Poly(ester imide)s with Low Linear Coefficients of Thermal Expansion and Low Water Uptake (VIII): Structure–Flame Retardancy Relationship
Abstract
1. Introduction
2. Experimental Section
2.1. Materials
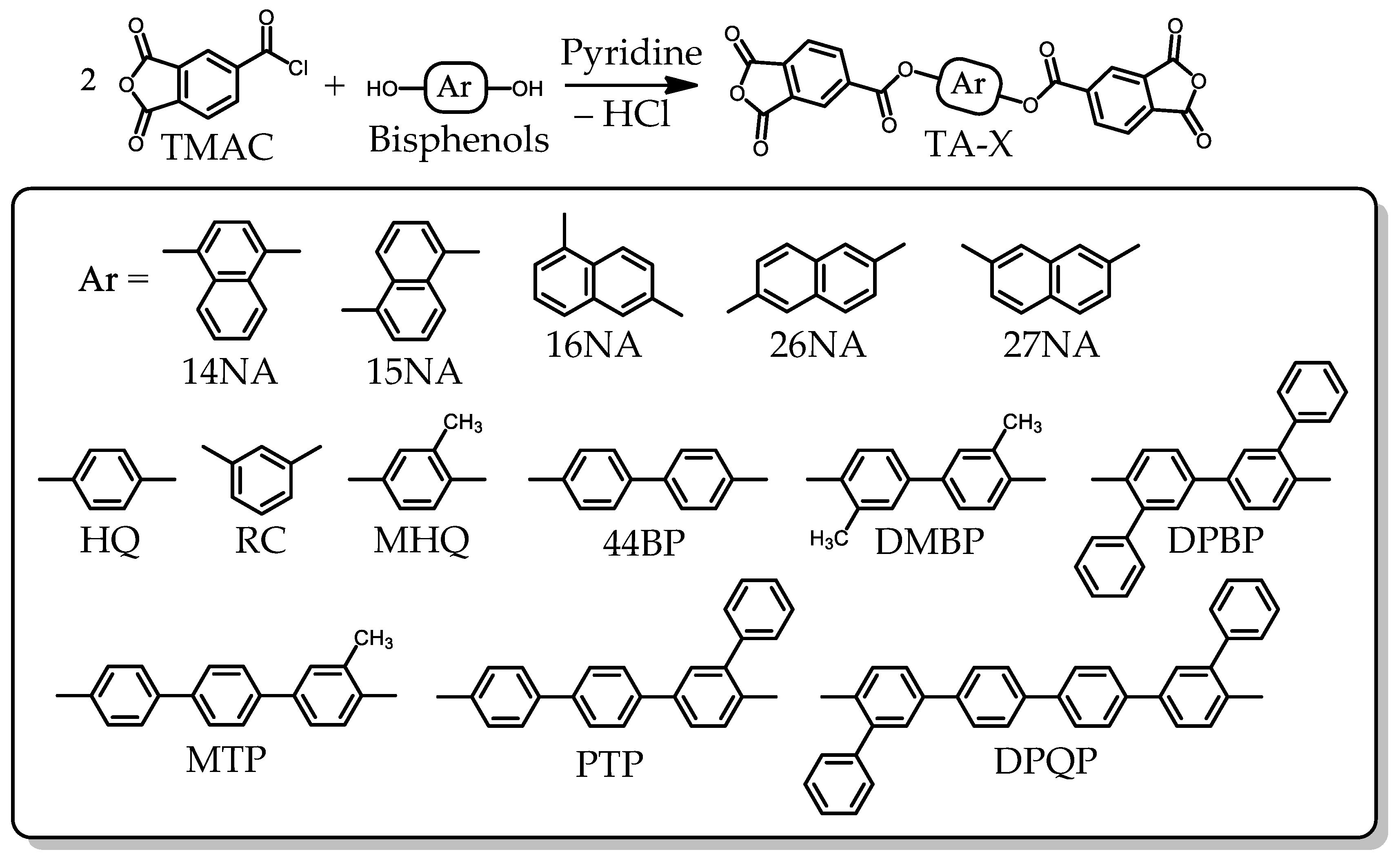
2.1.1. Monomer Synthesis
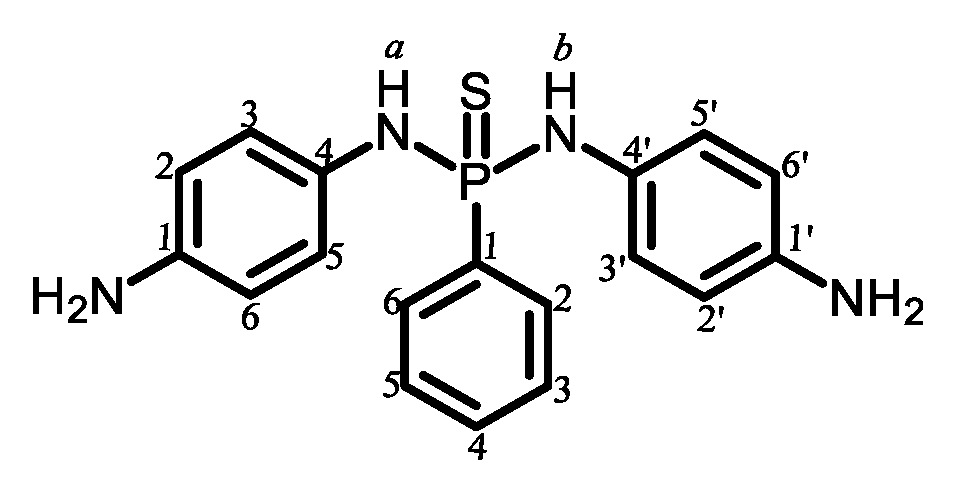
