Methallylsulfonate Polymeric Antiscalants for Application in Thermal Desalination Processes
Abstract
1. Introduction
2. Materials and Methods
2.1. Copolymere
2.2. Other Materials
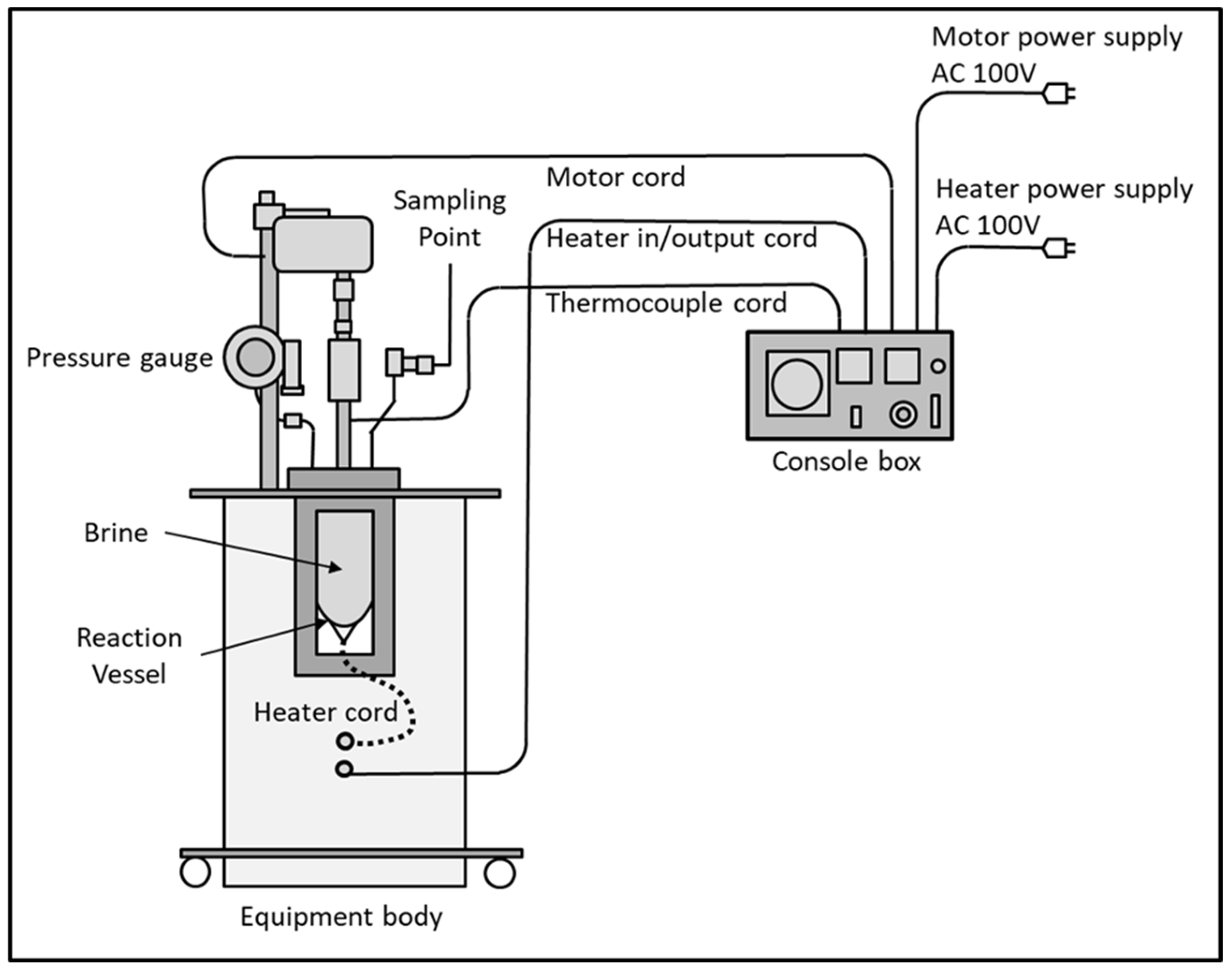
2.3. Description of Experimental Units
2.3.1. Pressure Measurement and Control (P-MAC) Test Unit
2.3.2. High Temperature-High Pressure Test Unit
3. Results
3.1. Series One: Preliminary Evaluation Tests on the P-MAC Unit
3.2. Series Two: Evaluation Tests on the High-Temperature–High-Pressure Unit
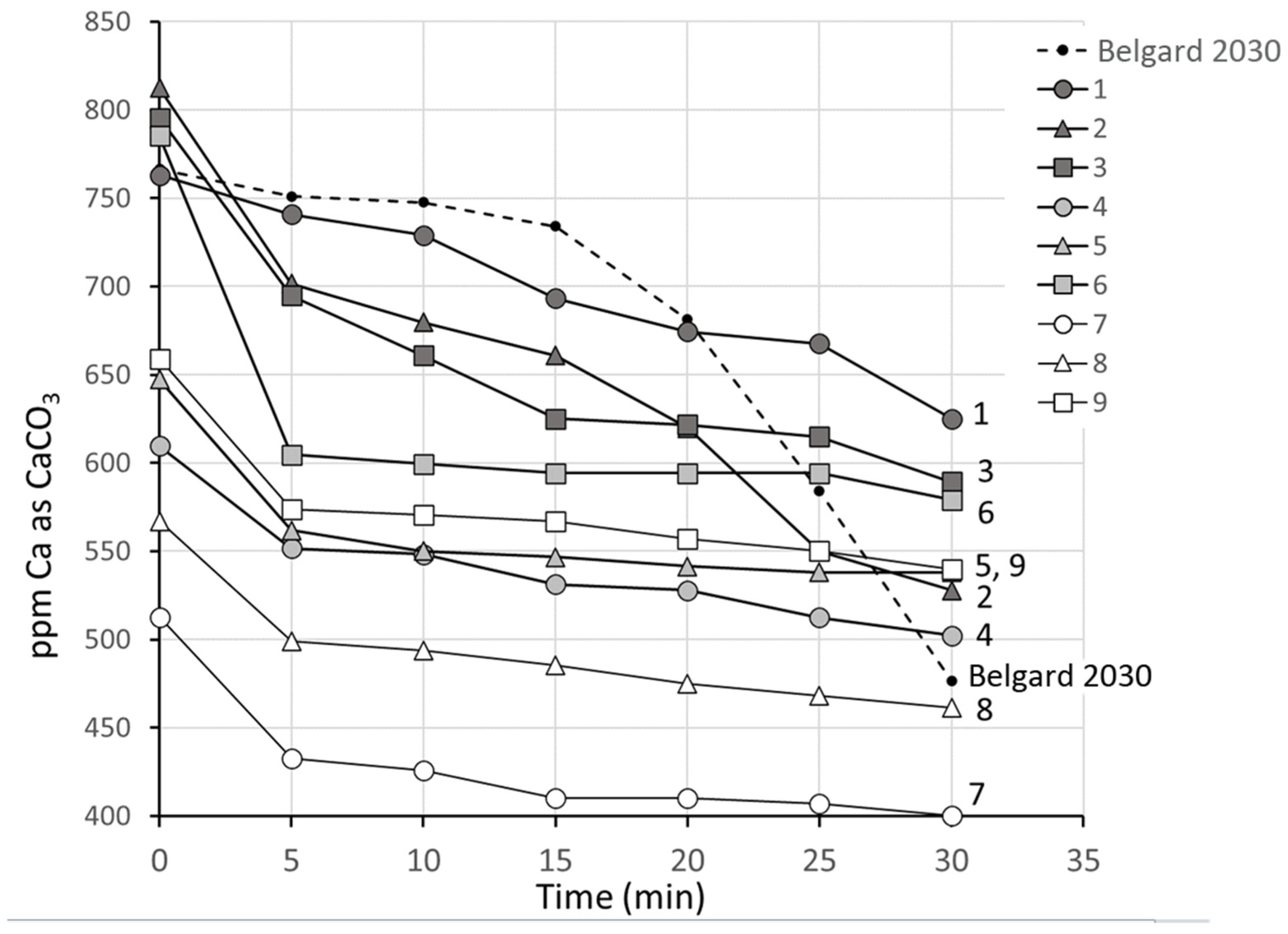
3.2.1. Series 2, Group 1: Direct Comparison of the Performances of the Nine Antiscalants
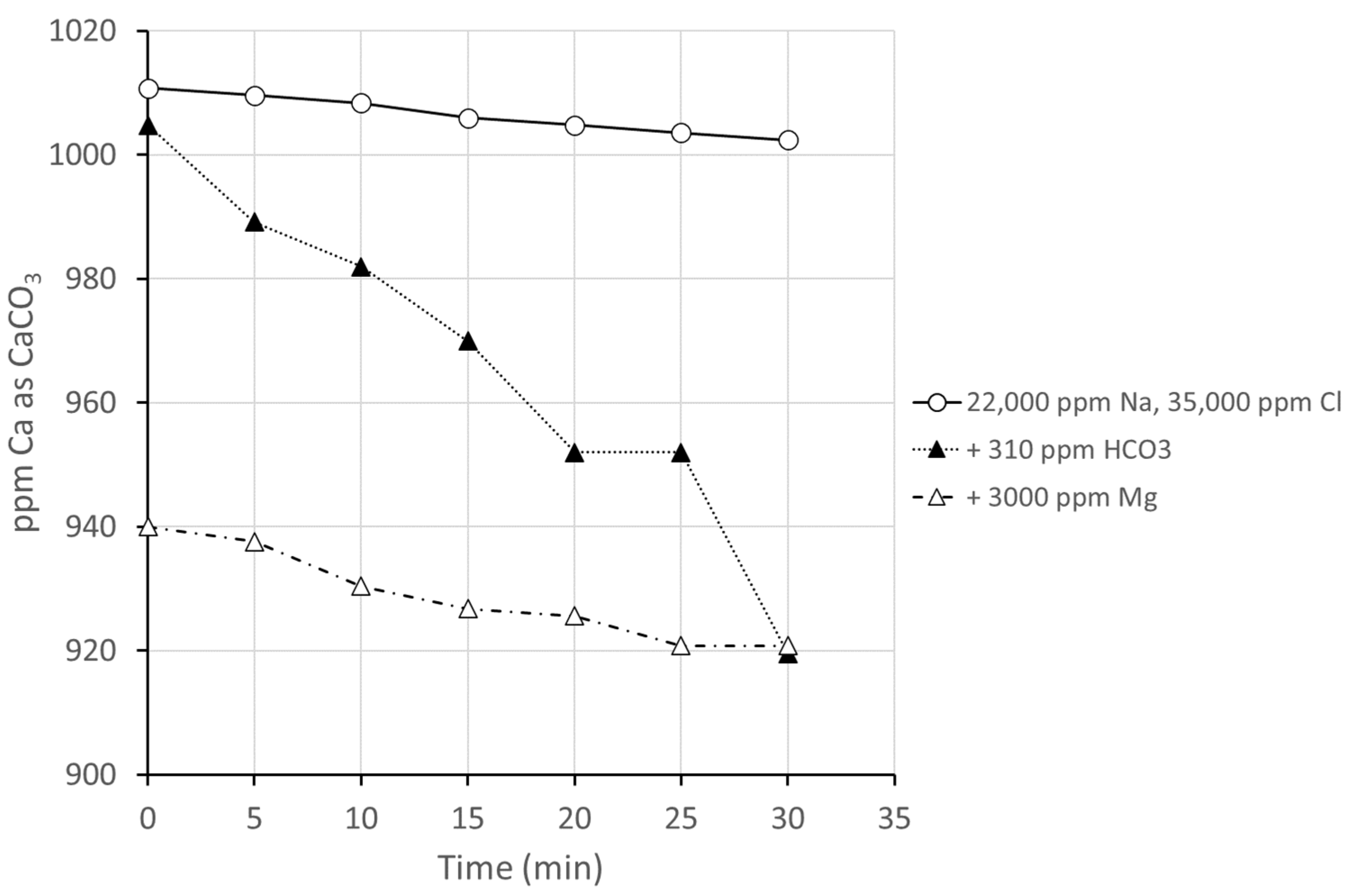
3.2.2. Series 2, Group 2: Impact of Other Ions on Antiscalant Performance
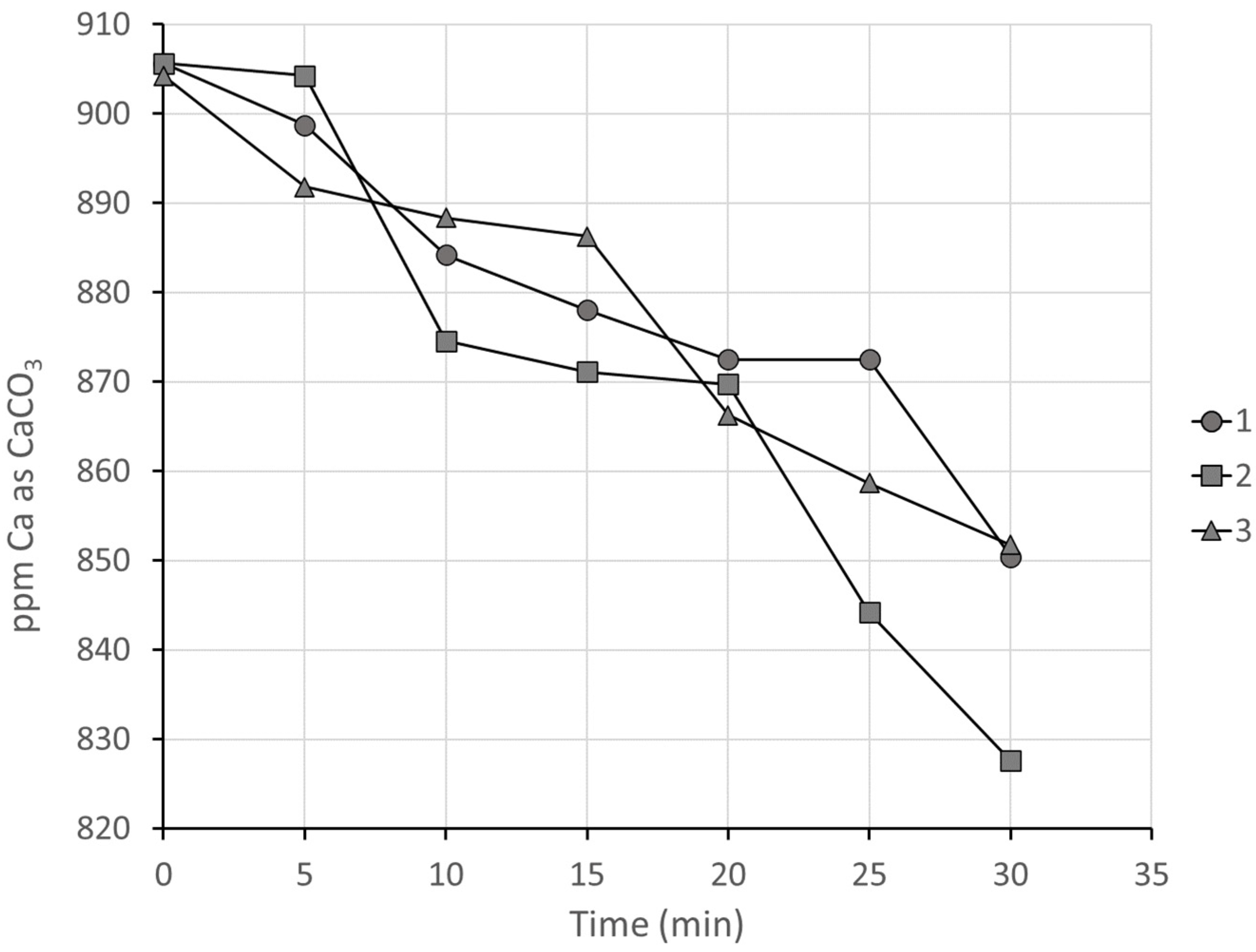
3.2.3. Series 2, Group 3: Comparison of Antiscalants on Synthetic Concentrated Seawater
3.2.4. Series 2, Group 4: Comparison of Antiscalants in Concentrated Seawater
3.3. Series Three: Performance of Low Molar Mass Antiscalants in the P-MAC Unit
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fellows, C.M.; Alhamzah, A.A.; East, C.P. Scale Control in Thermal Desalination. In Water-Formed Deposits: Fundamentals and Mitigation Strategies; Amjad, Z., Demadis, K., Eds.; Elsevier: London, UK, 2022; pp. 457–476. [Google Scholar]
