Bio-Based Materials as a Sustainable Solution for the Remediation of Contaminated Marine Sediments: An LCA Case Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Material Synthesis and Characterization
2.2. Life Cycle Assessment Methodology
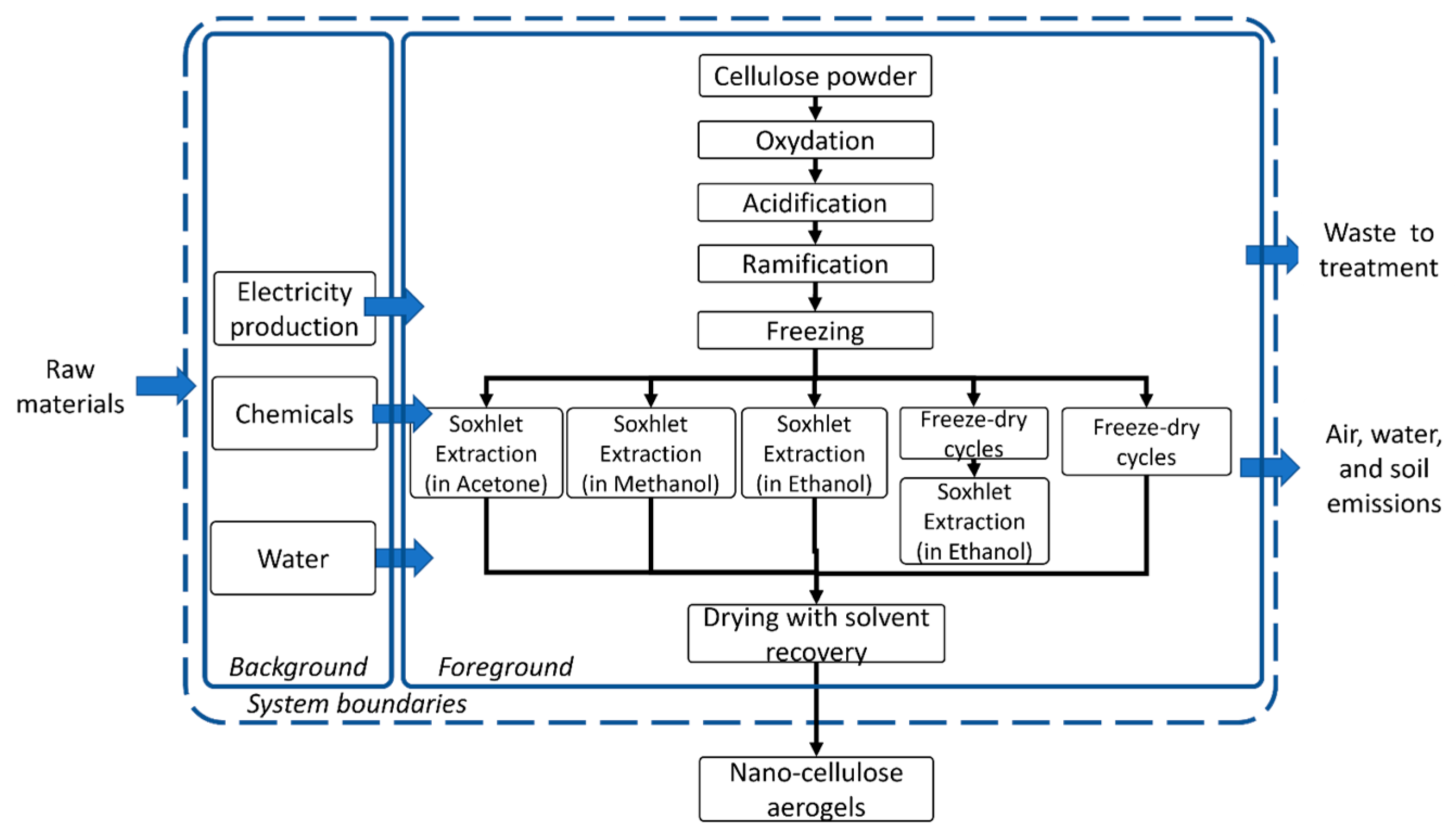
2.2.1. LCA Planning: System Limits, Functional Unit
2.2.2. Life Cycle Inventory
3. Results
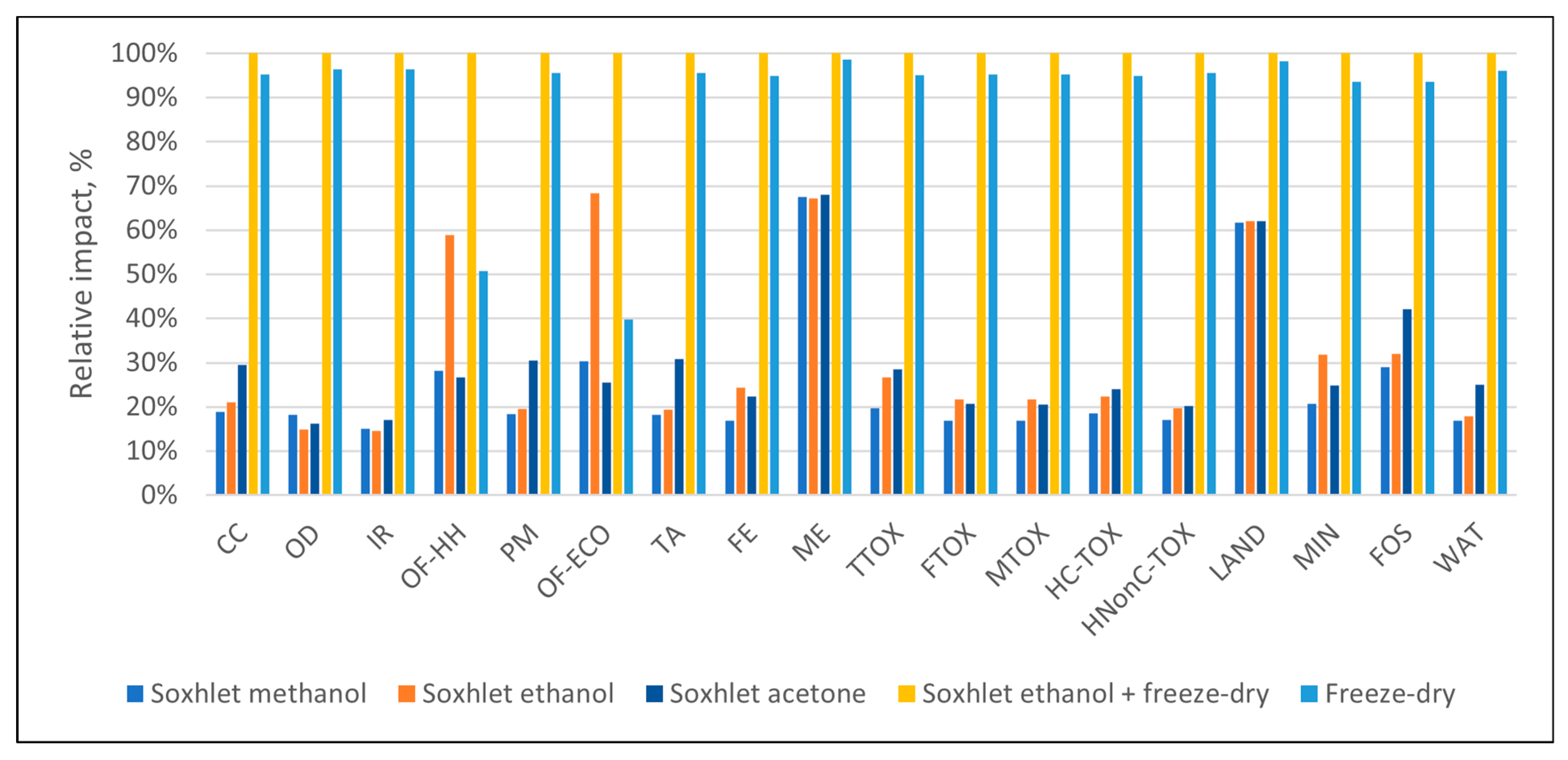
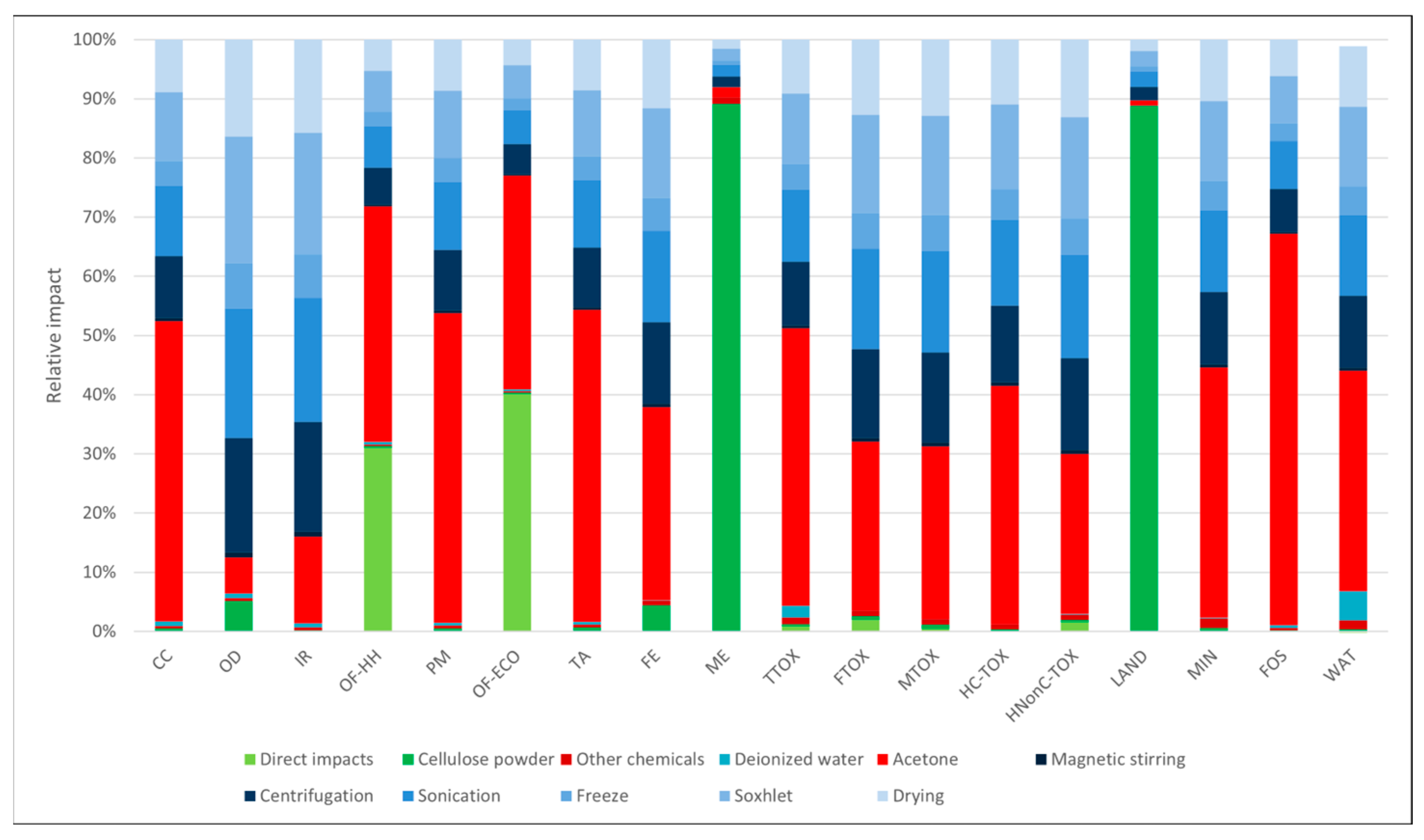
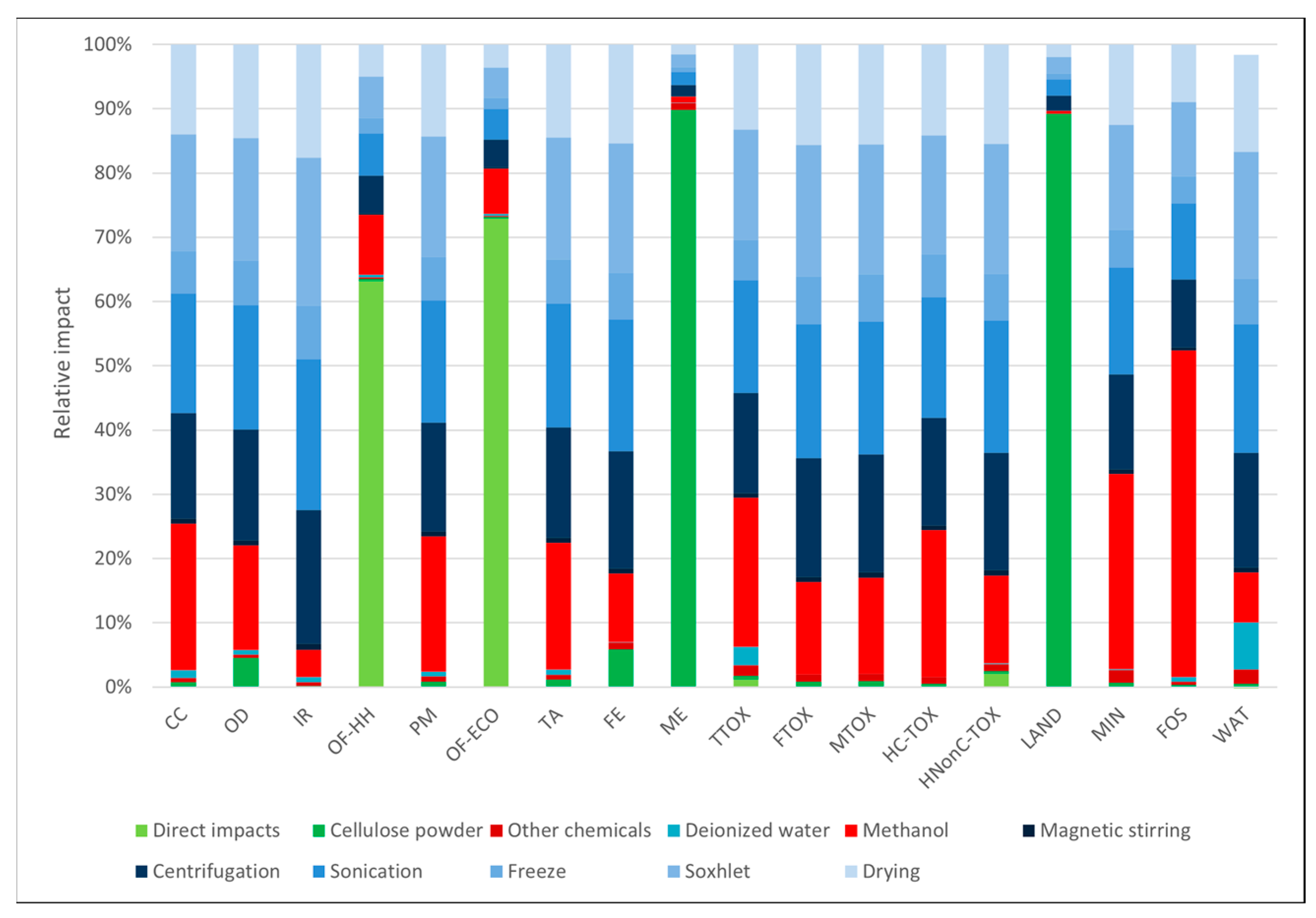
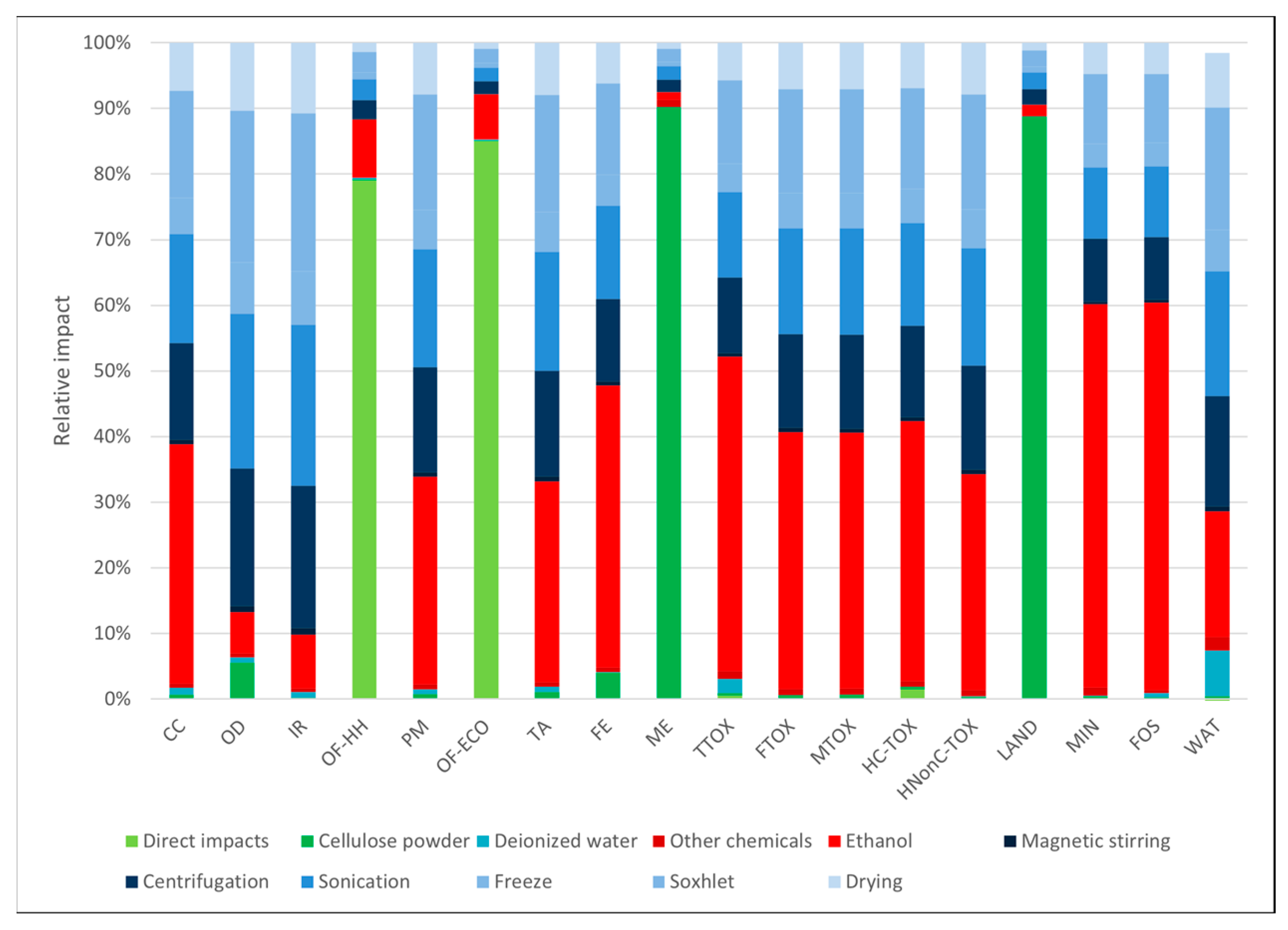
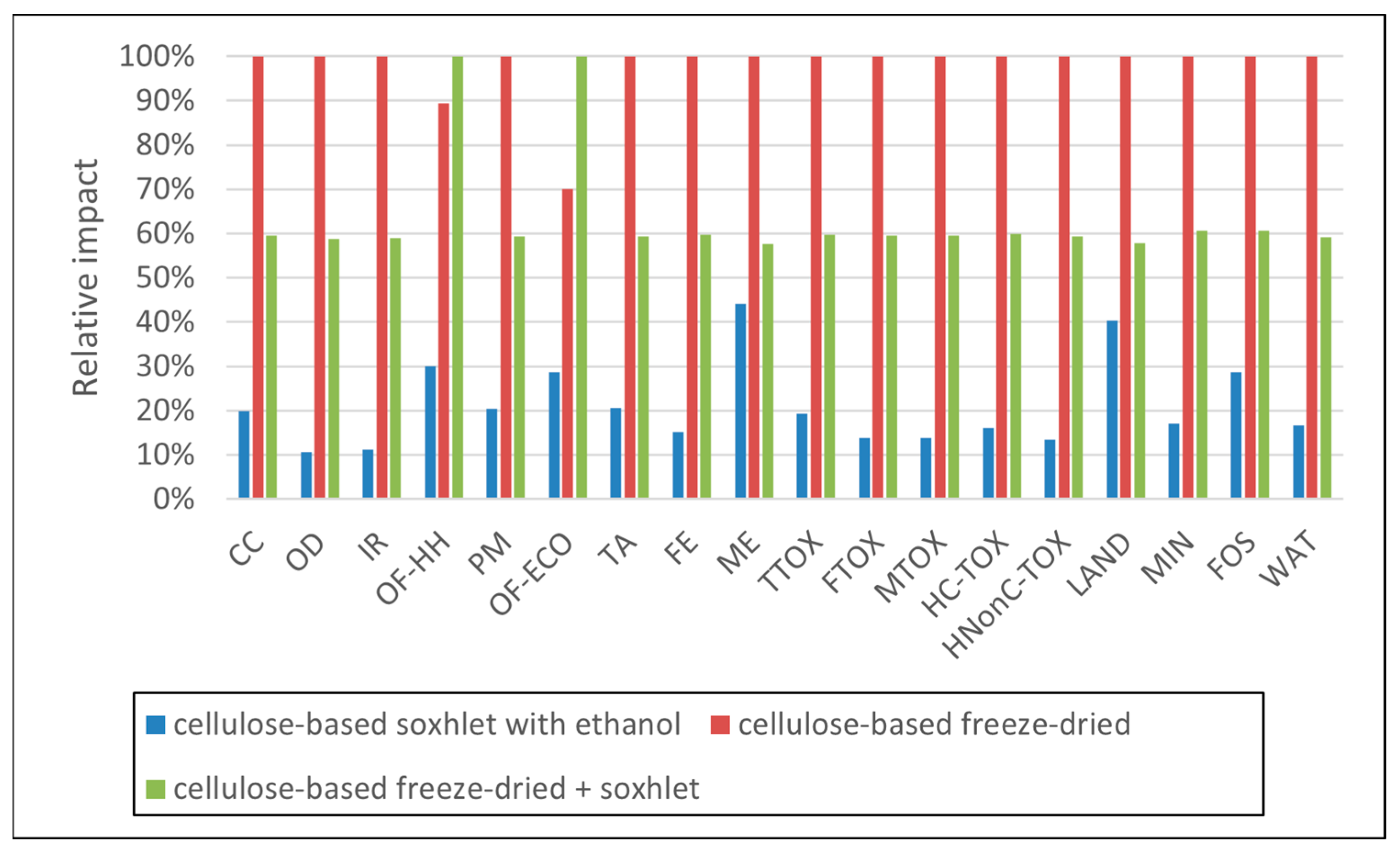
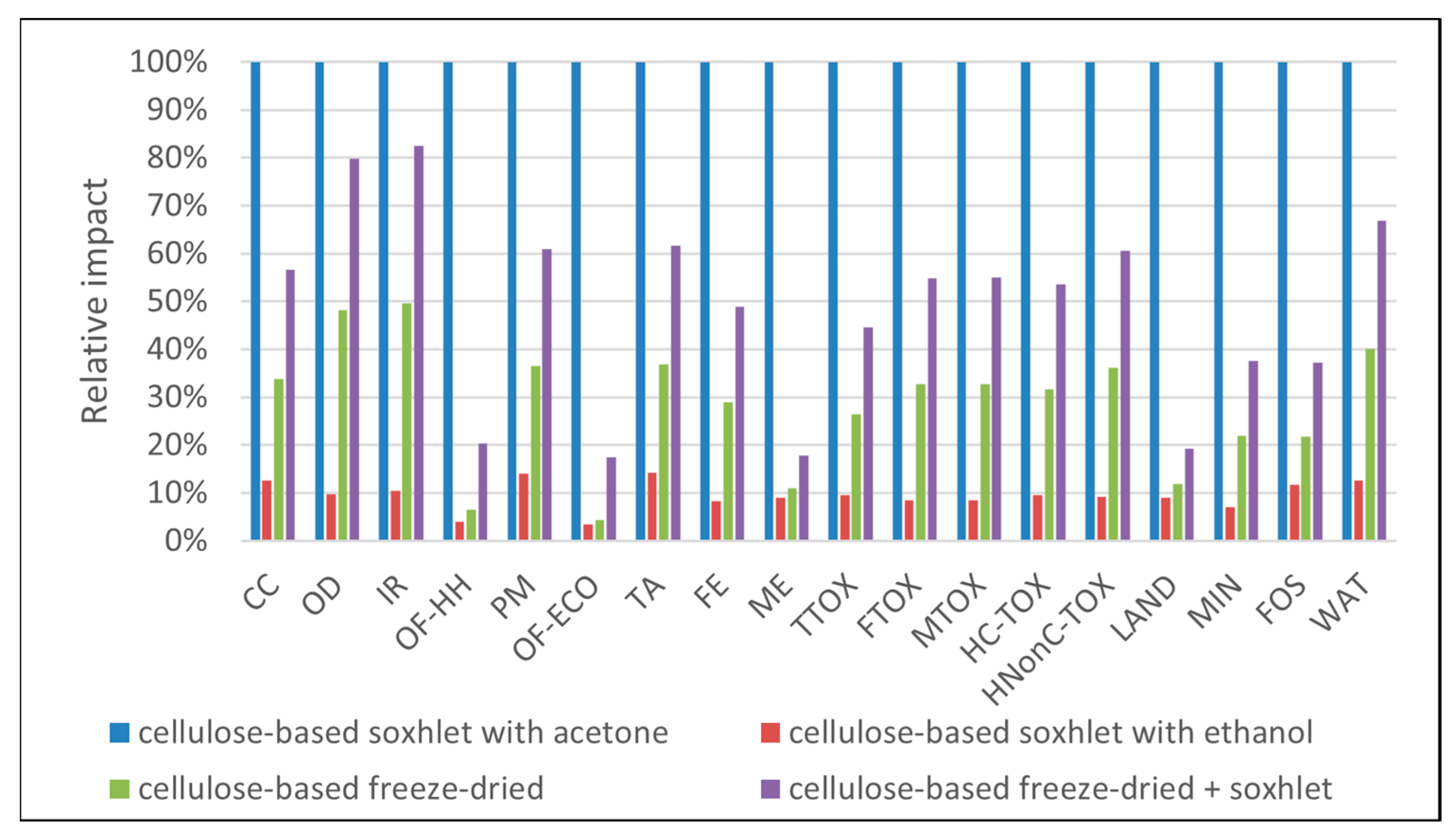
3.1. Environmental Profiles
3.2. Material Screening
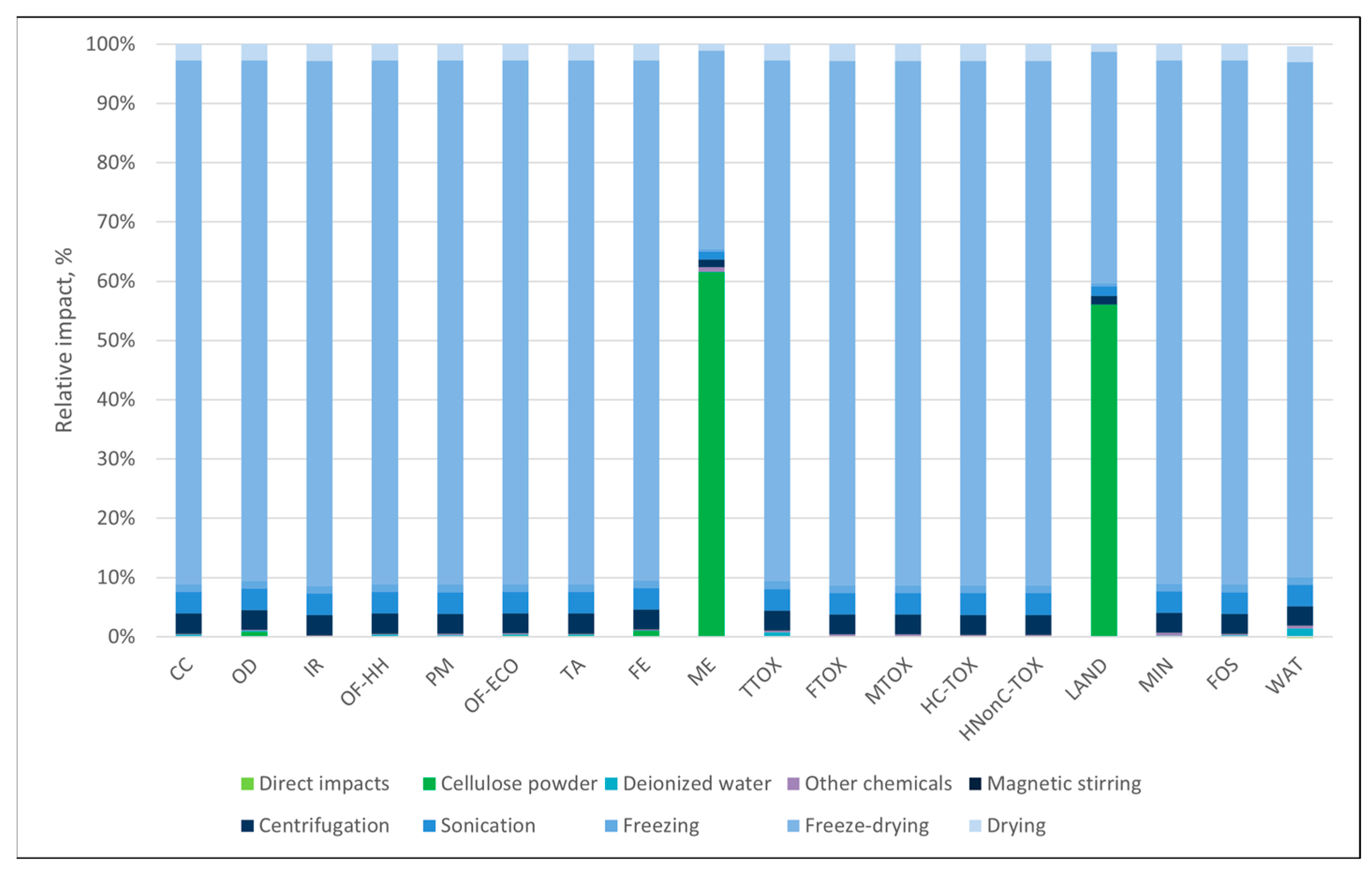
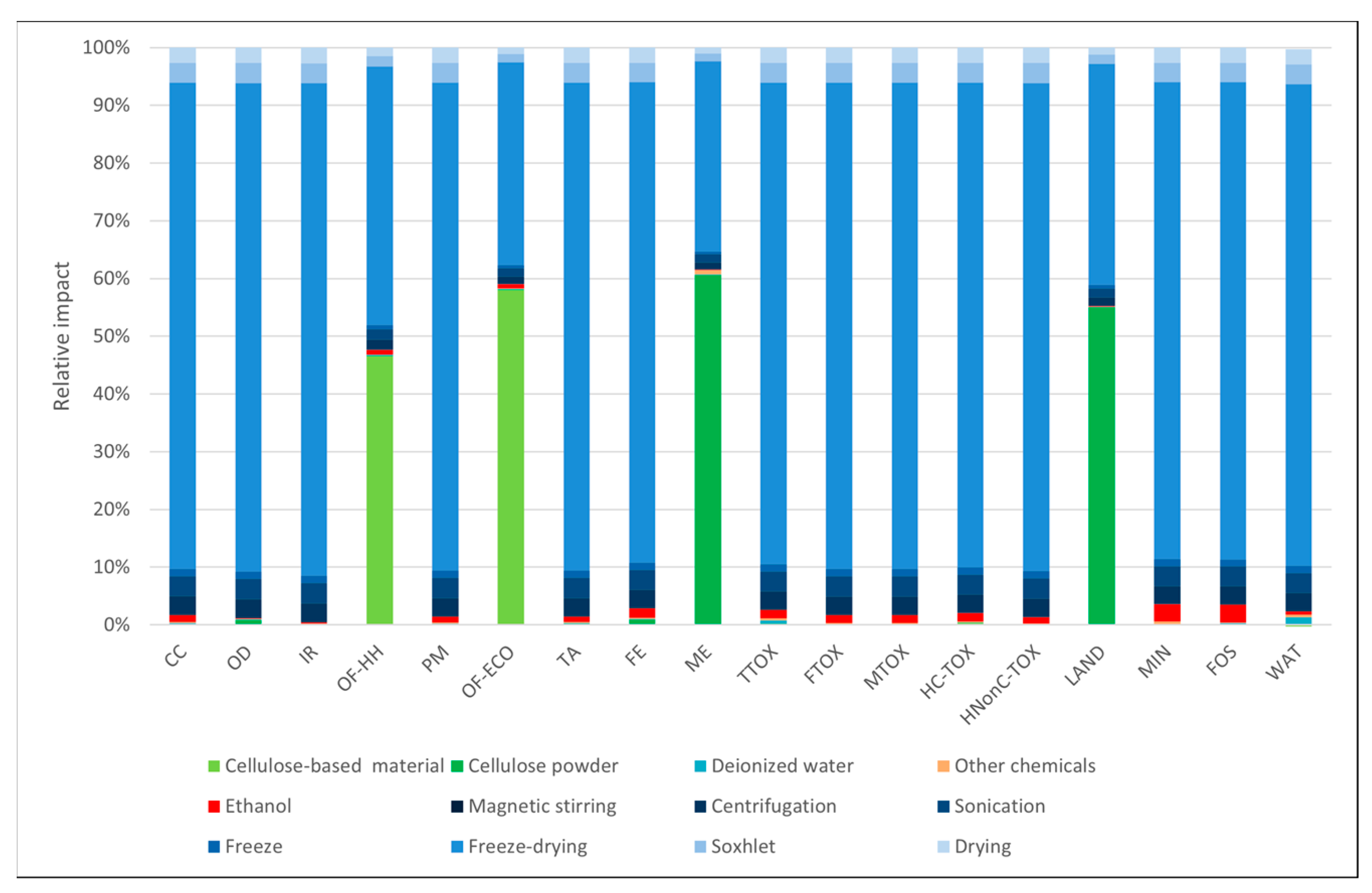
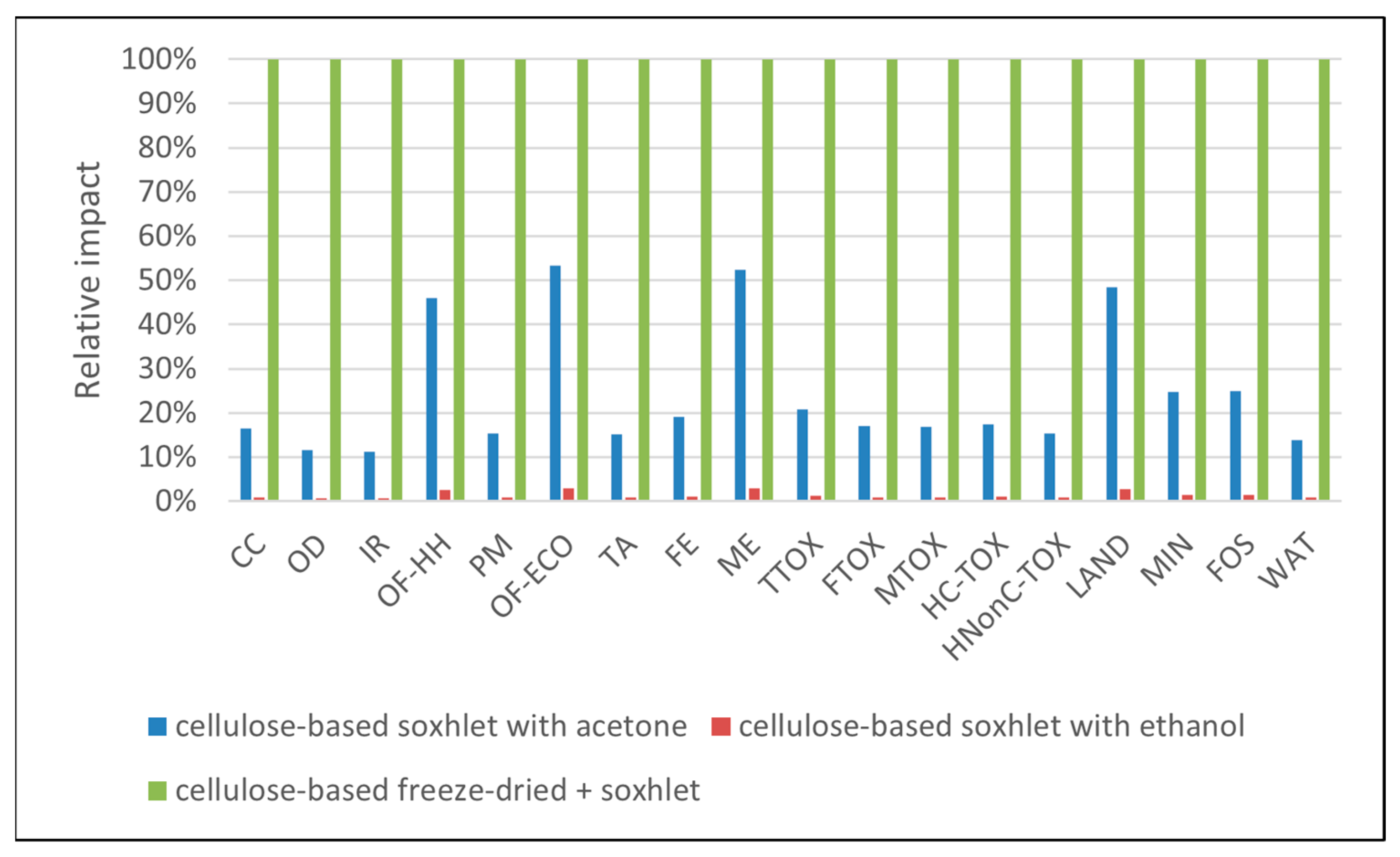
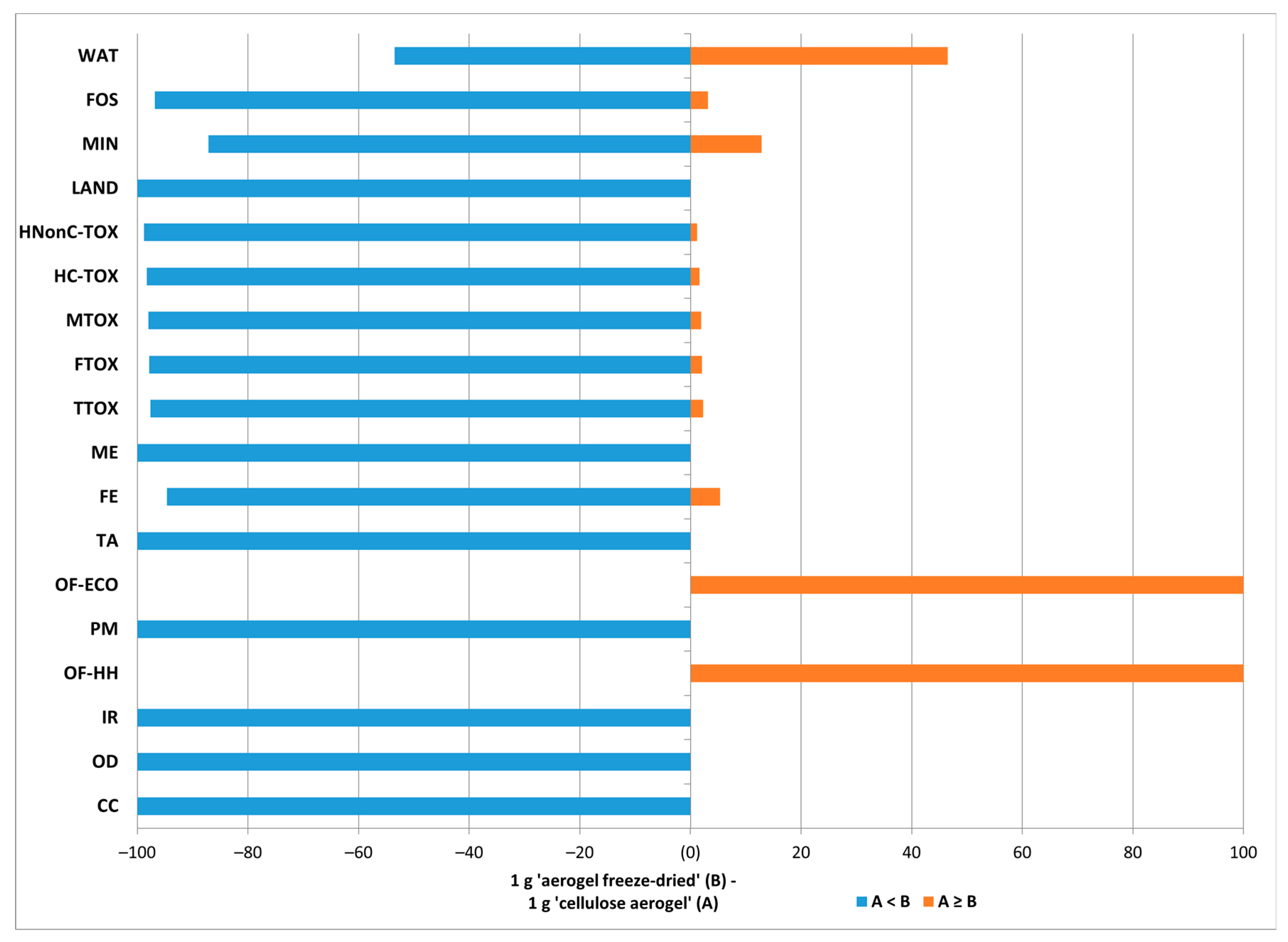
3.3. Sensitivity Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Albarano, L.; Costantini, M.; Zupo, V.; Lofrano, G.; Guida, M.; Libralato, G. Marine Sediment Toxicity: A Focus on Micro- and Mesocosms towards Remediation. Sci. Total Environ. 2020, 708, 134837. [Google Scholar] [CrossRef] [PubMed]
- Barjoveanu, G.; De Gisi, S.; Casale, R.; Todaro, F.; Notarnicola, M.; Teodosiu, C. A Life Cycle Assessment Study on the Stabilization/Solidification Treatment Processes for Contaminated Marine Sediments. J. Clean. Prod. 2018, 201, 391–402. [Google Scholar] [CrossRef]
- Bortone, I.; Labianca, C.; Todaro, F.; De Gisi, S.; Coulon, F.; Notarnicola, M. Experimental Investigations and Nu-merical Modelling of In-Situ Reactive Caps for PAH Contaminated Marine Sediments. J. Hazard. Mater. 2020, 387, 121724. [Google Scholar] [CrossRef] [PubMed]