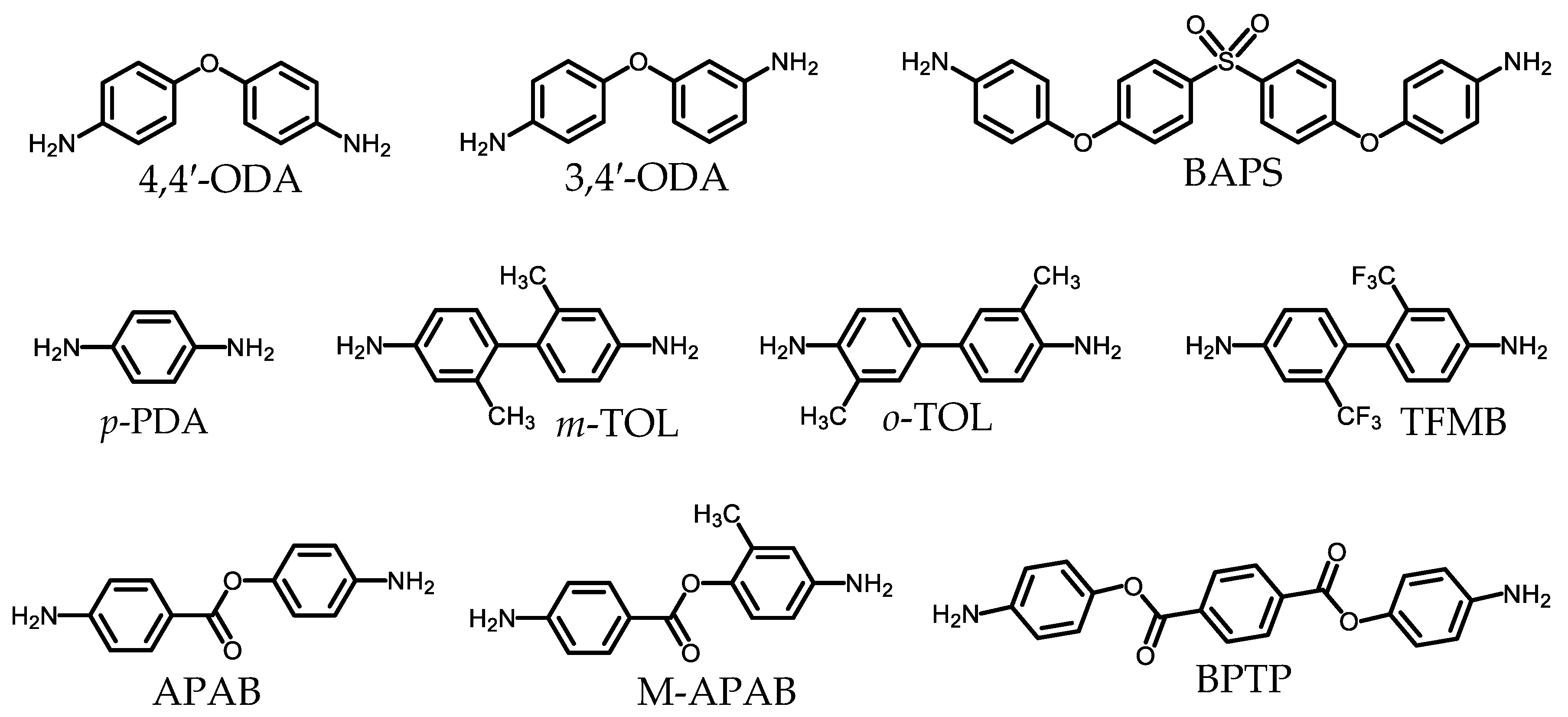
2.1.2. Common Monomers
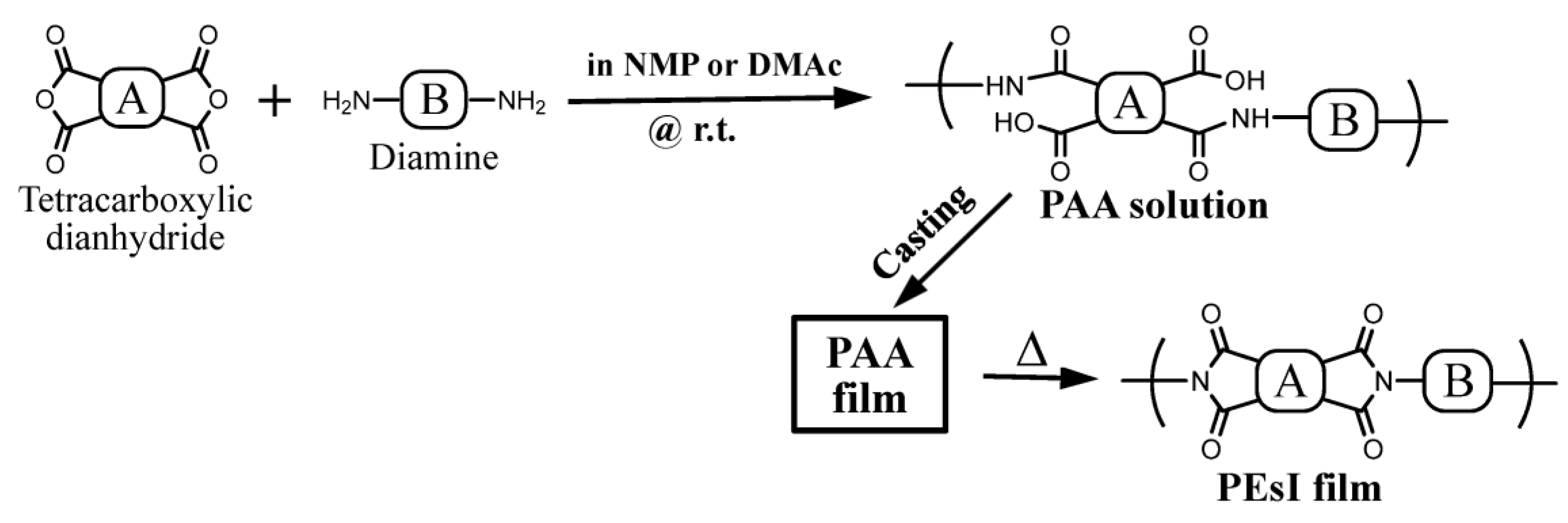
2.1.3. Polyaddition and Thermal Imidization for PEsI Film Preparation
2.2. Measurements and Characterization
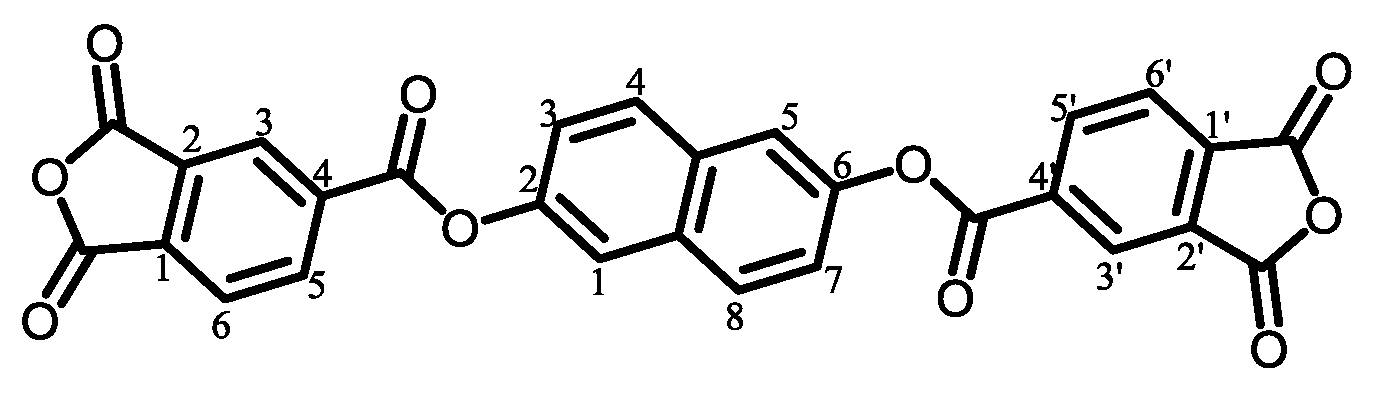
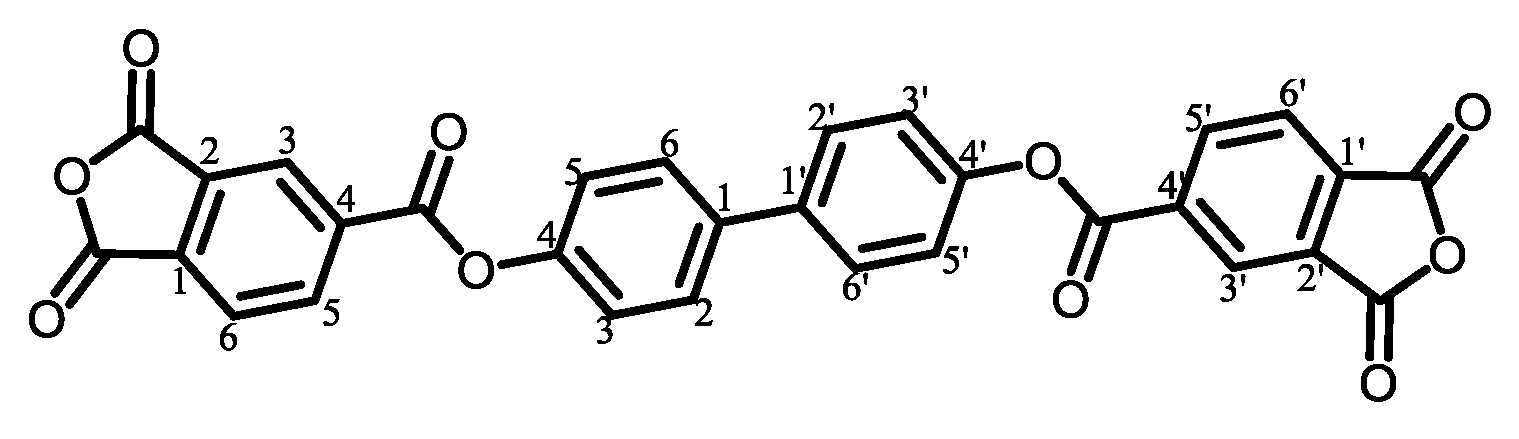
