Benefits of Core–Shell Particles over Single-Metal Coatings: Mechanical and Chemical Exposure and Antimicrobial Efficacy
Abstract
1. Introduction
2. Materials and Methods
2.1. Coating and Samples
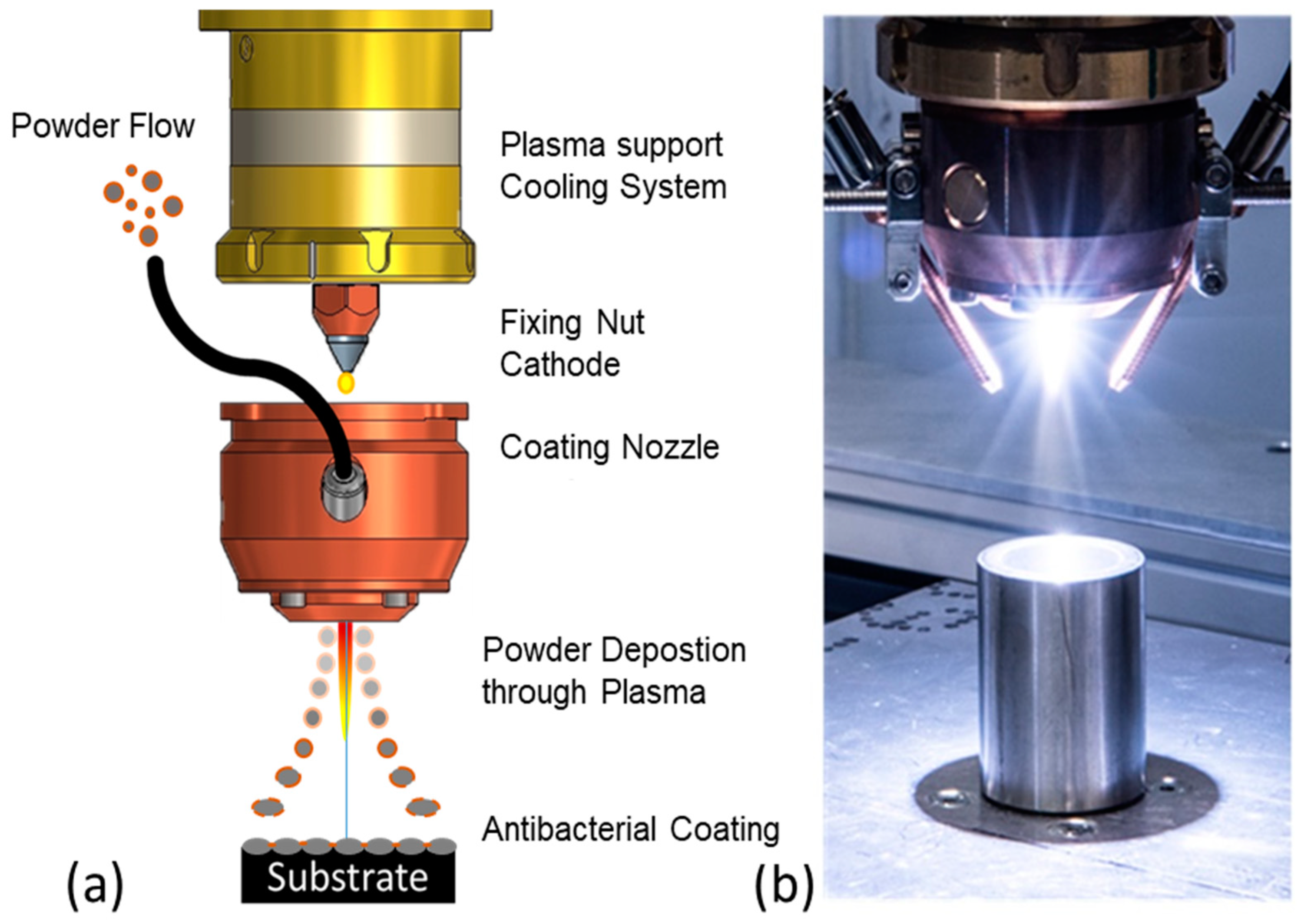
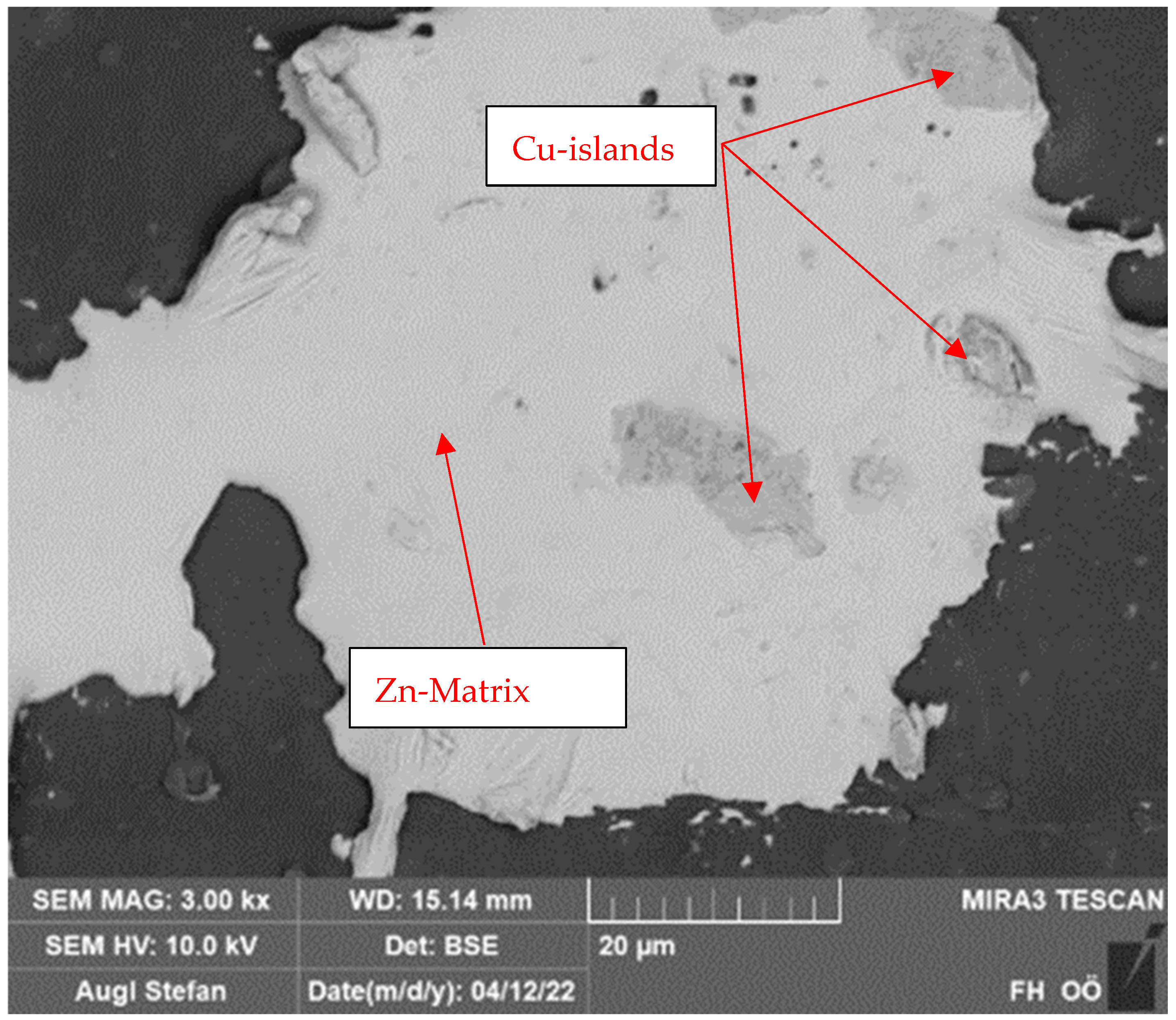
