar] [CrossRef]
- Li, Z.; Guo, F.; Tian, S.; Liu, R.; Yang, D.; Wang, X.; Hu, H.; Wang, Y.; Zhao, J. Hydroxyl Boron Nitrid e/Natural Rubber Composites with Enhanced Mechanical and Thermal Conduction Properties: Implications for Heat Dissipative Tires or Conveyor Belts. ACS Appl. Nano. Mater. 2023, 6, 5365–5373. [Google Scholar] [CrossRef]
- Aswathy, T.R.; Dash, B.; Dey, P.; Nair, S.; Naskar, K. Synergistic Effect of Graphene with Graphene Oxide, Nanoclay, and Nanosilica in Enhancing the Mechanical and Barrier Properties of Bromobutyl Rubber/Epoxidized Natural Rubber Composites. J. Appl. Polym. Sci. 2021, 138, 50746. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, M.; Lu, J.; Han, F.; Yan, P. Amine-Functionalized Fibrous Sepiolite and Graphene Oxide in Situ Self-Assembled Hybrid Network for Reinforcement of Natural Rubber Composite. J. Polym. Res. 2022, 29, 526. [Google Scholar] [CrossRef]
- Yang, F.; Hao, L.; Zhu, Y.; Liu, H.; Chen, S.; Shao, Y. Preparation of Graphene Modified Melamine Sponge and Solar-Assisted Cleanup of Heavy Oil Spills. J. Environ. Chem. Eng. 2022, 10, 107779. [Google Scholar] [CrossRef]
- Zhou, A.; Bai, J.; Hong, W.; Bai, H. Electrochemically Reduced Graphene Oxide: Preparation, Composites, and Applications. Carbon 2022, 191, 301–332. [Google Scholar] [CrossRef]
- Khakbaz, F.; Razzaghi-Kashani, M. Effect of Reduced Graphene Oxide on Vulcanization of Polydimethylsiloxane Composites. Polym. Compos. 2024, pc.28572. [Google Scholar] [CrossRef]
- Zhong, B.; Dong, H.; Luo, Y. Antioxidant Modified Graphene Oxide for Robust and Highly Aging Resistant Rubber Composites. Compos. Commun. 2023, 37, 101443. [Google Scholar] [CrossRef]
- Li, F.; Wang, H.; Ke, Y.; Hu, Y.; Xia, R. Preparation of GNP/SBR Thermal Conductivity Composites with High Strength and Toughness by Synergistic Construction of Interfacial Multiple Non-covalent and Covalent Bonds. Polym. Compos. 2024, 45, 5633–5642. [Google Scholar] [CrossRef]
- Siriwas, T.; Pichaiyut, S.; Nakason, C. Enhancing the Properties of Epoxidized Natural Rubber-Graphene Nanocomposites through Crosslinking with Imidazole-Activated Dicarboxylic Acid-Cured System. Ind. Crops Prod. 2024, 219, 119040. [Google Scholar] [CrossRef]
- Chu, L.; Kan, M.; Jerrams, S.; Zhang, R.; Xu, Z.; Liu, L.; Wen, S.; Zhang, L. Constructing Chemical Interface Layers by Using Ionic Liquid in Graphene Oxide/Rubber Composites to Achieve High-Wear Resistance in Environmental-Friendly Green Tires. ACS Appl. Mater. Interfaces 2022, 14, 5995–6004. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhou, Q.; Lu, Q.; Han, C.; Zhou, Z.; Liang, Z.; Liu, R.; Nie, Y. Reinforcement and Toughening of Rubber by Bridging Graphene and Nanosilica. J Inorg Organomet P. 2020, 30, 337–348. [Google Scholar] [CrossRef]
- Zhao, S.; Xie, S.; Liu, X.; Shao, X.; Zhao, Z.; Xin, Z.; Li, L. Covalent Hybrid of Graphene and Silicon Dioxide and Reinforcing Effect in Rubber Composites. J. Polym. Res. 2018, 25, 225. [Google Scholar] [CrossRef]
- Zhong, B.; Gou, W.; Tang, J.; Li, Q.; He, Q. Constructing Efficient and Recyclable Composite Absorbent Based on the Modification of Polymer Skeleton with in Situ Assembled Mesoporous Silica/Graphene Oxide Nanohybrid. Compos. Sci. Technol. 2022, 220, 109295. [Google Scholar] [CrossRef]
- Cheng, S.; Duan, X.; Zhang, Z.; An, D.; Zhao, G.; Liu, Y. Preparation of a Natural Rubber with High Thermal Conductivity, Low Heat Generation and Strong Interfacial Interaction by Using NS-Modified Graphene Oxide. J. Mater. Sci. 2021, 56, 4034–4050. [Google Scholar] [CrossRef]