2.2.1. Structural Characterization of Ester-Linked TCDAs
2.2.2. Reduced Viscosities
2.2.3. Linear Coefficients of Thermal Expansion
2.2.4. Linear Coefficients of Hygroscopic (Humidity) Expansion
2.2.5. Glass Transition Temperatures
2.2.6. Thermal Decomposition Temperatures
2.2.7. Mechanical Properties
2.2.8. Water Uptake
2.2.9. Contents of Imide, Methyl, Ester, and Phenylene Ether Groups in the Main Chains
2.2.10. Thickness-Direction Birefringence
2.2.11. Flame Retardancy
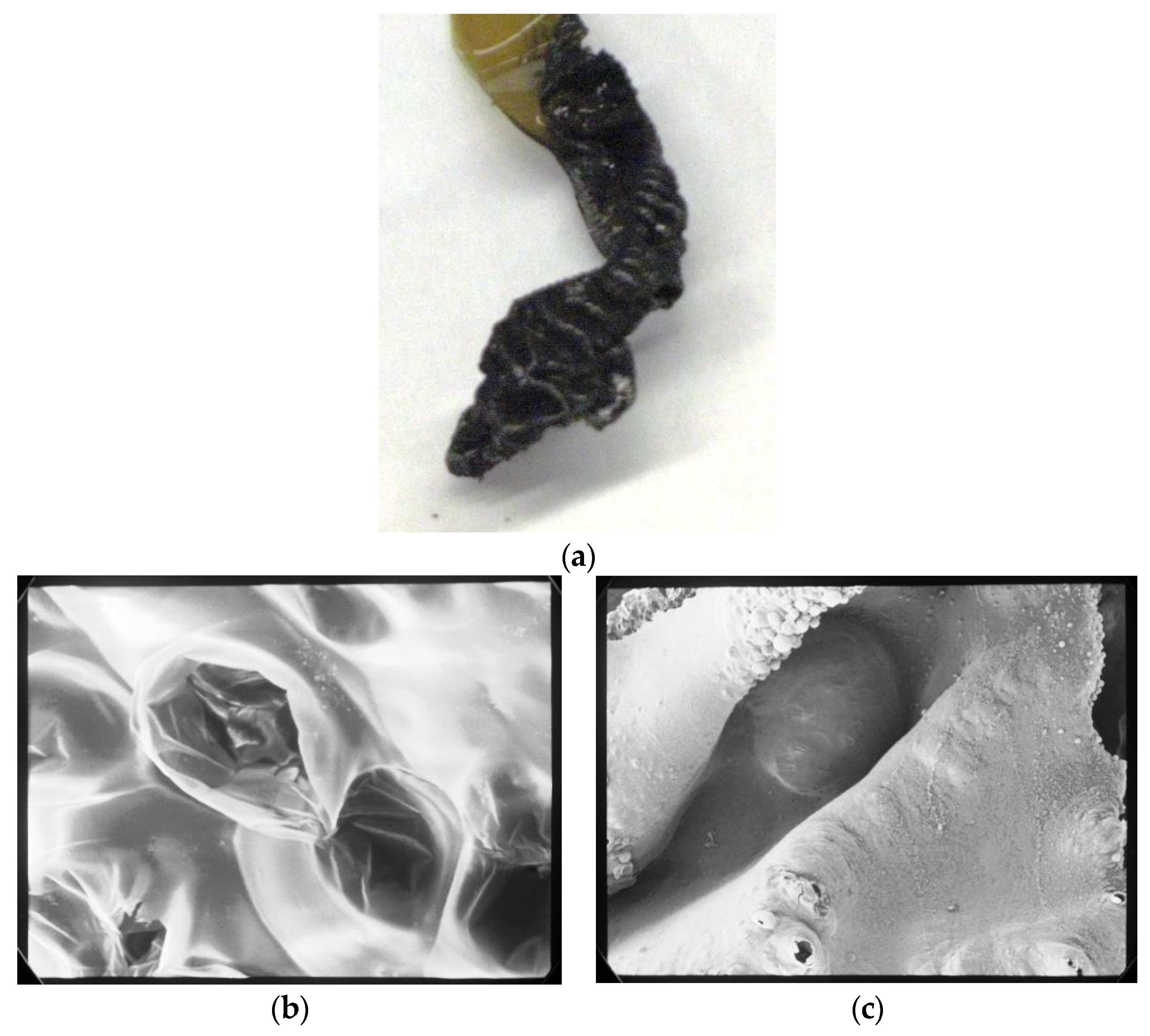
2.2.12. Morphological Observation of Carbonized Portion after Burning Test
3. Results and Discussion
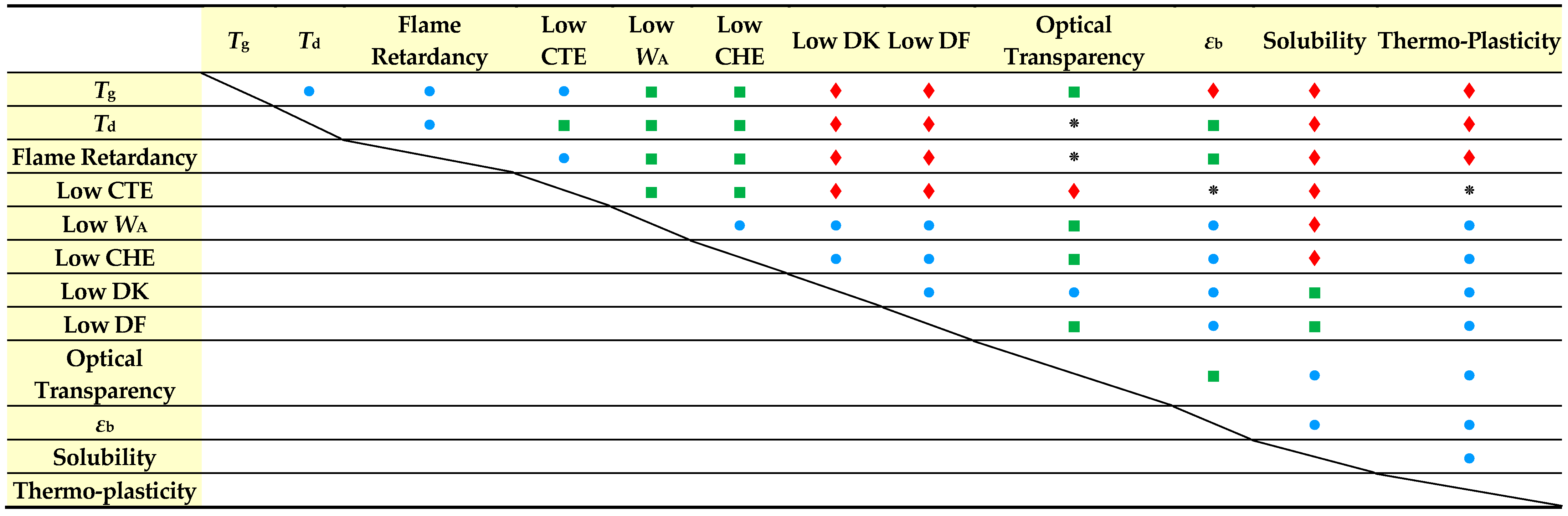
