The Effect of Polyethylene Glycol on the Non-Isothermal Crystallization of Poly(L-lactide) and Poly(D-lactide) Blends
Abstract
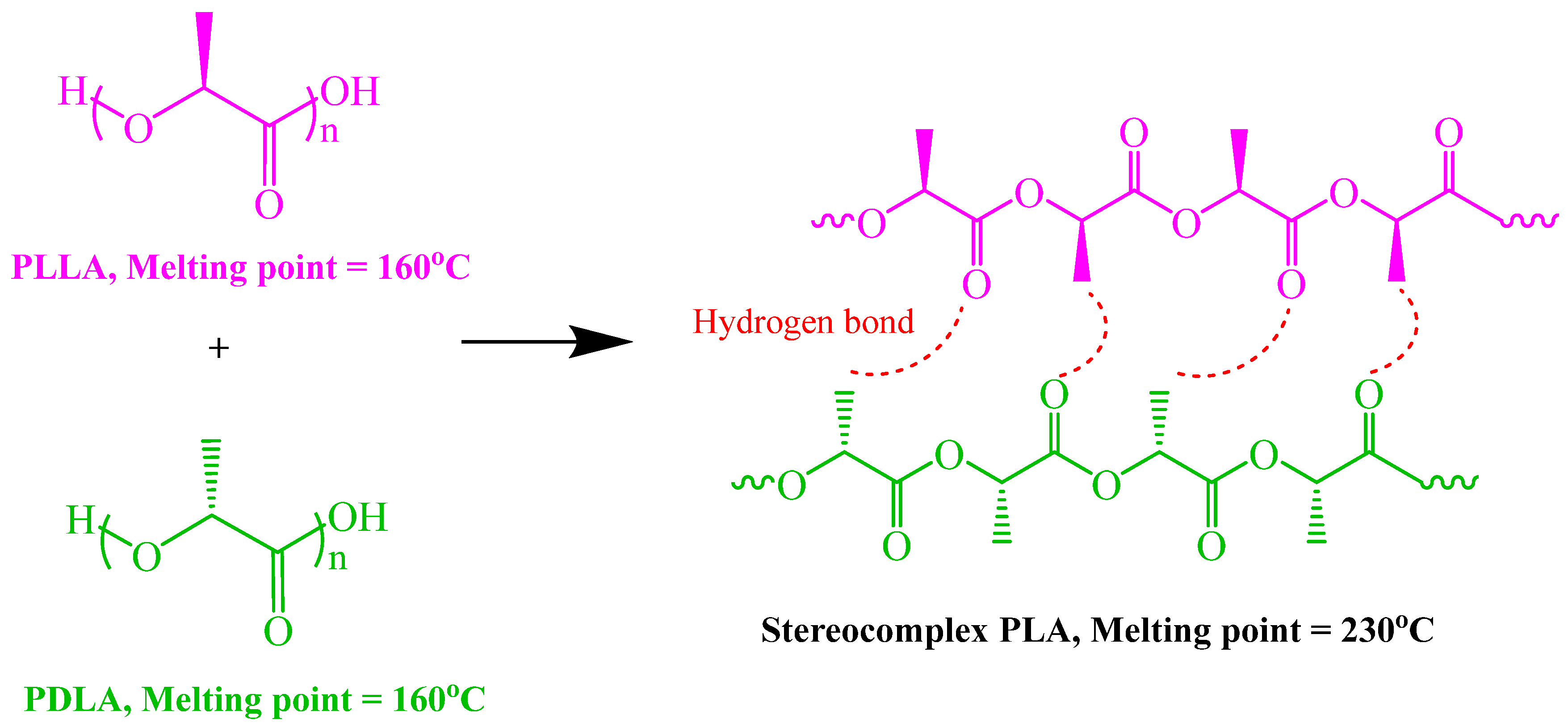
1. Introduction
2. Experimental
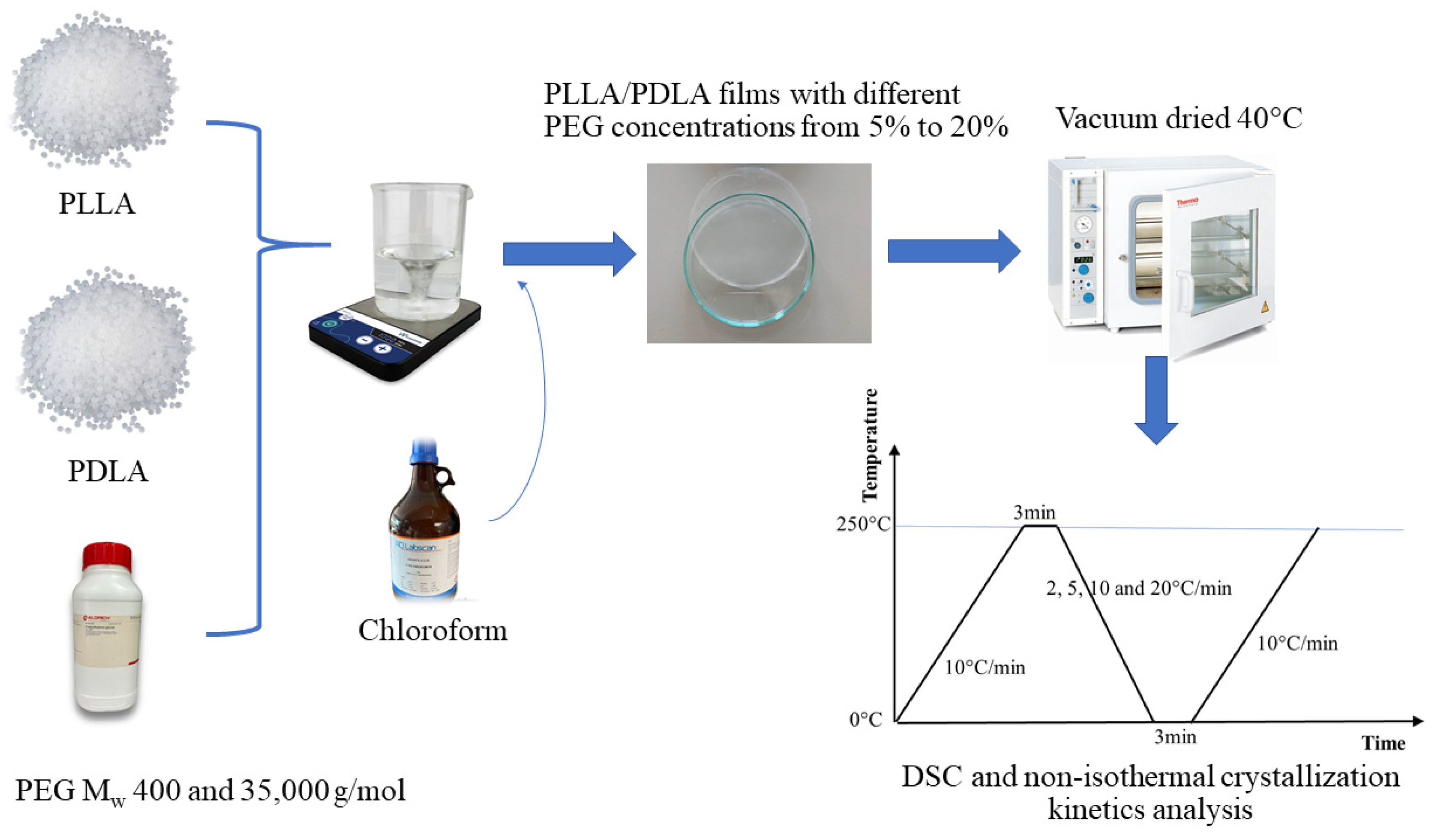
2.1. Materials
2.2. Sample Preparation
2.3. Differential Scanning Calorimetry (DSC)
3. Results and Discussion
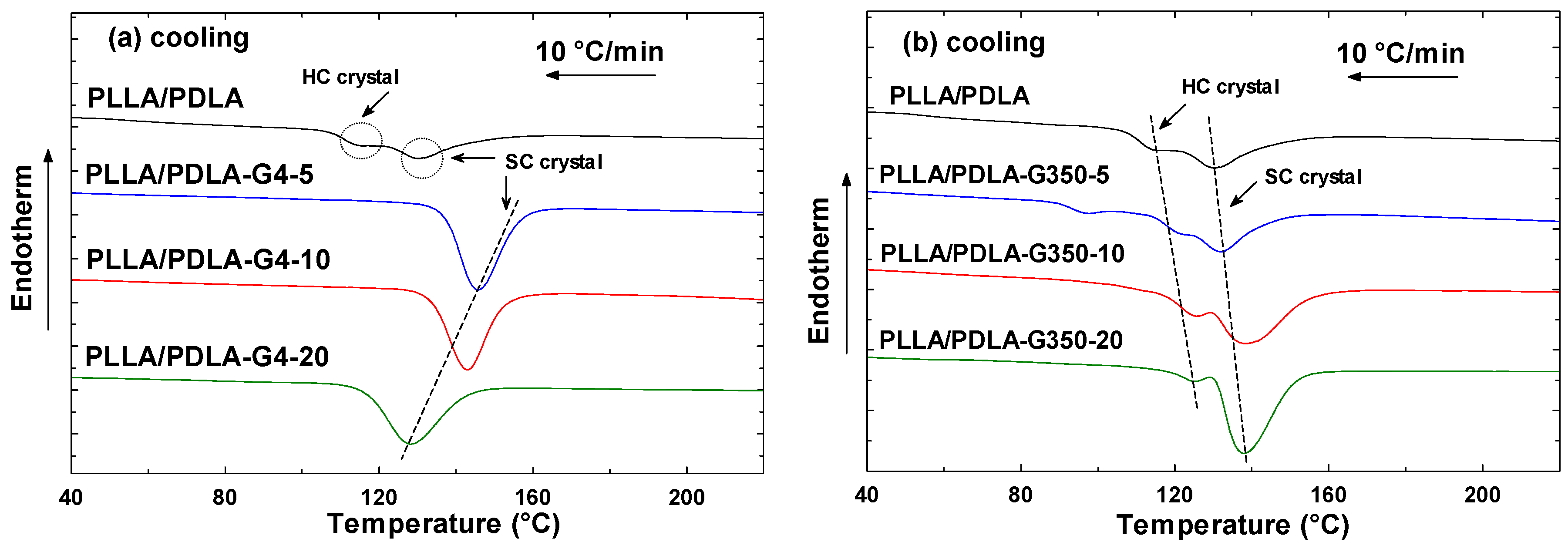
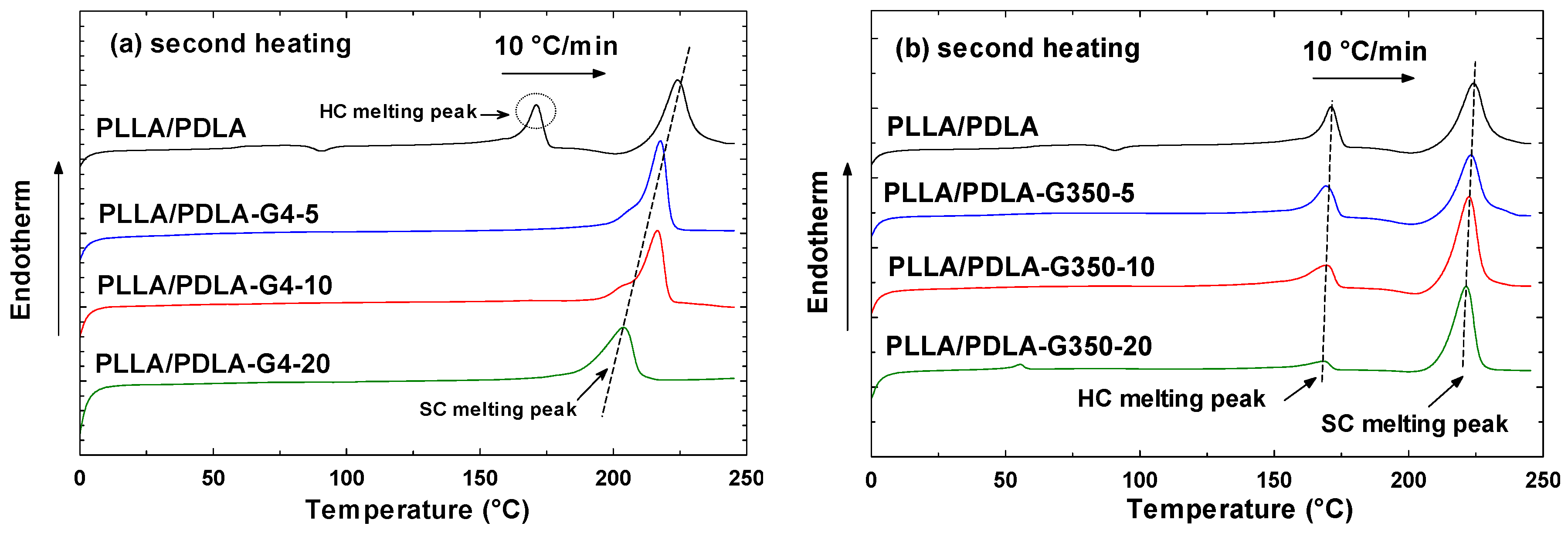
3.1. Effect of Different MWs of PEG on PLLA/PDLA Blends
3.1.1. Cooling Cycle
3.1.2. Second Heating Cycle
3.2. Effect of Different Cooling Rates on PLLA/PDLA Blends with PEG Incorporation
3.2.1. Cooling Cycle
3.2.2. Second Heating Cycle Following Non-Isothermal Crystallization
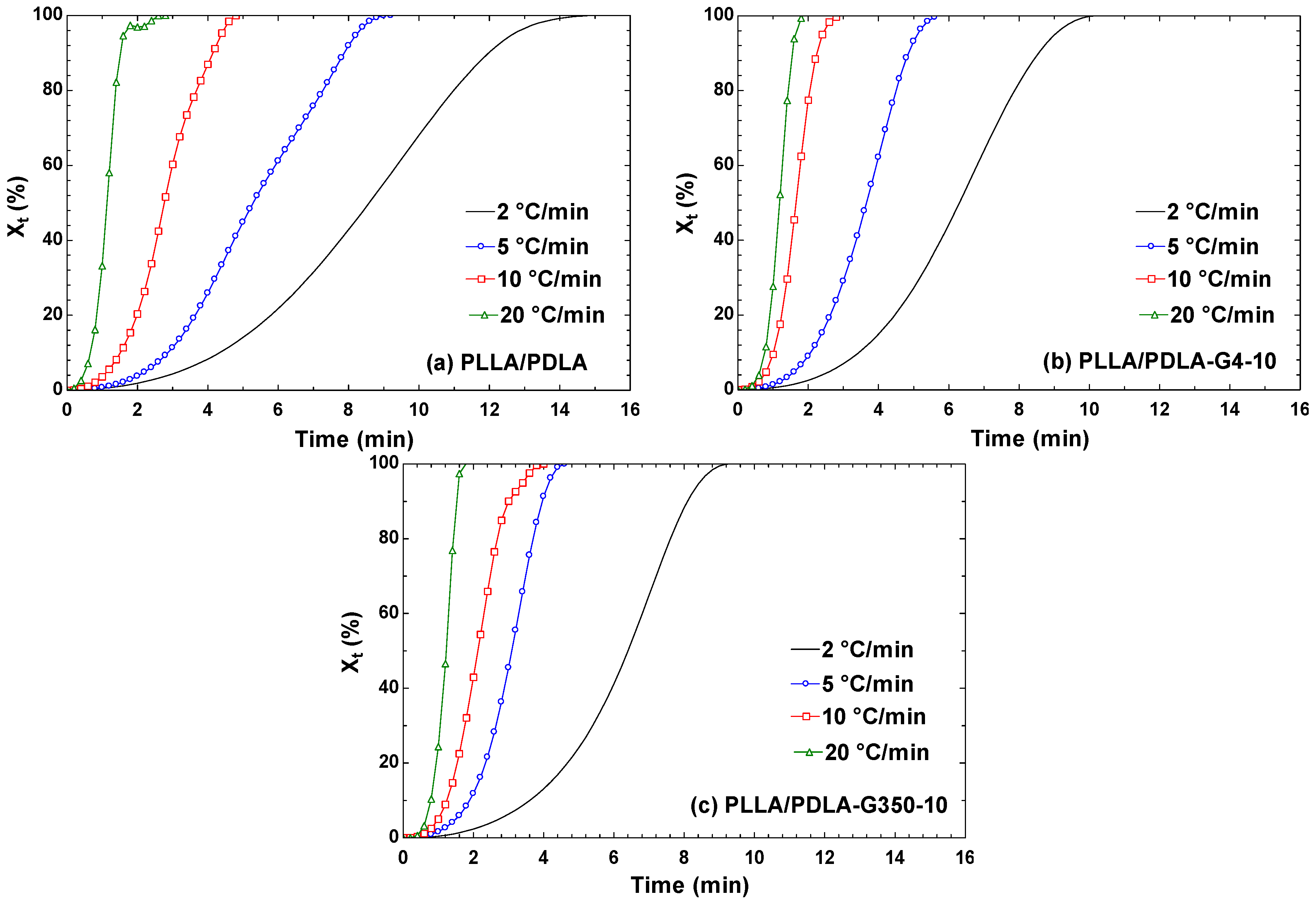
3.2.3. The Relationship between the Relative Crystallinity of PLLA/PDLA/PEG and Temperature and Time
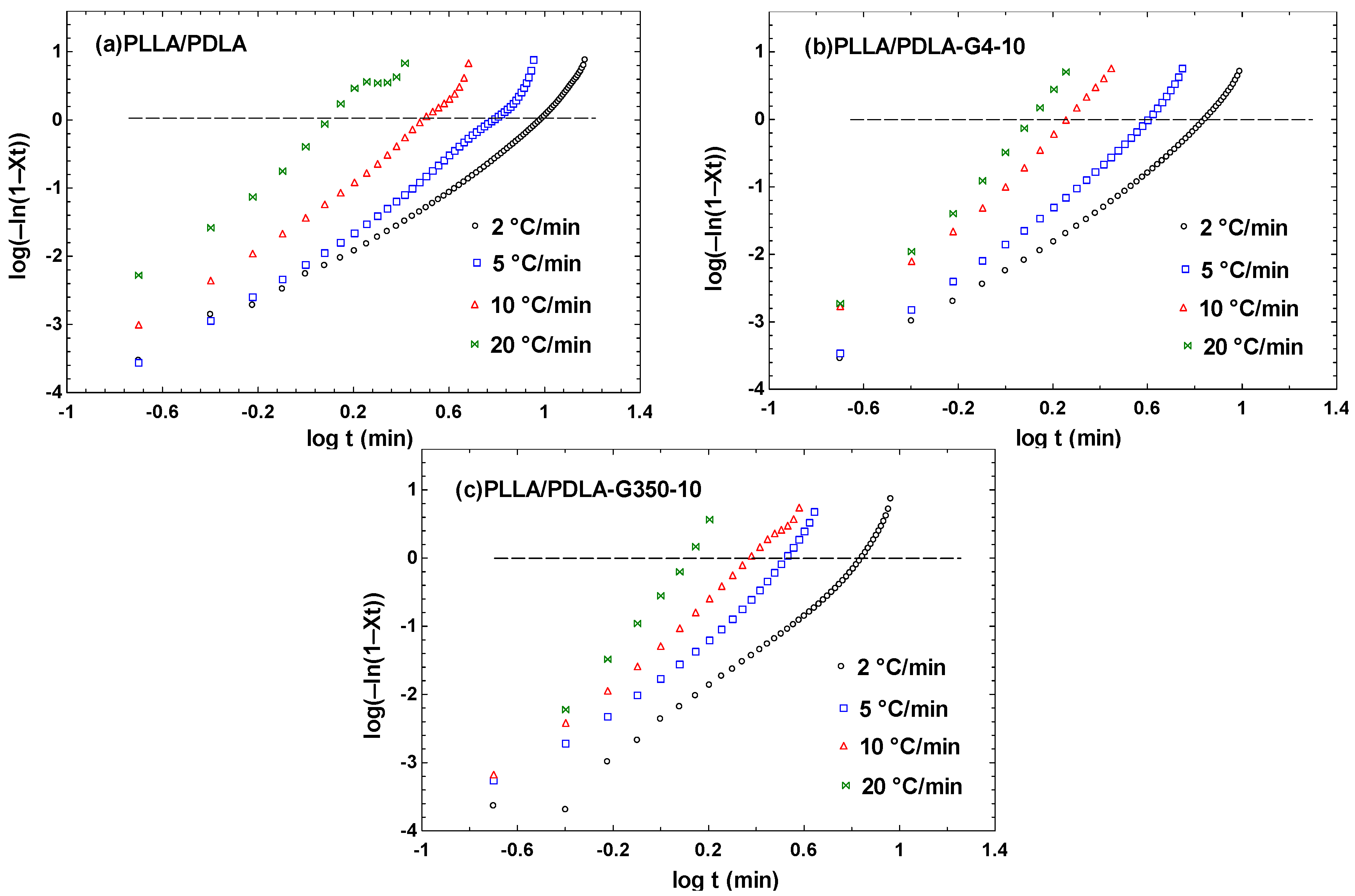
3.3. Non-Isothermal Crystallization Kinetics of PLLA/PDLA/PEG Blends
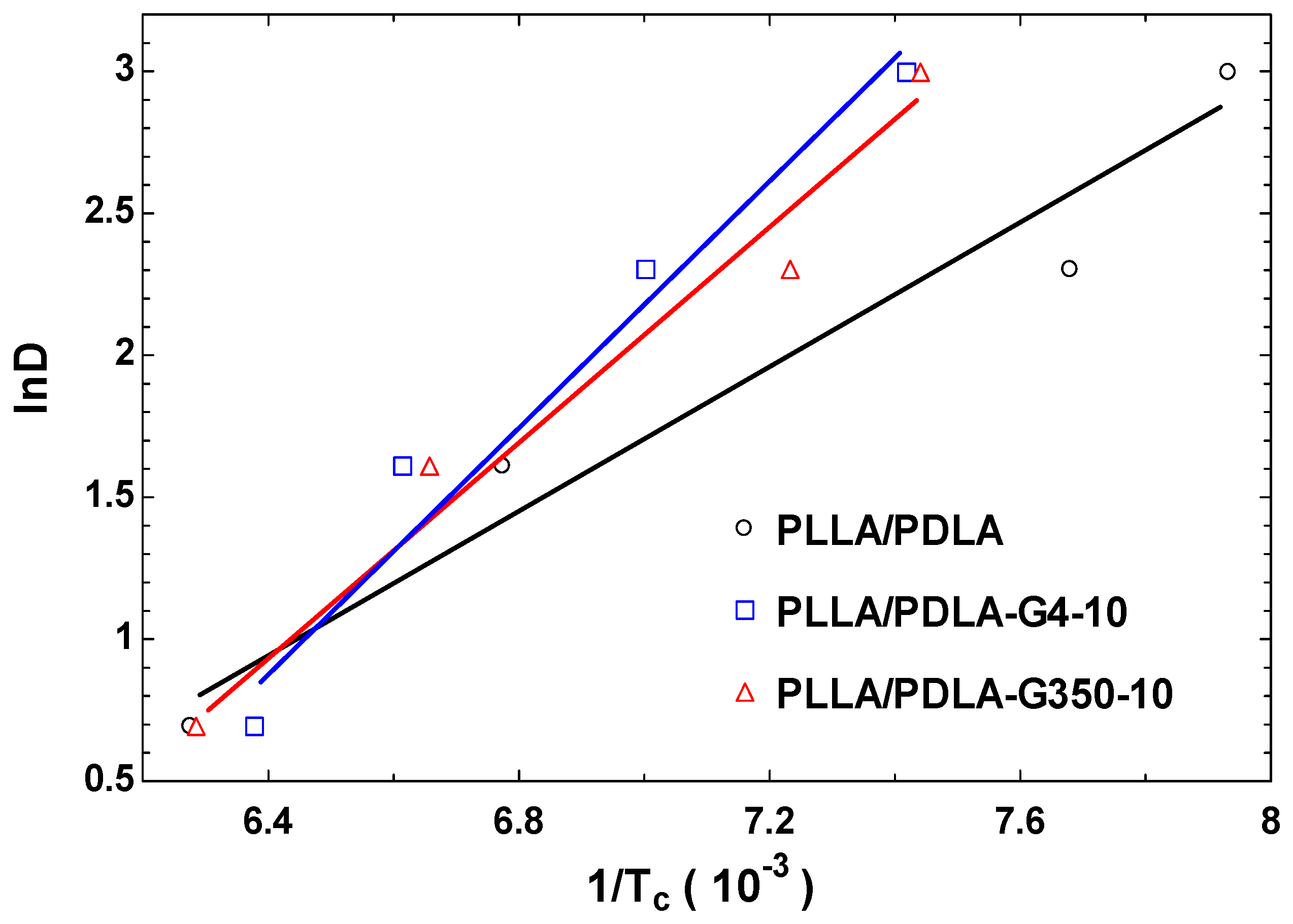
3.4. Non-Isothermal Crystallization Activation Energy
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Álvarez-Chávez, C.R.; Edwards, S.; Moure-Eraso, R.; Geiser, K. Sustainability of bio-based plastics: General comparative analysis and recommendations for improvement. J. Clean. Prod. 2012, 23, 47–56. [Google Scholar] [CrossRef]
- Iles, A.; Martin, A.N. Expanding bioplastics production: Sustainable business innovation in the chemical industry. J. Clean. Prod. 2013, 45, 38–49. [Google Scholar] [CrossRef]
- Zhou, L.; Ke, K.; Yang, M.-B.; Yang, W. Recent progress on chemical modification of cellulose for high mechanical-performance Poly (lactic acid)/Cellulose composite: A review. Comp. Comm. 2021, 23, 100548. [Google Scholar] [CrossRef]
- Kulkarni, R.K.; Moore, E.; Hegyeli, A.; Leonard, F. Biodegradable poly (lactic acid) polymers. J. Biomed. Mater. Res. 1971, 5, 169–181. [Google Scholar] [CrossRef]
- Datta, R.; Tsai, S.-P.; Bonsignore, P.; Moon, S.-H.; Frank, J.R. Technological and economic potential of poly (lactic acid) and lactic acid derivatives. FEMS Microbiol. Rev. 1995, 16, 221–231. [Google Scholar] [CrossRef]