- Ihm, S.; Al-Najdi, O.Y.; Hamed, O.A.; Jun, G.; Chung, H. Energy cost comparison between MSF, MED and SWRO: Case studies for dual purpose plants. Desalination 2016, 397, 116–125. [Google Scholar] [CrossRef]
- Zhou, L.; Jin, J.; Liu, Z. Controlling calcium sulfate scale in process of multi-stage distillation desalination of seawater. Sea-Lake Salt Chem. Ind. 2005, 34, 6–8. [Google Scholar]
- Glater, J.; Dooly, R.L.; McCutchan, J.W. Calcium Sulfate Scale Control in High Temperature Desalting Processes; US Department of Interior: Washington DC, USA, 1973; Report UCAL-ENG 7364.
- Sheikholeslami, R.; Ong, H.W.K. Kinetics and thermodynamics of calcium carbonate and calcium sulfate at salinities up to 1.5 M. Desalination 2003, 157, 217–234. [Google Scholar] [CrossRef]
- Fellows, C.M.; Al-Hamzah, A. Thermal Desalination: Current Challenges. In Mineral Scales and Deposits: Scientific and Technological Approaches; Amjad, Z., Demadis, K., Eds.; Elsevier: London, UK, 2015; pp. 583–602. [Google Scholar]
- Yu, B.; Miao, S.; Ding, M.; Ren, Y. Solubility and Physical Properties of α-Calcium Sulfate Hemihydrate in NaCl and Glycerol Aqueous Solutions at 303.15, 323.15, and 343.15 K. J. Chem. Eng. Data 2021, 66, 3686–3694. [Google Scholar] [CrossRef]
- Taherdangkoo, R.; Tian, M.; Sadighi, A.; Meng, T.; Yang, H.; Butscher, C. Experimental Data on Solubility of the Two Calcium Sulfates Gypsum and Anhydrite in Aqueous Solutions. Data 2022, 7, 140. [Google Scholar] [CrossRef]
