Effects of Boric Acid on Laminated Composites: An Experimental Study
Abstract
1. Introduction
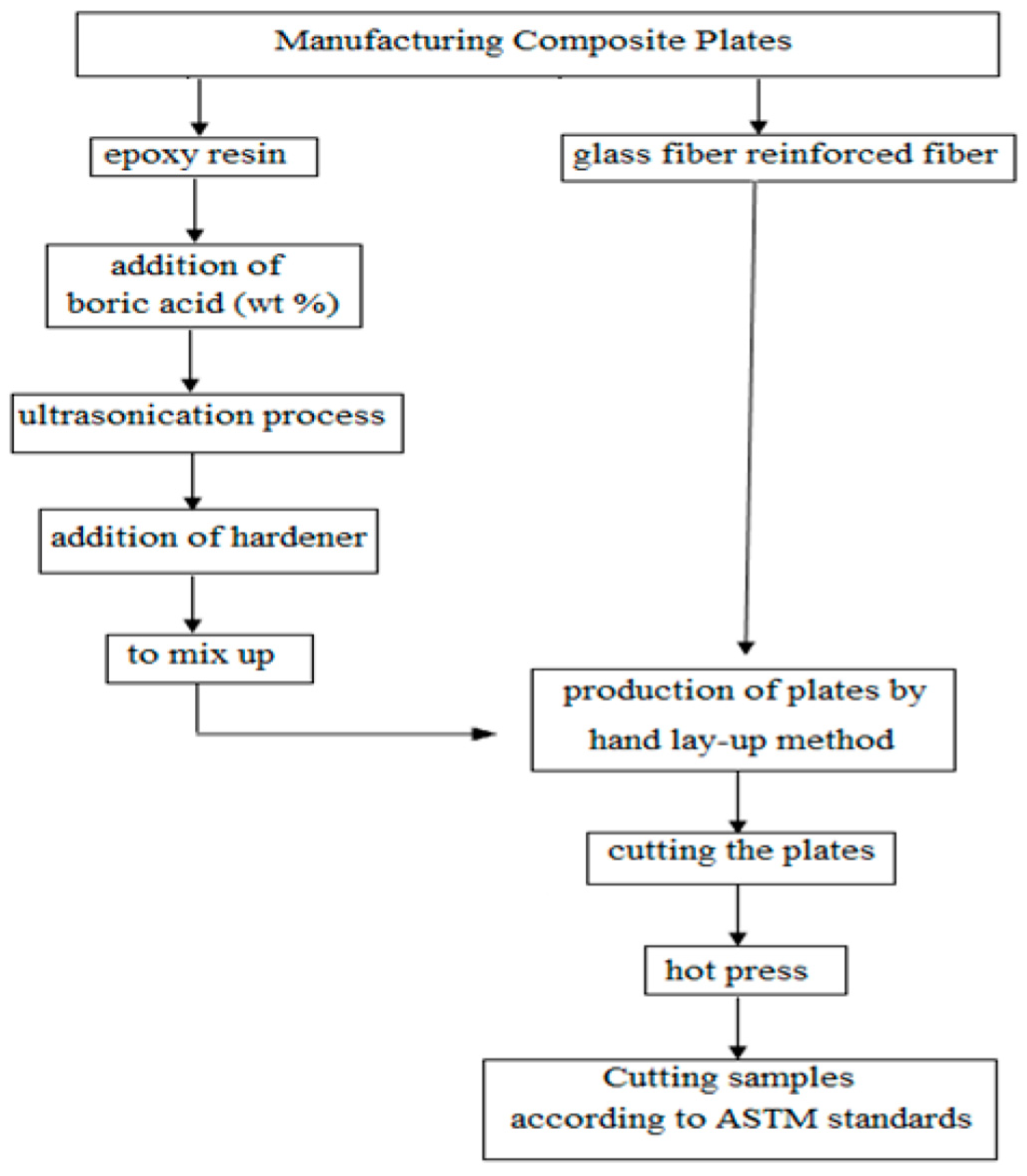
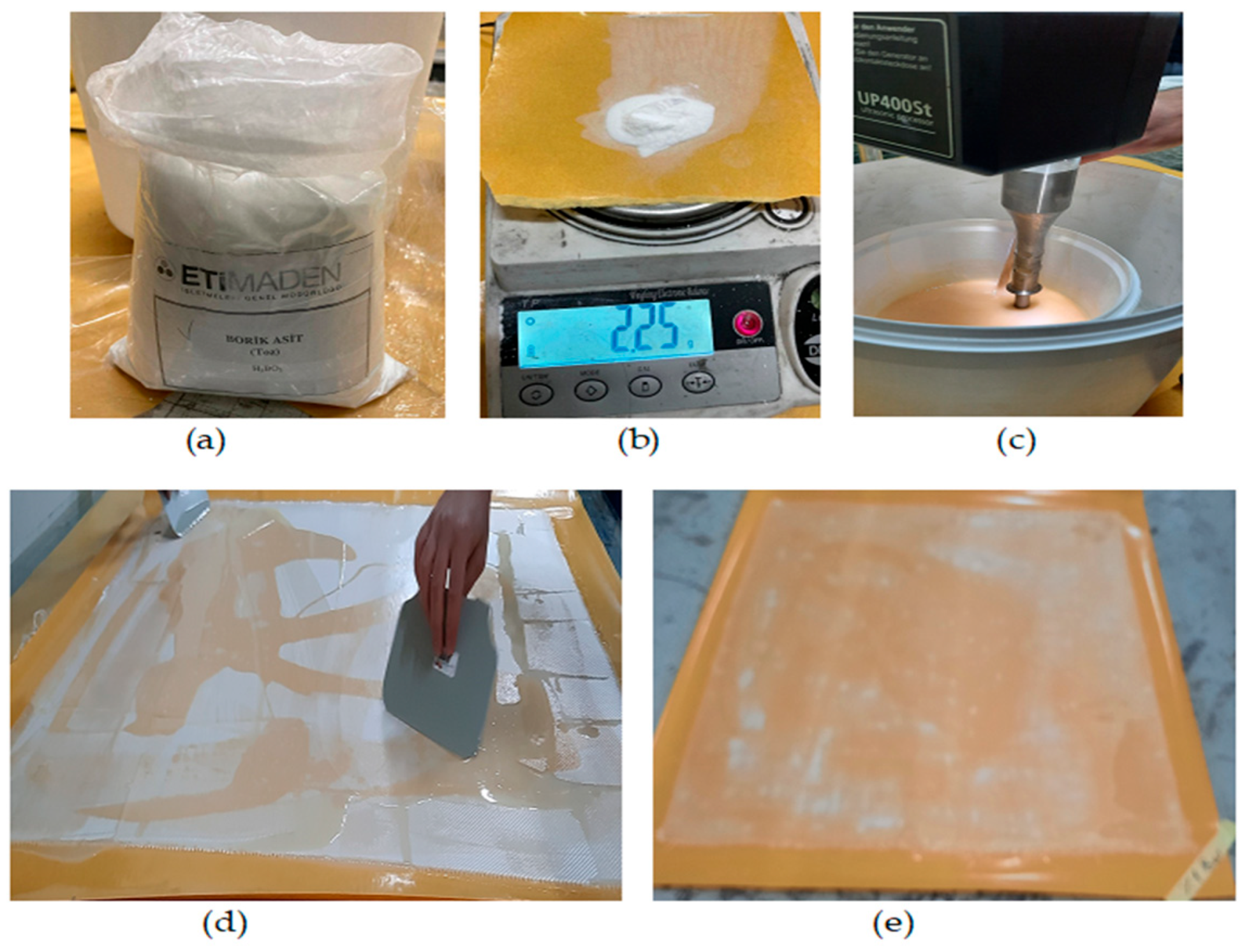
2. Materials and Methods
2.1. DMA Analysis
2.2. DSC Analysis
2.3. TGA Analysis
2.4. SEM and EDS Analysis
3. Results and Discussion
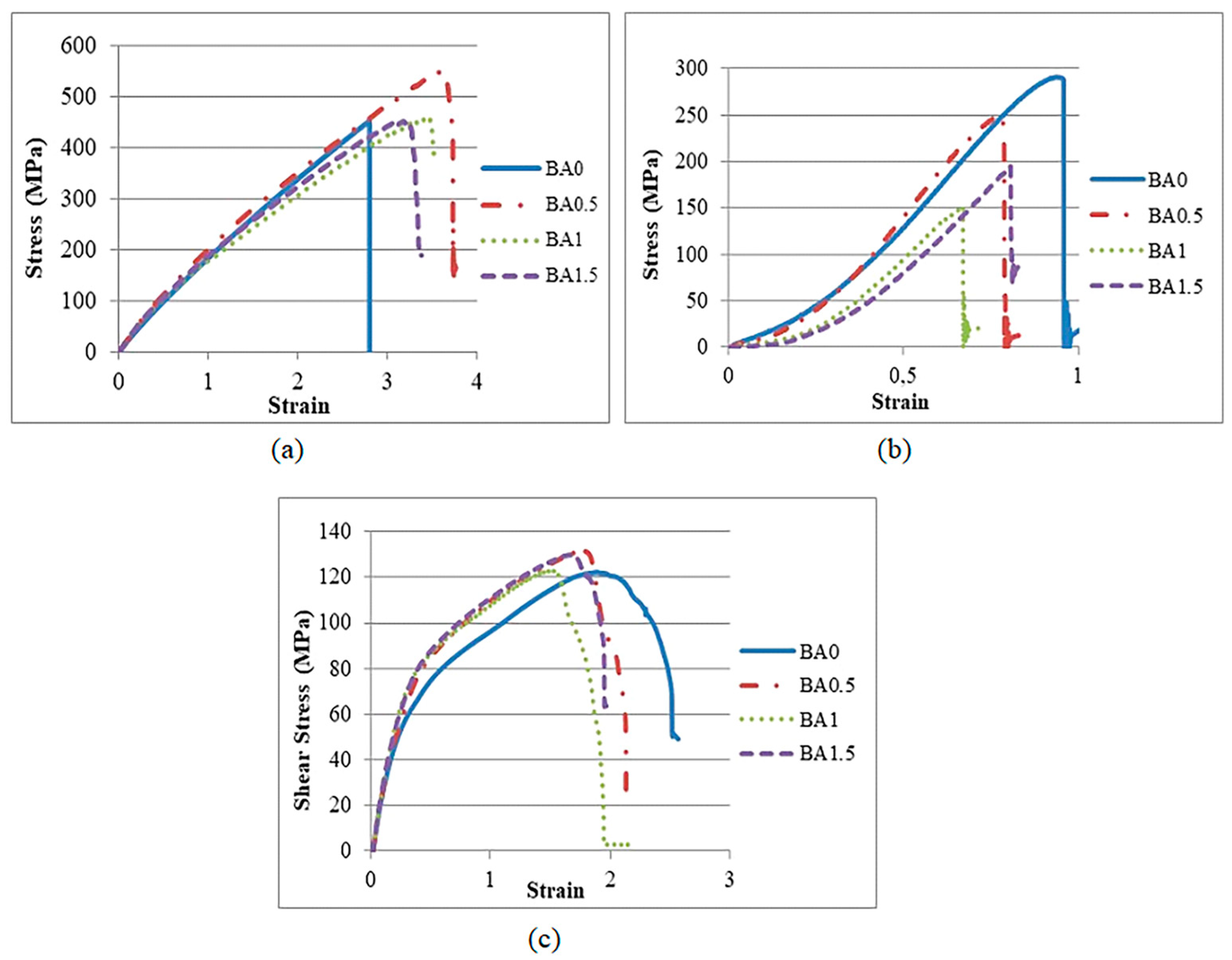
3.1. Mechanical Properties