- Tian, H.; Tagaya, H. Preparation, characterization and mechanical properties of the polylactide/perlite and the polylactide/montmorillonite composites. J. Mater. Sci. 2007, 42, 3244–3250. [Google Scholar] [CrossRef]
- Ambrosio-Martín, J.; Fabra, M.; Lopez-Rubio, A.; Lagaron, J. An effect of lactic acid oligomers on the barrier properties of polylactide. J. Mater. Sci. 2014, 49, 2975–2986. [Google Scholar] [CrossRef]
- Qin, Y.; Yang, J.; Xue, J. Characterization of antimicrobial poly (lactic acid)/poly (trimethylene carbonate) films with cinnamaldehyde. J. Mater. Sci. 2015, 50, 1150–1158. [Google Scholar] [CrossRef]
- Nofar, M. Multiphase Polylactide Blends: Toward a Sustainable and Green Environment; Elsevier: Amsterdam, The Netherlands, 2021. [Google Scholar]
- Nofar, M.; Sacligil, D.; Carreau, P.J.; Kamal, M.R.; Heuzey, M.-C. Poly (lactic acid) blends: Processing, properties and applications. Int. J. Biol. Macromol. 2019, 125, 307–360. [Google Scholar]
- Nofar, M.; Mohammadi, M.; Carreau, P.J. Super enhancement of rheological properties of amorphous PLA through generation of a fiberlike oriented crystal network. J. Rheol. 2021, 65, 493–505. [Google Scholar] [CrossRef]
- Pan, P.; Yang, J.; Shan, G.; Bao, Y.; Weng, Z.; Cao, A.; Yazawa, K.; Inoue, Y. Temperature-variable FTIR and solid-state 13C NMR investigations on crystalline structure and molecular dynamics of polymorphic poly (L-lactide) and poly (L-lactide)/poly (D-lactide) stereocomplex. Macromolecules 2012, 45, 189–197. [Google Scholar] [CrossRef]
- Gupta, A.; Mulchandani, N.; Shah, M.; Kumar, S.; Katiyar, V. Functionalized chitosan mediated stereocomplexation of poly (lactic acid): Influence on crystallization, oxygen permeability, wettability and biocompatibility behavior. Polymer 2018, 142, 196–208. [Google Scholar] [CrossRef]
- Srithep, Y.; Pholharn, D.; Worajittiphon, P.; Sriprateep, K.; Veang-In, O.; Morris, J. Toughening Polylactide Stereocomplex by Injection Molding with Thermoplastic Starch and Chain Extender. Polymers 2023, 15, 2055. [Google Scholar] [CrossRef] [PubMed]