- Labianca, C.; De Gisi, S.; Todaro, F.; Notarnicola, M.; Bortone, I. A Review of the In-Situ Capping Amendments and Modeling Approaches for the Remediation of Contaminated Marine Sediments. Sci. Total Environ. 2022, 806, 151257. [Google Scholar] [CrossRef] [PubMed]
- Peng, W.; Li, X.; Xiao, S.; Fan, W. Review of Remediation Technologies for Sediments Contaminated by Heavy Metals. J. Soils Sediments 2018, 18, 1701–1719. [Google Scholar] [CrossRef]
- Todaro, F.; Barjoveanu, G.; De Gisi, S.; Teodosiu, C.; Notarnicola, M. Sustainability Assessment of Reactive Capping Alternatives for the Remediation of Contaminated Marine Sediments. J. Clean. Prod. 2021, 286, 124946. [Google Scholar] [CrossRef]
- Lofrano, G.; Libralato, G.; Minetto, D.; De Gisi, S.; Todaro, F.; Conte, B.; Calabrò, D.; Quatraro, L.; Notarnicola, M. In Situ Remediation of Contaminated Marinesediment: An Overview. Environ. Sci. Pollut. Res. 2017, 24, 5189–5206. [Google Scholar] [CrossRef] [PubMed]
- Khan, F.I.; Husain, T.; Hejazi, R. An Overview and Analysis of Site Remediation Technologies. J. Environ. Manag. 2004, 71, 95–122. [Google Scholar] [CrossRef] [PubMed]
- Ting, Y.; Ch’ng, B.L.; Chen, C.; Ou, M.Y.; Cheng, Y.H.; Hsu, C.J.; Hsi, H.C. A Simulation Study of Mercury Immobilization in Estuary Sediment Microcosm by Activated Carbon/Clay-Based Thin-Layer Capping under Artificial Flow and Turbation. Sci. Total Environ. 2020, 708, 135068. [Google Scholar] [CrossRef]
- Bortone, I.; Di Natale, M.; Musmarra, D. Predicting the Effects of Capping Contaminated Sediments via Numerical Simulations. Desalination Water Treat. 2018, 133, 327–335. [Google Scholar] [CrossRef]