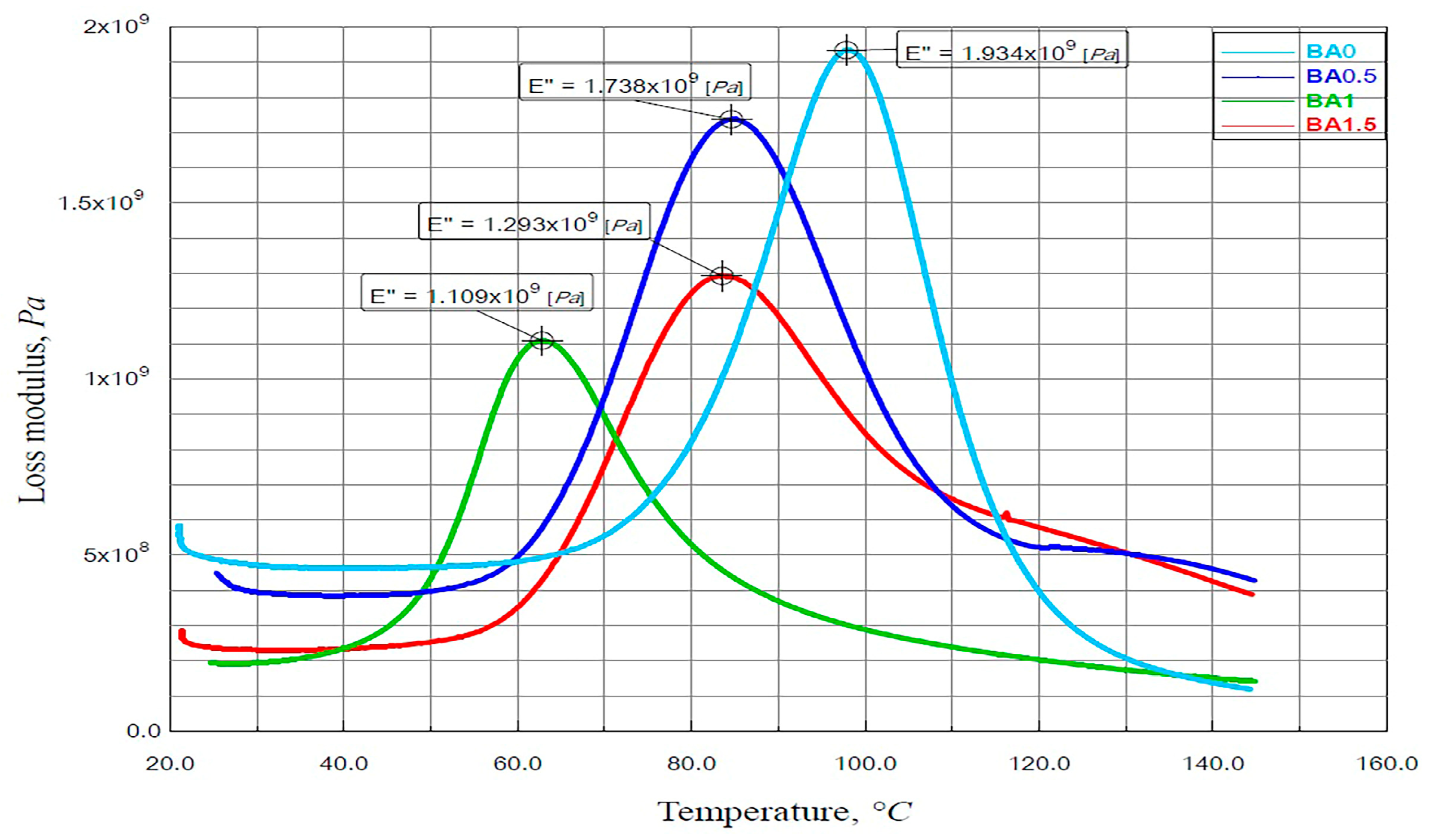
3.2. Findings of the DMA Analysis
3.3. Findings of the TGA Analysis
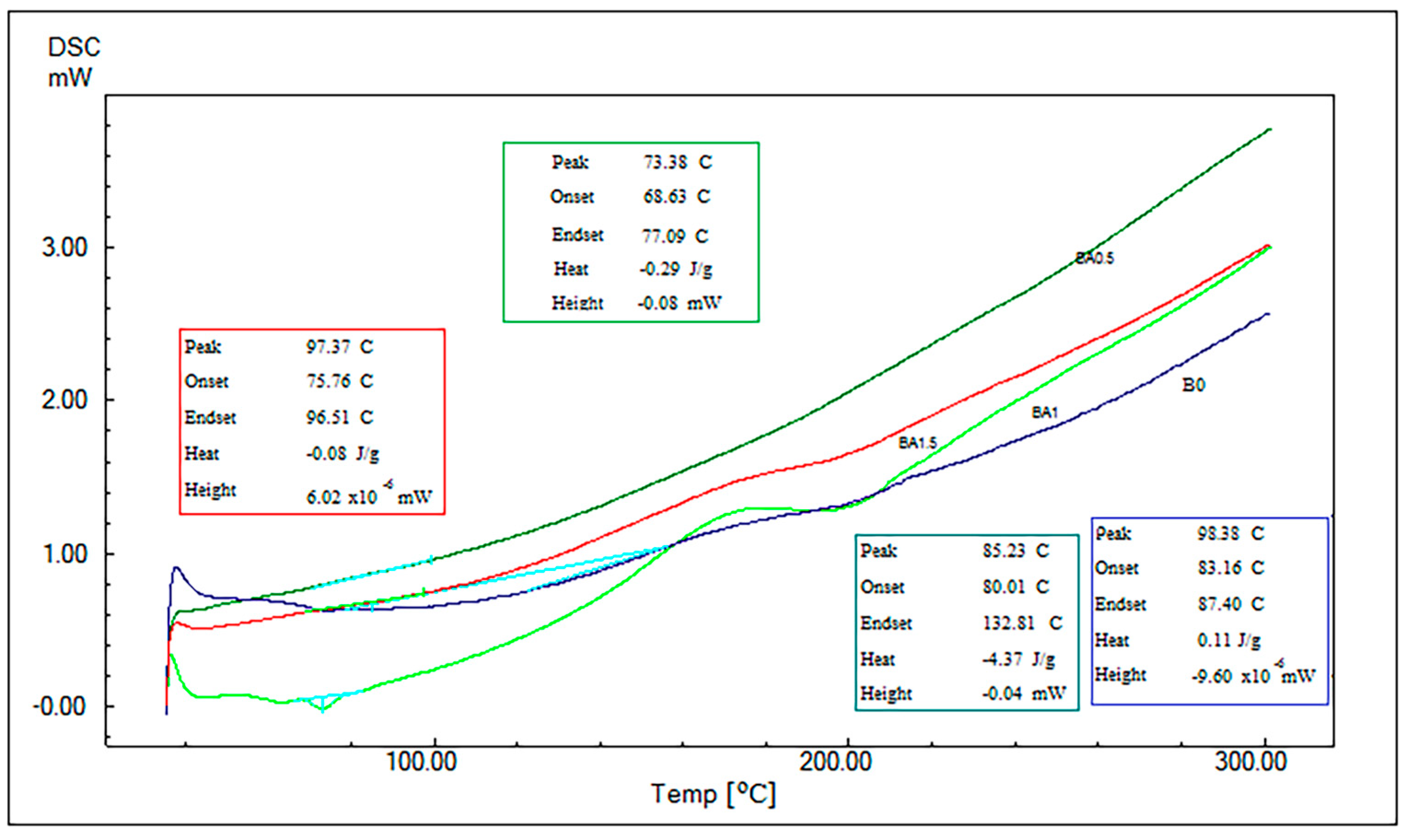
3.4. Results of DSC Analysis
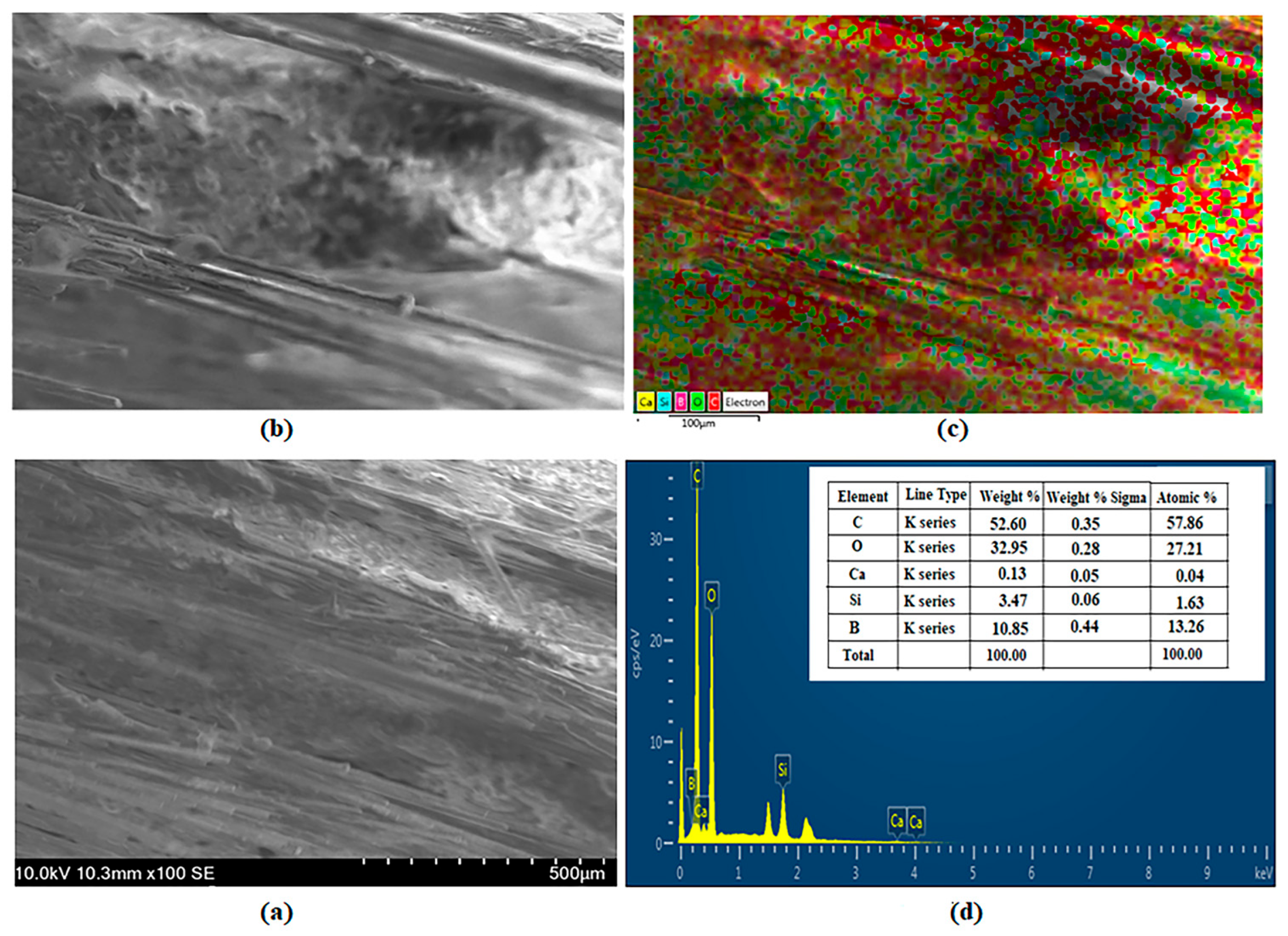
3.5. SEM and EDS Images
3.6. Effect on Failure Modes
4. Conclusions
- -
- With the exception of compressive strength, BA improved its mechanical properties. The highest values were obtained from the BA0.5 specimen.
- -
- As the BA ratio increased, the hardness value increased.
- -
- Tg obtained with the help of DMA and DSC analyses have been found to be compatible. The addition of BA decreased the Tg.
- -
- The TGA analysis revealed that, when the BA ratio increased, weight loss decreased.
- -
- The lowest value for both E′ and E″ was obtained from specimen BA1.
- -
- The E′ value obtained from the BA0.5 specimen was 22.65% higher than that obtained from the BA0 specimen.
- -
- The E″ value obtained from the BA0 specimen was 11.28%, 74.36%, and 49.46% higher than the values obtained from the BA0.5, BA1, and BA1.5 specimens, respectively.
- -
- The highest and lowest tan δ values were obtained from BA0 and BA1.5 specimens, respectively. The value obtained from specimen BA0 was 102.14% higher than the value obtained from specimen BA1.5.
- -
- All specimens with the addition of BA had fiber/matrix failures and interlayer separation, distinctly.
- -
- This study can be improved by using BA in different ratios in different fabrics and resins. Also, the effect of BA on peeling and impact tests can be investigated.
