Enhancing the Biodegradability, Water Solubility, and Thermal Properties of Polyvinyl Alcohol through Natural Polymer Blending: An Approach toward Sustainable Polymer Applications
Abstract
1. Introduction
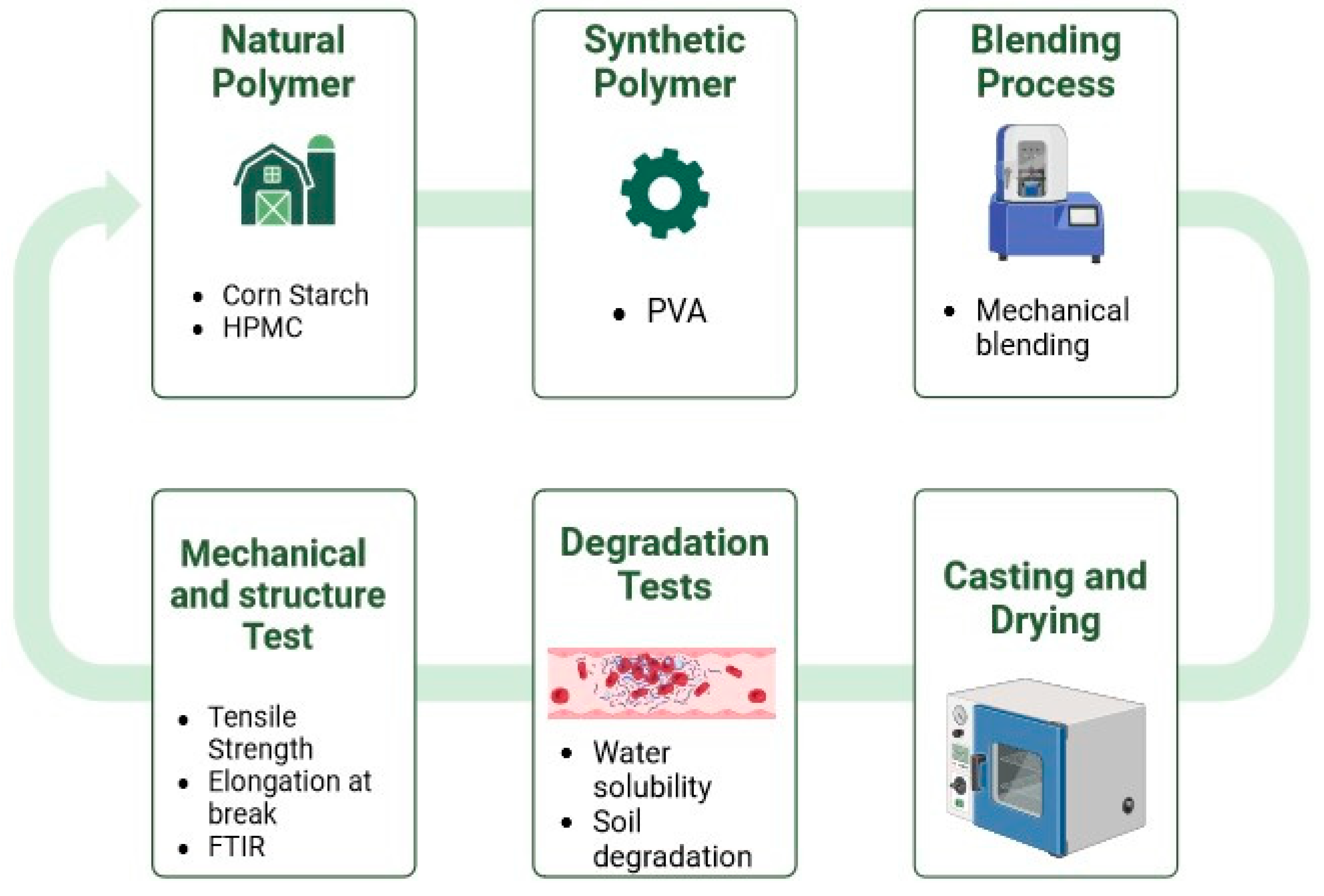
2. Materials and Methods
2.1. Materials
2.2. Method
2.2.1. Preparation of Polyvinyl Alcohol (PVA)
2.2.2. Preparation of Corn Starch-Polyvinyl Alcohol Blend Films (Csp)
2.2.3. Preparation of Hydroxypropyl Methylcellulose–Polyvinyl Alcohol (HPMCP) Blend Films
2.2.4. Characterization of Blend Films Made of Csp and HPMCP
Surface Morphology
Mechanical Properties
Water Solubility (WS) Test
Biodegradability of Blend Films
Fourier-Transform Infrared Spectroscopy (FTIR)
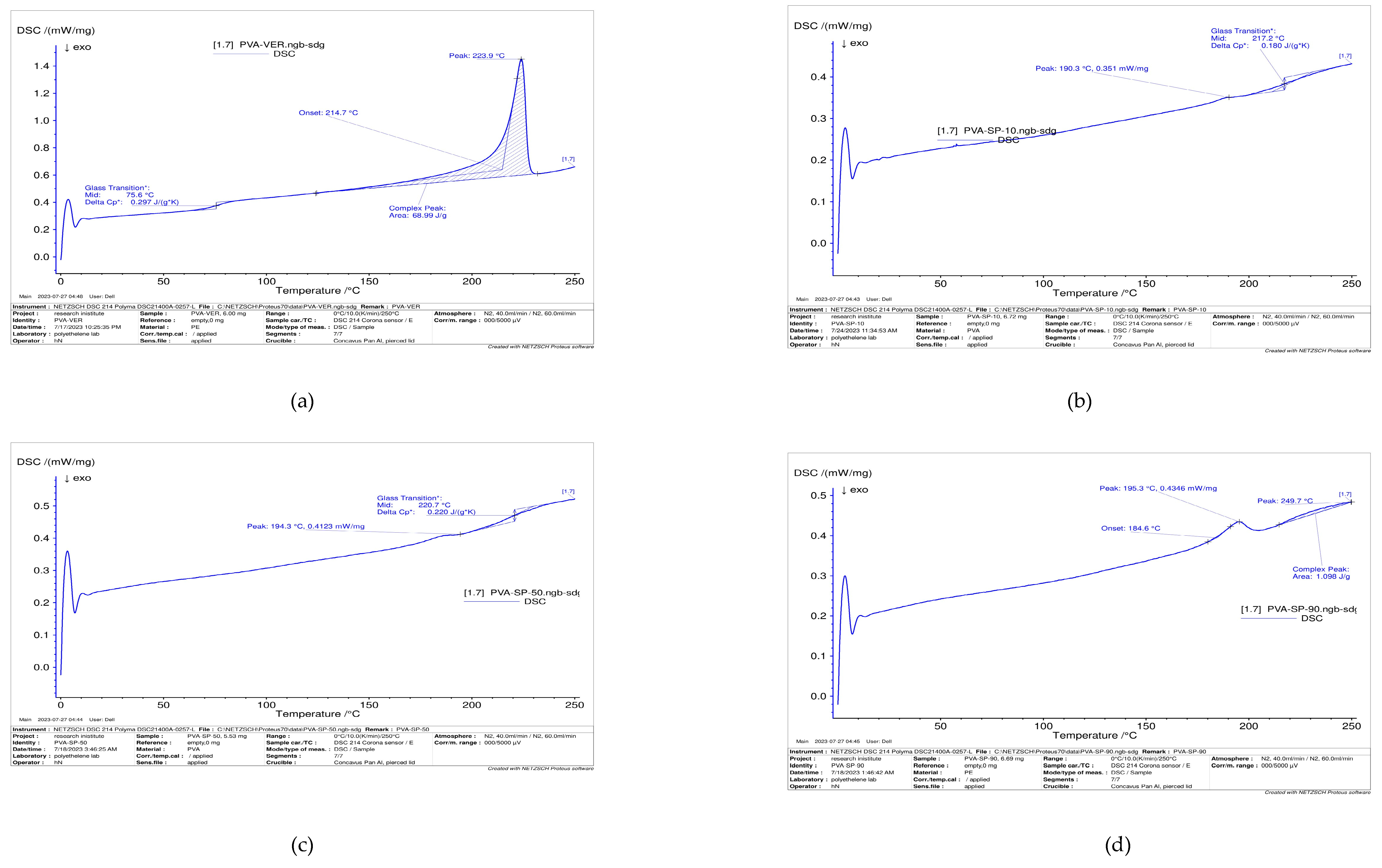
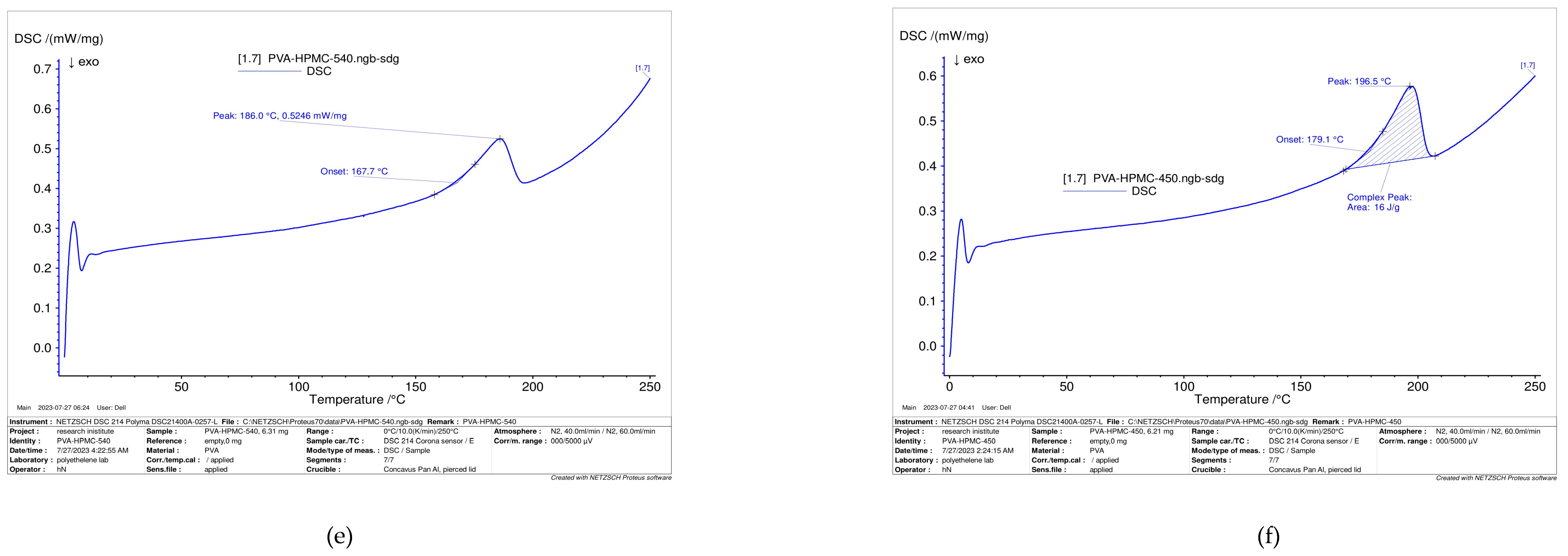
Diffraction Scanning Calorimetry (DSC)
3. Results and Discussion
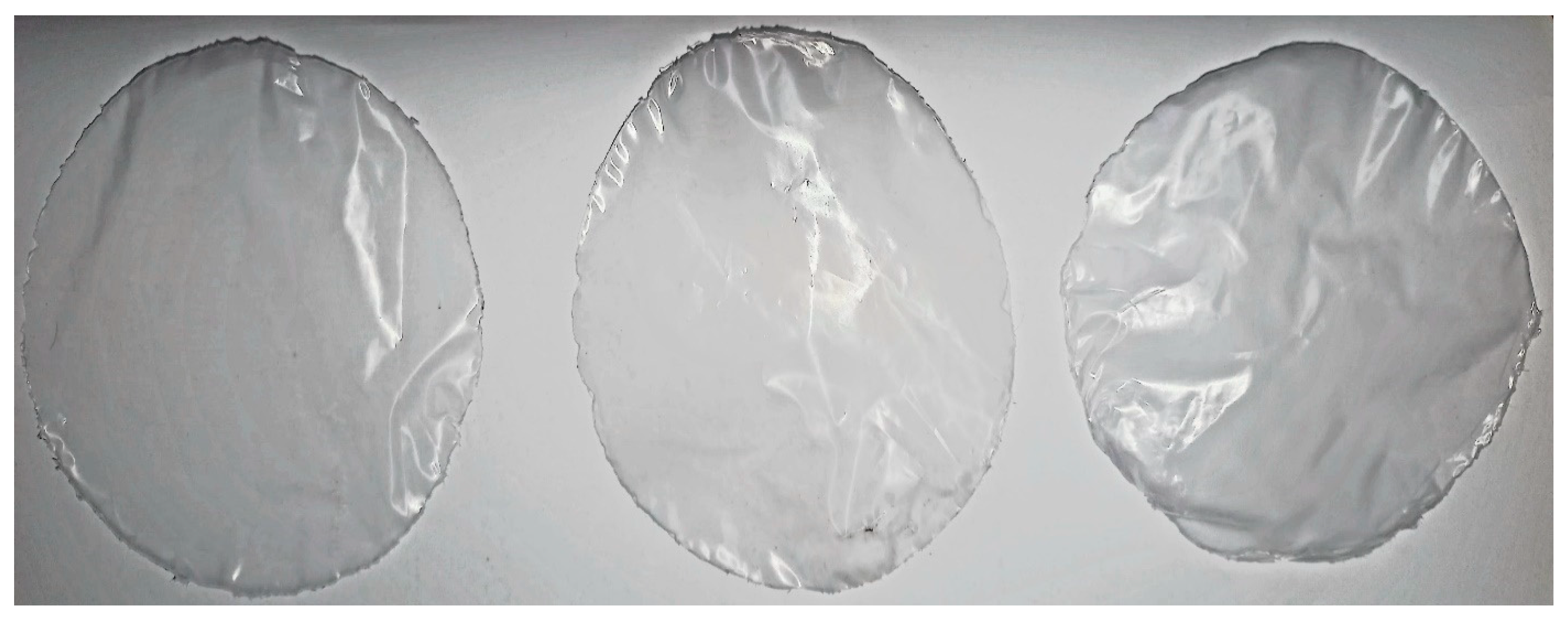
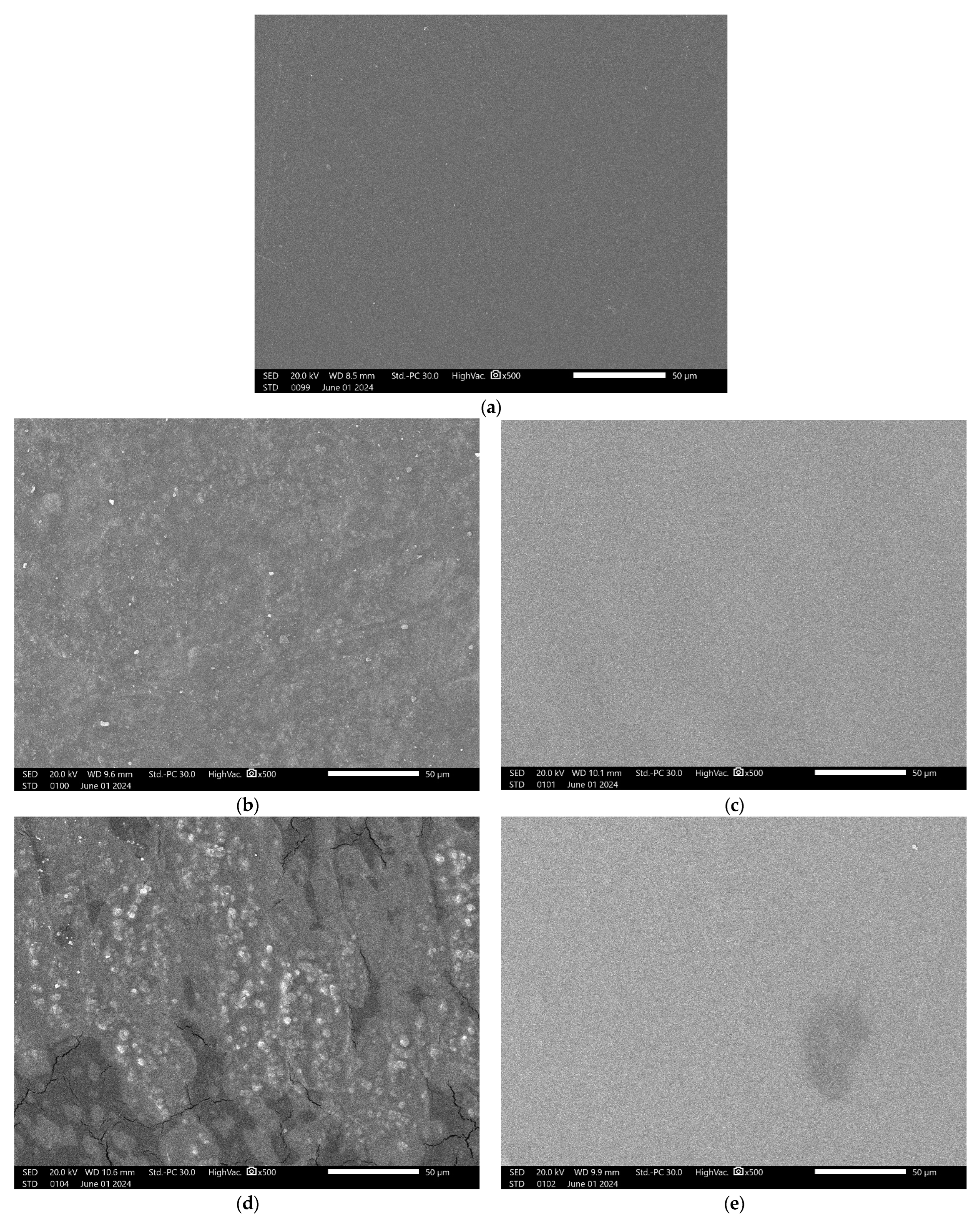
3.1. Surface Morphology of Blend Films
3.1.1. Csp Blend Films
3.1.2. HPMC Blend Films
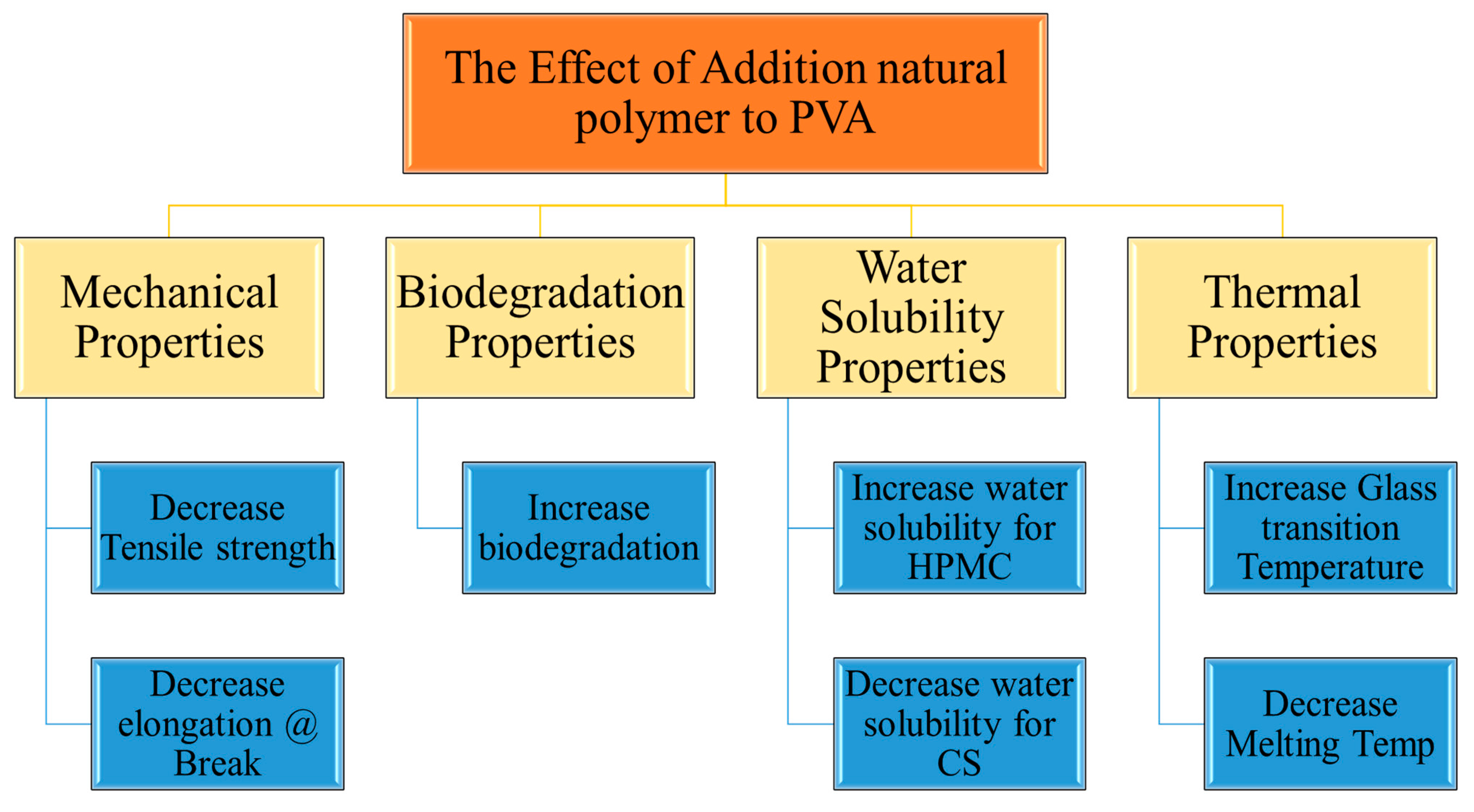
3.2. Mechanical Properties of Blend Films
3.2.1. Csp Blend Films
3.2.2. HPMC Blend Films
- Hydrogen bonds formed directly through hydroxyl groups [40].
- Water-mediated hydrogen bonding via water molecule plasticization [41]
3.3. Water Solubility (WS) of Blended Films
3.3.1. Csp Blended Films
3.3.2. HPMCP Blended Films
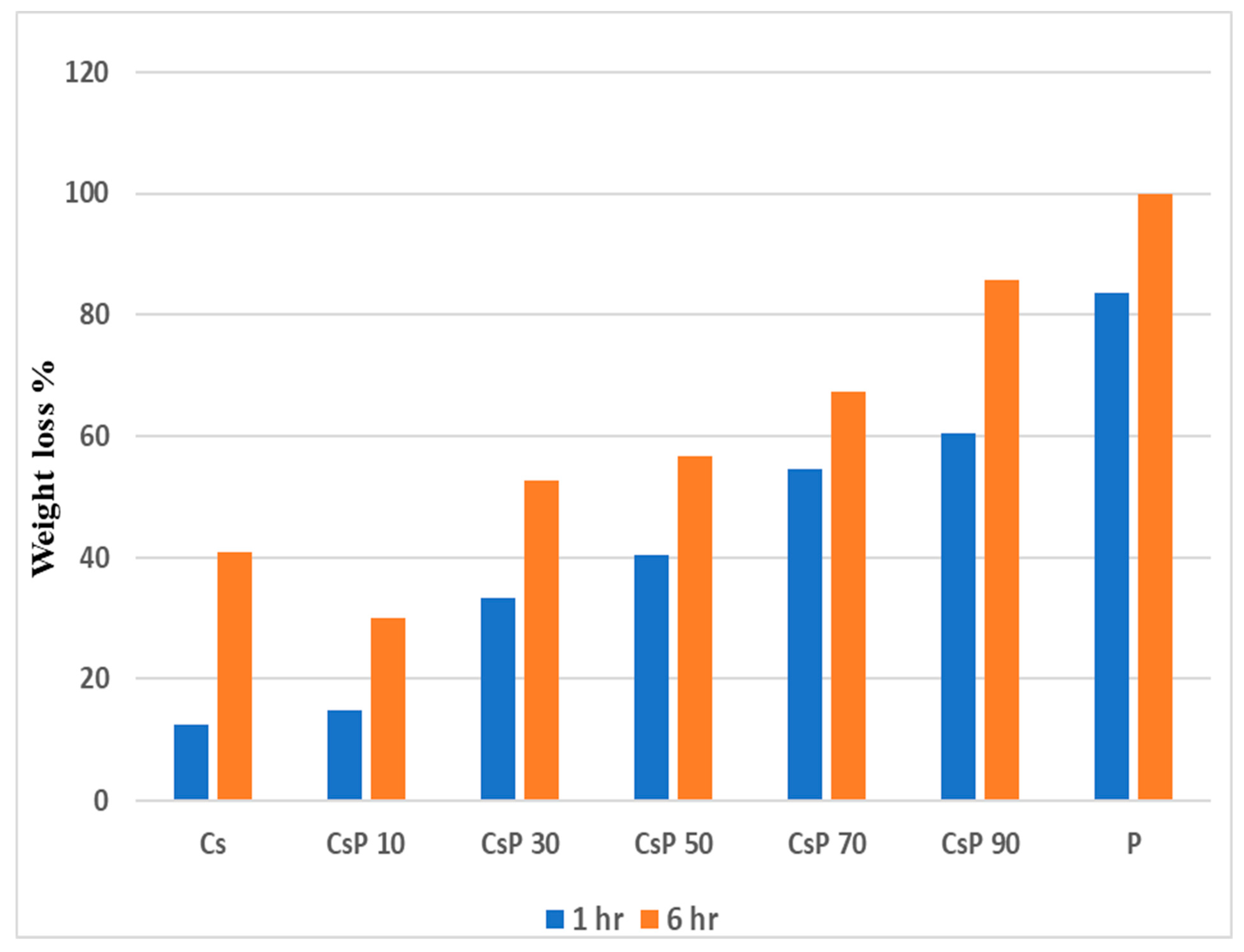
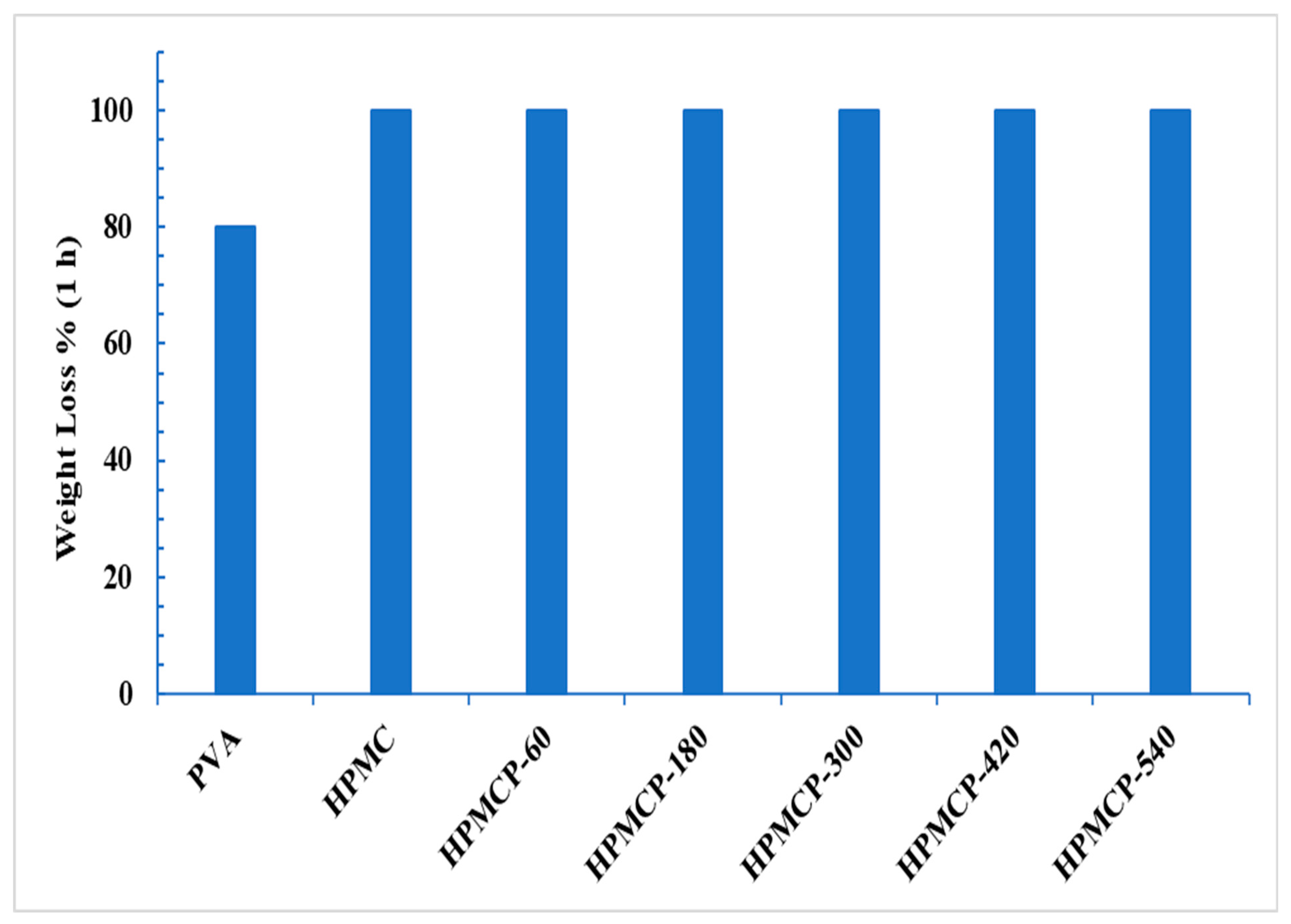
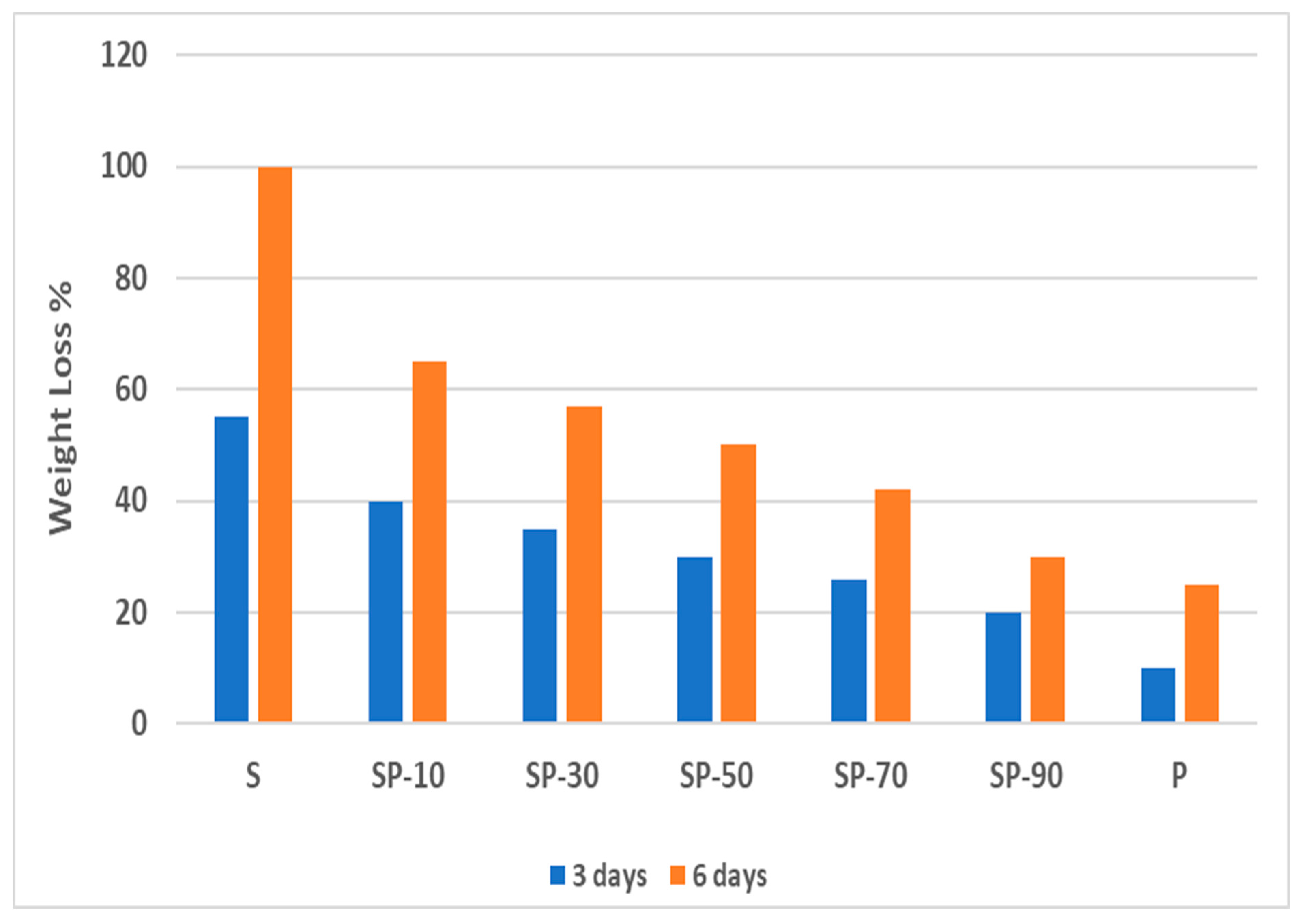
3.4. Biodegradability of Polymer Blend Films
3.4.1. Csp Blend Films
3.4.2. HPMCP Composite Films
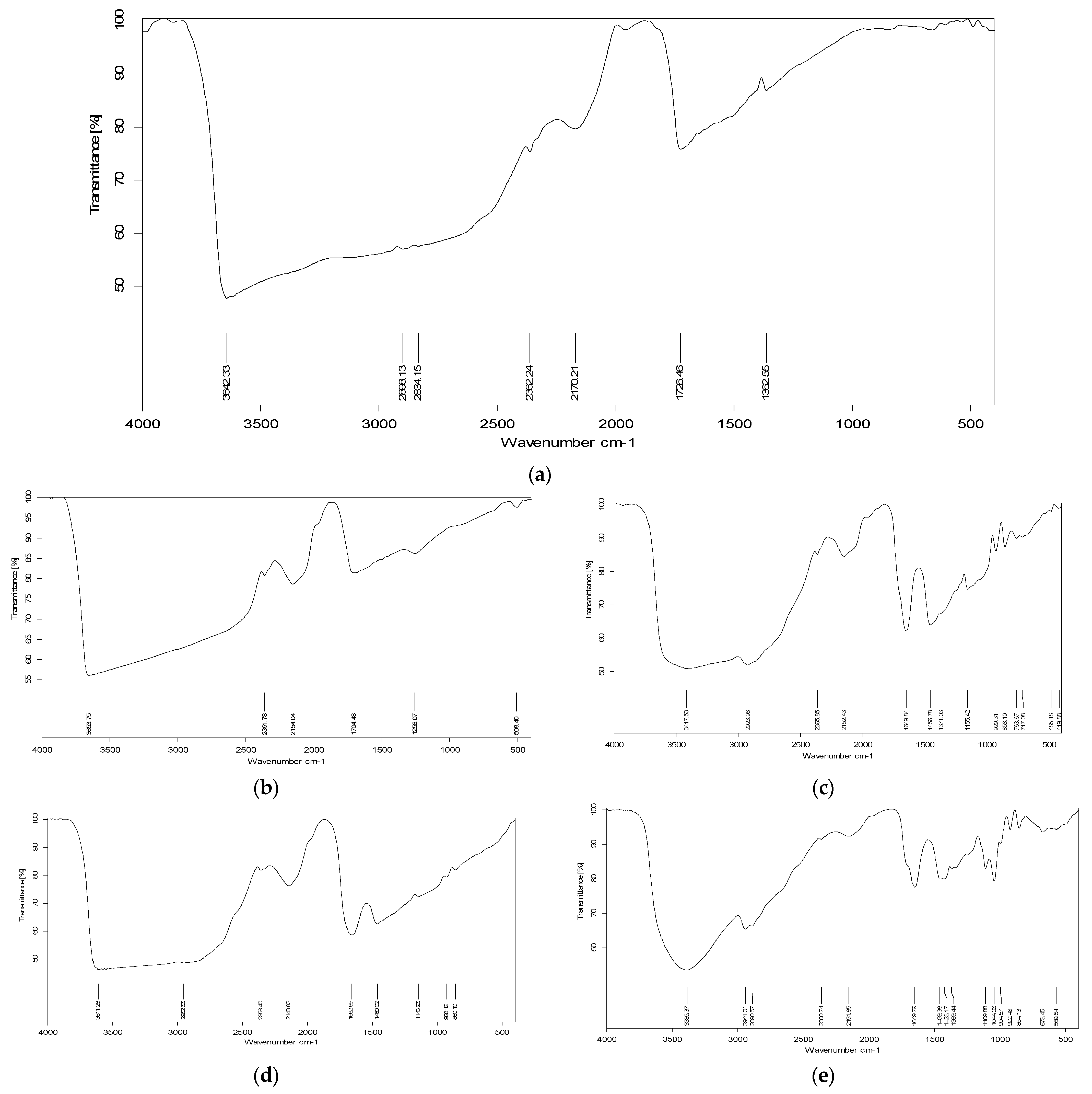
3.5. Fourier-Transform Infrared Spectroscopy
3.6. Thermal Properties of Polymer Blends
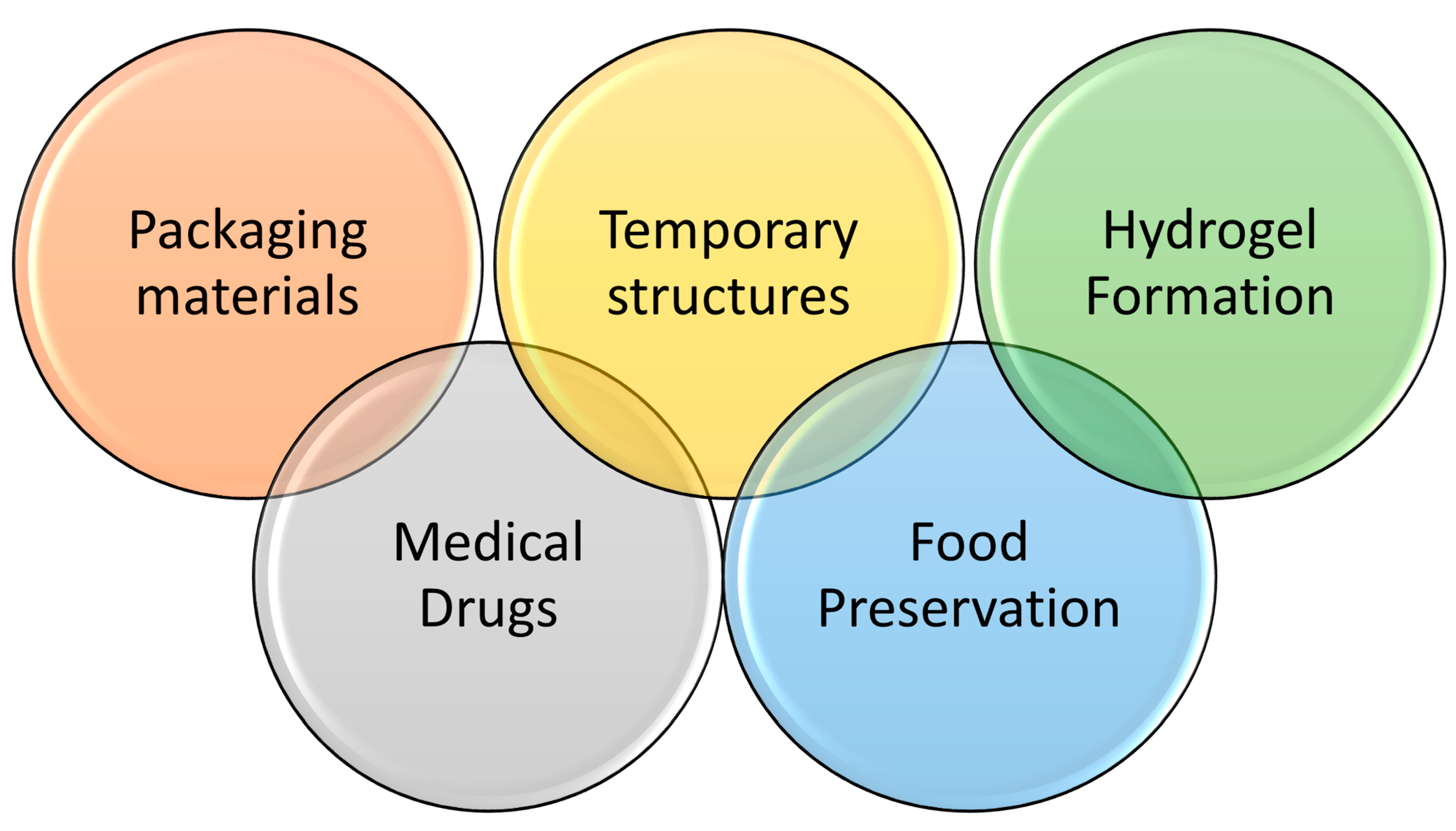
4. The Proposed Applications of the New Blends
5. Future Directions for Advancing Biodegradable Polymer Industries
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lim, W.S.; Kim, M.H.; Park, H.J.; Lee, M.H. Characterization of Polyvinyl Alcohol (PVA)/Polyacrylic Acid (PAA) Composite Film-Forming Solutions and Resulting Films as Affected by Beeswax Content. Polymers 2024, 16, 310. [Google Scholar] [CrossRef] [PubMed]
- Fong, R.J.; Robertson, A.; Mallon, P.E.; Thompson, R.L. The impact of plasticizer and degree of hydrolysis on free volume of poly (vinyl alcohol) films. Polymers 2018, 10, 1036. [Google Scholar] [CrossRef] [PubMed]
- Couți, N.; Porfire, A.; Iovanov, R.; Crișan, A.G.; Iurian, S.; Casian, T.; Tomuță, I. Polyvinyl Alcohol, a Versatile Excipient for Pharmaceutical 3D Printing. Polymers 2024, 16, 517. [Google Scholar] [CrossRef]
- Muñoz-Gimena, P.F.; Oliver-Cuenca, V.; Peponi, L.; López, D. A review on reinforcements and additives in starch-based composites for food packaging. Polymers 2023, 15, 2972. [Google Scholar] [CrossRef]
- Ojogbo, E.; Ogunsona, E.O.; Mekonnen, T.H. Chemical and physical modifications of starch for renewable polymeric materials. Mater. Today Sustain. 2020, 7, 100028. [Google Scholar] [CrossRef]
- Jiang, T.; Duan, Q.; Zhu, J.; Liu, H.; Yu, L. Starch-based biodegradable materials: Challenges and opportunities. Adv. Ind. Eng. Polym. Res. 2020, 3, 8–18. [Google Scholar] [CrossRef]