- Zhang, C.; Zhu, M.Y.; Zeng, G.M.; Yu, Z.G.; Cui, F.; Yang, Z.Z.; Shen, L.Q. Active Capping Technology: A New Environmental Remediation of Contaminated Sediment. Environ. Sci. Pollut. Res. 2016, 23, 4370–4386. [Google Scholar] [CrossRef] [PubMed]
- Hristozov, D.; Malsch, I. Hazards and Risks of Engineered Nanoparticles for the Environment and Human Health. Sustainability 2009, 1, 1161–1194. [Google Scholar] [CrossRef]
- Stone, V.; Nowack, B.; Baun, A.; van den Brink, N.; von der Kammer, F.; Dusinska, M.; Handy, R.; Hankin, S.; Hassellöv, M.; Joner, E.; et al. Nanomaterials for Environmental Studies: Classification, Reference Material Issues, and Strategies for Physico-Chemical Characterisation. Sci. Total Environ. 2010, 408, 1745–1754. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.W.; Zeng, G.M.; Gong, J.L.; Liang, J.; Xu, P.; Zhang, C.; Huang, B. Bin Impact of Humic/Fulvic Acid on the Removal of Heavy Metals from Aqueous Solutions Using Nanomaterials: A Review. Sci. Total Environ. 2014, 468–469, 1014–1027. [Google Scholar] [CrossRef] [PubMed]
- Guerra, F.D.; Attia, M.F.; Whitehead, D.C.; Alexis, F. Nanotechnology for Environmental Remediation: Materials and Applications. Molecules 2018, 23, 1760. [Google Scholar] [CrossRef] [PubMed]
- Crane, R.A.; Scott, T.B. Nanoscale Zero-Valent Iron: Future Prospects for an Emerging Water Treatment Technology. J. Hazard. Mater. 2012, 211–212, 112–125. [Google Scholar] [CrossRef] [PubMed]
- Silva, M.M.; Pérez, D.V.; Wasserman, J.C.; Santos-Oliveira, R.; Wasserman, M.A.V. The Effect of Nanohydroxyapatite on the Behavior of Metals in a Microcosm Simulating a Lentic Environment. Environ. Nanotechnol. Monit. Manag. 2017, 8, 219–227. [Google Scholar] [CrossRef]