- Rathi, S.R.; Coughlin, E.B.; Hsu, S.L.; Golub, C.S.; Ling, G.H.; Tzivanis, M.J. Effect of midblock on the morphology and properties of blends of ABA triblock copolymers of PDLA-mid-block-PDLA with PLLA. Polymer 2012, 53, 3008–3016. [Google Scholar] [CrossRef]
- Tsuji, H.; Ikada, Y. Stereocomplex formation between enantiomeric poly (lactic acid) s. XI. Mechanical properties and morphology of solution-cast films. Polymer 1999, 40, 6699–6708. [Google Scholar] [CrossRef]
- Tsuji, H.; Fukui, I. Enhanced thermal stability of poly (lactide) s in the melt by enantiomeric polymer blending. Polymer 2003, 44, 2891–2896. [Google Scholar] [CrossRef]
- Pan, P.; Han, L.; Bao, J.; Xie, Q.; Shan, G.; Bao, Y. Competitive stereocomplexation, homocrystallization, and polymorphic crystalline transition in poly (L-lactic acid)/poly (D-lactic acid) racemic blends: Molecular weight effects. J. Phys. Chem. B 2015, 119, 6462–6470. [Google Scholar] [CrossRef]
- Bao, J.; Xue, X.; Li, K.; Chang, X.; Xie, Q.; Yu, C.; Pan, P. Competing stereocomplexation and homocrystallization of poly (L-lactic acid)/poly (D-lactic acid) racemic mixture: Effects of miscible blending with other polymers. J. Phys. Chem. B 2017, 121, 6934–6943. [Google Scholar] [CrossRef] [PubMed]
- Alhaj, M.; Narayan, R. Scalable continuous manufacturing process of stereocomplex PLA by twin-screw extrusion. Polymers 2023, 15, 922. [Google Scholar] [CrossRef]
- Lai, W.-C.; Liau, W.-B.; Lin, T.-T. The effect of end groups of PEG on the crystallization behaviors of binary crystalline polymer blends PEG/PLLA. Polymer 2004, 45, 3073–3080. [Google Scholar] [CrossRef]
- Kulinski, b.; Piorkowska, E. Crystallization, structure and properties of plasticized poly (L-lactide). Polymer 2005, 46, 10290–10300. [Google Scholar] [CrossRef]
- Rasal, R.M.; Janorkar, A.V.; Hirt, D.E. Poly (lactic acid) modifications. Prog. Polym. Sci. 2010, 35, 338–356. [Google Scholar] [CrossRef]
- Saeidlou, S.; Huneault, M.A.; Li, H.; Park, C.B. Poly (lactic acid) crystallization. Prog. Polym. Sci. 2012, 37, 1657–1677. [Google Scholar] [CrossRef]