- Krystyjan, M.; Khachatryan, G.; Grabacka, M.; Krzan, M.; Witczak, M.; Grzyb, J.; Woszczak, L. Physicochemical, bacteriostatic, and biological properties of starch/chitosan polymer composites modified by graphene oxide, designed as new bionanomaterials. Polymers 2021, 13, 2327. [Google Scholar] [CrossRef] [PubMed]
- Khater, E.-S.; Bahnasawy, A.; Gabal, B.A.; Abbas, W.; Morsy, O. Effect of adding nano-materials on the properties of hydroxypropyl methylcellulose (HPMC) edible films. Sci. Rep. 2023, 13, 5063. [Google Scholar] [CrossRef]
- Pirsa, S.; Hafezi, K. Hydrocolloids: Structure, preparation method, and application in food industry. Food Chem. 2023, 399, 133967. [Google Scholar] [CrossRef]
- Hirun, N.; Kraisit, P. Drug-polymers composite matrix tablets: Effect of hydroxypropyl methylcellulose (hpmc) k-series on porosity, compatibility, and release behavior of the tablet containing a bcs class i drug. Polymers 2022, 14, 3406. [Google Scholar] [CrossRef]
- Degli-Innocenti, F.; Barbale, M.; Chinaglia, S.; Esposito, E.; Pecchiari, M.; Razza, F.; Tosin, M. Analysis of the microplastic emission potential of a starch-based biodegradable plastic material. Polym. Degrad. Stab. 2022, 199, 109934. [Google Scholar] [CrossRef]
- Elgharbawy, A.S.; El Demerdash, A.-G.M.; Sadik, W.A.; Kasaby, M.A.; Lotfy, A.H.; Osman, A.I. Synthetic Degradable Polyvinyl Alcohol Polymer and Its Blends with Starch and Cellulose—A Comprehensive Overview. Polymers 2024, 16, 1356. [Google Scholar] [CrossRef]
- Cano, A.; Fortunati, E.; Cháfer, M.; Kenny, J.M.; Chiralt, A.; González-Martínez, C. Properties and ageing behaviour of pea starch films as affected by blend with poly (vinyl alcohol). Food Hydrocoll. 2015, 48, 84–93. [Google Scholar] [CrossRef]
- Gómez-Aldapa, C.A.; Velazquez, G.; Gutierrez, M.C.; Rangel-Vargas, E.; Castro-Rosas, J.; Aguirre-Loredo, R.Y. Effect of polyvinyl alcohol on the physicochemical properties of biodegradable starch films. Mater. Chem. Phys. 2020, 239, 122027. [Google Scholar] [CrossRef]
- Negim, E.; Rakhmetullayeva, R.; Yeligbayeva, G.Z.; Urkimbaeva, P.; Primzharova, S.; Kaldybekov, D.; Khatib, J.; Mun, G.; Craig, W. Improving biodegradability of polyvinyl alcohol/starch blend films for packaging applications. Int. J. Basic Appl. Sci. 2014, 3, 263. [Google Scholar] [CrossRef]
- Song, J.; Lin, X.; Wu, H.; Huang, Z.; Gan, T.; Hu, H.; Qin, Y.; Zhang, Y. Fabrication of biodegradable and cold-water-soluble starch/polyvinyl alcohol films as inner packaging materials of pesticides: Enhanced emulsification, dispersibility, and efficacy. Carbohydr. Polym. 2024, 328, 121713. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Tao, H.; Tan, C.; Xie, J.; Yuan, F.; Guo, L.; Cui, B.; Zou, F.; Gao, W.; Liu, P. Intermolecular interactions between starch and polyvinyl alcohol for improving mechanical properties of starch-based straws. Int. J. Biol. Macromol. 2023, 239, 124211. [Google Scholar] [CrossRef] [PubMed]
