A Novel, Dual-Initiator, Continuous-Suspension Grafting Strategy for the Preparation of PP-g-AA-MAH Fibers to Remove of Indigo from Wastewater
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of PP-g-AA-MAH Fibers
2.3. Determination of Product Grafting Ratio and Monomer Grafting Efficiency
2.4. Characterization Methods
2.5. Adsorption and Regeneration of Indigo Using the PP-g-AA-MAH Fibers
3. Results and Discussion
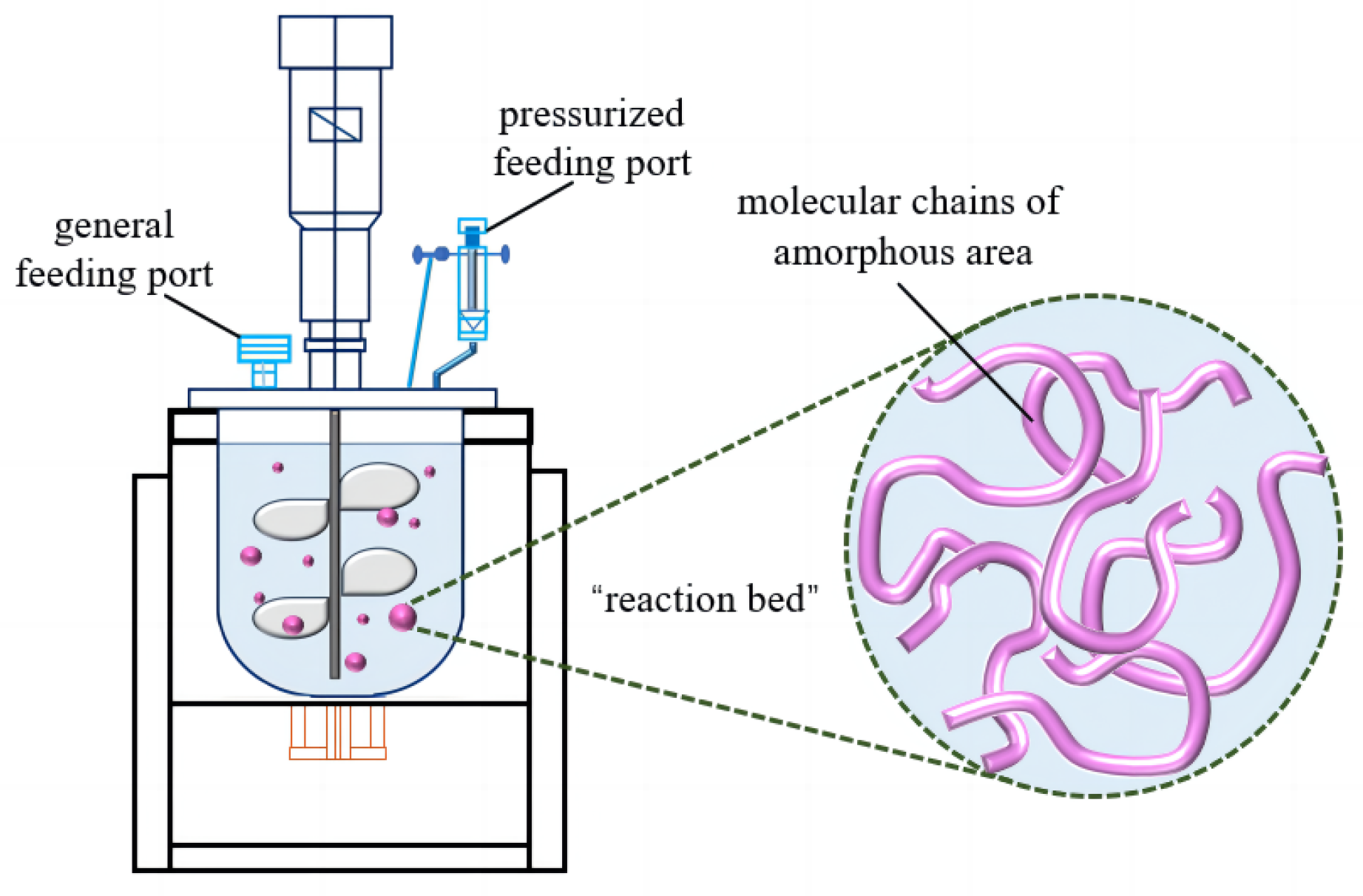
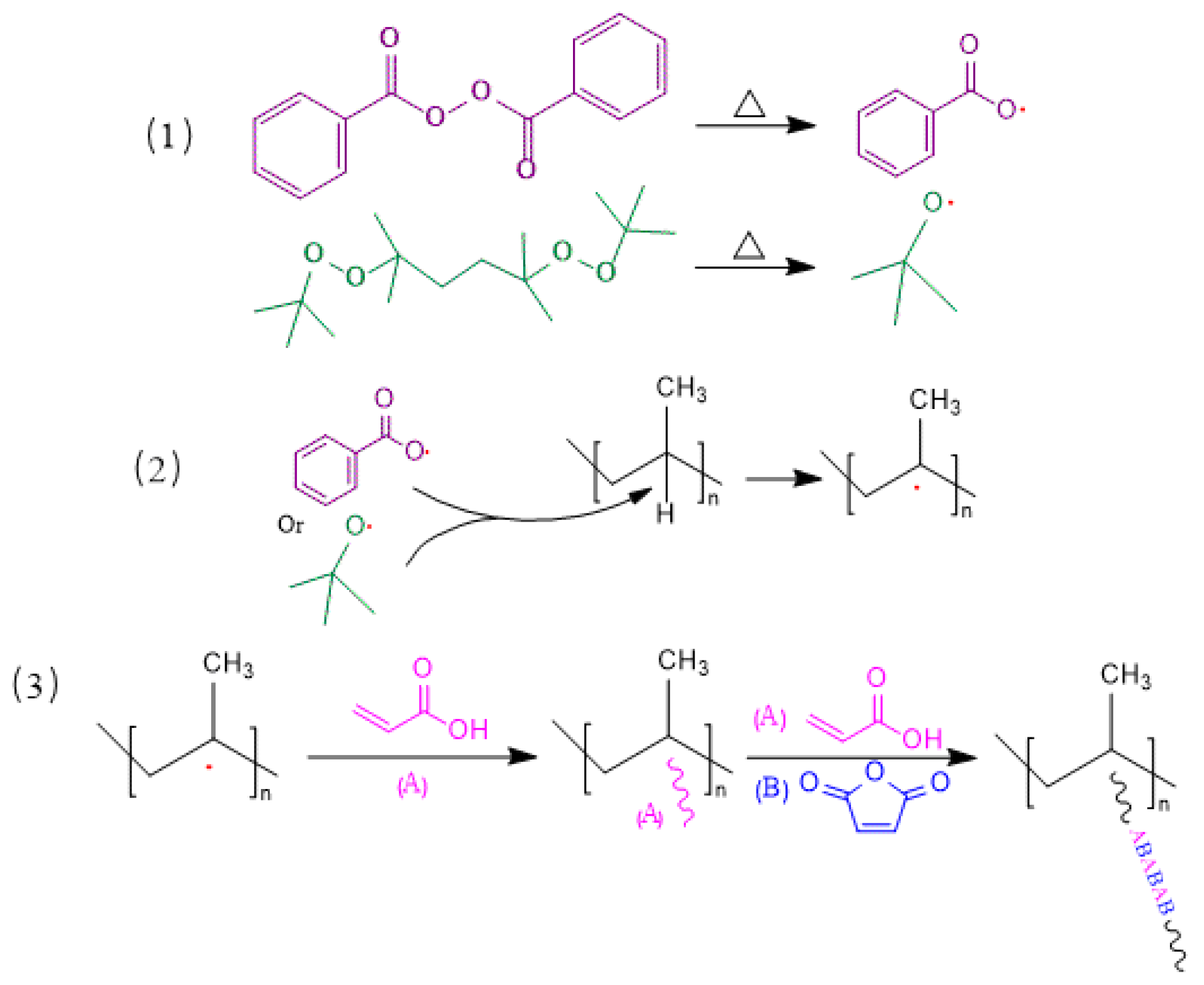
3.1. Mechanism of Suspension Grafting Polymerization
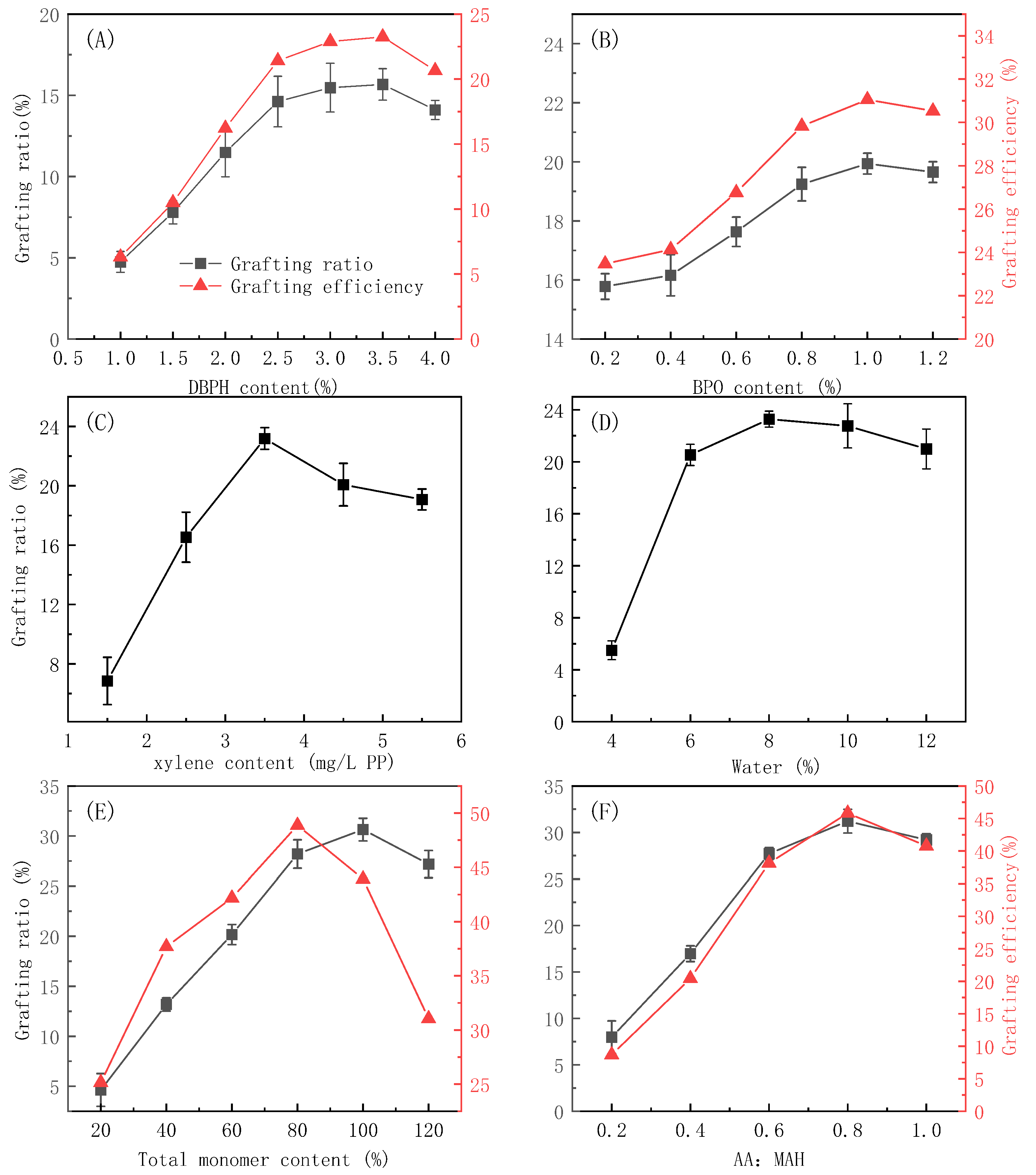
3.2. Influence of Reagent Concentrations on the Grafting Reaction
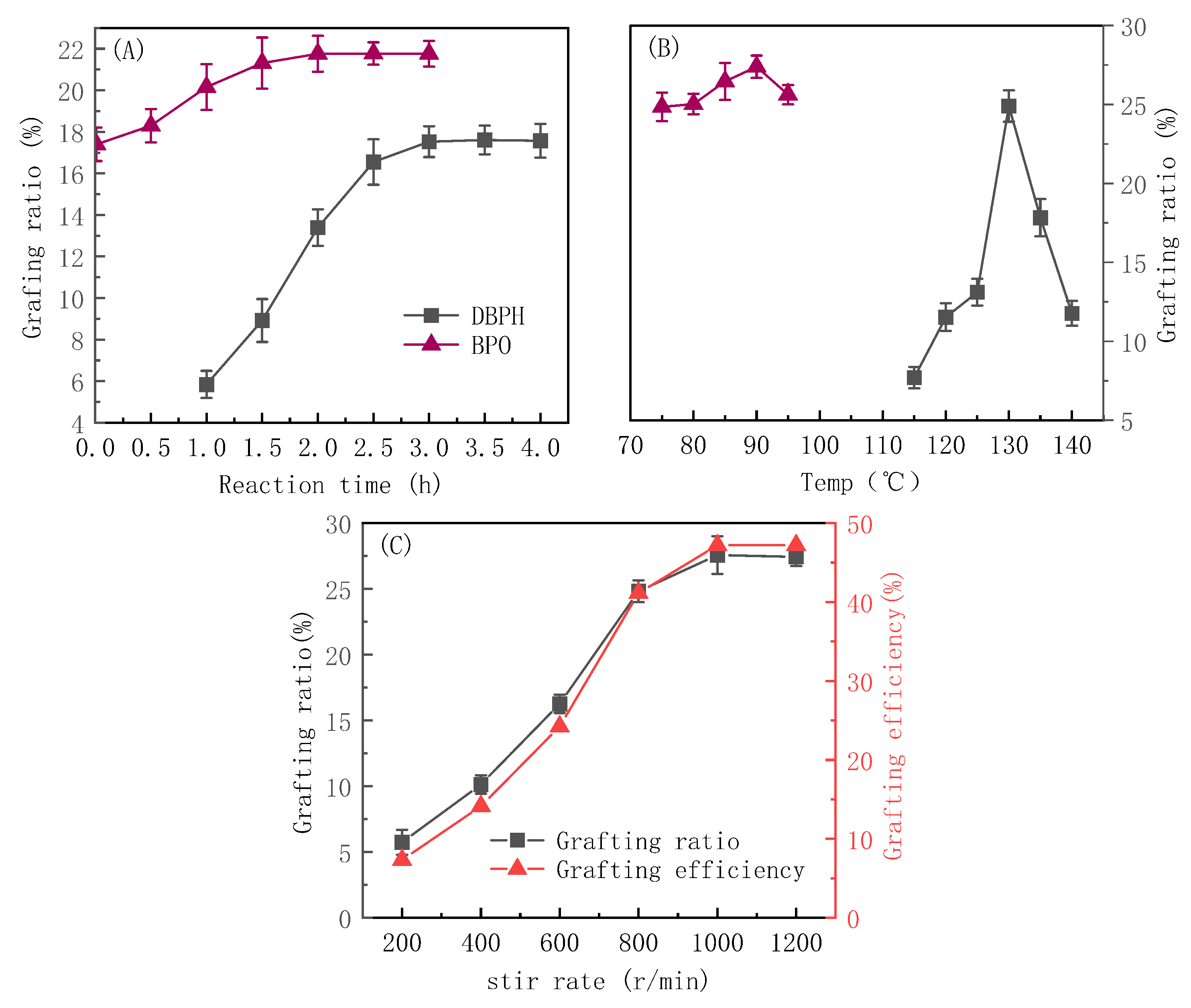
3.3. Influence of Reaction Conditions on the Grafting Modification
3.4. Characteristics of the PP-g-AA-MAH Fibers
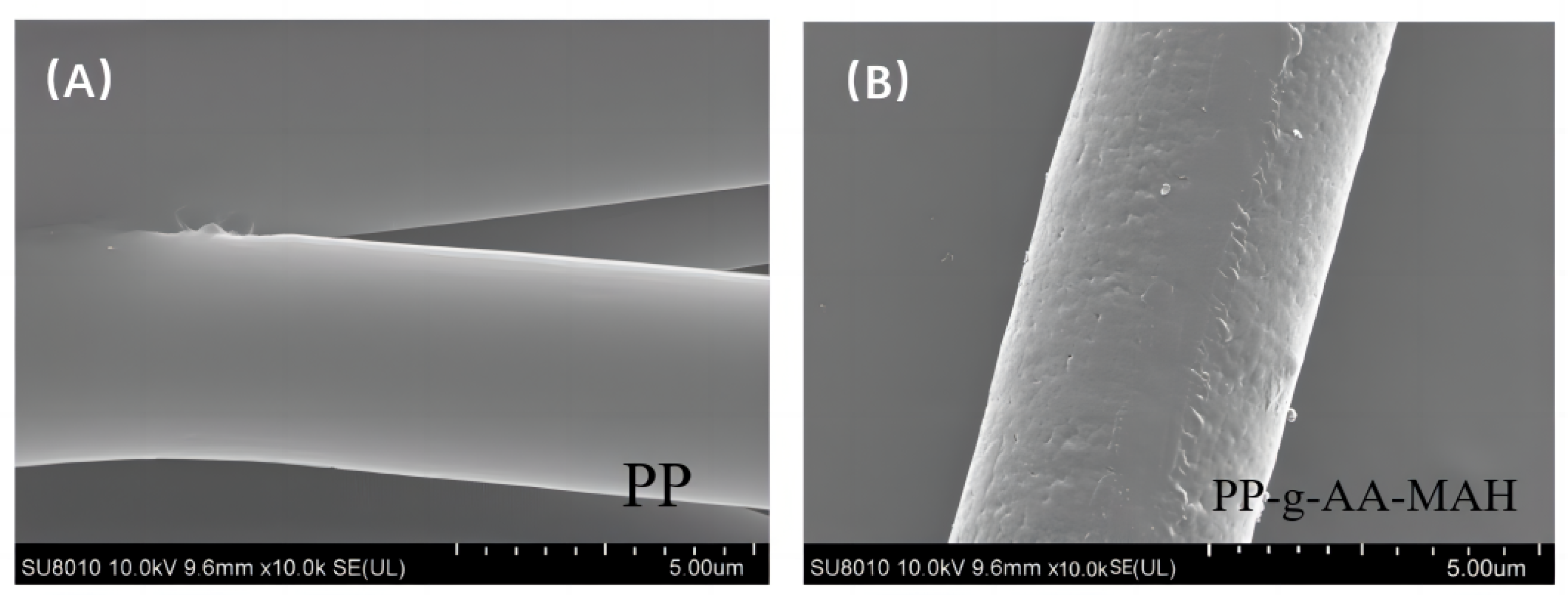
3.4.1. SEM Analysis of the Modified Fibers
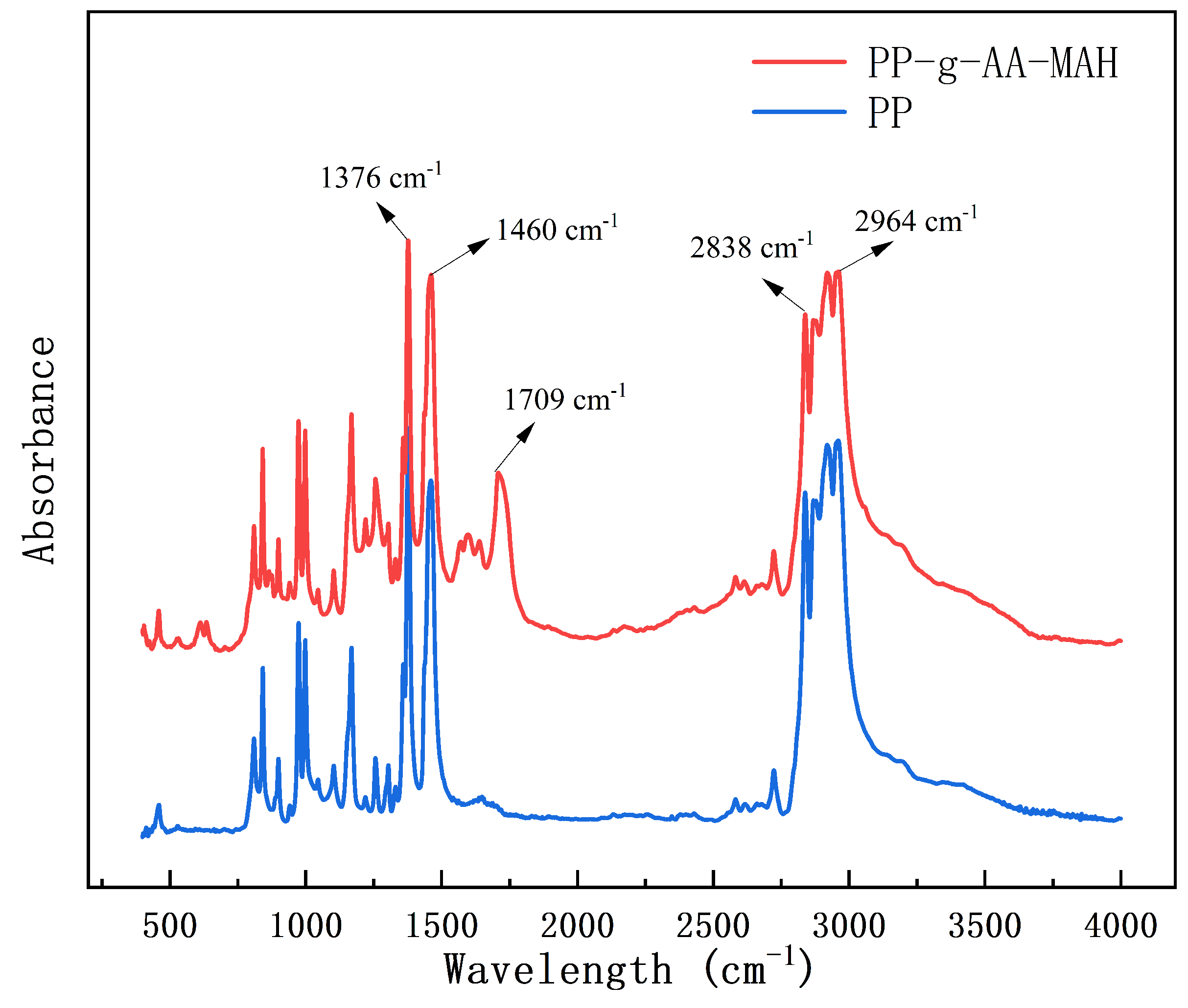
3.4.2. Fourier-Transform Infrared (FT-IR) Analysis of the Modified Fibers
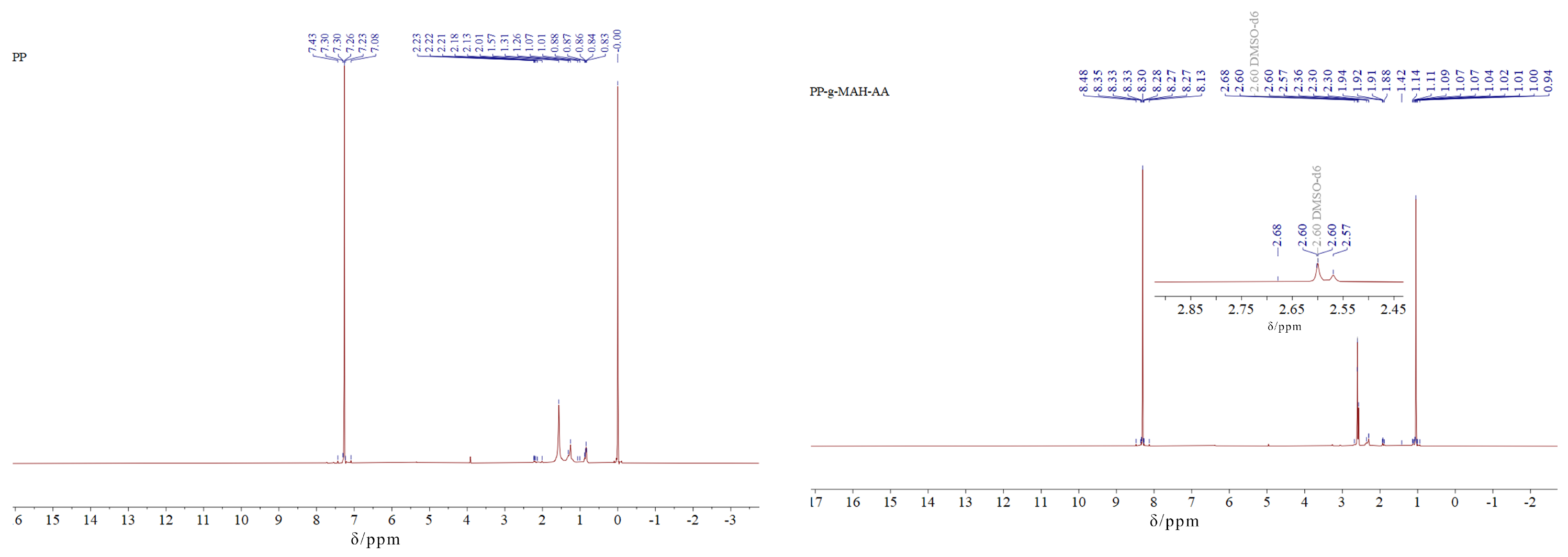
3.4.3. Nuclear Magnetic Resonance (1H-NMR) Analysis of the Modified Fibers
3.4.4. Surface-Property Analysis of the Modified Fibers
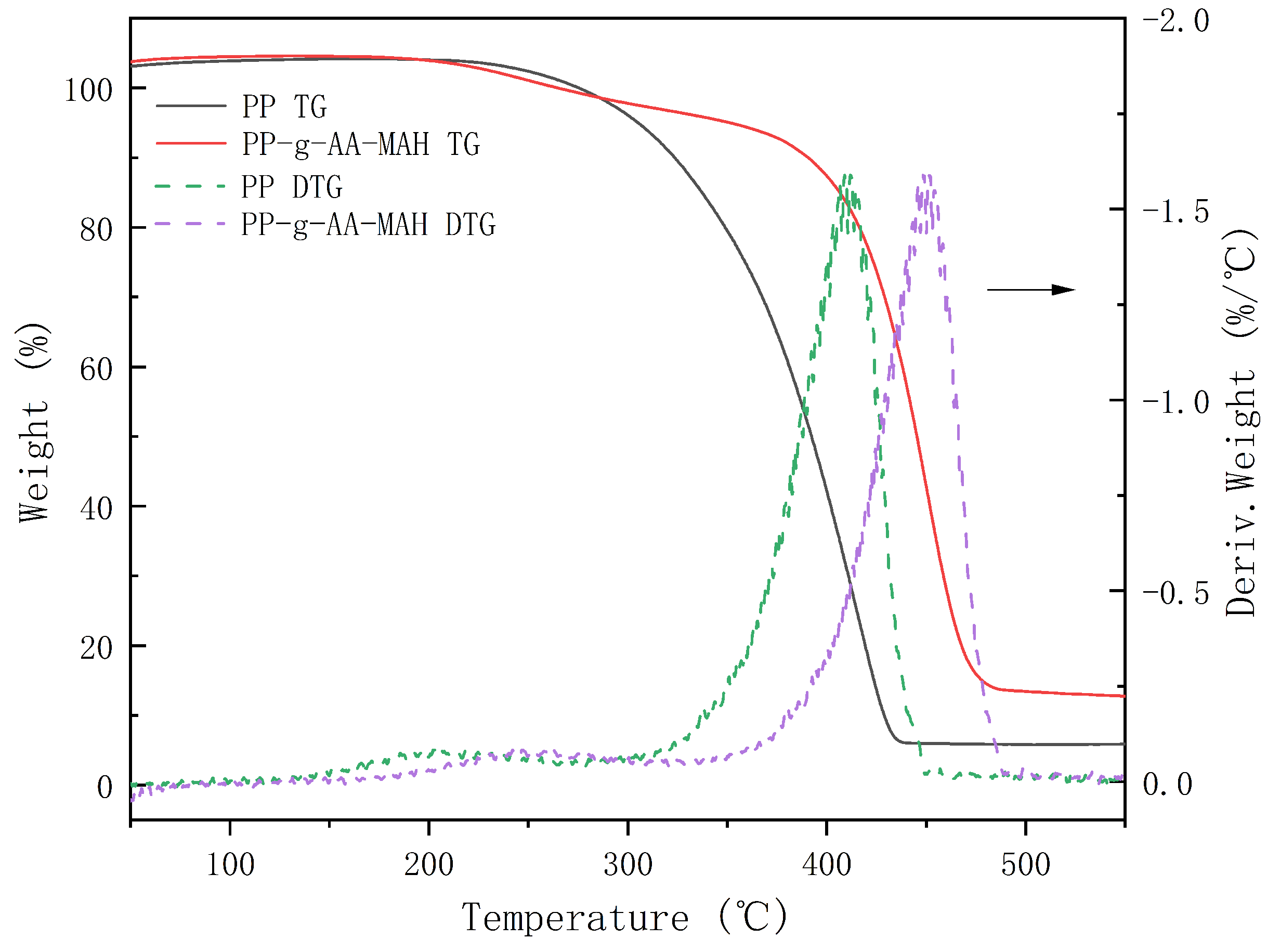
3.4.5. Thermal Stability Analysis of the Modified Fibers
3.5. Adsorption and Regeneration of Indigo Dye Using the PP-g-AA-MAH Fibers
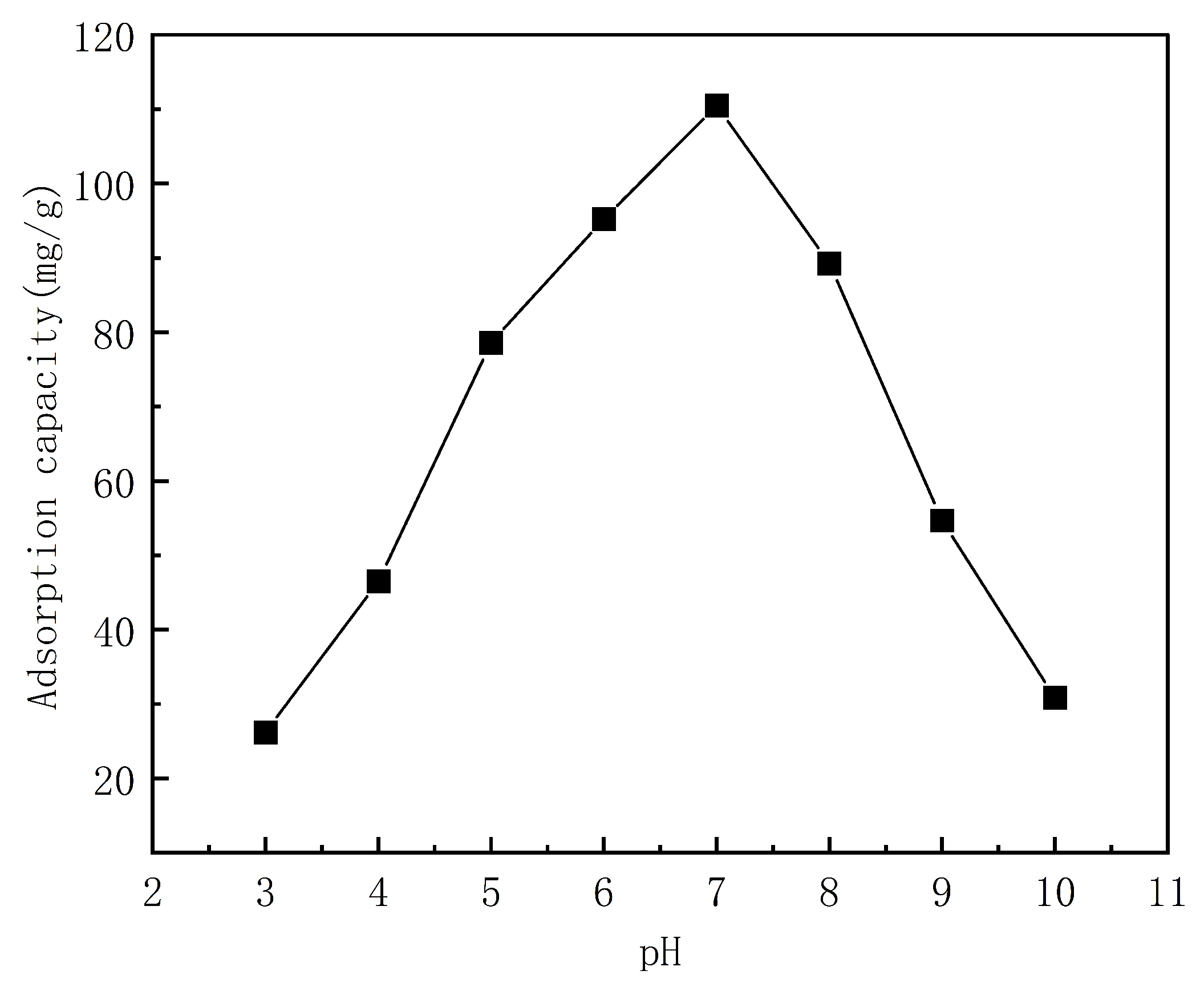
3.5.1. Effect of pH on the Fiber Adsorption Capacity for Indigo
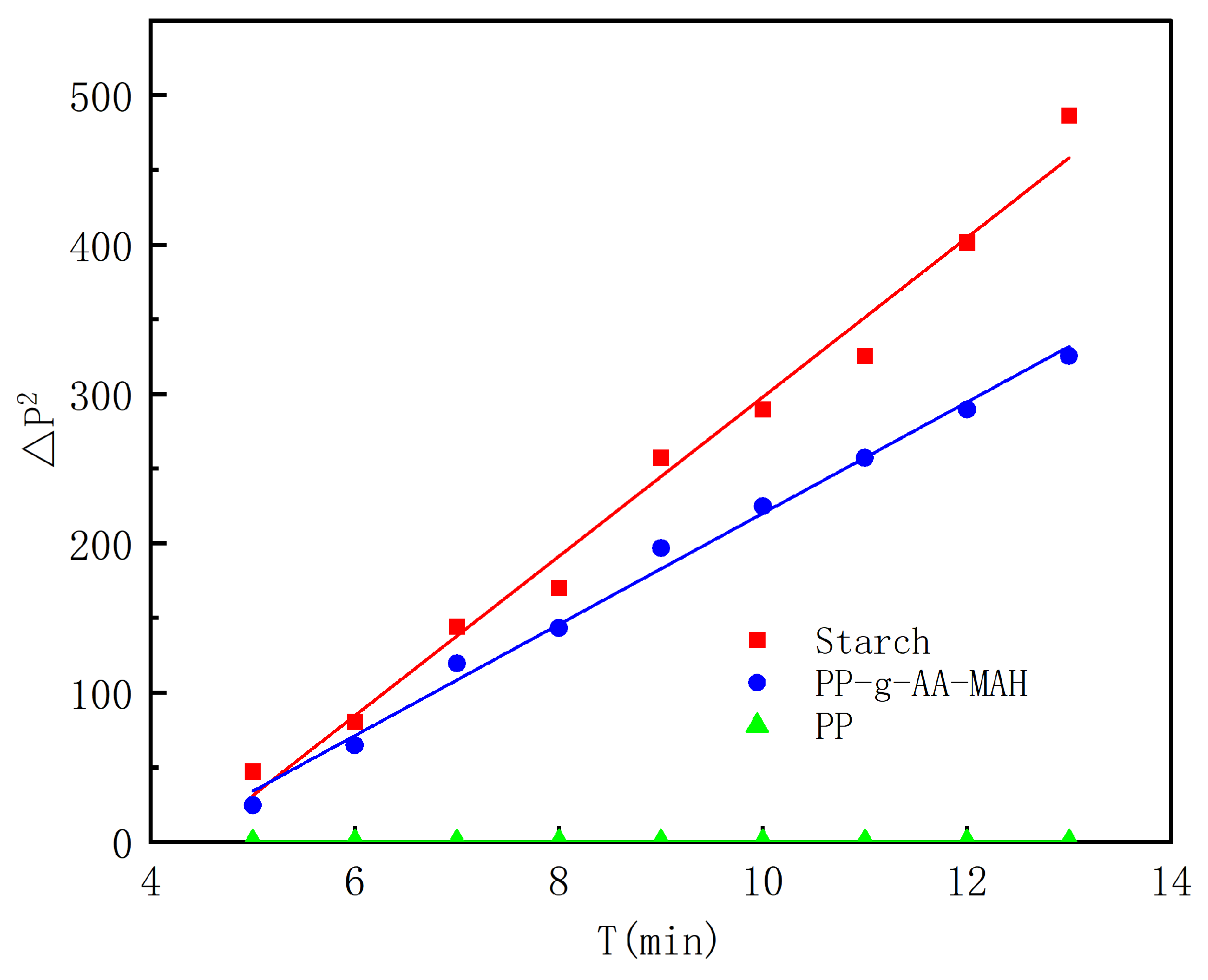
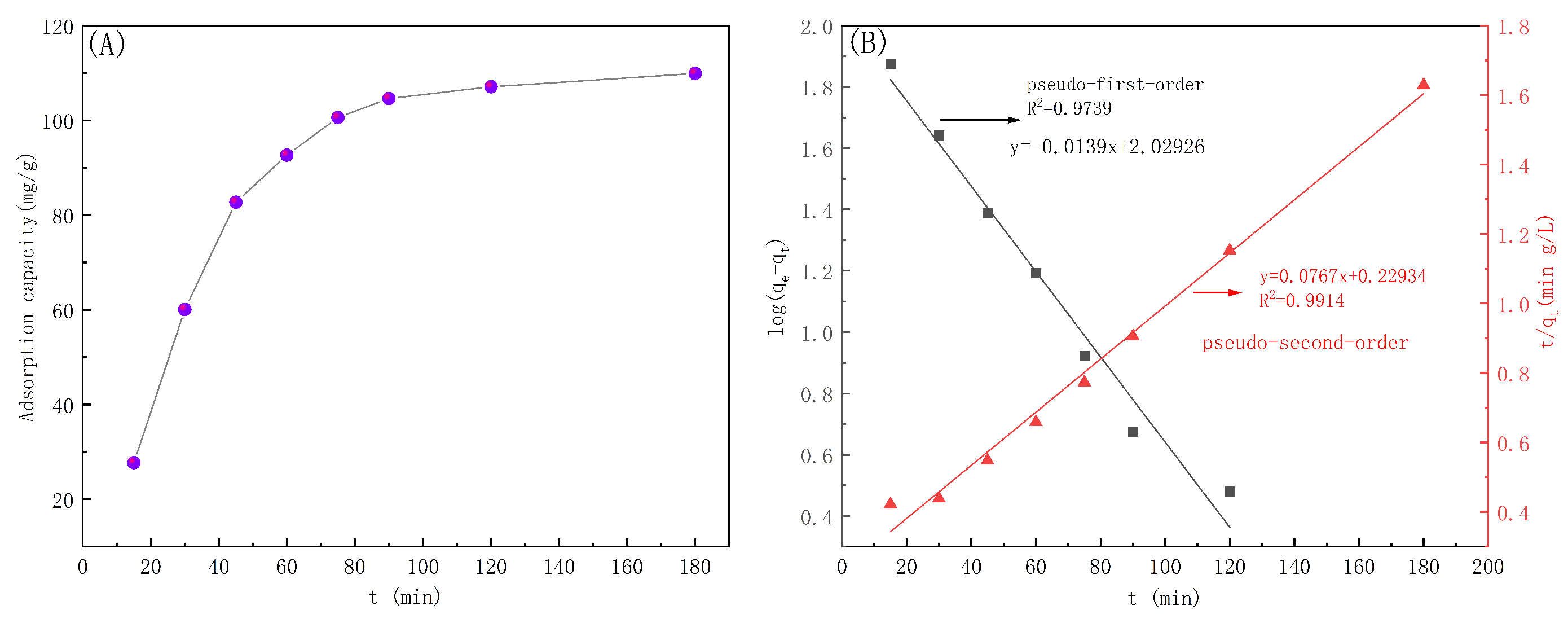
3.5.2. Adsorption Kinetics
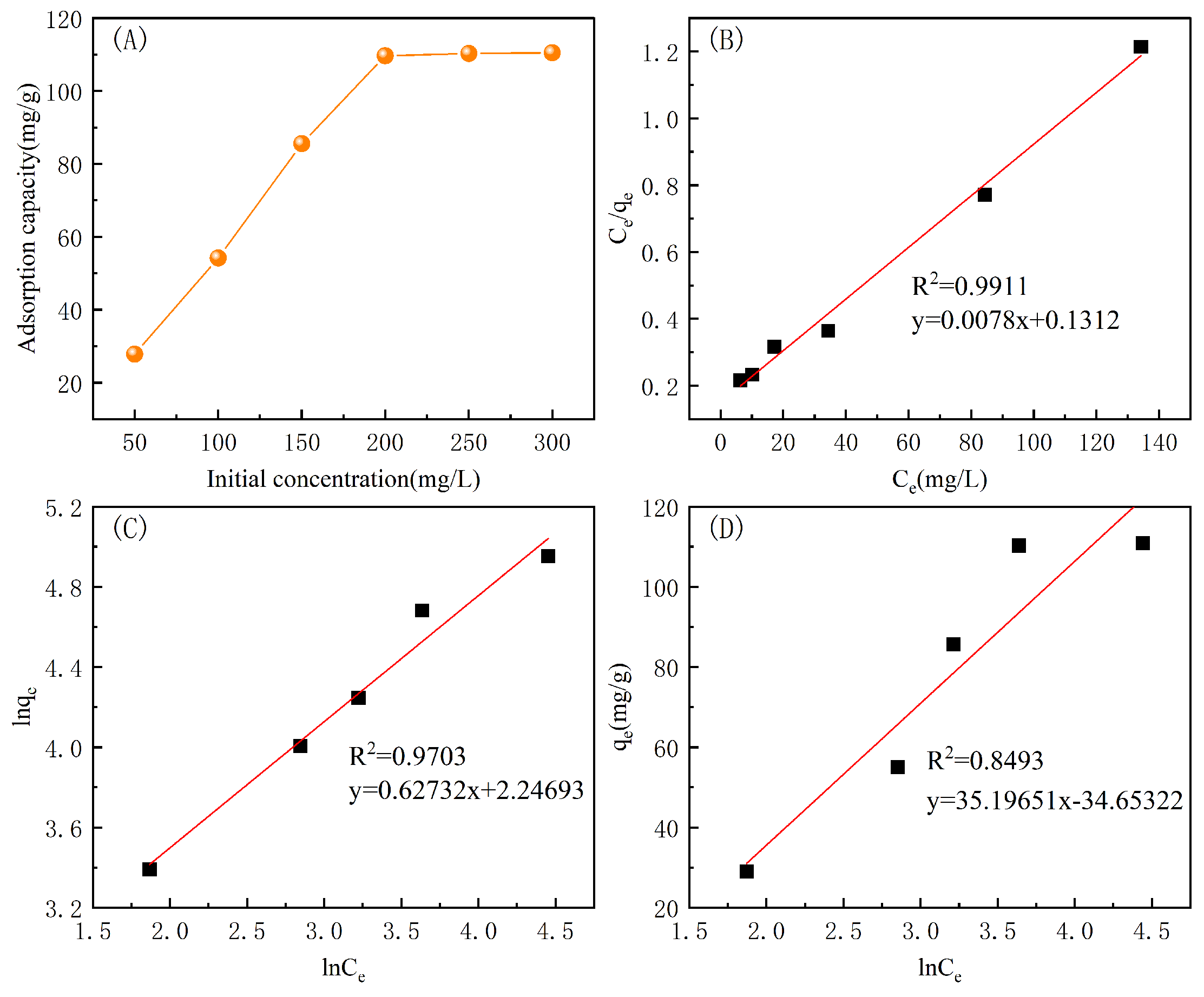
3.5.3. Adsorption Isotherms
3.5.4. Adsorption Performance of Regenerated PP-g-AA-MAH Fibers
3.5.5. Comparison of Adsorption Capacity with Other Adsorbents
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, Y.; Zhang, Y.; Yuan, Y.; Li, H.; Dong, K.; Li, X. Progress Research on Treatment Methods of Printing and Dyeing Wastewater. IOP Conf. Ser. Earth Environ. Sci. 2019, 330, 032073. [Google Scholar] [CrossRef]