- Kodal, M.; Sirin, H.; Ozkoc, G. Long-and short-term stability of plasticized poly (lactic acid): Effects of plasticizers type on thermal, mechanical and morphological properties. Polym. Bull. 2019, 76, 423–445. [Google Scholar] [CrossRef]
- Takhulee, A.; Takahashi, Y.; Vao-soongnern, V. Molecular simulation and experimental studies of the miscibility of polylactic acid/polyethylene glycol blends. J. Polym. Res. 2017, 24, 8. [Google Scholar] [CrossRef]
- Bao, R.-Y.; Yang, W.; Wei, X.-F.; Xie, B.-H.; Yang, M.-B. Enhanced formation of stereocomplex crystallites of high molecular weight poly (l-lactide)/poly (d-lactide) blends from melt by using poly (ethylene glycol). ACS Sustain. Chem. Eng. 2014, 2, 2301–2309. [Google Scholar] [CrossRef]
- Di Lorenzo, M.; Silvestre, C. Non-isothermal crystallization of polymers. Prog. Polym. Sci. 1999, 24, 917–950. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, L.; He, Y.; Fan, Z.; Li, S. Non-isothermal crystallization kinetics of poly (L-lactide). Polym. Int. 2010, 59, 1616–1621. [Google Scholar] [CrossRef]
- Nofar, M.; Zhu, W.; Park, C.B.; Randall, J. Crystallization kinetics of linear and long-chain-branched polylactide. Ind. Eng. Chem. Res. 2011, 50, 13789–13798. [Google Scholar] [CrossRef]
- Gao, P.; Kundu, A.; Coulter, J. Vibration-assisted injection molding: An efficient process for enhanced crystallinity development and mechanical characteristics for poly lactic acid. Int. J. Adv. Manuf. Technol. 2022, 121, 3111–3124. [Google Scholar] [CrossRef]
- Srithep, Y.; Pholharn, D. Plasticizer effect on melt blending of polylactide stereocomplex. e-Polymers 2017, 17, 409–416. [Google Scholar] [CrossRef]
- Yang, B.; Wang, H.; Wan, X.; Fan, B.; Sun, H. Nonisothermal crystallization of poly (L-lactic acid) promoted by polyols. Polym. Adv. Technol. 2023, 34, 2189–2197. [Google Scholar] [CrossRef]
- Ikada, Y.; Jamshidi, K.; Tsuji, H.; Hyon, S.H. Stereocomplex formation between enantiomeric poly (lactides). Macromolecules 1987, 20, 904–906. [Google Scholar] [CrossRef]