- Abbas, A.; Al-Amer, A.M.; Laoui, T.; Al-Marri, M.J.; Nasser, M.S.; Khraisheh, M.; Atieh, M.A. Heavy Metal Removal from Aqueous Solution by Advanced Carbon Nanotubes: Critical Review of Adsorption Applications. Sep. Purif. Technol. 2016, 157, 141–161. [Google Scholar] [CrossRef]
- Zhang, S.; Shi, Q.; Christodoulatos, C.; Meng, X. Lead and Cadmium Adsorption by Electrospun PVA/PAA Nano-fibers: Batch, Spectroscopic, and Modeling Study. Chemosphere 2019, 233, 405–413. [Google Scholar] [CrossRef]
- Fiorati, A.; Grassi, G.; Graziano, A.; Liberatori, G.; Pastori, N.; Melone, L.; Bonciani, L.; Pontorno, L.; Punta, C.; Corsi, I. Eco-Design of Nanostructured Cellulose Sponges for Sea-Water Decontamination from Heavy Metal Ions. J. Clean. Prod. 2020, 246, 119009. [Google Scholar] [CrossRef]
- Qiao, L.; Li, S.; Li, Y.; Liu, Y.; Du, K. Fabrication of Superporous Cellulose Beads via Enhanced Inner Cross-Linked Linkages for High Efficient Adsorption of Heavy Metal Ions. J. Clean. Prod. 2020, 253, 120017. [Google Scholar] [CrossRef]
- Riva, L.; Fiorati, A.; Punta, C. Synthesis and Application of Cellulose-Polyethyleneimine Composites and Nano-composites: A Concise Review. Materials 2021, 14, 473. [Google Scholar] [CrossRef] [PubMed]
- Tarekegn, M.M.; Hiruy, A.M.; Dekebo, A.H. Nano Zero Valent Iron (NZVI) Particles for the Removal of Heavy Metals (Cd2+, Cu2+ and Pb2+) from Aqueous Solutions. RSC Adv. 2021, 11, 18539–18551. [Google Scholar] [CrossRef] [PubMed]