- Reigl, S.; Van Driessche, A.E.S.; Mehringer, J.; Koltzenburg, S.; Kunz, W.; Kellermeier, M. Revisiting the Roles of Salinity, Temperature and Water Activity in Phase Selection during Calcium Sulfate Precipitation. CrystEngComm 2022, 24, 1529–1536. [Google Scholar] [CrossRef]
- Solomon, D.H.; Rolfe, P.F. Polymers that inhibit the deposition of calcium sulfate. Desalination 1966, 1, 260–266. [Google Scholar] [CrossRef]
- Amjad, Z. Calcium sulfate dihydrate (gypsum) scale formation on heat exchanger surfaces: The influence of scale inhibitors. J. Coll. Interf. Sci. 1988, 123, 523–536. [Google Scholar] [CrossRef]
- Oner, M.; Dogan, O.G.; Oner, G. The influence of polyelectrolytes architecture on calcium sulfate dihydrate growth retardation. J. Crystal Growth 1998, 186, 427–437. [Google Scholar] [CrossRef]
- Xue, X.; Change, F.; Li, N.; Zheng, F.; Yang, W.; Yang, X. Performance of a non-phosphorus antiscalant on inhibition of calcium-sulfate precipitation. Water Sci. Technol. 2012, 66, 193–200. [Google Scholar] [CrossRef] [PubMed]
- Popov, K.; Rudakova, G.; Larchenko, V.; Tusheva, M.; Kamagurov, S.; Dikareva, J.; Kovaleva, N. A comparative performance evaluation of some novel (Green) and traditional antiscalants in calcium sulfate scaling. Adv. Mater. Sci. Eng. 2016, 2016, 7635329. [Google Scholar] [CrossRef]