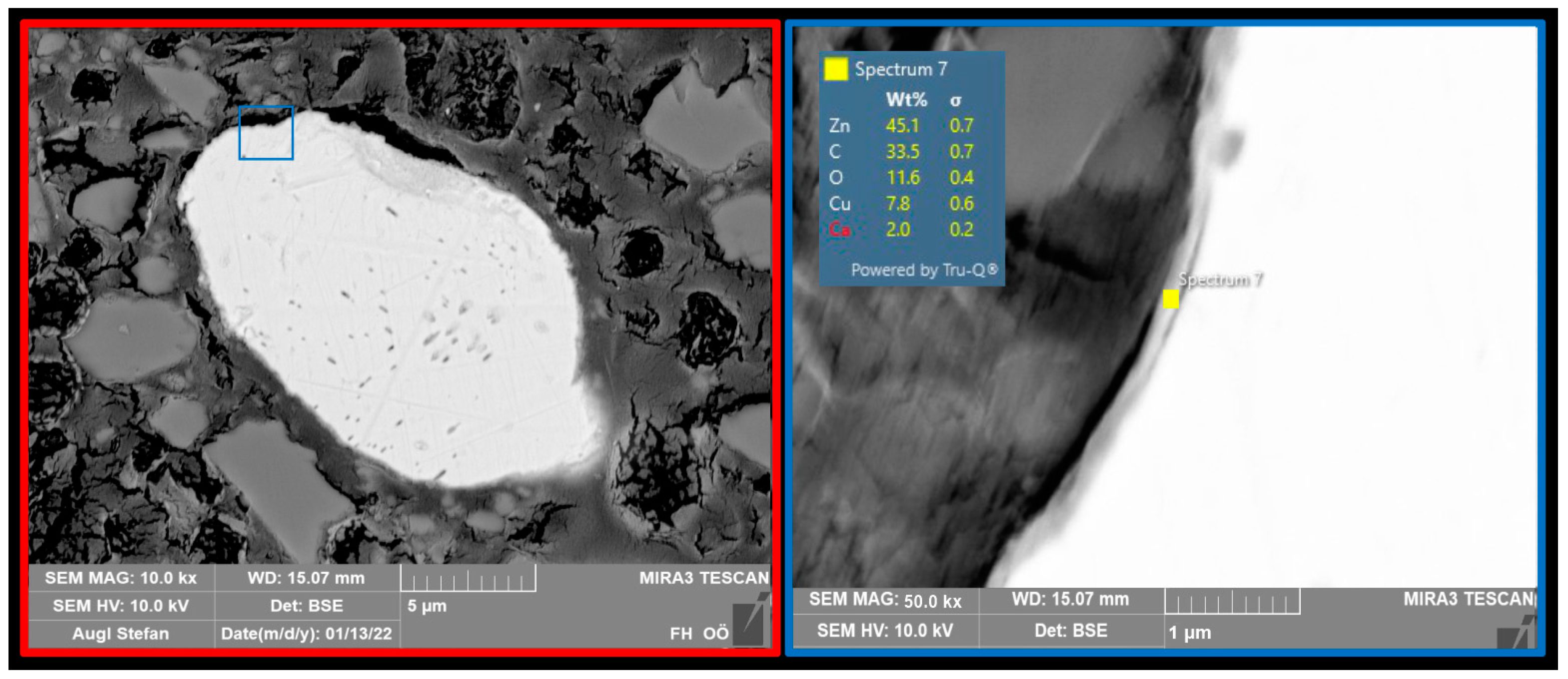
2.2. Particle Composition and Coating
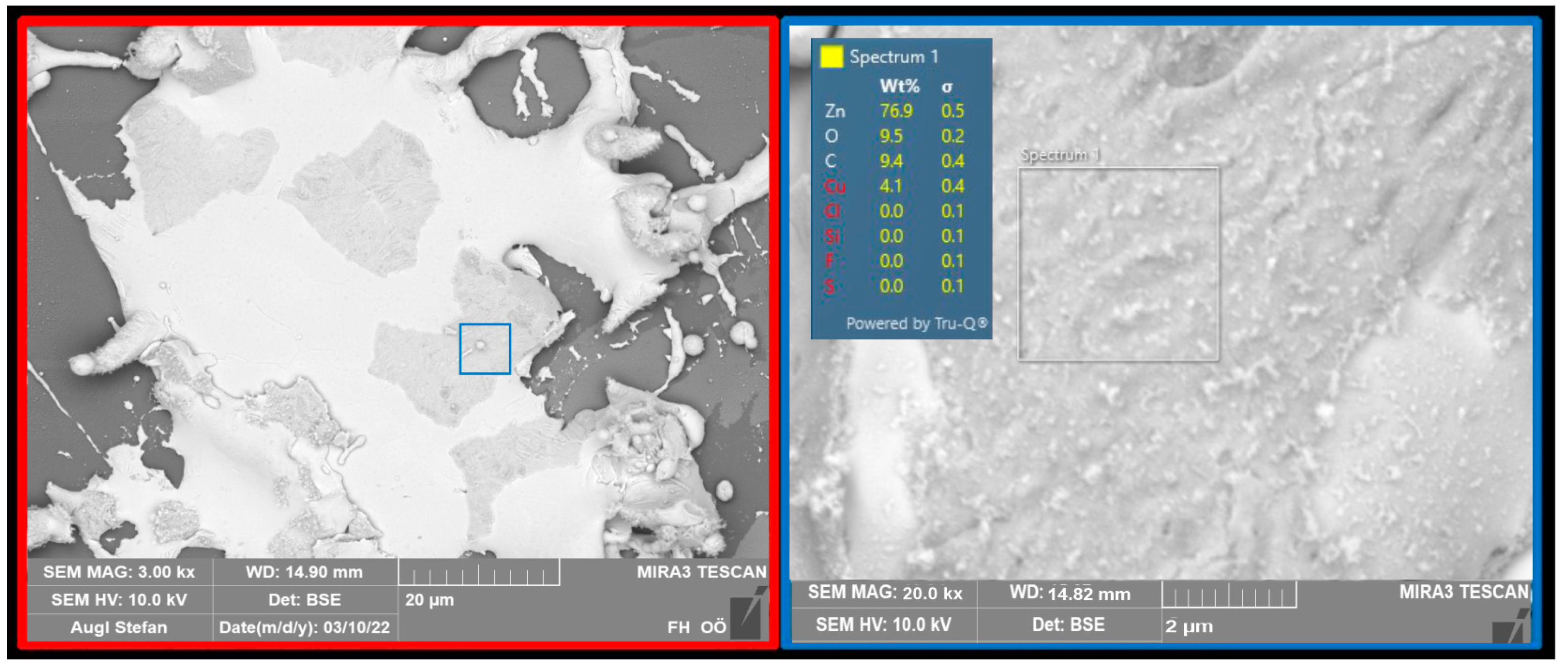
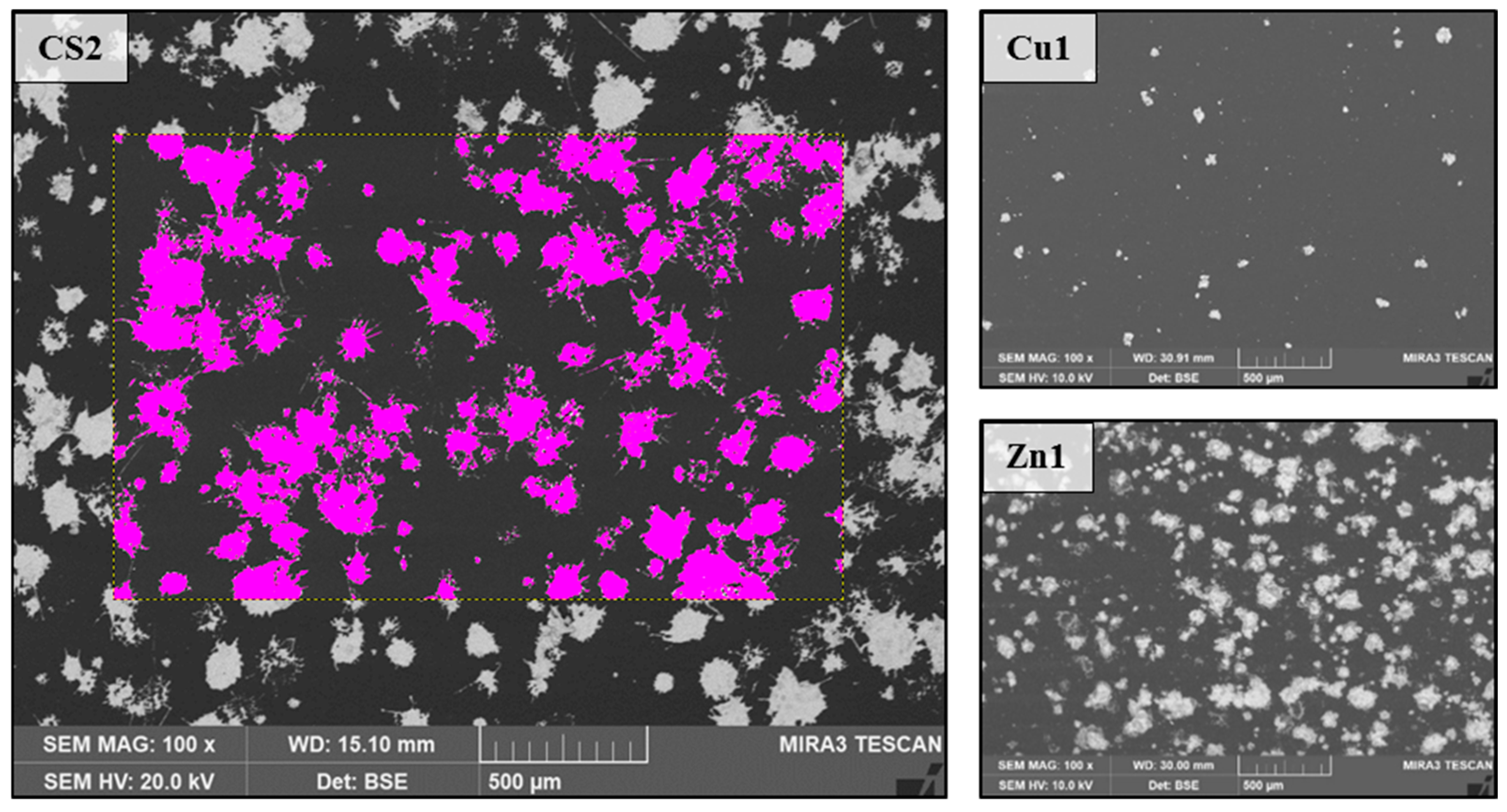
2.3. Durability Analyses of the Coating
2.3.1. Mechanical Exposure—Crockmeter Test
2.3.2. Chemical Exposure—Cleaning Agents