- Egbosiuba, T.C.; Abdulkareem, A.S.; Tijani, J.O.; Ani, J.I.; Krikstolaityte, V.; Srinivasan, M.; Veksha, A.; Lisak, G. Taguchi Optimization Design of Diameter-Controlled Synthesis of Multi Walled Carbon Nanotubes for the Adsorption of Pb(II) and Ni(II) from Chemical Industry Wastewater. Chemosphere 2021, 266, 128937. [Google Scholar] [CrossRef] [PubMed]
- Barjoveanu, G.; Pătrăuțanu, O.A.; Teodosiu, C.; Volf, I. Life Cycle Assessment of Polyphenols Extraction Processes from Waste Biomass. Sci. Rep. 2020, 10, 13632. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.; Xiang, Z.; Liu, Q.; Chen, Y.; Lu, F. Polyethyleneimine-Bacterial Cellulose Bioadsorbent for Effective Removal of Copper and Lead Ions from Aqueous Solution. Bioresour. Technol. 2017, 244, 844–849. [Google Scholar] [CrossRef]
- Morosanu, I.; Bucatariu, F.; Fighir, D.; Paduraru, C.; Mihai, M.; Teodosiu, C. Optimization of Lead and Diclofenac Removal from Aqueous Media Using a Composite Sorbent of Silica Core and Polyelectrolyte Coacervate Shell. Polymers 2023, 15, 1948. [Google Scholar] [CrossRef] [PubMed]
- Melone, L.; Rossi, B.; Pastori, N.; Panzeri, W.; Mele, A.; Punta, C. TEMPO-Oxidized Cellulose Cross-Linked with Branched Polyethyleneimine: Nanostructured Adsorbent Sponges for Water Remediation. Chempluschem 2015, 80, 1408–1415. [Google Scholar] [CrossRef]
- Fiorati, A.; Turco, G.; Travan, A.; Caneva, E.; Pastori, N.; Cametti, M.; Punta, C.; Melone, L. Mechanical and Drug Release Properties of Sponges from Cross-Linked Cellulose Nanofibers. Chempluschem 2017, 82, 848–858. [Google Scholar] [CrossRef]