- Zhang, S.; Qu, H.; Yang, Z.; Fu, C.-E.; Tian, Z. Scale inhibition performance and mechanism of sulfamic/amino acids modified polyaspartic acid against calcium sulfate. Desalination 2017, 419, 152–159. [Google Scholar] [CrossRef]
- Singh, Y.B.; Ng, K.C. Elucidation of dual-mode inhibition mechanism of a typical polymer-based antiscalant on Red seawater for thermal desalination at higher temperatures and higher concentration factors. J. Petrol. Sci. Eng. 2019, 183, 10638. [Google Scholar] [CrossRef]
- Reigl, S.; Van Driessche, A.E.S.; Wagner, E.; Montes-Hernandez, G.; Mehringer, J.; Koltzenburg, S.; Kunz, W.; Kellermeier, M. Toward more sustainable hydraulic binders: Controlling calcium sulfate phase selection via specific additives. ACS Sustain. Chem. Eng. 2023, 11, 8450–8461. [Google Scholar] [CrossRef]
- Al-Hamzah, A.; Wallace, A.G.; East, C.P.; Fellows, C.M.; Doherty, W.O.S. Inhibition by poly(acrylic acid) and morphological changes in calcium carbonate and calcium carbonate/calcium sulfate crystallization on silica fibers. Ind. Eng. Chem. Res. 2014, 53, 8793–8803. [Google Scholar] [CrossRef]
- Emmons, D.H. Low Molecular Weight Polyvinyl Sulfonate for Low pH Barium Sulfate Scale Control. United States Patent US4710303A, 1 December 1987. [Google Scholar]