- Laoasoke, W.; Choipang, C.; Chaiarwut, S.; Suwantong, O.; Chuysinuan, P.; Techasakul, S.; Sangsanoh, P.; Supaphol, P. Preparation and Characterization of Hydroxypropyl Methylcellulose/Polyvinyl Alcohol Mucoadhesive Buccal Film of Cannabidiol. J. Polym. Environ. 2024, 1–10. [Google Scholar] [CrossRef]
- Huang, J.; Hu, Z.; Chin, Y.; Li, G.; Hu, L.; Yuan, C.; Chen, J.; Hu, Y. The Preservation Behavior and Modification Effect of Roselle Anthocyanin–Based Film on Penaeus Vannamei: Biochemical Property Evaluation and Flavor Composition Characterization. Food Bioprocess Technol. 2024, 1–21. [Google Scholar] [CrossRef]
- Yu, H.; Zhou, Q.; He, D.; Yang, J.; Wu, K.; Chai, X.; Xiang, Y.; Duan, X.; Wu, X. Enhanced mechanical and functional properties of chitosan/polyvinyl alcohol/hydroxypropyl methylcellulose/alizarin composite film by incorporating cinnamon essential oil and tea polyphenols. Int. J. Biol. Macromol. 2023, 253, 126859. [Google Scholar] [CrossRef]
- Chen, J.; Su, X.; Zheng, M.; Lin, J.; Tan, K.B. Enhanced properties of chitosan/hydroxypropyl methylcellulose/polyvinyl alcohol green bacteriostatic film composited with bamboo fiber and silane-modified bamboo fiber. Polym. Compos. 2022, 43, 2440–2449. [Google Scholar] [CrossRef]
- Raj, S.S.; Kuzmin, A.; Subramanian, K.; Sathiamoorthyi, S.; Kandasamy, K.T. Philosophy of selecting ASTM standards for mechanical characterization of polymers and polymer composites. Mater. Plast. 2021, 58, 247–256. [Google Scholar] [CrossRef]
- Rhim, J.-W.; Hong, S.-I.; Ha, C.-S. Tensile, water vapor barrier and antimicrobial properties of PLA/nanoclay composite films. LWT-Food Sci. Technol. 2009, 42, 612–617. [Google Scholar] [CrossRef]
- Patil, S.; Bharimalla, A.K.; Mahapatra, A.; Dhakane-Lad, J.; Arputharaj, A.; Kumar, M.; Raja, A.; Kambli, N. Effect of polymer blending on mechanical and barrier properties of starch-polyvinyl alcohol based biodegradable composite films. Food Biosci. 2021, 44, 101352. [Google Scholar] [CrossRef]
- Medina-Jaramillo, C.; Ochoa-Yepes, O.; Bernal, C.; Famá, L. Active and smart biodegradable packaging based on starch and natural extracts. Carbohydr. Polym. 2017, 176, 187–194. [Google Scholar] [CrossRef] [PubMed]
- Graziano, A.; Jaffer, S.; Sain, M. Review on modification strategies of polyethylene/polypropylene immiscible thermoplastic polymer blends for enhancing their mechanical behavior. J. Elastomers Plast. 2019, 51, 291–336. [Google Scholar] [CrossRef]
- Dejen, K.D.; Zereffa, E.A.; Murthy, H.A.; Merga, A. Synthesis of ZnO and ZnO/PVA nanocomposite using aqueous Moringa oleifeira leaf extract template: Antibacterial and electrochemical activities. Rev. Adv. Mater. Sci. 2020, 59, 464–476. [Google Scholar] [CrossRef]
- Mittal, A.; Garg, S.; Kohli, D.; Maiti, M.; Jana, A.K.; Bajpai, S. Effect of cross linking of PVA/starch and reinforcement of modified barley husk on the properties of composite films. Carbohydr. Polym. 2016, 151, 926–938. [Google Scholar] [CrossRef]
- Chaléat, C.; Halley, P.; Truss, R. Study on the phase separation of plasticised starch/poly (vinyl alcohol) blends. Polym. Degrad. Stab. 2012, 97, 1930–1939. [Google Scholar] [CrossRef]