- Yang, Q.; Ren, S.; Zhao, Q.; Lu, R.; Hang, C.; Chen, Z.; Zheng, H. Selective separation of methyl orange from water using magnetic ZIF-67 composites. Chem. Eng. J. 2018, 333, 49–57. [Google Scholar] [CrossRef]
- Ren, Z.; Yang, X.; Zhang, W.; Zhao, Z. Preparation, characterization and performance of a novel magnetic Fe-Zn activated carbon for efficient removal of dyes from wastewater. J. Mol. Struct. 2023, 1274, 134407. [Google Scholar] [CrossRef]
- Shukla, A.K.; Alam, J.; Rahaman, M.; Alrehaili, A.; Alhoshan, M.; Aldalbahi, A. A Facile Approach for Elimination of Electroneutral/Anionic Organic Dyes from Water Using a Developed Carbon-Based Polymer Nanocomposite Membrane. Water Air Soil Pollut. 2020, 231, 104. [Google Scholar] [CrossRef]
- Olivo-Alanís, D.; Atilano-Camino, M.M.; García-González, A.; Humberto-Álvarez, L.; García-Reyes, R.B. Chlorophyll-sensitized phenolic resins for the photocatalytic degradation of methylene blue and synthetic blue wastewater. J. Sol.-Gel Sci. Technol. 2021, 100, 538–554. [Google Scholar] [CrossRef]
- Chowdhury, M.F.; Khandaker, S.; Sarker, F.; Islam, A.; Rahman, M.T.; Awual, M.R. Current treatment technologies and mechanisms for removal of indigo carmine dyes from wastewater: A review. J. Mol. Liq. 2020, 318, 114061. [Google Scholar] [CrossRef]
- Gao, J.Y.; Gao, F.Z.; Zhu, F.; Luo, X.H.; Jiang, J.; Feng, L. Synergistic coagulation of bauxite residue-based polyaluminum ferric chloride for dyeing wastewater treatment. J. Cent. South Univ. 2019, 26, 449–457. [Google Scholar] [CrossRef]
- Wang, Z.; Chu, Y.; Zhao, G.; Yin, Z.; Kuang, T.; Yan, F.; Zhang, L.; Zhang, L. Study of surface wettability of mineral rock particles by an improved Washburn method. ACS Omega 2023, 8, 15721–15729. [Google Scholar] [CrossRef]
- Yang, Q.; Zhang, W.; Zhang, H.; Li, Y.; Li, C. Wastewater treatment by alkali bacteria and dynamics of microbial communities in two bioreactors. Bioresour. Technol. 2011, 102, 3790–3798. [Google Scholar] [CrossRef]