- Falk, D.O.; Dormish, F.L.; Beazley, P.M.; Thompson, R.G. Polyvinyl Sulfonate Scale Inhibitor. United States Patent US5092404A, 3 March 1992. [Google Scholar]
- Crossman, M.; Holt, S.P.R. Scale Control Composition for High Scaling Environments. United States Patent 6995120B2, 7 February 2006. [Google Scholar]
- Shen, L.; Sippola, H.; Li, X.; Lindberg, D.; Taskinen, P. Thermodynamic modeling of calcium sulfate hydrates in the CaSO4-H2O system from 273.15 to 473.15 K with extension to 548.15 K. J. Chem. Eng. Data 2019, 64, 2697–2709. [Google Scholar] [CrossRef] [PubMed]
- Pitzer, K.S. Theoretical considerations of solubility with emphasis on mixed aqueous electrolytes. Pure Appl. Chem. 1986, 58, 1599–1610. [Google Scholar] [CrossRef]
- Zannoni, R.; Resini, I.; Liberti, L.; Santori, M.; Boari, G. Desulphation pretreatment for 138 °C (290 °F) operation and performance test of a 1 MGD plant at Doha East (Kuwait) power station. Desalination 1987, 66, 431–442. [Google Scholar] [CrossRef]
- Amjad, Z.; Zuhl, R.W.; Zibrida, J.F. Factors influencing the precipitation of calcium-inhibitor salts in industrial water systems. In Proceedings of the Association of Water Technologies Annual Convention, Phoenix, AZ, USA, 3–6 November 2003. [Google Scholar]
- Schweins, R.; Huber, K. Collapse of sodium polyacrylate chains in calcium salt solutions. Eur. Phys. J. E 2001, 5, 117–126. [Google Scholar] [CrossRef]