2.3.3. Mechanical and Chemical Exposure—Washability Test
2.4. Antimicrobial Surface Tests
2.4.1. Testing of Antibacterial Activity
2.4.2. Testing of Antiviral Activity
2.5. Graphics and Statistical Analysis
3. Results
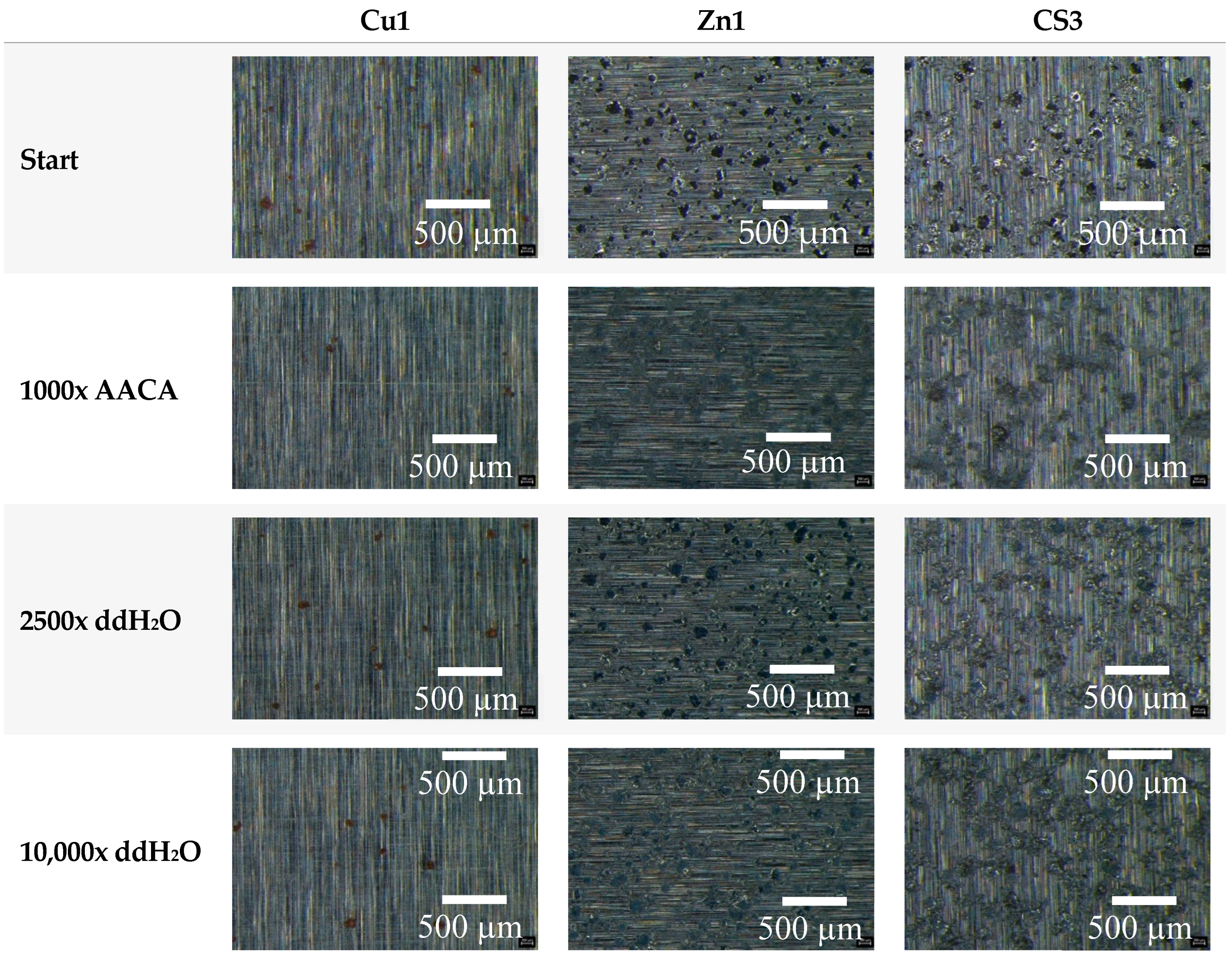
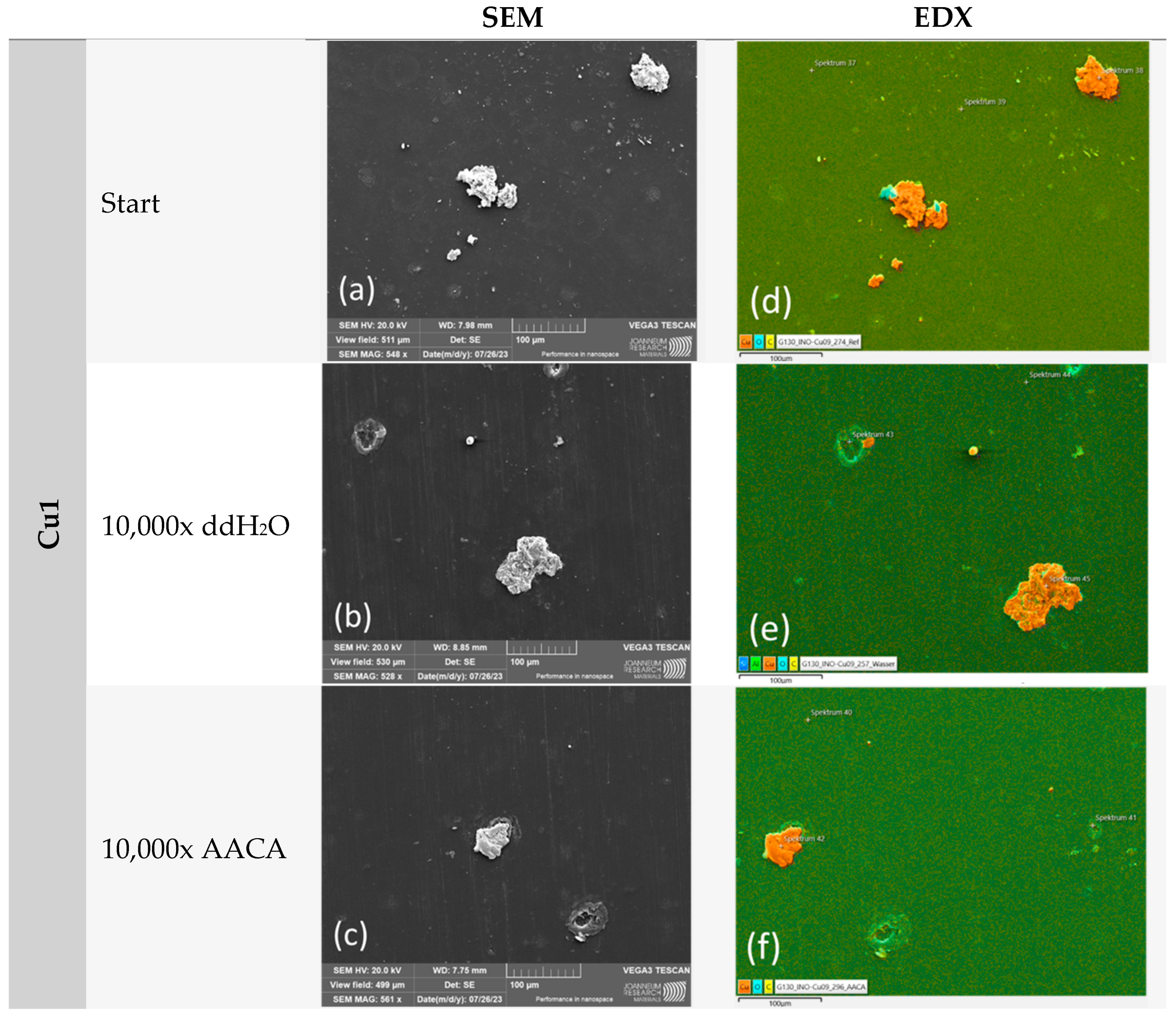