- Zanela, J.; Casagrande, M.; Reis, M.O.; Grossmann, M.V.E.; Yamashita, F. Biodegradable sheets of starch/polyvinyl alcohol (PVA): Effects of PVA molecular weight and hydrolysis degree. Waste Biomass Valorization 2019, 10, 319–326. [Google Scholar] [CrossRef]
- Tian, H.; Yan, J.; Rajulu, A.V.; Xiang, A.; Luo, X. Fabrication and properties of polyvinyl alcohol/starch blend films: Effect of composition and humidity. Int. J. Biol. Macromol. 2017, 96, 518–523. [Google Scholar] [CrossRef]
- Michen, B.; Geers, C.; Vanhecke, D.; Endes, C.; Rothen-Rutishauser, B.; Balog, S.; Petri-Fink, A. Avoiding drying-artifacts in transmission electron microscopy: Characterizing the size and colloidal state of nanoparticles. Sci. Rep. 2015, 5, 9793. [Google Scholar] [CrossRef] [PubMed]
- Rahmadiawan, D.; Abral, H.; Railis, R.M.; Iby, I.C.; Mahardika, M.; Handayani, D.; Natrana, K.D.; Juliadmi, D.; Akbar, F. The enhanced moisture absorption and tensile strength of PVA/Uncaria gambir extract by boric acid as a highly moisture-resistant, anti-UV, and strong film for food packaging applications. J. Compos. Sci. 2022, 6, 337. [Google Scholar] [CrossRef]
- Barbosa, J.A.; Al-Kauraishi, M.M.; Smith, A.M.; Conway, B.R.; Merchant, H.A. Achieving gastroresistance without coating: Formulation of capsule shells from enteric polymers. Eur. J. Pharm. Biopharm. 2019, 144, 174–179. [Google Scholar] [CrossRef]
- Ray, R.; Das, S.N.; Das, A. Mechanical, thermal, moisture absorption and biodegradation behaviour of date palm leaf reinforced PVA/starch hybrid composites. Mater. Today Proc. 2021, 41, 376–381. [Google Scholar] [CrossRef]
- Ashraf, R.; Sofi, H.S.; Malik, A.; Beigh, M.A.; Hamid, R.; Sheikh, F.A. Recent trends in the fabrication of starch nanofibers: Electrospinning and non-electrospinning routes and their applications in biotechnology. Appl. Biochem. Biotechnol. 2019, 187, 47–74. [Google Scholar] [CrossRef]
- Phattarateera, S.; Xin, L.; Amphong, C.; Limsamran, V.; Threepopnatkul, P. Comparative studies of starch blends on the properties of PVA films. Carbohydr. Polym. Technol. Appl. 2023, 6, 100340. [Google Scholar] [CrossRef]
- Jiang, G.; Hou, X.; Zeng, X.; Zhang, C.; Wu, H.; Shen, G.; Li, S.; Luo, Q.; Li, M.; Liu, X. Preparation and characterization of indicator films from carboxymethyl-cellulose/starch and purple sweet potato (Ipomoea batatas (L.) lam) anthocyanins for monitoring fish freshness. Int. J. Biol. Macromol. 2020, 143, 359–372. [Google Scholar] [CrossRef]
- Sin, L.T.; Rahman, W.; Rahmat, A.; Khan, M. Detection of synergistic interactions of polyvinyl alcohol–cassava starch blends through DSC. Carbohydr. Polym. 2010, 79, 224–226. [Google Scholar] [CrossRef]
- Tang, X.; Alavi, S. Recent advances in starch, polyvinyl alcohol based polymer blends, nanocomposites and their biodegradability. Carbohydr. Polym. 2011, 85, 7–16. [Google Scholar] [CrossRef]
- Kahvand, F.; Fasihi, M. Plasticizing and anti-plasticizing effects of polyvinyl alcohol in blend with thermoplastic starch. Int. J. Biol. Macromol. 2019, 140, 775–781. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Chen, M.; Zhou, Y.; Li, Y.; Hu, Y. Functional characteristics improvement by structural modification of hydroxypropyl methylcellulose modified polyvinyl alcohol films incorporating roselle anthocyanins for shrimp freshness monitoring. Int. J. Biol. Macromol. 2020, 162, 1250–1261. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Huang, T.-S.; Yan, H.; Hu, X.; Ren, T. Novel pH-sensitive films based on starch/polyvinyl alcohol and food anthocyanins as a visual indicator of shrimp deterioration. Int. J. Biol. Macromol. 2020, 145, 768–776. [Google Scholar] [CrossRef]