- De Arenaza, I.M.; Sarasua, J.R.; Amestoy, H.; Lopez-Rodriguez, N.; Zuza, E.; Meaurio, E.; Meyer, F.; Santos, J.I.; Raquez, J.M.; Dubois, P. Polylactide stereocomplex crystallization prompted by multiwall carbon nanotubes. J. Appl. Polym. Sci. 2013, 130, 4327–4337. [Google Scholar] [CrossRef]
- Yu, S.; Zhang, Y.; Hu, H.; Li, J.; Zhou, W.; Zhao, X.; Peng, S. Effect of maleic anhydride grafted poly (lactic acid) on rheological behaviors and mechanical performance of poly (lactic acid)/poly (ethylene glycol)(PLA/PEG) blends. RSC Adv. 2022, 12, 31629–31638. [Google Scholar] [CrossRef] [PubMed]
- Han, L.; Pan, P.; Shan, G.; Bao, Y. Stereocomplex crystallization of high-molecular-weight poly (l-lactic acid)/poly (d-lactic acid) racemic blends promoted by a selective nucleator. Polymer 2015, 63, 144–153. [Google Scholar] [CrossRef]
- Ren, Q.; Wu, M.; Weng, Z.; Zhu, X.; Li, W.; Huang, P.; Wang, L.; Zheng, W.; Ohshima, M. Promoted formation of stereocomplex in enantiomeric poly (lactic acid) s induced by cellulose nanofibers. Carbohydr. Polym. 2022, 276, 118800. [Google Scholar] [CrossRef] [PubMed]
- Chang, W.-W.; Niu, J.; Peng, H.; Rong, W. Preferential formation of stereocomplex crystals in poly (L-lactic acid)/poly (D-lactic acid) blends by a fullerene nucleator. Int. J. Biol. Macromol. 2023, 253, 127230. [Google Scholar] [CrossRef]
- Mohapatra, A.K.; Mohanty, S.; Nayak, S. Effect of PEG on PLA/PEG blend and its nanocomposites: A study of thermo-mechanical and morphological characterization. Polym. Compos. 2014, 35, 283–293. [Google Scholar] [CrossRef]
- Chalid, M.; Gustiraharjo, G.; Pangesty, A.I.; Adyandra, A.; Whulanza, Y.; Supriadi, S. Effect of PEG Incorporation on Physicochemical and in vitro Degradation of PLLA/PDLLA Blends: Application in Biodegradable Implants. J. Renew. Mater. 2023, 11, 3043–3056. [Google Scholar] [CrossRef]
- Xiao, H.; Yang, L.; Ren, X.; Jiang, T.; Yeh, J.T. Kinetics and crystal structure of poly (lactic acid) crystallized nonisothermally: Effect of plasticizer and nucleating agent. Polym. Compos. 2010, 31, 2057–2068. [Google Scholar] [CrossRef]