3.1. Durability Analyses of the Coatings
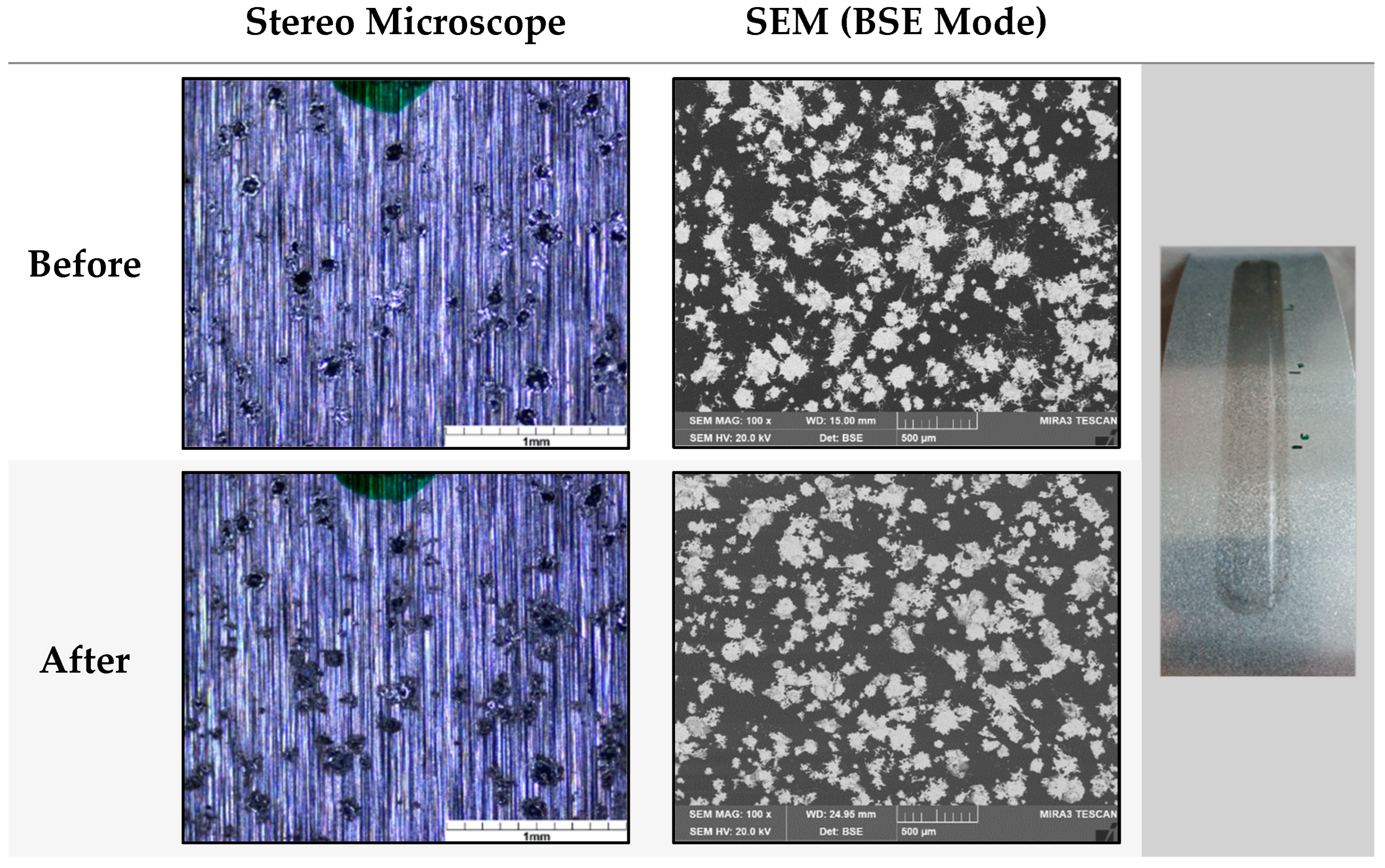
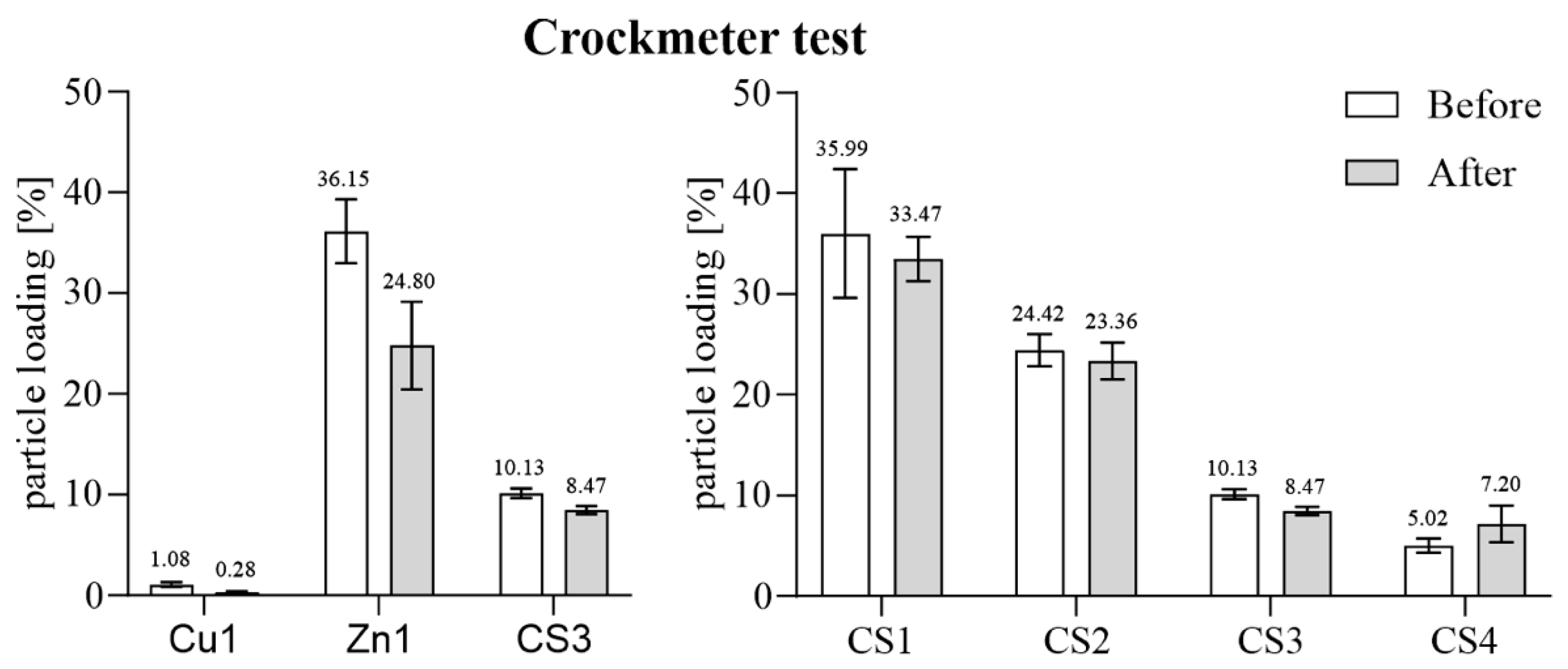
3.1.1. Mechanical Exposure—Crockmeter Test
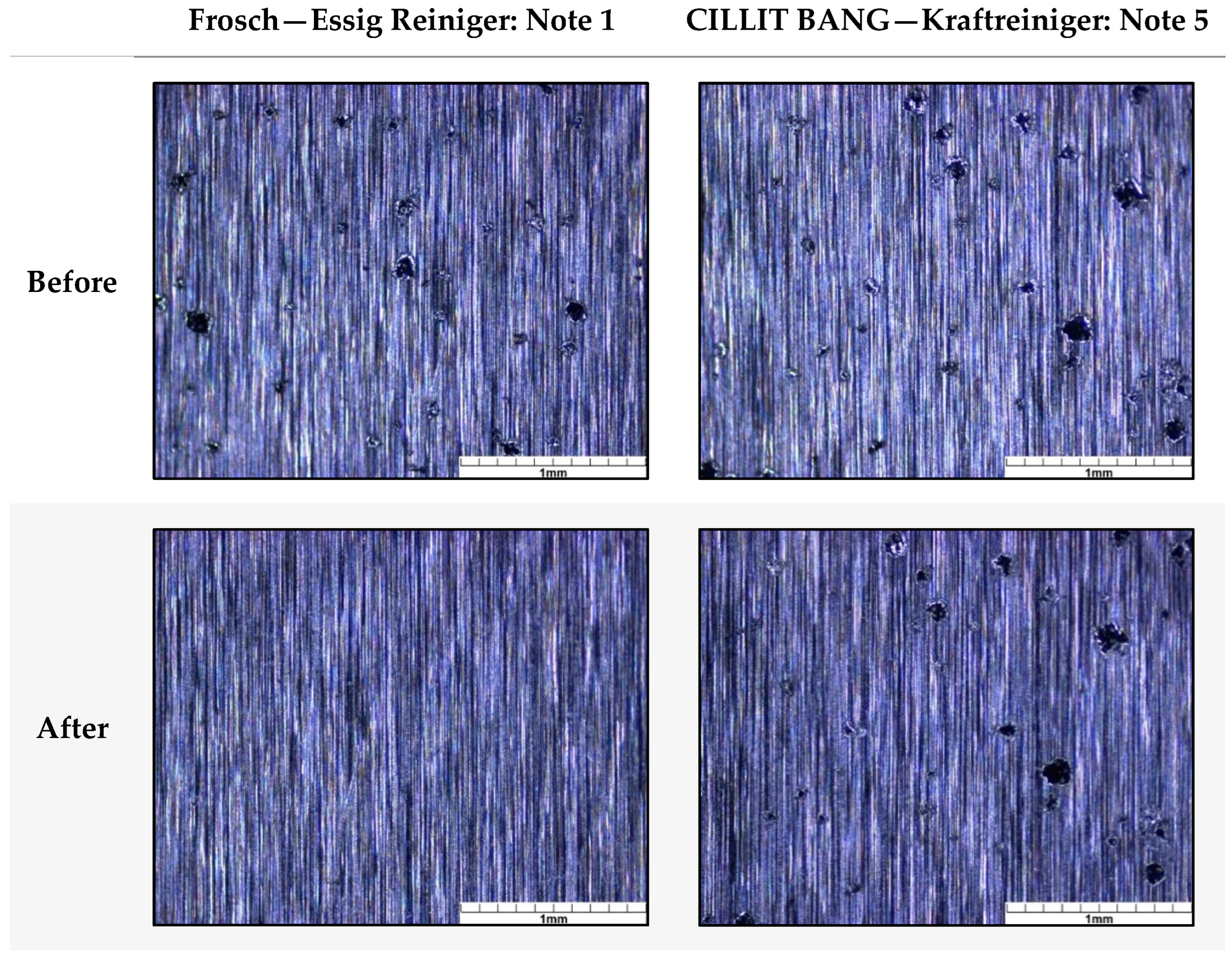
3.1.2. Chemical Exposure—Cleaning Agents
3.1.3. Mechanical and Chemical Exposure—Washability Test