- Yurong, G.; Dapeng, L. Preparation and characterization of corn starch/PVA/glycerol composite films incorporated with ε-polylysine as a novel antimicrobial packaging material. e-Polymers 2020, 20, 154–161. [Google Scholar] [CrossRef]
- Hiremani, V.D.; Sataraddi, S.; Bayannavar, P.K.; Gasti, T.; Masti, S.P.; Kamble, R.R.; Chougale, R.B. Mechanical, optical and antioxidant properties of 7-Hydroxy-4-methyl coumarin doped polyvinyl alcohol/oxidized maize starch blend films. SN Appl. Sci. 2020, 2, 1877. [Google Scholar] [CrossRef]
- Taghizadeh, M.T.; Sabouri, N. Biodegradation behaviors and water adsorption of poly (vinyl alcohol)/starch/carboxymethyl cellulose/clay nanocomposites. Int. Nano Lett. 2013, 3, 51. [Google Scholar] [CrossRef]
- Abral, H.; Soni Satria, R.; Mahardika, M.; Hafizulhaq, F.; Affi, J.; Asrofi, M.; Handayani, D.; Sapuan, S.M.; Stephane, I.; Sugiarti, E. Comparative study of the physical and tensile properties of jicama (Pachyrhizus erosus) starch film prepared using three different methods. Starch-Stärke 2019, 71, 1800224. [Google Scholar] [CrossRef]
- Savadekar, N.; Mhaske, S. Synthesis of nano cellulose fibers and effect on thermoplastics starch based films. Carbohydr. Polym. 2012, 89, 146–151. [Google Scholar] [CrossRef] [PubMed]
- Tundisi, L.; Mostaço, G.; Carricondo, P.C.; Petri, D. Hydroxypropyl methylcellulose: Physicochemical properties and ocular drug delivery formulations. Eur. J. Pharm. Sci. 2021, 159, 105736. [Google Scholar] [CrossRef]
- Guirguis, O.W.; Moselhey, M.T. Optical study of poly (vinyl alcohol)/hydroxypropyl methylcellulose blends. J. Mater. Sci. 2011, 46, 5775–5789. [Google Scholar] [CrossRef]
- Ghadermazi, R.; Hamdipour, S.; Sadeghi, K.; Ghadermazi, R.; Khosrowshahi Asl, A. Effect of various additives on the properties of the films and coatings derived from hydroxypropyl methylcellulose—A review. Food Sci. Nutr. 2019, 7, 3363–3377. [Google Scholar] [CrossRef] [PubMed]
- El-Naas, M.H.; Mourad, A.-H.I.; Surkatti, R. Evaluation of the characteristics of polyvinyl alcohol (PVA) as matrices for the immobilization of Pseudomonas putida. Int. Biodeterior. Biodegrad. 2013, 85, 413–420. [Google Scholar] [CrossRef]
- Sau, S.; Pandit, S.; Kundu, S. Crosslinked poly (vinyl alcohol): Structural, optical and mechanical properties. Surf. Interfaces 2021, 25, 101198. [Google Scholar] [CrossRef]
- Mathew, S.; Snigdha, S.; Mathew, J.; Radhakrishnan, E. Poly (vinyl alcohol): Montmorillonite: Boiled rice water (starch) blend film reinforced with silver nanoparticles; characterization and antibacterial properties. Appl. Clay Sci. 2018, 161, 464–473. [Google Scholar] [CrossRef]