- Ghanbari, M.H.; Shahdost-Fard, F.; Salehzadeh, H.; Ganjali, M.R.; Iman, M.; Rahimi-Nasrabadi, M.; Ahmadi, F. A Nanocomposite Prepared from Reduced Graphene Oxide, Gold Nanoparticles and Poly(2-Amino-5-Mercapto-1,3,4-Thiadiazole) for Use in an Electrochemical Sensor for Doxorubicin. Microchim. Acta. 2019, 186, 641. [Google Scholar] [CrossRef] [PubMed]
- Shahdan, D.; Chen, R.S.; Ahmad, S. Optimization of Graphene Nanoplatelets Dispersion and Nano-Filler Loading in Bio-Based Polymer Nanocomposites Based on Tensile Thermogravim. Analysis. J. Mater. Res. Technol. 2021, 15, 1284–1299. [Google Scholar] [CrossRef]
- Abuhasel, K.; Jeng, Y.T.; Munusamy, Y.; Kchaou, M.; Alquraish, M. Latex-Based Membrane for Oily Wastewater Filtration: Study on the Sulfur Concentration Effect. Appl. Sci. 2021, 11, 1779. [Google Scholar] [CrossRef]
- Li, X.-Y.; Zhao, P.-P.; Han, L.-X.; Deng, C. Hierarchically Structured Elastomer for Absorption-Dominated Electromagnetic Interference Shielding in an Ultra-Wide Band. Compos. Sci. Technol. 2022, 219, 109221. [Google Scholar] [CrossRef]
- Han, D.; Wang, K.; Yan, G.; Pan, Y.; Xue, J.; Wang, C.; Bian, H. Effect of the Ratio of Graphene Oxide(GO) and Multi-Walled Carbon Nanotubes(MWCNTs) on Metal Friction and Wear during Mixing. Polym. Test. 2022, 106, 107441. [Google Scholar] [CrossRef]
- Li, J.; Lyu, Y.; Li, C.; Zhang, F.; Li, K.; Li, X.; Li, J.; Kim, K.-H. Development of Strong, Tough and Flame-Retardant Phenolic Resins by Using Acacia Mangium Tannin-Functionalized Graphene Nanoplatelets. Int. J. Biol. Macromol. 2023, 227, 1191–1202. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Li, H.; Zhang, X.; Li, Q.; Luo, S.; Liu, F. Antibiofouling Performance of a Metal-Organic Gel Based on 2-Amino-5-Mercapto-1,3,4-Thiadiazole and CuI. Adv. Mater. Interfaces. 2022, 9, 2201688. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, K.; Fei, G.; Salzano De Luna, M.; Lavorgna, M.; Xia, H. High Silica Content Graphene/Natural Rubber Composites Prepared by a Wet Compounding and Latex Mixing Process. Polym. 2020, 12, 2549. [Google Scholar] [CrossRef]
- Zhang, L.; Li, H.; Lai, X.; Liao, X.; Wang, J.; Su, X.; Liu, H.; Wu, W.; Zeng, X. Functionalized Graphene as an Effective Antioxidant in Natural Rubber. ACS Symp. Ser. 2018, 107, 47–54. [Google Scholar] [CrossRef]
- Xue, X.; Yin, Q.; Jia, H.; Zhang, X.; Wen, Y.; Ji, Q.; Xu, Z. Enhancing Mechanical and Thermal Properties of Styrene-Butadiene Rubber/Carboxylated Acrylonitrile Butadiene Rubber Blend by the Usage of Graphene Oxide with Diverse Oxidation Degrees. Appl. Surf. Sci. 2017, 423, 584–591. [Google Scholar] [CrossRef]
- Zhong, B.; Luo, Y.; Chen, W.; Luo, Y.; Hu, D.; Dong, H.; Jia, Z.; Jia, D. Immobilization of Rubber Additive on Graphene for High-Performance Rubber Composites. J. Colloid Interface Sci. 2019, 550, 190–198. [Google Scholar] [CrossRef] [PubMed]
- Mensah, B.; Gupta, K.C.; Kang, G.; Lee, H.; Nah, C. A Comparative Study on Vulcanization Behavior of Acrylonitrile-Butadiene Rubber Reinforced with Graphene Oxide and Reduced Graphene Oxide as Fillers. Polym. Test. 2019, 76, 127–137. [Google Scholar] [CrossRef]
- Zheng, L.; Jerrams, S.; Xu, Z.; Zhang, L.; Liu, L.; Wen, S. Enhanced Gas Barrier Properties of Graphene Oxide/Rubber Composites with Strong Interfaces Constructed by Graphene Oxide and Sulfur. Chem. Eng. J. 2020, 383, 123100. [Google Scholar] [CrossRef]
- Duan, H.; Duan, X.; Miao, X.; Cheng, H.; Liang, C.; Zhao, G.; Liu, Y.; Cheng, S. Synergistically Improving Mechanical Properties and Lowering Build-up Heat in Natural Rubber Tires through Nano-Zinc Oxide on Graphene Oxide and Strong Cross-Linked Interfaces Derived from Thiol-Ene Click Reaction. Adv. Compos. Hybrid. Ma. 2024, 7, 7. [Google Scholar] [CrossRef]