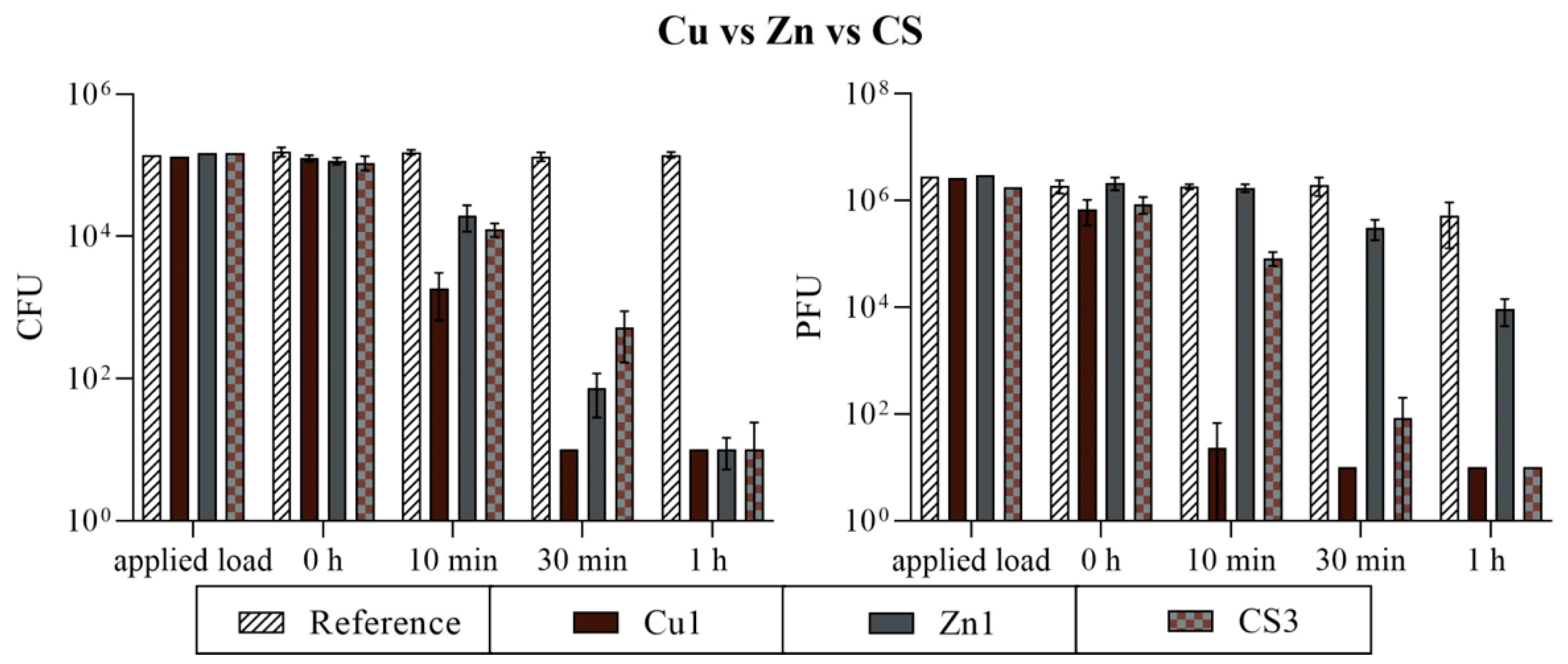
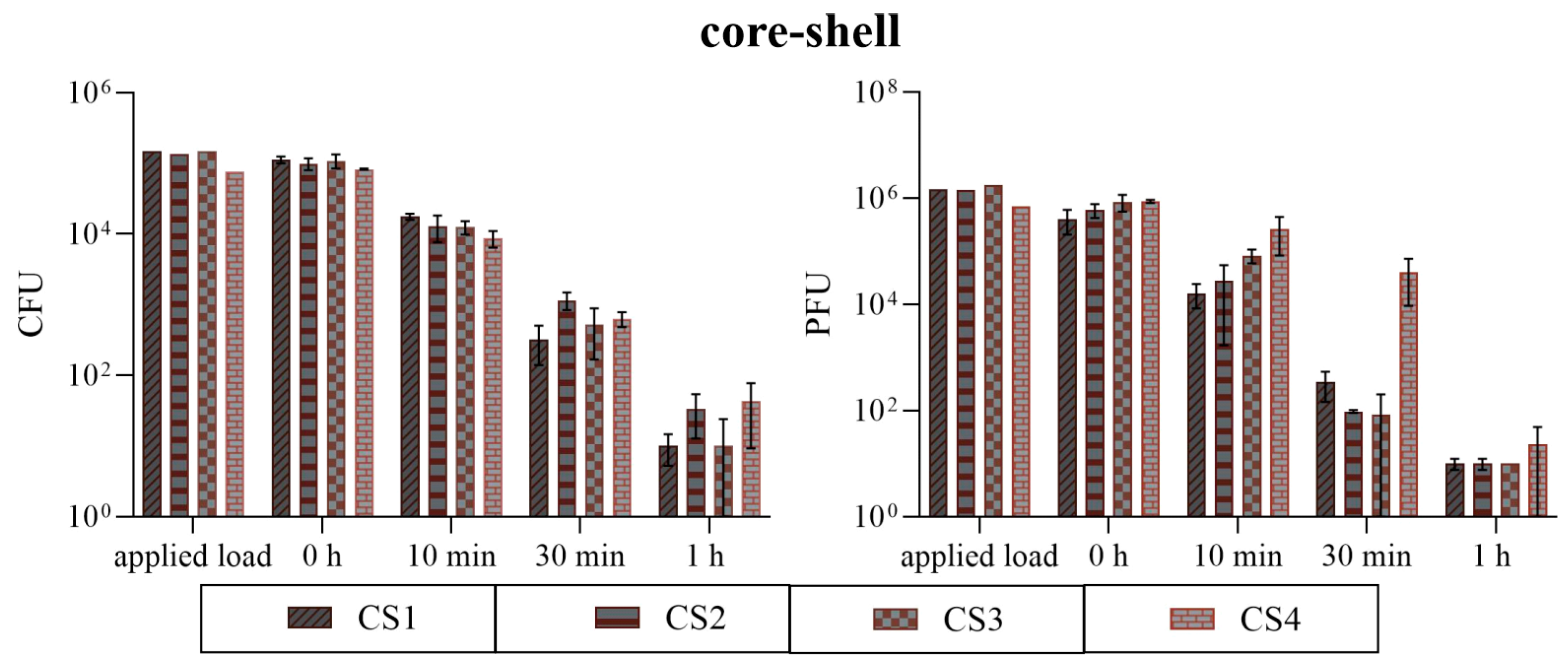
3.2. Antimicrobial Efficiency
3.2.1. Comparison of Three Different Coatings
3.2.2. Comparison of the CS Coatings
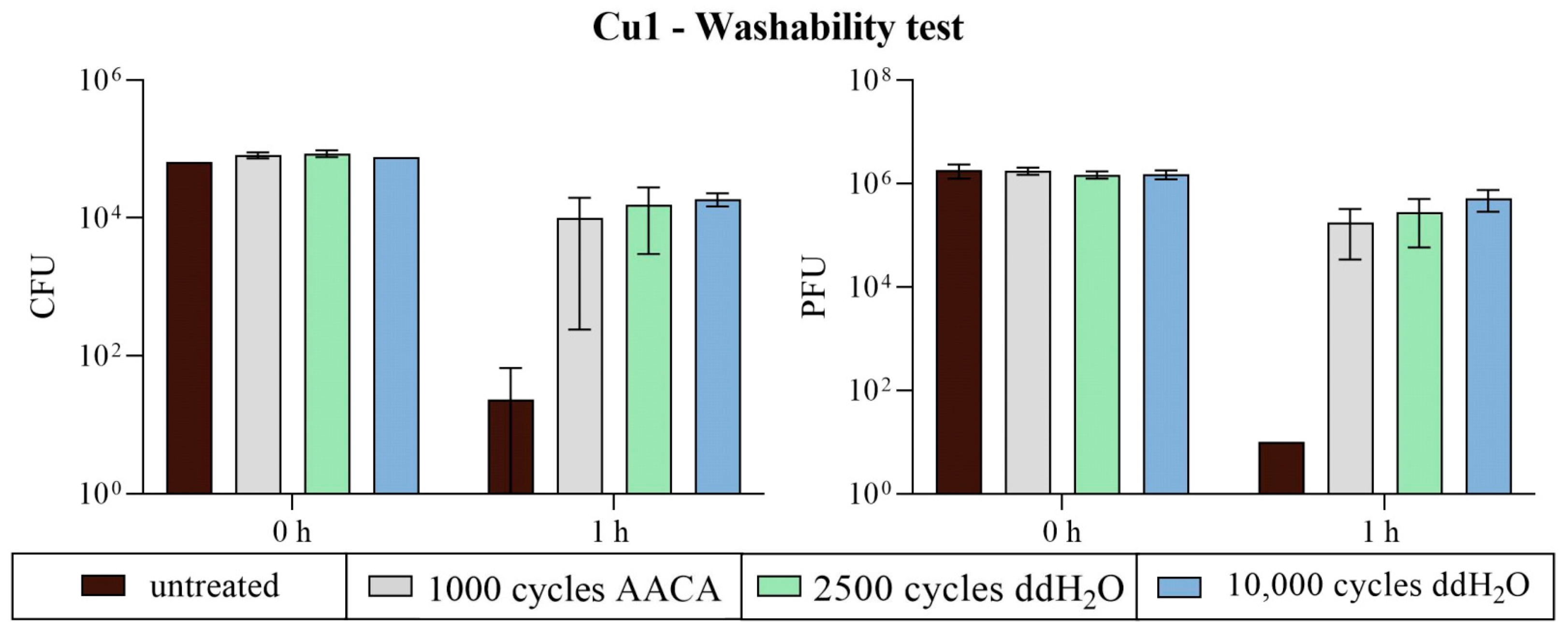
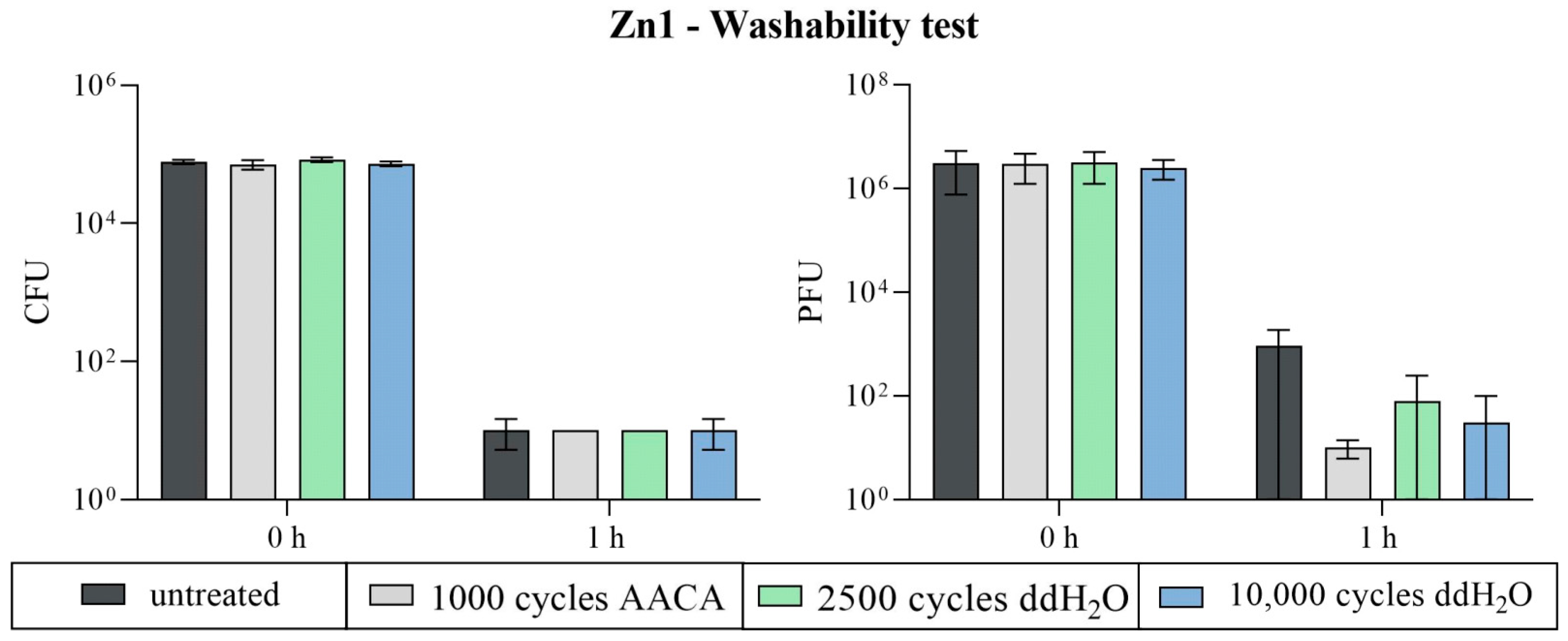