- Hoffman, J.D.; Weeks, J.J. Melting process and the equilibrium melting temperature of polychlorotrifluoroethylene. J. Res. Natl. Bur. Stand. Sect. A Phys. Chem. 1962, 66, 13. [Google Scholar] [CrossRef]
- Venkitaraj, K.; Suresh, S. Experimental study on the thermal storage performance and non-isothermal crystallization kinetics of pentaerythritol blended with low melting metal. Thermochim. Acta 2018, 662, 75–89. [Google Scholar]
- Soleimanpour, A.; Khonakdar, H.; Mousavi, S.R.; Hemmati, F.; Arjmand, M.; Arnhold, K.; Reuter, U.; Khonakdar, H.A. Dynamic crystallization kinetics and morphology of carbonaceous nanofillers-reinforced poly (lactic acid) foams. Thermochim. Acta 2022, 716, 179308. [Google Scholar] [CrossRef]
- Friedman, H.L. Kinetics of thermal degradation of char-forming plastics from thermogravimetry. J. Polym. Sci. Part C Polym. Symp. 1964, 6, 183–195. [Google Scholar] [CrossRef]
- Zhou, K.C.T.Z.Z.; Cao, K.S.Z. Study on Nonisothermal Crystallization Kinetics of Glass Fiber Reinforced PA66 Composites. China Plast. 2012, 26, 31. [Google Scholar]

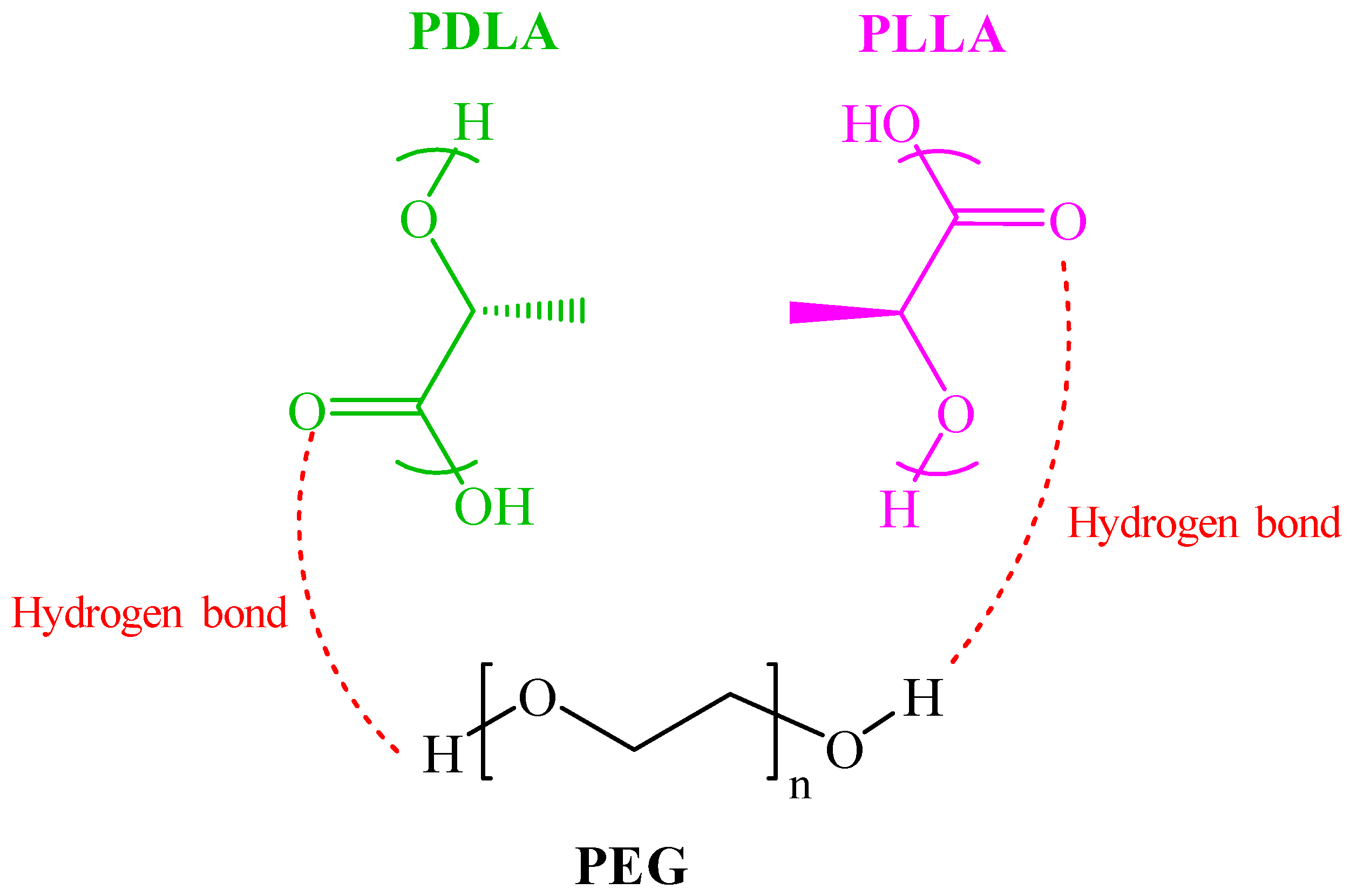




| PLLA | PDLA | |
|---|---|---|
| Code | L130 | D070 |
| Optical Purity | ≥99% (L-isomer) | ≥99% (D-isomer) |
| Weight-average molecular weight (Mw) (GPC) *1 | 1.68 × 105 | 0.87 × 105 |
| Mw/Mn | 2.02 | 1.73 |
| Sample Code | Average MW (Mn) (g/mol) of PEG | Concentration of PEGs (wt%) | Composition (wt%) | ||
|---|---|---|---|---|---|
| PLLA | PDLA | PEG | |||
| PLLA/PDLA | - | 0 | 50 | 50 | 0 |
| PLLA/PDLA-G4-5 | 400 | 5 | 47.5 | 47.5 | 5 |
| PLLA/PDLA-G4-10 | 400 | 10 | 45 | 45 | 10 |
| PLLA/PDLA-G4-20 | 400 | 20 | 40 | 40 | 20 |
| PLLA/PDLA-G350-5 | 35,000 | 5 | 47.5 | 47.5 | 5 |
| PLLA/PDLA-G350-10 | 35,000 | 10 | 45 | 45 | 10 |
| PLLA/PDLA-G350-20 | 35,000 | 20 | 40 | 40 | 20 |
| Samples | ∆Hc | Tc | Tonset − Tc | t1/2 |
|---|---|---|---|---|
| (J/g) | (°C) | (°C) | (min) | |
| PLLA/PDLA | −48.4 | 130.2 | 28.3 | 5.6 |
| PLLA/PDLA-G4-5 | −77.5 | 145.8 | 17.3 | 3.4 |
| PLLA/PDLA-G4-10 | −68.2 | 142.8 | 20.8 | 4.1 |