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jin, F.L.; Li, X.; Park, S.J. Synthesis and application of epoxy resins: A review. J. Ind. Eng. Chem. 2015, 29, 1–11. [Google Scholar] [CrossRef]
- Shao, Z.; Wang, H.; Li, M.; Chen, T.; Xu, Y.; Yuan, C.; Zeng, B.; Dai, L. Effect of functionalized graphene oxide with phosphaphenanthrene and isocyanurate on flammability, mechanical properties, and thermal stability of epoxy composites. J. Appl. Polym. Sci. 2020, 137, 48761. [Google Scholar] [CrossRef]
- Rakhman, A.; Diharjo, K.; Raharjo, W.W.; Suryanti, V.; Kaleg, S. Improvement of Fire Resistance and Mechanical Properties of Glass Fiber Reinforced Plastic (GFRP) Composite Prepared from Combination of Active Nano Filler of Modified Pumice and Commercial Active Fillers. Polymers 2023, 15, 51. [Google Scholar] [CrossRef]
- Ullah, S.; Ahmad, F.; Shariff, A.M.; Bustam, M.A.; Gonfa, G.; Gillani, Q.F. Effects of ammonium polyphosphate and boric acid on the thermal degradation of an intumescent fire retardant coating. Prog. Org. Coat. 2017, 109, 70–82. [Google Scholar] [CrossRef]
- Dixit, A.C.; Achutha, M.V.; Sridhara, B.K. Elastic properties of aluminum boron carbide metal matrix composites. Mater. Today Proc. 2021, 43, 1253–1257. [Google Scholar] [CrossRef]
- Sathiyaraj, S.; Senthilkumar, A.; Muhammed, A.P.; Sundar, R.; Saseendran, V. Experimental investigations on mechanical properties of Al-B4C metal matrix composites. Mater. Today Proc. 2021, 45, 6372–6376. [Google Scholar] [CrossRef]
- Zaki, M.U.; Hussain, S. Impact of addition of manganese and boron carbide on aluminium metal matrix composites using powder metallurgy process. Mater. Today Proc. 2021, 44, 4364–4368. [Google Scholar] [CrossRef]
- Santha, R.D.; Simhadri, T.S. Improvements in mechanical properties of aluminium alloy-titanium di boride and boron carbide hybrid composite produced through friction stir processing route. Mater. Today Proc. 2021, 45, 5231–5236. [Google Scholar] [CrossRef]
- Guo, H.; Li, J.; Liu, N.; Wei, X.; Fan, M.; Shang, Y.; Jiang, W.; Zhang, Y.; Cui, Y.; Sun, L.; et al. Strengthening and toughening B4C/Al composites via optimizing the Al2O3 distribution during hot Rolling. J. Alloys Compd. 2022, 902, 163773. [Google Scholar] [CrossRef]
- Shreenivasaiah, P.H.; Gowda, T.; Kuldeep, B.; Ravikumar, K.P.; Muthanna, K.P. Experimental investigation of cubic boron nitride reinforced Al2014 composites. Mater. Today Proc. 2021, 46, 7760–7763. [Google Scholar] [CrossRef]
- Mohanavel, V.; Sundar, M.; Pugazhendhi, L.; Ranganathan, K. Thermal and mechanical properties of titanium matrix composites synthesized through solid state technique. Mater. Today Proc. 2021, 37, 497–503. [Google Scholar] [CrossRef]
- Kumar, G.; Saravanan, R.; Nagaral, M. Dry sliding wear behavior of nano boron carbide particulates reinforced Al2214 alloy composites. Mater. Today Proc. 2023, 81, 191–195. [Google Scholar] [CrossRef]
- Tasgin, Y. The effects of boron minerals on the microstructure and abrasion resistance of babbitt metal (sn–sb–cu) used as coating materials in hydroelectric power plants. Int. J. Metalcast. 2020, 14, 257–265. [Google Scholar] [CrossRef]
- Tasyurek, M.; Duzcukoglu, H. Improving the wear behavior of epoxy resin with boron carbide reinforcement. Iran. Polym. J. 2022, 31, 169–184. [Google Scholar] [CrossRef]
- Vaidya, S.A.; Rangaswamy, T. Identification of mechanical characteristics of Boron Carbide filled glass–epoxy composite treated to low temperature. Mater. Today Proc. 2021, 46, 8980–8984. [Google Scholar] [CrossRef]
- Zhang, C.; Xia, Q.; Han, L.; Zhao, Y.; Huang, N.; Ren, Q.; Zhang, X.; Ru, H. Fabrication of carbon-coated boron carbide particle and its role in the reaction bonding of boron carbide by silicon infiltration. J. Eur. Ceram. 2022, 42, 860–868. [Google Scholar] [CrossRef]
- Kiran, M.S.R.N.; Raidongia, K.; Ramamurty, U.; Rao, C.N.R. Improved mechanical properties of polymer nanocomposites incorporating graphene-like BN: Dependence on the number of BN layers. Scr. Mater. 2011, 64, 592–595. [Google Scholar] [CrossRef]