- Ogunmolasuyi, A.; Egwim, E.; Adewoyin, M.; Nkop, E. Physicochemical and structural characterization of yam starch modified by potassium dihydrogen phosphate treatment in aqueous glycerol. Edorium J. Cell Biol. 2016, 2, 6–14. [Google Scholar]
- Kędzierska-Matysek, M.; Matwijczuk, A.; Florek, M.; Barłowska, J.; Wolanciuk, A.; Matwijczuk, A.; Chruściel, E.; Walkowiak, R.; Karcz, D.; Gładyszewska, B. Application of FTIR spectroscopy for analysis of the quality of honey. BIO Web Conf. 2018, 10, 02008. [Google Scholar] [CrossRef]
- Mahesh, B.; Kathyayani, D.; Channe Gowda, D.; Mrutunjaya, K. Blends of synthetic plastic-derived polypeptide with Hydroxypropylmethylcellulose and polyvinyl alcohol: Unraveling the specific interaction parameters, morphology and thermal stability of the polymers couple. J. Polym. Res. 2020, 27, 278. [Google Scholar] [CrossRef]
- Bhajantri, R.F.; Chavan, C.; Cyriac, V.; Bulla, S.S. Investigation on the structural and ion transport properties of magnesium salt doped HPMC-PVA based polymer blend for energy storage applications. J. Non-Cryst. Solids 2023, 609, 122276. [Google Scholar]
- Garavand, F.; Cacciotti, I.; Vahedikia, N.; Rehman, A.; Tarhan, Ö.; Akbari-Alavijeh, S.; Shaddel, R.; Rashidinejad, A.; Nejatian, M.; Jafarzadeh, S. A comprehensive review on the nanocomposites loaded with chitosan nanoparticles for food packaging. Crit. Rev. Food Sci. Nutr. 2022, 62, 1383–1416. [Google Scholar] [CrossRef]
- Garavand, F.; Rouhi, M.; Razavi, S.H.; Cacciotti, I.; Mohammadi, R. Improving the integrity of natural biopolymer films used in food packaging by crosslinking approach: A review. Int. J. Biol. Macromol. 2017, 104, 687–707. [Google Scholar] [CrossRef]
- Tian, H.; Yuan, L.; Zhou, D.; Niu, J.; Cong, H.; Xiang, A. Improved mechanical properties of poly (vinyl alcohol) films with glycerol plasticizer by uniaxial drawing. Polym. Adv. Technol. 2018, 29, 2612–2618. [Google Scholar] [CrossRef]
- Wu, W.; Tian, H.; Xiang, A. Influence of polyol plasticizers on the properties of polyvinyl alcohol films fabricated by melt processing. J. Polym. Environ. 2012, 20, 63–69. [Google Scholar] [CrossRef]
- Balani, K.; Verma, V.; Agarwal, A.; Narayan, R. Biosurfaces: A Materials Science and Engineering Perspective; John Wiley & Sons: Hoboken, NJ, USA, 2015. [Google Scholar]
- Tsioptsias, C.; Fardis, D.; Ntampou, X.; Tsivintzelis, I.; Panayiotou, C. Thermal Behavior of Poly (vinyl alcohol) in the Form of Physically Crosslinked Film. Polymers 2023, 15, 1843. [Google Scholar] [CrossRef] [PubMed]
- Abedi-Firoozjah, R.; Chabook, N.; Rostami, O.; Heydari, M.; Kolahdouz-Nasiri, A.; Javanmardi, F.; Abdolmaleki, K.; Khaneghah, A.M. PVA/starch films: An updated review of their preparation, characterization, and diverse applications in the food industry. Polym. Test. 2023, 118, 107903. [Google Scholar] [CrossRef]
- Ge, C.; Lansing, B.; Lewis, C.L. Thermoplastic starch and poly (vinyl alcohol) blends centered barrier film for food packaging applications. Food Packag. Shelf Life 2021, 27, 100610. [Google Scholar] [CrossRef]
- Francis, D.V.; Thaliyakattil, S.; Cherian, L.; Sood, N.; Gokhale, T. Metallic nanoparticle integrated ternary polymer blend of PVA/starch/glycerol: A promising antimicrobial food packaging material. Polymers 2022, 14, 1379. [Google Scholar] [CrossRef] [PubMed]