| PLLA/PDLA-G4-20 | −67.3 | 128.1 | 20.8 | 4.1 |
| PLLA/PDLA-G350-5 | −51.6 | 131.9 | 19.9 | 3.9 |
| PLLA/PDLA-G350-10 | −76.5 | 138.3 | 23.8 | 4.7 |
| PLLA/PDLA-G350-20 | −66.0 | 137.9 | 18.0 | 3.6 |
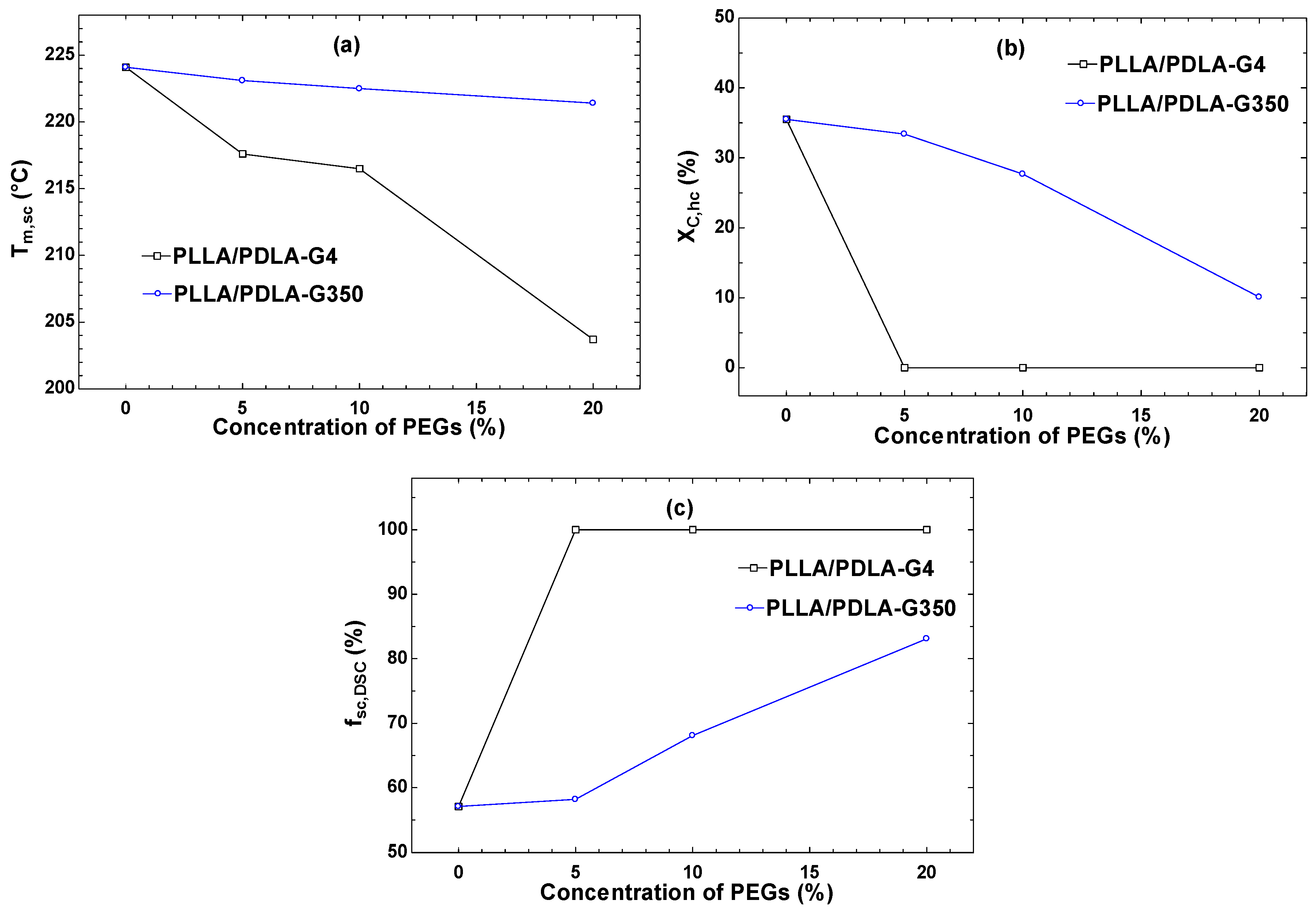
| Samples | Cold Crystallization | Homocrystal Melting (HC) | Stereocomplex Melting (SC) | Xc,hc | Xc,sc | fsc,DSC | |||
|---|---|---|---|---|---|---|---|---|---|
| ∆Hcc | Tcc | ∆Hm,hc | Tm,hc | ∆Hm,sc | Tm,sc | (%) | (%) | (%) | |
| (J/g) | (°C) | (J/g) | (°C) | (J/g) | (°C) | ||||
| PLLA/PDLA | −3.6 | 90.5 | 33.0 | 171.1 | 67.1 | 224.1 | 31.6 | 47.3 | 59.9 |
| PLLA/PDLA-G4-5 | - | - | - | - | 74.7 | 217.6 | 0.0 | 52.6 | 100.0 |
| PLLA/PDLA-G4-10 | - | - | - | - | 62.3 | 216.5 | 0.0 | 43.9 | 100.0 |
| PLLA/PDLA-G4-20 | - | - | - | - | 67.0 | 203.7 | 0.0 | 47.2 | 100.0 |
| PLLA/PDLA-G350-5 | - | - | 31.0 | 169.1 | 66.1 | 223.1 | 33.4 | 46.5 | 58.2 |
| PLLA/PDLA-G350-10 | - | - | 25.7 | 169.5 | 84.1 | 222.5 | 27.7 | 59.2 | 68.1 |
| PLLA/PDLA-G350-20 | - | - | 9.4 | 168.3 | 70.8 | 221.4 | 10.1 | 49.8 | 83.1 |
| Samples | Cooling Rate (°C/min) | ∆Hc | Tc | Tonset − Tc |
|---|---|---|---|---|
| (J/g) | (°C) | (°C) | ||
| PLLA/PDLA | 2 | −70.4 | 159.3 | 20.1 |
| 5 | −52.3 | 147.6 | 21.3 | |
| 10 | −48.4 | 130.2 | 28.3 | |
| 20 | −34.6 | 126.1 | 28.5 | |
| PLLA/PDLA-G4-10 | 2 | −60.9 | 151.8 | 11.9 |
| 5 | −72.3 | 151.2 | 17.5 | |
| 10 | −68.2 | 142.8 | 20.8 | |
| 20 | −70.3 | 134.8 | 18.2 | |
| PLLA/PDLA-G350-10 | 2 | −67.4 | 159.1 | 10.4 |
| 5 | −58.6 | 150.2 | 15.5 | |
| 10 | −76.5 | 138.3 | 23.8 | |
| 20 | −59.8 | 134.4 | 22.5 |
| Samples | Cooling Rate (°C/min) | Cold Crystallization | Homocrystal Melting (HC) | Stereocomplex Melting (SC) | |||
|---|---|---|---|---|---|---|---|
| ∆Hcc | Tcc | ∆Hm,hc | Tm,hc | ∆Hm,sc | Tm,sc | ||
| (J/g) | (°C) | (J/g) | (°C) | (J/g) | (°C) | ||
| PLLA/PDLA | 2 | - | - | 13.5 | 170.1 | 64.9 | 225.2 |
| 5 | - | - | 21.3 | 169.2 | 52.6 | 223.6 | |
| 10 | −3.6 | 90.5 | 33.0 | 171.1 | 67.1 | 224.1 | |
| 20 | −13.0 | 89.9 | 30.0 | 170.1 | 85.6 | 224.3 | |