- Liu, C.; Jin, R.N.; Ouyang, X.K.; Wang, Y.G. Adsorption Behavior of Carboxylated Cellulose Nanocrystal—Polyethyleneimine Composite for Removal of Cr(VI) Ions. Appl. Surf. Sci. 2017, 408, 77–87. [Google Scholar] [CrossRef]
- Riva, L.; Pastori, N.; Panozzo, A.; Antonelli, M.; Punta, C. Nanostructured Cellulose-Based Sorbent Materials for Water Decontamination from Organic Dyes. Nanomaterials 2020, 10, 1570. [Google Scholar] [CrossRef] [PubMed]
- Sparrevik, M.; Saloranta, T.; Cornelissen, G.; Eek, E.; Fet, A.M.; Breedveld, G.D.; Linkov, I. Use of Life Cycle Assessments to Evaluate the Environmental Footprint of Contaminated Sediment Remediation. Environ. Sci. Technol. 2011, 45, 4235–4241. [Google Scholar] [CrossRef] [PubMed]
- Visentin, C.; da Silva Trentin, A.W.; Braun, A.B.; Thomé, A. Life Cycle Sustainability Assessment of the Nanoscale Zero-Valent Iron Synthesis Process for Application in Contaminated Site Remediation. Environ. Pollut. 2021, 268, 115915. [Google Scholar] [CrossRef] [PubMed]
- Ingrao, C.; Vesce, E.; Evola, R.S.; Rebba, E.; Arcidiacono, C.; Martra, G.; Beltramo, R. Chemistry behind Leather: Life Cycle Assessment of Nano-Hydroxyapatite Preparation on the Lab-Scale for Fireproofing Applications. J. Clean. Prod. 2021, 279, 123837. [Google Scholar] [CrossRef]
- Bartolozzi, I.; Daddi, T.; Punta, C.; Fiorati, A.; Iraldo, F. Life Cycle Assessment of Emerging Environmental Tech-nologies in the Early Stage of Development: A Case Study on Nanostructured Materials. J. Ind. Ecol. 2020, 24, 101–115. [Google Scholar] [CrossRef]
- Gavankar, S.; Suh, S.; Keller, A.A. The Role of Scale and Technology Maturity in Life Cycle Assessment of Emerging Technologies: A Case Study on Carbon Nanotubes. J. Ind. Ecol. 2015, 19, 51–60. [Google Scholar] [CrossRef]