3.1. Difficulty in Simultaneously Achieving Several Target Properties
3.2. Difficulty in Enhancing the Flame Retardancy of PEsI Systems
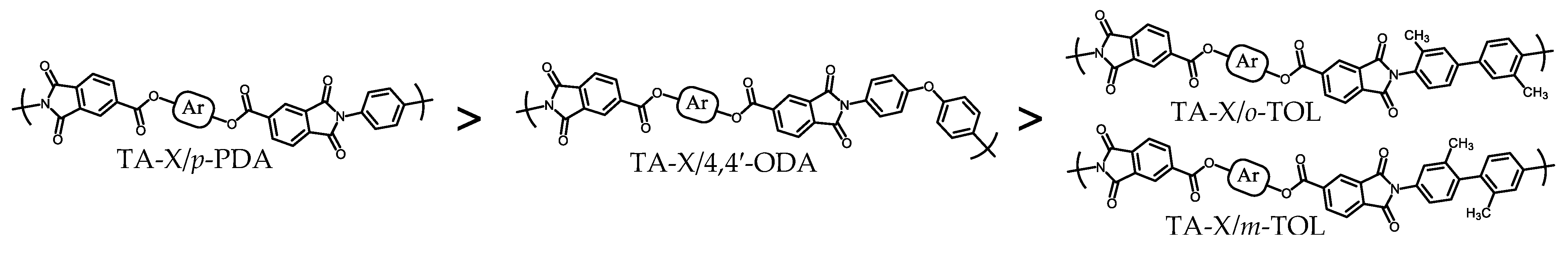
3.3. Strategies for Mitigating Flame Retardancy Deterioration
3.4. Approaches to Improve the Flame Retardancy of PEsIs Using Reactive Modifiers