- Pan, Z.; Tao, D.; Ren, M.; Cheng, L. A Combinational Optimization Method for Efficient Production of Indigo by the Recombinant Escherichia coli with Expression of Monooxygenase and Malate Dehydrogenase. Foods 2023, 12, 502. [Google Scholar] [CrossRef]
- Dhas, P.G.T.N.; Gulyas, H.; Otterpohl, R. Impact of powdered activated carbon and anion exchange resin on photocatalytic treatment of textile wastewater. J. Environ. Prot. 2015, 6, 54421. [Google Scholar] [CrossRef]
- Hashemian, S.; Hidarian, M. Synthesize and characterization of sawdust/MnFe2O4 nano composite for removal of indigo carmine from aqueous solutions. Orient. J. Chem. 2014, 30, 1753. [Google Scholar] [CrossRef][Green Version]
- Qiu, M.; Huang, C. Removal of dyes from aqueous solution by activated carbon from sewage sludge of the municipal wastewater treatment plant. Desalin. Water Treat. 2015, 53, 3641–3648. [Google Scholar] [CrossRef]
- Peng, S.; Zhang, D.; Huang, H.; Jin, Z.; Peng, X. Ionic polyacrylamide hydrogel improved by graphene oxide for efficient adsorption of methylene blue. Res. Chem. Intermed. 2018, 45, 1545–1563. [Google Scholar] [CrossRef]
- Mostafa, M.M.H.; Nkudede, E.; Sulemana, H.; Zhang, B.; Zhu, K.; Hu, S.; Gblinwon, R.T.; Kosiba, A.A.; Manickam, S. Treatment of simulated printing and dyeing wastewater using ozone microbubble. E3S Web Conf. 2021, 261, 04005. [Google Scholar] [CrossRef]
- Benhalima, T.; Sadi, A.; Dairi, N.; Ferfera-Harrar, H. Multifunctional carboxymethyl cellulose-dextran sulfate/AgNPs@ zeolite hydrogel beads for basic red 46 and methylene blue dyes removal and water disinfection control. Sep. Purif. Technol. 2024, 342, 127001. [Google Scholar] [CrossRef]
- Qian, J.; Han, X.; Yang, S.; Kuang, L.; Hua, D. A strategy for effective cesium adsorption from aqueous solution by polypentacyanoferrate-grafted polypropylene fabric under γ-ray irradiation. J. Taiwan Inst. Chem. Eng. 2018, 89, 162–168. [Google Scholar] [CrossRef]