- Sreekumar, P.; Al-Harthi, M.A.; De, S. Studies on compatibility of biodegradable starch/polyvinyl alcohol blends. Polym. Eng. Sci. 2012, 52, 2167–2172. [Google Scholar] [CrossRef]
- Perez-Robles, S.; Carotenuto, C.; Minale, M. HPMC hydrogel formation mechanisms unveiled by the evaluation of the activation energy. Polymers 2022, 14, 635. [Google Scholar] [CrossRef]
- Huang, J.; Liu, J.; Chen, M.; Yao, Q.; Hu, Y. Immobilization of roselle anthocyanins into polyvinyl alcohol/hydroxypropyl methylcellulose film matrix: Study on the interaction behavior and mechanism for better shrimp freshness monitoring. Int. J. Biol. Macromol. 2021, 184, 666–677. [Google Scholar] [CrossRef]



| Sample Code | Starch (Cs) (g) | PVA (P) (g) | PVA w/w | Glycerol (g) |
|---|---|---|---|---|
| Cs | 3 | - | - | 1 |
| Csp 10 | 3 | 0.3 | 10 | 1 |
| Csp 30 | 3 | 0.9 | 30 | 1 |
| Csp 50 | 3 | 1.5 | 50 | 1 |
| Csp 70 | 3 | 2.1 | 70 | 1 |
| Csp 90 | 3 | 2.7 | 90 | 1 |
| PVA | 0 | 3 | 100 | 1 |
| Sample Code | HPMC (g) | PVA (P) (g) | PVA w/w | Glycerol (g) |
|---|---|---|---|---|
| HPMC | 0.5 | - | - | 1 |
| HPMCP 60 | 0.5 | 0.3 | 60 | 1 |
| HPMCP 180 | 0.5 | 0.9 | 180 | 1 |
| HPMCP 300 | 0.5 | 1.5 | 300 | 1 |
| HPMCP 420 | 0.5 | 2.1 | 420 | 1 |
| HPMCP 540 | 0.5 | 2.7 | 540 | 1 |
| PVA | - | 3 | 100% | 1 |
| Organic Material % | Main Elements (ppm) | Anions (ppm) | Cations (ppm) | Electrical Conductivity | PH | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0.5 | N | P | SO4 | HCO3 | CO3 | Cl | Ca | Mg | K | Na | P.P.M | Us/cm | 7.67 |
| 105 | 0.9 | 19.4 | 183 | -- | 106.5 | 28 | 7.2 | 34.7 | 23 | 106.2 | 166 | ||
| Factors | Effects | Relation |
|---|---|---|
| Chain flexibility | The nature of the polymer backbone and the groups that are directly connected to it define the intrinsic chain flexibility. While ringed structures cause the chain to stiffen, the aliphatic C-C and C-O bonds exhibit remarkable flexibility. The glass transition temperature rises when the chain becomes stiffer. |  |
| Intermolecular interactions | Intermolecular interactions and secondary bonding, such as hydrogen bonding, van der Waals force, induction forces, and dipole–dipole interactions, also have an impact on segmental rotations. These kinds of interactions raise the glass transition temperature because they make polymeric materials stiffer. |  |
| Molecular weight | The glass transition temperature of a polymer decreases with increasing molecular weight, and it is logical to assume that this temperature increases linearly with increasing chain-end concentration. On the other hand, we can say that this temperature decreases linearly as the molecular weight of the polymer increases. |  |
| Crosslinking | The polymer becomes rigid and restricted due to crosslinking, which raises the glass transition temperature. |  |
| Plasticizer | Plasticizers separate polymer chains, reduce cohesive forces, and generally promote molecular mobility when added to polymers. The glass transition temperature is lowered, and the polymer brittleness is decreased by the plasticizer. |  |