3.5. Strategies to Improve the Flame Retardancy of PEsIs without Flame Retardants
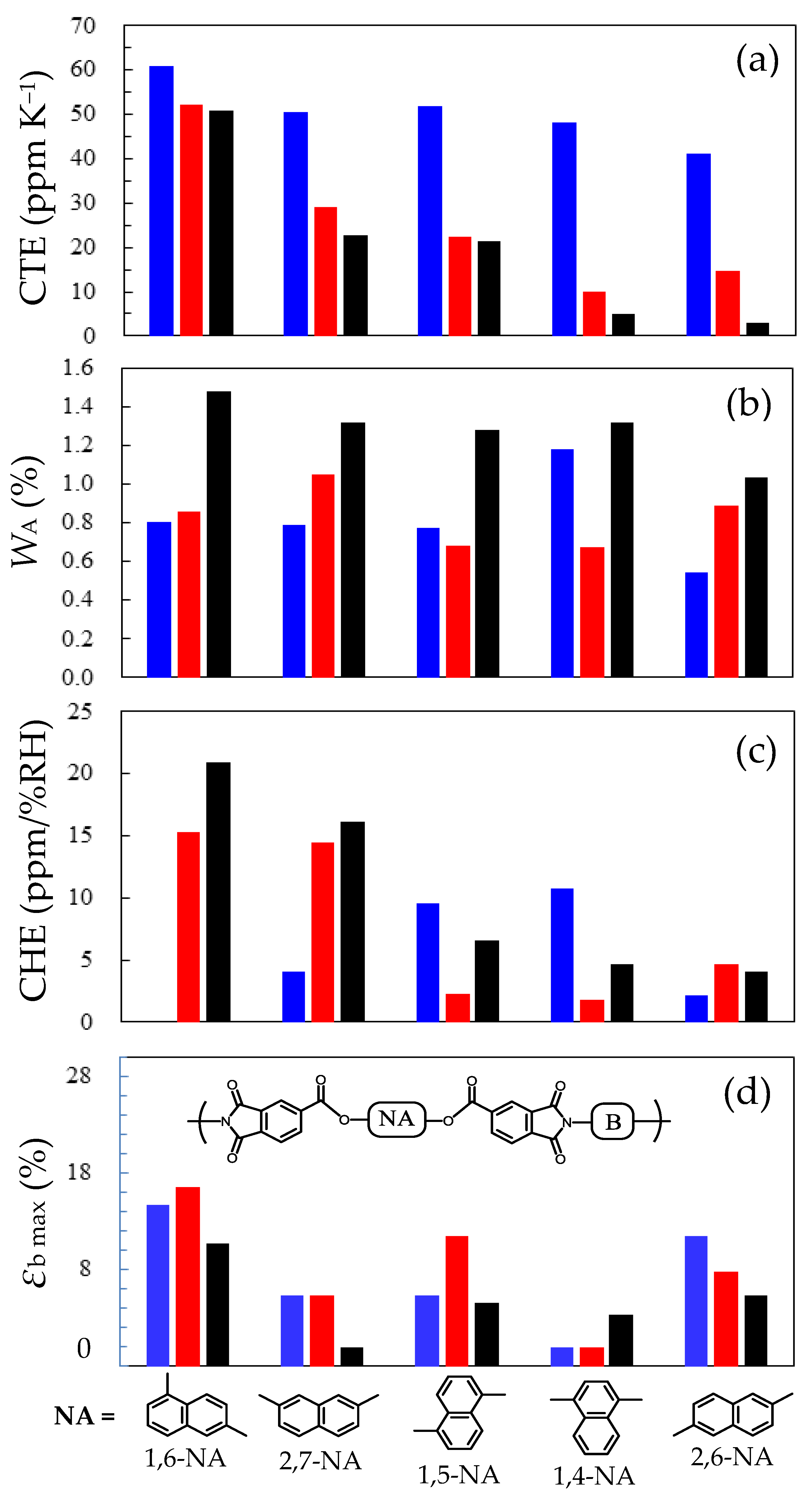
3.5.1. PEsIs Derived from Isomeric TA-NAs
- (a)
- TA-14NA-based PEsIs
- (b)
- TA-15NA-based PEsIs
- (c)
- TA-16NA-based PEsIs
- (d)
- TA-27NA-based PEsIs
- (e)
- TA-26NA-based PEsIs