- Mahboubizadeh, S.; Hasanzadeh, M.; Panahi, F.; Arzaqi, Z. A Review of Processing Methods and Modification of Reinforcements Used in Polypropylene Composites. J. Iran. Chem. Eng. 2021, 20, 36–63. [Google Scholar]
- Correa-Aguirre, J.P.; Luna-Vera, F.; Caicedo, C.; Vera-Mondragón, B.; Hidalgo-Salazar, M.A. The Effects of Reprocessing and Fiber Treatments on the Properties of Polypropylene-Sugarcane Bagasse Biocomposites. Polymers 2020, 12, 1440. [Google Scholar] [CrossRef]
- Mandal, D.K.; Bhunia, H.; Bajpai, P.K.; Chaudhari, C.V.; Dubey, K.A.; Varshney, L. Radiation-induced grafting of acrylic acid onto polypropylene film and its biodegradability. Radiat. Phys. Chem. 2016, 123, 37–45. [Google Scholar] [CrossRef]
- Abdel Ghaffar, A.; El-Arnaouty, M.; Aboulfotouh, M.E.; Taher, N.; Taha, A.A. Radiation graft copolymerization of butyl methacrylate and acrylamide onto low density polyethylene and polypropylene films, and its application in wastewater treatment. Radiat. Eff. Defects Solids 2014, 169, 741–753. [Google Scholar] [CrossRef]
- Nosova, N.; Roiter, Y.; Samaryk, V.; Varvarenko, S.; Stetsyshyn, Y.; Minko, S.; Stamm, M.; Voronov, S. Polypropylene surface peroxidation with heterofunctional polyperoxides. Macromol. Symp. 2004, 210, 339–348. [Google Scholar] [CrossRef]
- Basuki, B.; Suyitno, S.; Kristiawan, B. Absorbance and electrochemical properties of natural indigo dye. In AIP Conference Proceedings; AIP Publishing: Long Island, NY, USA, 2018; Volume 1931. [Google Scholar]
- Li, Z.; Ma, Y.; Yang, W. A facile, green, versatile protocol to prepare polypropylene-g-poly(methyl methacrylate) copolymer by water-solid phase suspension grafting polymerization using the surface of reactor granule technology polypropylene granules as reaction loci. J. Appl. Polym. Sci. 2013, 129, 3170–3177. [Google Scholar] [CrossRef]
- Ji, L.; Shi, B. A novel method for determining surface free energy of powders using Washburn’s equation without calculating capillary factor and contact angle. Powder Technol. 2015, 271, 88–92. [Google Scholar] [CrossRef]