- Hop, T.T.T.; Mai, D.T.; Cong, T.D.; Nhi, T.T.Y.; Loi, V.D.; Huong, N.T.M.; Tung, N.T. A Comprehensive Study on Preparation of Nanocellulose from Bleached Wood Pulps by TEMPO-Mediated Oxidation. Results Chem. 2022, 4, 100540. [Google Scholar] [CrossRef]
- Velardi, S.A.; Barresi, A.A. Development of Simplified Models for the Freeze-Drying Process and Investigation of the Optimal Operating Conditions. Chem. Eng. Res. Des. 2008, 86, 9–22. [Google Scholar] [CrossRef]
- Shukla, S. Freeze Drying Process: A Review. Int. J. Pharm. Sci. Res. 2011, 2, 3061–3068. [Google Scholar]
- López-Bascón-Bascon, M.A.; Luque de Castro, M.D. Soxhlet Extraction. In Handbooks in Separation Science; Liquid-Phase Extraction; Elsevier: Amsterdam, The Netherlands, 2020; pp. 327–354. [Google Scholar] [CrossRef]
- Patrice Didion, Y.; Gijsbert Tjalsma, T.; Su, Z.; Malankowska, M.; Pinelo, M. What Is next? The Greener Future of Solid Liquid Extraction of Biobased Compounds: Novel Techniques and Solvents Overpower Traditional Ones. Sep. Purif. Technol. 2023, 320, 124147. [Google Scholar] [CrossRef]
- Tornero, V.; Hanke, G. Potential Chemical Contaminants in the Marine Environment an Overview of Main Contaminant Lists; European Commission: Brussels, Belgium, 2018. [Google Scholar] [CrossRef]
- Reichelt-Brushett, A. (Ed.) Marine Pollution-Monitoring, Management and Mitigation; Springer: Berlin/Heidelberg, Germany, 2023. [Google Scholar]
- ISO ISO 14040; Environmental Management—Life Cycle Assessment—Part 1: Principles and Framework. International Organization for Standardization: Geneva, Switzerland, 2006; p. 3.