| The chemical structure of the polymer | The melting temperatures of various plastics will vary due to their distinct chemical structures. For example, plastics with more hydrocarbon groups often melt at a higher temperature than plastics with various functional groups. |
| Degree of crystallinity | The melting point of crystalline polymers is higher than that of amorphous plastics. This is because crystalline plastics have molecules arranged in a certain arrangement that makes them more resilient to disintegration. |
| The mass ratio of components in the plastic | The mass ratio of a plastic’s constituent parts can also affect the plastic’s melting point. |
| Additives | The plastic’s melting temperature may change if additives are added. For example, thermal stabilizers can be added to increase the plastic’s melting temperature. |
| Sample | Tg (°C) | Tm (°C) |
|---|---|---|
| PVA | 75.6 | 223.9 |
| CSP10 | 190.3 | 217.2 |
| CSP70 | 194.3 | 220.7 |
| CSP90 | 195.3 | 221 |
| HPMCP 420 | 167.7 | 168 |
| HPMCP 540 | 179.1 | 196.5 |
| Application | Types of Blended Films Used | Reason |
|---|---|---|
| Packaging Materials (single-use plastic) | The blend of starch/PVA can used as a biodegradable film that can be employed in packaging and does not need high strength. |
|
| Medical drugs |
|
|
| Temporary Structures |
|
|
| Food preservation | The blend of HPMC/PVA can used in applications for food preservation and used as a natural film for monitoring the freshness of food [70]. |
|
| Hydrogel Formation | It is possible to make use of the polymer’s high solubility in water to create hydrogels, materials with a high capacity to absorb and hold water. |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elgharbawy, A.S.; El Demerdash, A.-G.M.; Sadik, W.A.; Kasaby, M.A.; Lotfy, A.H.; Osman, A.I. Enhancing the Biodegradability, Water Solubility, and Thermal Properties of Polyvinyl Alcohol through Natural Polymer Blending: An Approach toward Sustainable Polymer Applications. Polymers 2024, 16, 2141. https://doi.org/10.3390/polym16152141
Elgharbawy AS, El Demerdash A-GM, Sadik WA, Kasaby MA, Lotfy AH, Osman AI. Enhancing the Biodegradability, Water Solubility, and Thermal Properties of Polyvinyl Alcohol through Natural Polymer Blending: An Approach toward Sustainable Polymer Applications. Polymers. 2024; 16(15):2141. https://doi.org/10.3390/polym16152141
Chicago/Turabian StyleElgharbawy, Abdallah S., Abdel-Ghaffar M. El Demerdash, Wagih A. Sadik, Mosaad A. Kasaby, Ahmed H. Lotfy, and Ahmed I. Osman. 2024. "Enhancing the Biodegradability, Water Solubility, and Thermal Properties of Polyvinyl Alcohol through Natural Polymer Blending: An Approach toward Sustainable Polymer Applications" Polymers 16, no. 15: 2141. https://doi.org/10.3390/polym16152141
APA StyleElgharbawy, A. S., El Demerdash, A.-G. M., Sadik, W. A., Kasaby, M. A., Lotfy, A. H., & Osman, A. I. (2024). Enhancing the Biodegradability, Water Solubility, and Thermal Properties of Polyvinyl Alcohol through Natural Polymer Blending: An Approach toward Sustainable Polymer Applications. Polymers, 16(15), 2141. https://doi.org/10.3390/polym16152141