- Qing, C.; Bigui, W.; Yingdong, H. Capillary pressure method for measuring lipophilic hydrophilic ratio of filter media. Chem. Eng. J. 2009, 150, 323–327. [Google Scholar] [CrossRef]
- Lian, Z.; Xu, Y.; Zuo, J.; Qian, H.; Luo, Z.; Wei, W. Preparation of PP-g-(AA-MAH) Fibers Using Suspension Grafting and Melt-Blown Spinning and its Adsorption for Aniline. Polymers 2020, 12, 2157. [Google Scholar] [CrossRef]
- Bora, D.; Dutta, H.; Saha, B.; Reddy, Y.A.K.; Patel, R.; Verma, S.K.; Sellamuthu, P.S.; Sadiku, R.; Jayaramudu, J. A review on modification of polypropylene carbonate (PPC) using different types of blends/composites and its advanced uses. Mater. Today Commun. 2023, 37, 107304. [Google Scholar] [CrossRef]
- Yang, M.; Sun, L.; Liu, Q.; Deng, Y.; Liu, C.; Li, N.; Xu, J.; Chen, Y.; Jian, X. Ultra high density carbon fiber derived from isotactic polypropylene fiber without skin-core structure. Polym. Adv. Technol. 2024, 35, e6300. [Google Scholar] [CrossRef]
- George, G.; Joseph, K.; Saritha, A.; Nagarajan, E.R. Influence of fiber content and chemical modifications on the transport properties of PP/jute commingled biocomposites. Polym. Compos. 2017, 39, E250–E260. [Google Scholar] [CrossRef]
- Jiao, W.; Liu, W.; Yang, F.; Jiang, L.; Jiao, W.; Wang, R. Improving the interfacial property of carbon fiber/vinyl ester resin composite by grafting modification of sizing agent on carbon fiber surface. J. Mater. Sci. 2017, 52, 13812–13828. [Google Scholar] [CrossRef]
- Liang, F.; Yuan, H.; Shao, Q.; Song, W. Study of suspension grafting process of polypropylene. Des. Monomers Polym. 2018, 21, 130–136. [Google Scholar] [CrossRef]
- Wang, S.; Muiruri, J.K.; Soo, X.Y.D.; Liu, S.; Thitsartarn, W.; Tan, B.H.; Suwardi, A.; Li, Z.; Zhu, Q.; Loh, X.J. Bio-Polypropylene and Polypropylene-based Biocomposites: Solutions for a Sustainable Future. Chem. Asian J. 2023, 18, e202200972. [Google Scholar] [CrossRef] [PubMed]