- Panda, J.N.; Bijwe, J.; Pandey, R.K. Role of micro and nano-particles of hBN as a secondary solid lubricant for improving tribo-potential of PAEK composite. Tribol. Int. 2019, 130, 400–412. [Google Scholar] [CrossRef]
- Li, G.; Ma, Y.; Xu, H.; Chen, L.; An, Y.; Gao, M.; Zhou, H.; Chen, J. Hydroxylated hxagonal boron nitride nanoplatelets enhance the mechanical and tribological properties of epoxy-based composite coatings. Prog. Org. Coat. 2022, 165, 106731. [Google Scholar] [CrossRef]
- Chen, B.; Gong, J.; Huang, W.; Gao, N.; Deng, C.; Gao, X. Constructing a parallel aligned shish kebab structure of HDPE/BN composites: Toward improved two-way thermal conductivity and tensile strength. Compos. Part B Eng. 2023, 259, 110699. [Google Scholar] [CrossRef]
- Zulkifli, M.A.A.; Mahmud, J. Failure analysis of boron/glass hybrid laminates under biaxial tension. Mater. Today Proc. 2021, 41, 72–76. [Google Scholar] [CrossRef]
- Wang, D.C.; Chang, G.W.; Chen, Y. Preparation and thermal stability of boron-containing phenolic resin/clay nanocomposites. Polym. Degrad. Stab. 2008, 93, 125–133. [Google Scholar] [CrossRef]
- Tuisov, A.G.; Kychkin, A.; Kychkin, A.K.; Anan’eva, E.S. Reinforced Epoxy Binder Modified with Borpolymer. Polymers 2023, 15, 2632. [Google Scholar] [CrossRef]
- Karua, P.; Arifuzzaman, M.d. Compressive behavior of perlite/sodium silicate composite foam modified by boric acid. Metall. Mater. Eng. 2022, 28, 103–124. [Google Scholar] [CrossRef] [PubMed]
- Pehlivanlı, Z.O. Manufacturing and characterization of polypropylene/boric acid composite. Polym. Bull. 2021, 78, 4033–4046. [Google Scholar] [CrossRef]
- Bagci, M.; Imrek, H. Solid particle erosion behaviour of glass fibre reinforced boric acid filled epoxy resin composites. Tribol. Int. 2011, 44, 1704–1710. [Google Scholar] [CrossRef]
- Polat, O.; Kaynak, C. Use of Boron Oxide and Boric Acid to Improve Flame Retardancy of an Organophosphorus Compound in Neat and Fiber Reinforced Polyamide-6. J. Vinyl Addit. Technol. 2016, 22, 300–310. [Google Scholar] [CrossRef]
- Jian, S.; Liu, S.; Chen, L.; Zhou, S.; Fan, P.; Zeng, Y.; Hou, H. Nano-boria reinforced polyimide composites with greatly enhanced thermal and mechanical properties via in-situ thermal conversion of boric acid. Compos. Commun. 2017, 3, 14–17. [Google Scholar] [CrossRef]
- Uddin, Z.; Yasin, T.; Shafiq, M. Development of novel silane modified boric acid/high density polyethylene composites for radiation shielding applications. Radiat. Phys. Chem. 2022, 192, 109909. [Google Scholar] [CrossRef]
- Visakh, P.M.; Nazarenko, O.B.; Amelkovich, Y.A.; Melnikova, T.V. Thermal properties of epoxy composites filled with boric acid. IOP Conf. Ser. Mater. Sci. Eng. 2015, 81, 012095. [Google Scholar] [CrossRef]
- Visakh, P.M.; Nazarenko, O.B.; Amelkovich, Y.A.; Melnikova, T.V. Effect of zeolite and boric acid on epoxy-based composites. Polym. Adv. Technol. 2016, 27, 1098–1101. [Google Scholar] [CrossRef]
- Nazarenko, O.B.; Melnikova, T.V.; Visakh, P.M. Thermal and Mechanical Characteristics of Polymer Composites Based on Epoxy Resin, Aluminium Nanopowders and Boric Acid. J. Phys. Conf. Ser. 2016, 671, 012040. [Google Scholar] [CrossRef]
- Nazarenko, O.B.; Amelkovich, Y.A.; Bannov, A.G.; Berdyugina, I.S.; Maniyan, V.P. Thermal Stability and Flammability of Epoxy Composites Filled with Multi-Walled Carbon Nanotubes, Boric Acid, and Sodium Bicarbonate. Polymers 2021, 13, 638. [Google Scholar] [CrossRef] [PubMed]
- Nazarenko, O.B.; Melnikova, T.V.; Visakh, P.M. Combined effect of zeolite and boric acid on thermal behavior of epoxy composites. J. Therm. Anal. Calorim. 2017, 128, 169–175. [Google Scholar] [CrossRef]
- Zhu, J.; Wang, Y.; Zhao, X.; Li, N.; Guo, X.; Zhao, L.; Yin, Y. Anisotropic composite aerogel with thermal insulation and flame retardancy from cellulose nanofibers, calcium alginate and boric acid. Int. J. Biol. Macromol. 2024, 267, 131450. [Google Scholar] [CrossRef] [PubMed]