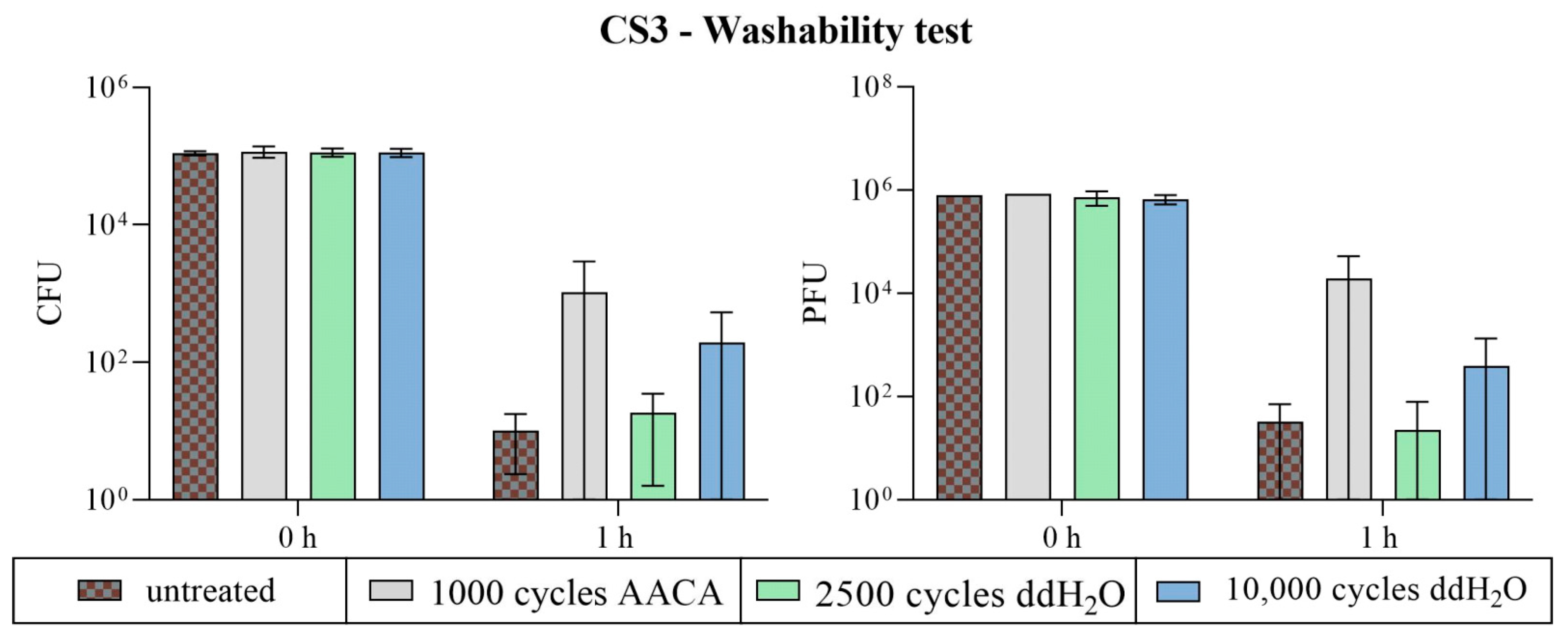
3.2.3. Washability Surfaces
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO. Int. The Top 10 Causes of Death. 2020. Available online: https://www.who.int/news-room/fact-sheets/detail/the-top-10-causes-of-death (accessed on 15. February 2024).