- Badertscher, M.; Bühlmann, P.; Pretsch, E. Structure Determination of Organic Compounds: Tables of Spectral Data; Springer: Berlin/Heidelberg, Germany, 2009; Volume 443. [Google Scholar]
- Wang, J.; Sun, C.; Xia, W.; Cao, Z.; Sheng, G.; Xie, X. Pd/BN catalysts for highly efficient hydrogenation of maleic anhydride to succinic anhydride. Appl. Catal. A Gen. 2022, 630, 118471. [Google Scholar] [CrossRef]
- Musah, M.; Azeh, Y.; Mathew, J.T.; Umar, M.T.; Abdulhamid, Z.; Muhammad, A.I. Adsorption kinetics and isotherm models: A review. CaJoST 2022, 4, 20–26. [Google Scholar] [CrossRef]
- Yuan, H.; Shao, Q.; Liang, F.; Shi, H.; Song, W. Mechanism of crosslinking in benzoyl peroxide-initiated functionalization of vinyltriethoxysilane onto polypropylene in the water medium. J. Appl. Polym. Sci. 2020, 137, 49534. [Google Scholar] [CrossRef]
- Zhao, D.; Wang, L.; Zhang, R.; Ma, Y.; Chen, D.; Zhao, C.; Yang, W. Preparation of toughened polypropylene-g-poly (butyl acrylate-co-acrylated castor oil) by suspension grafting polymerization. Polym. Eng. Sci. 2018, 58, 86–93. [Google Scholar] [CrossRef]
- Zhong, J.; Yang, B.; Feng, Y.; Chen, Y.; Wang, L.G.; You, W.D.; Ying, G.G. Enhanced photo–Fenton removal efficiency with core-shell magnetic resin catalyst for textile dyeing wastewater treatment. Water 2021, 13, 968. [Google Scholar] [CrossRef]
- Okoya, A.A.; Diisu, D.O.; Olaiya, O.O.; Adegbaju, O.S. Adsorption Efficiency of Flamboyant Pods for Indigo Dye Removal from Textile Industrial Wastewater. Environ. Pollut. 2020, 9, 224371109. [Google Scholar] [CrossRef]
- Kadiri, L.; Ouass, A.; Hsissou, R.; Safi, Z.; Wazzan, N.; Essaadaoui, Y.; Lebkiri, I.; El Khattabi, O.; Housseine Rifi, E.; Lebkiri, A. Adsorption properties of coriander seeds: Spectroscopic kinetic thermodynamic and computational approaches. J. Mol. Liq. 2021, 343, 116971. [Google Scholar] [CrossRef]
- Basta, A.H.; Lotfy, V.F. Impact of pulping routes of rice straw on cellulose nanoarchitectonics and their behavior toward Indigo dye. Appl. Nanosci. 2022, 13, 4455–4469. [Google Scholar] [CrossRef]