- Dong, H.; Luo, Y.; Zhong, B.; Bai, J.; Jia, D. Effects of Vulcanization Accelerator Functionalized Graphene on the Co-Vulcanization Kinetics and Mechanical Strength of NR/SBR Blends. Polym. Test. 2020, 81, 106169. [Google Scholar] [CrossRef]
- Zhao, X.; Liu, C.; He, J.; Zhang, W.; Duan, X.; Liang, C. Graphene Rubber toward High Content and Energy Saving Enabled by Spray Drying. Diam. Relat. Mater. 2024, 141, 110594. [Google Scholar] [CrossRef]
- Cheng, S.; Duan, X.; Cui, Y.; Liang, C.; Zhang, Z.; Zhao, G.; Liu, Y. Facile Strategy for the Preparation of Green Graphene Rubber with Enhanced Interfacial Interaction and Thermal Management Capability. J. Appl. Polym. Sci. 2022, 139, e52882. [Google Scholar] [CrossRef]
- Bijina, V.; Jandas, P.J.; Jose, J.; Muhammed Ajnas, N.; Abhitha, K.P.; John, H. Tuning the Low Rolling Resistance, Wet Grip, and Heat Build-up Properties of Tyre Tread Formulations Using Carbon Black/Thermally Exfoliated Graphite Hybrid Fillers. J. Appl. Polym. Sci. 2023, 140, e53508. [Google Scholar] [CrossRef]
- Liu, H.; Yang, L.; Liu, X.; Cao, J.-P.; Zhang, J.; Luo, Z.; Gao, Z. Silicon Dioxide Nanoparticle Decorated Graphene with Excellent Dispersibility in Natural Rubber Composites via Physical Mixing for Application in Green Tires. Compos. Part B Eng. 2023, 258, 110700. [Google Scholar] [CrossRef]
- Duan, X.; Tao, R.; Chen, Y.; Zhang, Z.; Zhao, G.; Liu, Y.; Cheng, S. Improved Mechanical, Thermal Conductivity and Low Heat Build-up Properties of Natural Rubber Composites with Nano-Sulfur Modified Graphene Oxide/Silicon Carbide. Ceram. Int. 2022, 48, 22053–22063. [Google Scholar] [CrossRef]
- Limrungruengrat, S.; Chaikittiratana, A.; Pornpeerakeat, S.; Chantrasmi, T. Thermo-Mechanical Finite Element Simulation and Validation of Rubber Curing Process. J. Mech. Sci. Technol. 2022, 36, 3039–3046. [Google Scholar] [CrossRef]
- Goswami, M.; Mandloi, B.S.; Kumar, A.; Sharma, S.; Ghorai, S.K.; Sarkar, K.; Chattopadhyay, S. Optimization of Graphene in Carbon Black-filled Nitrile Butadiene Rubber: Constitutive Modeling and Verification Using Finite Element Analysis. Polym. Compos. 2020, 41, 1853–1866. [Google Scholar] [CrossRef]
- Barghamadi, M.; Ghoreishy, M.H.R.; Karrabi, M.; Mohammadian-Gezaz, S. Modeling of Nonlinear hyper-viscoelastic and Stress Softening Behaviors of Acrylonitrile Butadiene Rubber/Polyvinyl Chloride Nanocomposites Reinforced by Nanoclay and Graphene. Polym. Compos. 2021, 42, 583–596. [Google Scholar] [CrossRef]






| Materials | Content (phr) |
|---|---|
| NR | 100 |
| GO, AGO | 1.0 |
| ZnO | 5 |
| S | 2 |
| SA | 2 |
| RD | 2 |
| 4020 NA | 2 |
| NOBS | 2 |
| SiO2 | 30 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jia, H.; Miao, X.; Zhang, Z. Building Chemical Interface Layers in Functionalized Graphene Oxide/Rubber Composites to Achieve Enhanced Mechanical Properties and Thermal Control Capability of Tires. Polymers 2024, 16, 2234. https://doi.org/10.3390/polym16162234
Jia H, Miao X, Zhang Z. Building Chemical Interface Layers in Functionalized Graphene Oxide/Rubber Composites to Achieve Enhanced Mechanical Properties and Thermal Control Capability of Tires. Polymers. 2024; 16(16):2234. https://doi.org/10.3390/polym16162234
Chicago/Turabian StyleJia, Haixiang, Xiaohe Miao, and Zhiyi Zhang. 2024. "Building Chemical Interface Layers in Functionalized Graphene Oxide/Rubber Composites to Achieve Enhanced Mechanical Properties and Thermal Control Capability of Tires" Polymers 16, no. 16: 2234. https://doi.org/10.3390/polym16162234
APA StyleJia, H., Miao, X., & Zhang, Z. (2024). Building Chemical Interface Layers in Functionalized Graphene Oxide/Rubber Composites to Achieve Enhanced Mechanical Properties and Thermal Control Capability of Tires. Polymers, 16(16), 2234. https://doi.org/10.3390/polym16162234