- Martin, M.J.; Thottathil, S.E.; Newman, T.B. Antibiotics overuse in animal agriculture: A call to action for health care providers. Am. J. Public Health 2015, 105, 2409–2410. [Google Scholar] [CrossRef]
- Dadi, N.C.T.; Radochová, B.; Vargová, J.; Bujdáková, H. Impact of healthcare-associated infections connected to medical devices—An update. Microorganisms 2021, 9, 2332. [Google Scholar] [CrossRef]
- Shahid, A.; Aslam, B.; Muzammil, S.; Aslam, N.; Shahid, M.; Almatroudi, A.; Allemailem, K.S.; Saqalein, M.; Nisar, M.A.; Rasool, M.H.; et al. The prospects of antimicrobial coated medical implants. J. Appl. Biomater. Funct. Mater. 2021, 19. [Google Scholar] [CrossRef]
- Pietsch, F.; O’Neill, A.J.; Ivask, A.; Jenssen, H.; Inkinen, J.; Kahru, A.; Ahonen, M.; Schreiber, F. Selection of resistance by antimicrobial coatings in the healthcare setting. J. Hosp. Infect. 2020, 106, 115–125. [Google Scholar] [CrossRef]
- Tavman, S.; Otles, S.; Glaue, S.; Gogus, N. 4—Food preservation technologies. In Saving Food; Academic Press: Cambridge, MA, USA, 2019; pp. 117–140. [Google Scholar] [CrossRef]
- Barros-Velazquez, J. Antimicrobial Food Packaging; Elsevier: Amsterdam, The Netherlands, 2016. [Google Scholar] [CrossRef]
- Valencia-Chamorro, S.A.; Palou, L.; Delŕio, M.A.; Pérez-Gago, M.B. Antimicrobial edible films and coatings for fresh and minimally processed fruits and vegetables: A review. Crit. Rev. Food Sci. Nutr. 2011, 51, 872–900. [Google Scholar] [CrossRef]
- Schmid, P.J.; Maitz, S.; Plank, N.; Knaipp, E.; Pölzl, S.; Kittinger, C. Fiber-based food packaging materials in view of bacterial growth and survival capacities. Front. Microbiol. 2023, 14, 1099906. [Google Scholar] [CrossRef]
- Fu, Y.; Dudley, E.G. Antimicrobial-coated films as food packaging: A review. Compr. Rev. Food Sci. Food Saf. 2021, 20, 3404–3437. [Google Scholar] [CrossRef]
- Azlin-Hasim, S.; Cruz-Romero, M.C.; Morris, M.A.; Cummins, E.; Kerry, J.P. Spray coating application for the development of nanocoated antimicrobial low-density polyethylene films to increase the shelf life of chicken breast fillets. Food Sci. Technol. Int. 2018, 24, 688–698. [Google Scholar] [CrossRef]
- Al-Naamani, L.; Dutta, J.; Dobretsov, S. Nanocomposite zinc oxide-chitosan coatings on polyethylene films for extending storage life of okra (Abelmoschus esculentus). Nanomaterials 2018, 8, 479. [Google Scholar] [CrossRef]
- Al-Naamani, L.; Dobretsov, S.; Dutta, J. Chitosan-zinc oxide nanoparticle composite coating for active food packaging applications. Innov. Food Sci. Emerg. Technol. 2016, 38, 231–237. [Google Scholar] [CrossRef]
- Grass, G.; Rensing, C.; Solioz, M. Metallic copper as an antimicrobial surface. Appl. Environ. Microbiol. 2011, 77, 1541–1547. [Google Scholar] [CrossRef]
- Riduan, S.N.; Zhang, Y. Recent Advances of Zinc-based Antimicrobial Materials. Chem.—Asian J. 2021, 16, 2588–2595. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Hu, C.; Shao, L. The antimicrobial activity of nanoparticles: Present situation and prospects for the future. Int. J. Nanomedicine 2017, 12, 1227–1249. [Google Scholar] [CrossRef]
- Yu, J.; Zhang, W.; Li, Y.; Wang, G.; Yang, L.; Jin, J.; Chen, Q.; Huang, M. Synthesis, characterization, antimicrobial activity and mechanism of a novel hydroxyapatite whisker/nano zinc oxide biomaterial. Biomed. Mater. 2015, 10, 015001. [Google Scholar] [CrossRef]
- Sarghini, S.; Paulussen, S.; Terryn, H. Atmospheric Pressure Plasma Technology: A Straightforward Deposition of Antibacterial Coatings. Plasma Process. Polym. 2011, 8, 59–69. [Google Scholar] [CrossRef]
- Múgica-Vidal, R.; Sainz-García, E.; Álvarez-Ordóñez, A.; Prieto, M.; González-Raurich, M.; López, M.; López, M.; Rojo-Bezares, B.; Sáenz, Y.; Alba-Elías, F. Production of Antibacterial Coatings Through Atmospheric Pressure Plasma: A Promising Alternative for Combatting Biofilms in the Food Industry. Food Bioprocess Technol. 2019, 12, 1251–1263. [Google Scholar] [CrossRef]
- Garza-Cervantes, J.A.; Chávez-Reyes, A.; Castillo, E.C.; García-Rivas, G.; Ortega-Rivera, O.A.; Salinas, E.; Ortiz-Martínez, M.; Gómez-Flores, S.L.; Peña-Martínez, J.A.; Pepi-Molina, A.; et al. Synergistic antimicrobial effects of silver/transition-metal combinatorial treatments. Sci. Rep. 2017, 7, 903. [Google Scholar] [CrossRef]
- Chatterjee, K.; Sarkar, S.; Jagajjanani Rao, K.; Paria, S. Core/shell nanoparticles in biomedical applications. Adv. Colloid Interface Sci. 2014, 209, 8–39. [Google Scholar] [CrossRef]
- Rani, P.; Varma, R.S.; Singh, K.; Acevedo, R.; Singh, J. Catalytic and antimicrobial potential of green synthesized Au and Au@Ag core-shell nanoparticles. Chemosphere 2023, 317, 137841. [Google Scholar] [CrossRef]
- Chen, H.; Wang, R.; Zhang, J.; Hua, H.; Zhu, M. Synthesis of core-shell structured ZnO@m-SiO2 with excellent reinforcing effect and antimicrobial activity for dental resin composites. Dent. Mater. 2018, 34, 1846–1855. [Google Scholar] [CrossRef]
- ISO 105-X12:2016; Textiles—Tests for Colour Fastness—Part X12: Colour Fastness to Rubbing. ISO: Geneva, Switzerland, 2016.
- EN 12720:2009+A1:2013; Furniture—Assessment of Surface Resistance to Cold Liquids. BSI: London, UK, 2009.