- Lv, A.; Lv, X.; Xu, X.; Chen, Y.; Zhang, J.; Shao, Z.-B. Tailored multifunctional composite hydrogel based on chitosan and quaternary ammonium ionic liquids@montmorillonite with different chain lengths for effective removal of dyes and 4-nitrophenol. Sep. Purif. Technol. 2024, 342, 127019. [Google Scholar] [CrossRef]
- Ribeiro, J. Study of the Ziziphus Joazeiro Peel for Indigo Blue Adsorption. Int. J. Adv. Res. 2019, 7, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Missaoui, M.; Tahri, N.; Daramola, M.O.; Duplay, J.; Schäfer, G.; Ben Amar, R. Comparative investigation of indigo blue dye removal efficiency of activated carbon and natural clay in adsorption/ultrafiltration system. Desalin. Water Treat. 2019, 164, 326–338. [Google Scholar] [CrossRef]
- Radhi, I.K.; Hussein, M.A.; Kadhim, Z.N. Investigation of ni grosine, alizarin, indigo and acid fuchsin removal by modification of CaO derived from eggshell with AgI: Adsorption, kinetic and photocatalytic studies. Eur. J. Chem. 2019, 10, 64–71. [Google Scholar] [CrossRef]
- Sanda, O.; Amoo, K.I.; Taiwo, E.A. Studies on the adsorption of indigo dye from aqueous media onto carbonised sugarcane bagasse. Niger. J. Basic Appl. Sci. 2018, 25, 40–47. [Google Scholar] [CrossRef]
- Robledo-Padilla, F.; Aquines, O.; Silva-Núñez, A.; Alemán-Nava, G.S.; Castillo-Zacarías, C.; Ramirez-Mendoza, R.A.; Zavala-Yoe, R.; Iqbal, H.M.N.; Parra-Saldívar, R. Evaluation and Predictive Modeling of Removal Condition for Bioadsorption of Indigo Blue Dye by Spirulina platensis. Microorganisms 2020, 8, 82. [Google Scholar] [CrossRef] [PubMed]

| PP | Monomers | Xylene | DBPH | BPO | Water | RPM | Grafting Ratio | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AA | MAH | Amount | Temp | Time | Amount | Temp | Time | |||||
| 200 g | 71 g | 89 g | 700 mL | 7 g | 130 °C | 3 h | 2 g | 90 °C | 2 h | 1.6 L | 1000 r/min | 31.2% |
| Kinetic Model | (mg/g) | K | |
|---|---|---|---|