- Amjad, Z.; Zuhl, R.W.; Zibrida, J.F. The impact of thermal stress on deposit control polymer performance. In Proceedings of the Association of Water Technologies Annual Convention & Exposition, Uncasville, CT, USA, 19–22 September 2013. [Google Scholar]
- Senogles, E.; Doherty, W.O.S.; Crees, O.L.C. Scale Inhibitors. In Encyclopedia of Polymer Science and Technology, 2nd ed.; Mark, H.F., Bikales, N., Menges, G., Gaylord, N., Kroschwitz, J.I., Eds.; Wiley-Interscience: Hoboken, NJ, USA, 1996; pp. 7587–7594. [Google Scholar]


| Sample Number | w/w % SMS | Molar Mass/103 (Mm) Degussa/RI/UV | Molar Mass/103 (Mn) |
|---|---|---|---|
| 1 | 9 | 2.5/2.9/2.3 | 1.6/1.5/1.2 |
| 2 | 18 | 2.0/2.6/1.8 | 1.5/1.5/0.9 |
| 3 | 28 | 2.5// | 1.6// |
| 4 | 9 | 5.0/5.9/3.0 | 3.0/3.4/1.3 |
| 5 | 19 | 5.0/5.5/4.3 | 3.0/2.5/1.7 |
| 6 | 28 | 4.5/5.0/3.4 | 3.0/2.1/1.6 |
| 7 | 9 | 9.0/10.9/8.7 | 6.0/6.5/2.9 |
| 8 | 19 | 9.5// | 6.0// |
| 9 | 30 | 7.0/8.7/5.8 | 5.0/5.7/2.0 |
| Parameter | Value | Unit |
|---|---|---|
| TDS | 44,500 | ppm |
| pH | 8.1 | |
| Chloride (Cl−) | 23,500 | ppm |
| Sodium (Na+) | 12,400 | ppm |
| Sulfate (SO42−) | 3290 | ppm |
| Magnesium (Mg2+) | 1530 | ppm |
| Calcium (Ca2+) | 450 | ppm |
| Potassium (K+) | 470 | ppm |
| Hydrogen carbonate (HCO3−) | 98 | ppm |
| Antiscalant | 1 | 2 | 3 | 6 |
|---|---|---|---|---|