- ASTM D 2486:2017; Standard Test Methods for Scrub Resistance of Wall Paints. ASTM: West Conshohocken, PA, USA, 2017.
- ISO 22196:2011; Measurement of Antibacterial Activity on Plastics and Other Non-Porous Surfaces. ISO: Geneva, Switzerland, 2011.
- ISO 21702:2019; Measurement of Antiviral Activity on Plastics and Other Non-Porous Surfaces. ISO: Geneva, Switzerland, 2019.
- ISO 18071:2016; Fine Ceramics—Determination of Antiviral Activity of Semiconducting Photocatalytic Materials under Indoor Lighting Environment—Test Method Using Bacteriophage Q-Beta. ISO: Geneva, Switzerland, 2016.
- Turgeon, N.; Toulouse, M.J.; Martel, B.; Moineau, S.; Duchaine, C. Comparison of five bacteriophages as models for viral aerosol studies. Appl. Environ. Microbiol. 2014, 80, 4242–4250. [Google Scholar] [CrossRef]
- Poelzl, S.; Rieger, J.; Zatloukal, K.; Augl, S.; Stummer, M.; Hinterer, A.; Kittinger, C. Inactivation of Bacteriophage ϕ6 and SARS-CoV-2 in Antimicrobial Surface Tests. Viruses 2023, 15, 1833. [Google Scholar] [CrossRef] [PubMed]
- Biermaier, C.; Petz, P.; Bechtold, T.; Pham, T. Investigation of the Functional Ageing of Conductive Coated Fabrics under Simulated Washing Conditions. Materials 2023, 16, 912. [Google Scholar] [CrossRef]
- Sun, C.; Li, Y.; Li, Z.; Su, Q.; Wang, Y.; Liu, X. Durable and Washable Antibacterial Copper Nanoparticles Bridged by Surface Grafting Polymer Brushes on Cotton and Polymeric Materials. J. Nanomater. 2018, 2018. [Google Scholar] [CrossRef]
- Ye, W.; Xin, J.H.; Li, P.; Lee, K.L.D.; Kwong, T.L. Durable antibacterial finish on cotton fabric by using chitosan-based polymeric core-shell particles. J. Appl. Polym. Sci. 2006, 102, 1787–1793. [Google Scholar] [CrossRef]
- Azam, A.; Ahmed, A.S.; Oves, M.; Khan, M.S.; Habib, S.S.; Memic, A. Antimicrobial activity of metal oxide nanoparticles against Gram-positive and Gram-negative bacteria: A comparative study. Int. J. Nanomed. 2012, 7, 6003–6009. [Google Scholar] [CrossRef]
- Kubo, A.-L.; Rausalu, K.; Savest, N.; Žusinaite, E.; Vasiliev, G.; Viirsalu, M.; Plamus, T.; Krumme, A.; Merits, A.; Bondarenko, O. Antibacterial and Antiviral Effects of Ag, Cu and Zn Metals, Respective Nanoparticles and Filter Materials Thereof against Coronavirus SARS-CoV-2 and Influenza A Virus. Pharmaceutics 2022, 12, 2549. [Google Scholar] [CrossRef]
- Merkl, P.; Long, S.; McInerney, G.M.; Sotiriou, G.A. Antiviral activity of silver, copper oxide and zinc oxide nanoparticle coatings against SARS-CoV-2. Nanomaterials 2021, 11, 1312. [Google Scholar] [CrossRef] [PubMed]
- Glass, A.; Klinkhammer, K.E.; Christofferson, R.C.; Mores, C.N. Efficacy of copper blend coatings in reducing SARS-CoV-2 contamination. BioMetals 2023, 36, 217–225. [Google Scholar] [CrossRef]
- Padmavathy, N.; Vijayaraghavan, R. Enhanced bioactivity of ZnO nanoparticles—An antimicrobial study. Sci. Technol. Adv. Mater. 2008, 9, 035004. [Google Scholar] [CrossRef]
- Emami-Karvani, Z. Antibacterial activity of ZnO nanoparticle on Gram-positive and Gram-negative bacteria. Afr. J. Microbiol. Res. 2012, 5, 1368–1373. [Google Scholar] [CrossRef]
- Cunliffe, A.J.; Askew, P.D.; Stephan, I.; Iredale, G.; Cosemans, P.; Simmons, L.M.; Verran, J.; Redfern, J. How do we determine the efficacy of an antibacterial surface? A review of standardised antibacterial material testing methods. Antibiotics 2021, 10, 1069. [Google Scholar] [CrossRef] [PubMed]
- Maitz, S.; Poelzl, S.; Dreisiebner, D.; Zarschenas, E.; Kittinger, C. Antimicrobial non-porous surfaces: A comparison of the standards ISO 22196: 2011 and the recently published ISO 7581: 2023. Front. Microbiol. 2024, 15, 1400265. [Google Scholar] [CrossRef] [PubMed]



| Name | Coating | Particle Loading [%] | |
|---|---|---|---|
| PET-Al | Reference | - | - |
| Cu1 | Copper particles | 1.08 | |
| Zn1 | Zinc particles | 36.15 | |
| CS1 | Core–shell particles | 35.99 | |
| CS2 | 24.42 | ||
| CS3 | 10.13 | ||
| CS4 | 5.02 |
| Frosch Essig Reiniger | Cif Bad & Dusche Salle de Bain | Frosch Glas-Reiniger Spiritus | Frosch Aktiv-Soda Reiniger | CILLIT BANG FETTLÖSER Kraftreiniger | |
|---|---|---|---|---|---|
| Application | Removal of lime scale, water stains, soap residue, and dirt in all areas of the household | Cleaning of bathroom and shower | Cleaning of glass and smooth surfaces | Cleaning of almost all wipeable surfaces, cleaning of kitchen | Cleaning of all surfaces, cleaning of kitchen and bathroom |
| pH | 2.4 | 3.9 | 5.5 | 10.4 | 11.4 |
| Numerical Classification | Description |
|---|---|
| 5 | No change The test area cannot be distinguished from the adjacent area |
| 4 | Slight change The test area can only be distinguished from the adjacent area if the light source is reflected on the test surface and is reflected to the eye of the observer, e.g., a change in color or gloss No change in the surface structure, e.g., crack and blister formation |
| 3 | Moderate change The test area can be distinguished from the adjacent area, visible from several perspectives, e.g., a change in color or gloss No change in the surface structure, e.g., crack and blister formation |
| 2 | Substantial change The test area is clearly distinguishable from the adjacent area, visible from all perspectives, e.g., change in color or gloss and/or the surface structure has changed slightly, e.g., crack and blister formation |
| 1 | Major change The surface has changed considerably and/or change in color or gloss and/or the surface material has become partially or completely detached and/or filter paper adheres to the surface |
| Frosch | Cif | Frosch | Frosch | CILLIT BANG | |
|---|---|---|---|---|---|
| Essig Reiniger | Bad & Dusche Salle de Bain | Glas-Reiniger Spiritus | Aktiv-Soda Reiniger | FETTLÖSER Kraftreiniger | |
| CS1 | 1.8 | 3.0 | 3.0 | 5.0 | 5.0 |
| CS2 | 2.5 | 3.7 | 3.0 | 4.3 | 5.0 |
| CS3 | 1.3 | 2.7 | 2.8 | 4.8 | 5.0 |
| CS4 | 1.0 | 3.0 | 4.3 | 5.0 | 5.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Poelzl, S.; Augl, S.; Schwan, A.M.; Chwatal, S.; Lackner, J.M.; Kittinger, C. Benefits of Core–Shell Particles over Single-Metal Coatings: Mechanical and Chemical Exposure and Antimicrobial Efficacy. Polymers 2024, 16, 2209. https://doi.org/10.3390/polym16152209
Poelzl S, Augl S, Schwan AM, Chwatal S, Lackner JM, Kittinger C. Benefits of Core–Shell Particles over Single-Metal Coatings: Mechanical and Chemical Exposure and Antimicrobial Efficacy. Polymers. 2024; 16(15):2209. https://doi.org/10.3390/polym16152209
Chicago/Turabian StylePoelzl, Sabine, Stefan Augl, Alexander Michael Schwan, Simon Chwatal, Jürgen Markus Lackner, and Clemens Kittinger. 2024. "Benefits of Core–Shell Particles over Single-Metal Coatings: Mechanical and Chemical Exposure and Antimicrobial Efficacy" Polymers 16, no. 15: 2209. https://doi.org/10.3390/polym16152209
APA StylePoelzl, S., Augl, S., Schwan, A. M., Chwatal, S., Lackner, J. M., & Kittinger, C. (2024). Benefits of Core–Shell Particles over Single-Metal Coatings: Mechanical and Chemical Exposure and Antimicrobial Efficacy. Polymers, 16(15), 2209. https://doi.org/10.3390/polym16152209