- Hamciuc, C.; Vlad-Bubulac, T.; Serbezeanu, D.; Macsim, A.M.; Lisa, G.; Anghel, I.; Şofran, I.E. Effects of Phosphorus and Boron Compounds on Thermal Stability and Flame Retardancy Properties of Epoxy Composites. Polymers 2022, 14, 4005. [Google Scholar] [CrossRef] [PubMed]
- Avci, A.; Eker, A.A.; Bodur, M.S.; Küçükyildirim, B.O. The effects of various boron compounds on the thermal, microstructural and mechanical properties of PLA biocomposites. Thermochim. Acta 2024, 731, 179656. [Google Scholar] [CrossRef]
- Rudawska, A. Mechanical Properties of Epoxy Compounds Based on Unmodified Epoxy Resin Modified with Boric Acid as an Antiseptic. Materials 2024, 17, 259. [Google Scholar] [CrossRef]
- Rudawska, A.; Frigione, M.; Sarcinella, A.; Sarcinella, A.; Brunella, V.; Lorenzo, L.D.; Kruszkowska, E.O. Properties and Performance of Epoxy Resin/Boron Acid Composites. Materials 2024, 17, 2092. [Google Scholar] [CrossRef]
- Yoğurtçu, H.; Gürler, N. Evaluation of effect of boric acid on thermoplastic starch: Morphological, mechanical, barrier, and optical properties. Polym. Eng. Sci. 2024, 64, 2230–2240. [Google Scholar] [CrossRef]
- Örçen, G.; Bayram, D. Effect of nanoclay on the mechanical and thermal properties of glass fiber-reinforced epoxy composites. J. Mater. Sci. 2024, 59, 3467–3487. [Google Scholar] [CrossRef]
- ASTM D3039/D3039M-17; Standard Test Method for Tensile Properties of Polymer Matrix Composite Materials. ASTM International: West Conshohocken, PA, USA, 2017.
- ASTM D6641/D6641M; Standard Test Method for Compressive Properties of Polymer Matrix Composite Materials Using a Combined Loading Compression (CLC) Test Fixture. ASTM International: West Conshohocken, PA, USA, 2017.
- ASTM D7078/D7078M-20; Standard Test Method for Shear Properties of Composite Materials by V-Notched Rail Shear Method. ASTM International: West Conshohocken, PA, USA, 2021.
- ASTM D7028-07; Standard Test Method for Glass Transition Temperature (DMA Tg) of Polymer Matrix Composites by Dynamic Mechanical Analysis (DMA). ASTM International: West Conshohocken, PA, USA, 2015.
- Alexandra, L.; Yingtao, L.; Shreya, V. Effect of Immediate Curing at Elevated Temperatures on the Tensile and Interfacial Properties of Carbon Fiber-Epoxy Composites. Funct. Compos. Mater. 2024, 6, 035001. [Google Scholar]
- Suresha, B.; Saini, M.S. Influence of organo-modified montmorillonite nanolayers on static mechanical and dynamic mechanical behavior of carbon/epoxy composites. J. Compos. Mater. 2016, 50, 3589–3601. [Google Scholar] [CrossRef]








| Boric Acid (BA) | Mechanical Properties | ||||||
|---|---|---|---|---|---|---|---|
| Tensile Stress (MPa)-(Xt) | Compressive Stress (MPa)-(Xc) | Shear Stress (MPa)-(S12) | Modulus of Elasticity (MPa)-(E12) | Modulus of Shear (MPa)-(G12) | Poissons’s Ratio-ʋ12 | Hardness (HV0.3/10) | |
| 0% SD | 448.38 [41] 2.53 | 292.87 [41] 2.31 | 119.81 [41] 3.57 | 22,085.98 [41] 5.78 | 4087.78 [41] 6.57 | 0.16 [41] 0.01 | 31 [41] 0.40 |
| 0.5% SD | 559.49 2.32 | 246.92 2.64 | 130.29 1.06 | 27,636.93 4.51 | 4547.18 4.04 | 0.18 0.01 | 35.2 0.76 |
| 1% SD | 463.99 2.19 | 151.91 1.47 | 124.22 1.53 | 26,538.28 2.08 | 4211.93 2.65 | 0.17 0.01 | 38.0 0.58 |
| 1.5% SD | 463.49 2.05 | 195.46 2.80 | 126.95 1.25 | 25,320.46 2.65 | 4379.17 1.53 | 0.18 0.005 | 52.9 0.25 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Örçen, G.; Bayram, D. Effects of Boric Acid on Laminated Composites: An Experimental Study. Polymers 2024, 16, 2133. https://doi.org/10.3390/polym16152133
Örçen G, Bayram D. Effects of Boric Acid on Laminated Composites: An Experimental Study. Polymers. 2024; 16(15):2133. https://doi.org/10.3390/polym16152133
Chicago/Turabian StyleÖrçen, Gurbet, and Duygu Bayram. 2024. "Effects of Boric Acid on Laminated Composites: An Experimental Study" Polymers 16, no. 15: 2133. https://doi.org/10.3390/polym16152133
APA StyleÖrçen, G., & Bayram, D. (2024). Effects of Boric Acid on Laminated Composites: An Experimental Study. Polymers, 16(15), 2133. https://doi.org/10.3390/polym16152133