| Time to reach pressure drop of 2 psi (min) | 26.5 | 28.5 | 45 | 8.2 |
| 14.37 | 28 | 7.03 | 3.02 | |
| 11.18 | 13 | 4.75 | 3 | |
| 5.5 | 18.5 | 4.02 | 3 | |
| 4.0 | 7.83 | 2.33 | 2.82 | |
| 3.37 | 7.78 | 2.03 | 2.23 | |
| 3.0 | 6.5 | 2.06 | ||
| 2.93 | 3 | 2 | ||
| 2.72 | 2.33 | |||
| 2.5 | ||||
| 2.02 | ||||
| Average | 7.1 | 11.7 | 10.9 | 3.3 |
| Standard Deviation | 7.2 | 9.3 | 15.4 | 1.9 |
| Maximum | 26.5 | 28.5 | 45 | 8.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Hamzah, A.A.; Fellows, C.M.; Hamed, O.A. Methallylsulfonate Polymeric Antiscalants for Application in Thermal Desalination Processes. Polymers 2024, 16, 2838. https://doi.org/10.3390/polym16192838
Al-Hamzah AA, Fellows CM, Hamed OA. Methallylsulfonate Polymeric Antiscalants for Application in Thermal Desalination Processes. Polymers. 2024; 16(19):2838. https://doi.org/10.3390/polym16192838
Chicago/Turabian StyleAl-Hamzah, Ali A., Christopher M. Fellows, and Osman A. Hamed. 2024. "Methallylsulfonate Polymeric Antiscalants for Application in Thermal Desalination Processes" Polymers 16, no. 19: 2838. https://doi.org/10.3390/polym16192838
APA StyleAl-Hamzah, A. A., Fellows, C. M., & Hamed, O. A. (2024). Methallylsulfonate Polymeric Antiscalants for Application in Thermal Desalination Processes. Polymers, 16(19), 2838. https://doi.org/10.3390/polym16192838