| Pseudo-first-order kinetic model | 92.45 | 0.062 | 0.9739 |
| Pseudo-second-order kinetic model | 127.66 | 0.00078 | 0.9914 |
| Langmuir | Freundlich | Temkin | ||||||
|---|---|---|---|---|---|---|---|---|
| /mg/g | ||||||||
| 111.74 | 1.72 | 0.9911 | 2.0253 | 5.2069 | 0.9703 | 28.9843 | 0.5369 | 0.8493 |
| Adsorbents | qmax/mg·g−1 | Reference |
|---|---|---|
| Chitosan-modified flamboyant pods (CMFP) | 22.45 | [40] |
| Coriander seeds | 124 | [41] |
| Nanocelluloses using acid hydrolysis and oxidizing agents | 39.7 | [42] |
| A composite hydrogel (DMCHA) | 306.08 | [43] |
| Zizyphus joazeiro Mart peel (ZJP) | 50 | [44] |
| Natural clay (NC) | 57 | [45] |
| CaO nanoparticles made from eggshells | 260 | [46] |
| Carbonized sugarcane bagasse | 8.88 | [47] |
| Spirulina platensis | 91 | [48] |
| PP-g-AA-MAH fibers | 110.43 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xie, S.; Fang, Z.; Lian, Z.; Luo, Z.; Zhang, X.; Ma, S. A Novel, Dual-Initiator, Continuous-Suspension Grafting Strategy for the Preparation of PP-g-AA-MAH Fibers to Remove of Indigo from Wastewater. Polymers 2024, 16, 2144. https://doi.org/10.3390/polym16152144
Xie S, Fang Z, Lian Z, Luo Z, Zhang X, Ma S. A Novel, Dual-Initiator, Continuous-Suspension Grafting Strategy for the Preparation of PP-g-AA-MAH Fibers to Remove of Indigo from Wastewater. Polymers. 2024; 16(15):2144. https://doi.org/10.3390/polym16152144
Chicago/Turabian StyleXie, Sijia, Ziyang Fang, Zhouyang Lian, Zhengwei Luo, Xueying Zhang, and Shengxiu Ma. 2024. "A Novel, Dual-Initiator, Continuous-Suspension Grafting Strategy for the Preparation of PP-g-AA-MAH Fibers to Remove of Indigo from Wastewater" Polymers 16, no. 15: 2144. https://doi.org/10.3390/polym16152144
APA StyleXie, S., Fang, Z., Lian, Z., Luo, Z., Zhang, X., & Ma, S. (2024). A Novel, Dual-Initiator, Continuous-Suspension Grafting Strategy for the Preparation of PP-g-AA-MAH Fibers to Remove of Indigo from Wastewater. Polymers, 16(15), 2144. https://doi.org/10.3390/polym16152144