| PLLA/PDLA-G4-10 | 2 | - | - | - | - | 59.6 | 211.8 |
| 5 | - | - | - | - | 81.6 | 214.3 | |
| 10 | - | - | - | - | 62.3 | 216.5 | |
| 20 | - | - | - | - | 68.5 | 215.3 | |
| PLLA/PDLA-G350-10 | 2 | - | - | 0.6 | 164.3 | 74.3 | 223.6 |
| 5 | - | - | 8.4 | 166.0 | 60.0 | 223.2 | |
| 10 | - | - | 25.7 | 169.5 | 84.1 | 222.5 | |
| 20 | - | - | 17.2 | 168.5 | 70.1 | 222.3 | |
| D (°C/min) | Main Crystallization Stage | ||
|---|---|---|---|
| n | Zt | ||
| PLLA/PDLA | 2 | 2.49 | 0.0055 |
| 5 | 3.39 | 0.0075 | |
| 10 | 3.44 | 0.0367 | |
| 20 | 2.66 | 0.4033 | |
| PLLA/PDLA-G4-10 | 2 | 2.79 | 0.0057 |
| 5 | 3.60 | 0.0139 | |
| 10 | 4.00 | 0.0999 | |
| 20 | 4.75 | 0.3235 | |
| PLLA/PDLA-G350-10 | 2 | 3.44 | 0.0044 |
| 5 | 3.97 | 0.0168 | |
| 10 | 3.54 | 0.0511 | |
| 20 | 6.13 | 0.2794 | |
| Sample | ∆HT (KJ·mol−1) |
|---|---|
| PLLA/PDLA | −10.34 |
| PLLA/PDLA-G4-10 | −17.6 |
| PLLA/PDLA-G350-10 | −15.23 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Phuangthong, P.; Li, W.; Shen, J.; Nofar, M.; Worajittiphon, P.; Srithep, Y. The Effect of Polyethylene Glycol on the Non-Isothermal Crystallization of Poly(L-lactide) and Poly(D-lactide) Blends. Polymers 2024, 16, 2129. https://doi.org/10.3390/polym16152129
Phuangthong P, Li W, Shen J, Nofar M, Worajittiphon P, Srithep Y. The Effect of Polyethylene Glycol on the Non-Isothermal Crystallization of Poly(L-lactide) and Poly(D-lactide) Blends. Polymers. 2024; 16(15):2129. https://doi.org/10.3390/polym16152129
Chicago/Turabian StylePhuangthong, Panthima, Wenwei Li, Jun Shen, Mohammadreza Nofar, Patnarin Worajittiphon, and Yottha Srithep. 2024. "The Effect of Polyethylene Glycol on the Non-Isothermal Crystallization of Poly(L-lactide) and Poly(D-lactide) Blends" Polymers 16, no. 15: 2129. https://doi.org/10.3390/polym16152129
APA StylePhuangthong, P., Li, W., Shen, J., Nofar, M., Worajittiphon, P., & Srithep, Y. (2024). The Effect of Polyethylene Glycol on the Non-Isothermal Crystallization of Poly(L-lactide) and Poly(D-lactide) Blends. Polymers, 16(15), 2129. https://doi.org/10.3390/polym16152129