- Huijbregts, M.A.J.; Steinmann, Z.J.N.; Elshout, P.M.F.; Stam, G.; Verones, F.; Vieira, M.; Zijp, M.; Hollander, A.; van Zelm, R. ReCiPe2016: A Harmonised Life Cycle Impact Assessment Method at Midpoint and Endpoint Level. Int. J. Life Cycle Assess. 2017, 22, 138–147. [Google Scholar] [CrossRef]
- Husgafvel, R.; Vanhatalo, K.; Rodriguez-Chiang, L.; Linkosalmi, L.; Dahl, O. Comparative Global Warming Potential Assessment of Eight Microcrystalline Cellulose Manufacturing Systems. J. Clean. Prod. 2016, 126, 620–629. [Google Scholar] [CrossRef]
- Lavoine, N.; Bergström, L. Nanocellulose-Based Foams and Aerogels: Processing, Properties, and Applications. J. Mater. Chem. A Mater. 2017, 5, 16105–16117. [Google Scholar] [CrossRef]

| Material Type | Maximum Sorption Capacity (mg/g) | ||||
|---|---|---|---|---|---|
| NBA | NP | MB | Cd2+ | Cr2+ | |
| Cellulose-based freeze-dried (NC-FD) | X | 0.9 | 20 | X | X |
| Cellulose-based freeze-dried, washed with Soxhlet extractor (NC-FD-SOX) | 1.1 | 1.6 | 12 | X | X |
| Cellulose-based, Soxhlet extraction with acetone (NC-SOX-ACE) | 1.4 | X | 16 | X | X |
| Cellulose-based, Soxhlet extraction with ethanol (NC-SOX-ETH) | 1.4 | 1.5 | 25 | 38.9 | 42.3 |
| Inventory Entry | Eco-Invent Process | Unit | Quantity for Cellulose-Based Material | Data Source/Comments |
|---|---|---|---|---|
| Inputs | ||||
| Cellulose microcrystalline powder | Cellulose powder | g | 1.33 | Modeled according to [46] |
| Potassium Bromide (KBr) | Bromine {GLO}|market for|APOS, U | g | 0.20 | |
| 2,2,6,6-Tetramethylpiperidinyloxyl (TEMPO) | Dimethylamine (production from alcohols), at producer, 100% active substance/EU-27 | g | 0.03 | Modeled as dimethylamine |
| Deionized water | water, deionized {Europe without Switzerland}|market for water, deionized|APOS, U | g | 368.85 | |
| NaClO 10% | Sodium hypochlorite/RER | g | 15 | |
| NaOH 0.5 M | Neutralizing agent, sodium hydroxide-equivalent {GLO}| market for|APOS, U | g | 1.94 | |
| HCl 37% v/v | Hydrochloric acid, without water, in 30% solution {RER}|market for|APOS, U | g | 0.59 | |
| bPEI Mw = 25,000 | Ethylamine {RER}|production|APOS, U | g | 1.36 | |
| Ethanol | Ethanol, without water, in 99.7% solution, from ethylene {RER}| market for ethanol, without water, in 99.7% solution, from ethylene|APOS, U | g | 394.5 | |
| Magnetic stirring | Electricity, low voltage {IT}|electricity voltage transformation from medium to low voltage|APOS, U | KWh | 0.02 | |
| Centrifugation | KWh | 0.425 | ||
| Sonication | KWh | 0.48 | ||
| Freeze | KWh | 0.17 | ||
| Soxhlet | KWh | 0.468 | ||
| Drying process | KWh | 0.21 | ||
| Outputs | ||||
| Ethanol | Ethanol, in air | g | 40 | |
| Chemically polluted water | Chemically polluted water | g | 352.46 | |
| Alcohols | Alcohols, unspecified | g | 315 | |
| Amine | Amine, tertiary | g | 0.33 | |
| Impact Category | Unit | Abbrev. | Soxhlet Methanol | Soxhlet Ethanol | Soxhlet Acetone | Soxhlet Ethanol + Freeze-Dry | Freeze-Dry |
|---|---|---|---|---|---|---|---|
| Global warming | kg CO2 eq | CC | 1.20 × 10+0 | 1.35 × 10+0 | 1.89 × 10+0 | 6.39 × 10+0 | 6.09 × 10+0 |
| Stratospheric ozone depletion | kg CFC11 eq | OD | 8.92 × 10−7 | 7.35 × 10−7 | 7.94 × 10−7 | 4.91 × 10−6 | 4.74 × 10−6 |
| Ionizing radiation | kBq Co-60 eq | IR | 1.05 × 10−1 | 1.01 × 10−1 | 1.18 × 10−1 | 6.94 × 10−1 | 6.69 × 10−1 |
| Ozone formation, human health | kg NOx eq | OF-HH | 5.96 × 10−3 | 1.24 × 10−2 | 5.62 × 10−3 | 2.11 × 10−2 | 1.07 × 10−2 |
| Fine particulate matter formation | kg PM2.5 eq | PM | 1.22 × 10−3 | 1.29 × 10−3 | 2.02 × 10−3 | 6.61 × 10−3 | 6.32 × 10−3 |
| Ozone formation, terrestrial ecosystems | kg NOx eq | OF-ECO | 8.34 × 10−3 | 1.87 × 10−2 | 7.00 × 10−3 | 2.74 × 10−2 | 1.09 × 10−2 |
| Terrestrial acidification | kg SO2 eq | TA | 3.57 × 10−3 | 3.80 × 10−3 | 6.06 × 10−3 | 1.96 × 10−2 | 1.88 × 10−2 |
| Freshwater eutrophication | kg P eq | FE | 3.08 × 10−4 | 4.46 × 10−4 | 4.09 × 10−4 | 1.83 × 10−3 | 1.73 × 10−3 |
| Marine eutrophication | kg N eq | ME | 2.90 × 10−4 | 2.89 × 10−4 | 2.92 × 10−4 | 4.30 × 10−4 | 4.23 × 10−4 |
| Terrestrial ecotoxicity | kg 1,4-DCB | TTOX | 8.49 × 10−1 | 1.15 × 10+0 | 1.23 × 10+0 | 4.30 × 10+0 | 4.08 × 10+0 |
| Freshwater ecotoxicity | kg 1,4-DCB | FTOX | 2.85 × 10−2 | 3.68 × 10−2 | 3.50 × 10−2 | 1.69 × 10−1 | 1.61 × 10−1 |
| Marine ecotoxicity | kg 1,4-DCB | MTOX | 3.74 × 10−2 | 4.79 × 10−2 | 4.52 × 10−2 | 2.21 × 10−1 | 2.10 × 10−1 |
| Human carcinogenic toxicity | kg 1,4-DCB | HC-TOX | 4.57 × 10−2 | 5.49 × 10−2 | 5.90 × 10−2 | 2.46 × 10−1 | 2.33 × 10−1 |
| Human non-carcinogenic toxicity | kg 1,4-DCB | HNonC-TOX | 6.01 × 10−1 | 6.93 × 10−1 | 7.10 × 10−1 | 3.52 × 10+0 | 3.36 × 10+0 |
| Land use | m2a crop eq | LAND | 1.91 × 10+0 | 1.92 × 10+0 | 1.92 × 10+0 | 3.09 × 10+0 | 3.04 × 10+0 |
| Mineral resource scarcity | kg Cu eq | MIN | 1.22 × 10−3 | 1.87 × 10−3 | 1.46 × 10−3 | 5.88 × 10−3 | 5.51 × 10−3 |
| Fossil resource scarcity | kg oil eq | FOS | 5.87 × 10−1 | 6.47 × 10−1 | 8.50 × 10−1 | 2.02 × 10+0 | 1.89 × 10+0 |
| Water consumption | m3 | WAT | 2.08 × 10−2 | 2.21 × 10−2 | 3.09 × 10−2 | 1.24 × 10−1 | 1.19 × 10−1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Clemente, M.E.; Barjoveanu, G.; Todaro, F.; Notarnicola, M.; Teodosiu, C. Bio-Based Materials as a Sustainable Solution for the Remediation of Contaminated Marine Sediments: An LCA Case Study. Polymers 2024, 16, 2101. https://doi.org/10.3390/polym16152101
Di Clemente ME, Barjoveanu G, Todaro F, Notarnicola M, Teodosiu C. Bio-Based Materials as a Sustainable Solution for the Remediation of Contaminated Marine Sediments: An LCA Case Study. Polymers. 2024; 16(15):2101. https://doi.org/10.3390/polym16152101
Chicago/Turabian StyleDi Clemente, Milvia Elena, George Barjoveanu, Francesco Todaro, Michele Notarnicola, and Carmen Teodosiu. 2024. "Bio-Based Materials as a Sustainable Solution for the Remediation of Contaminated Marine Sediments: An LCA Case Study" Polymers 16, no. 15: 2101. https://doi.org/10.3390/polym16152101
APA StyleDi Clemente, M. E., Barjoveanu, G., Todaro, F., Notarnicola, M., & Teodosiu, C. (2024). Bio-Based Materials as a Sustainable Solution for the Remediation of Contaminated Marine Sediments: An LCA Case Study. Polymers, 16(15), 2101. https://doi.org/10.3390/polym16152101