- (f)
- Comparative Analysis of the Properties of TA-NA-based PEsIs
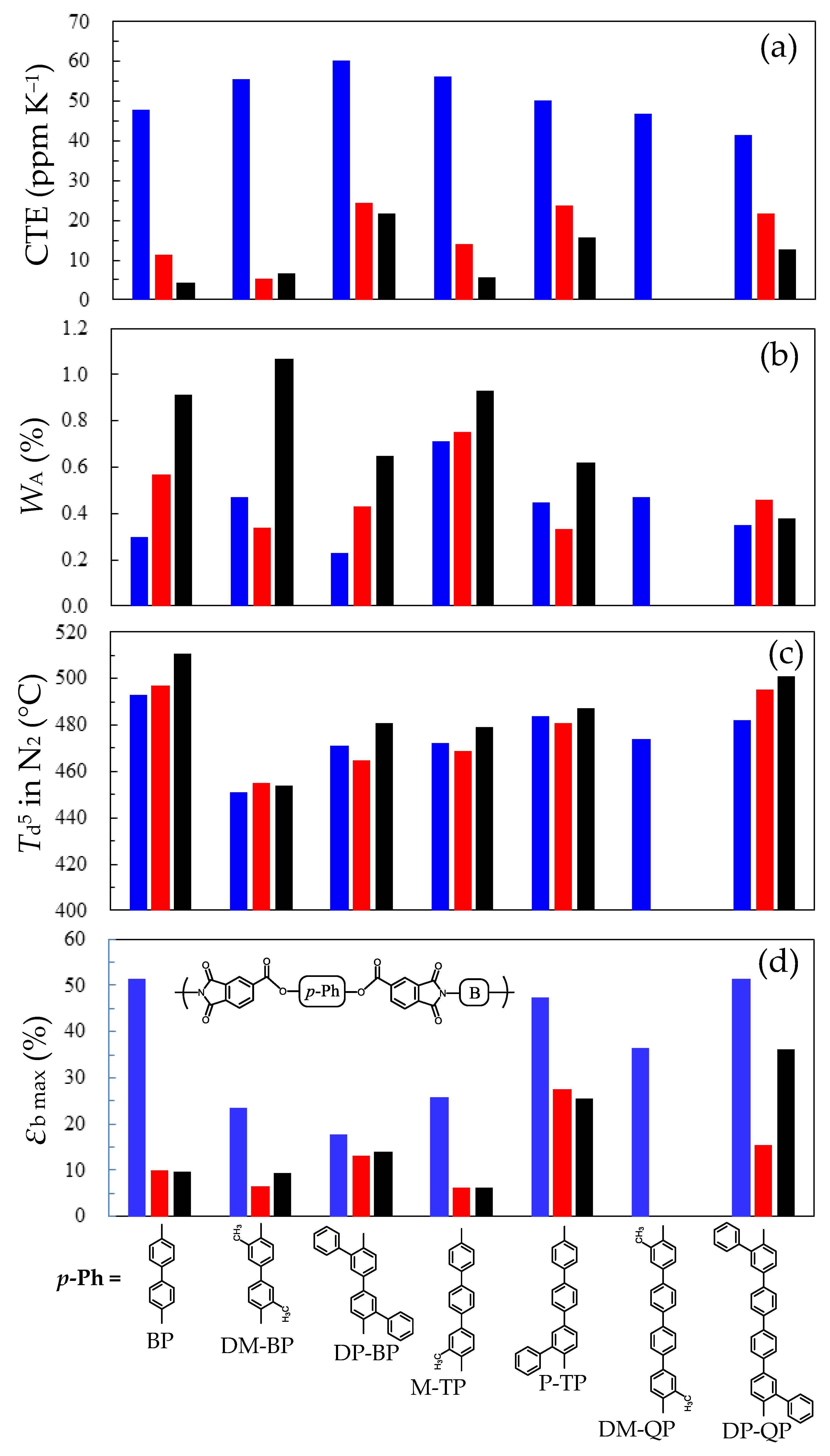
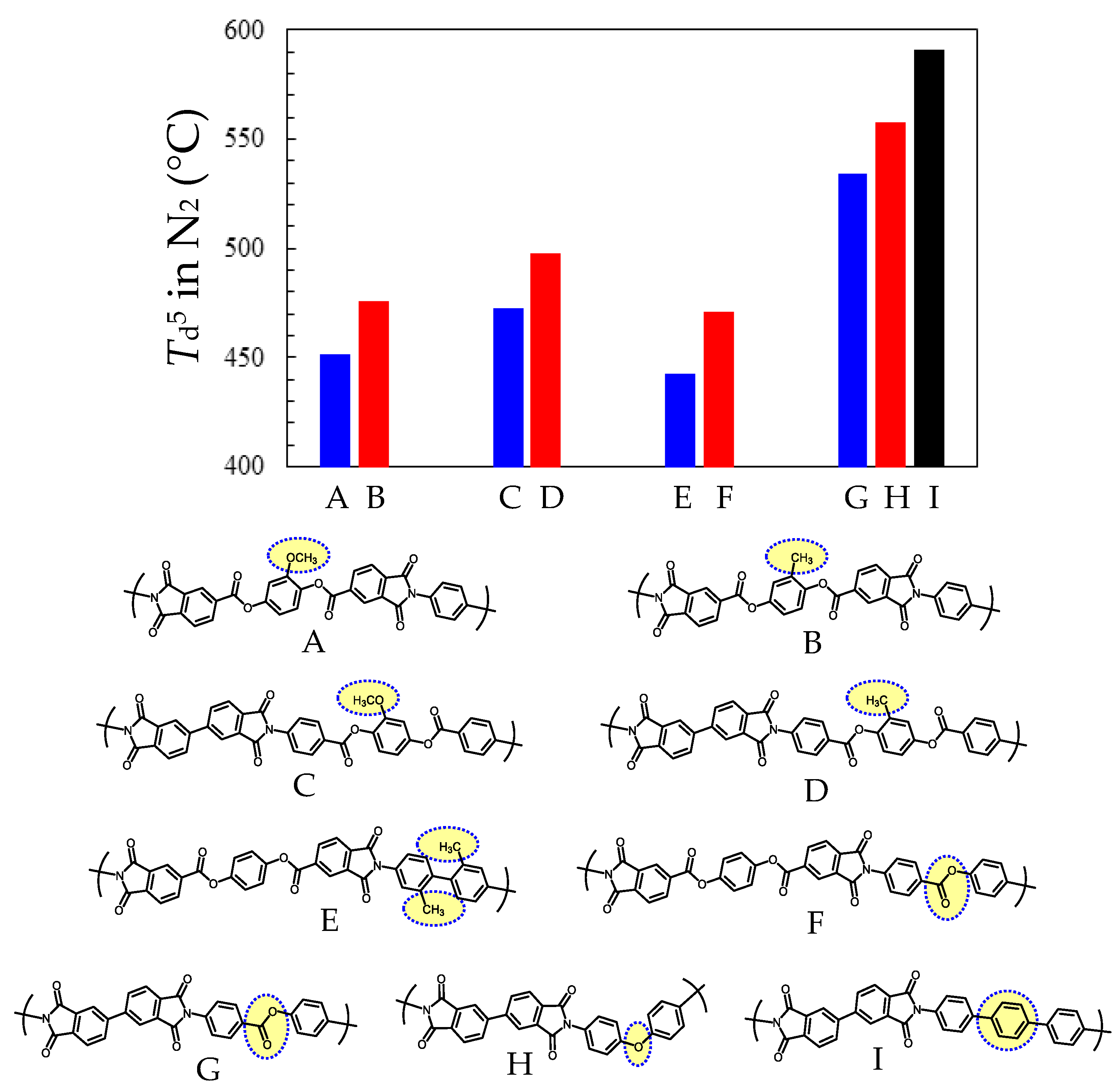
3.5.2. PEsIs Derived from para-Phenylene-containing TCDAs (TA-pPh)
- (a)
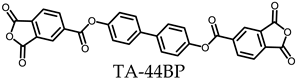
- TA-44BP-based PEsIs
- (b)
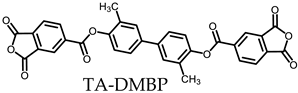
- TA-DMBP-based PEsIs
- (c)
- TA-DPBP-based PEsIs
- (d)
- TA-MTP-based PEsIs
- (e)
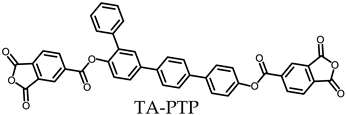
- TA-PTP-based PEsIs
- (f)
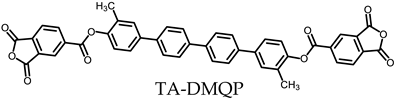
- TA-DMQP-based PEsIs
- (g)
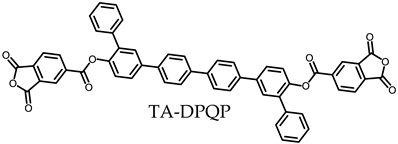
- TA-DPQP-based PEsIs
- (h)
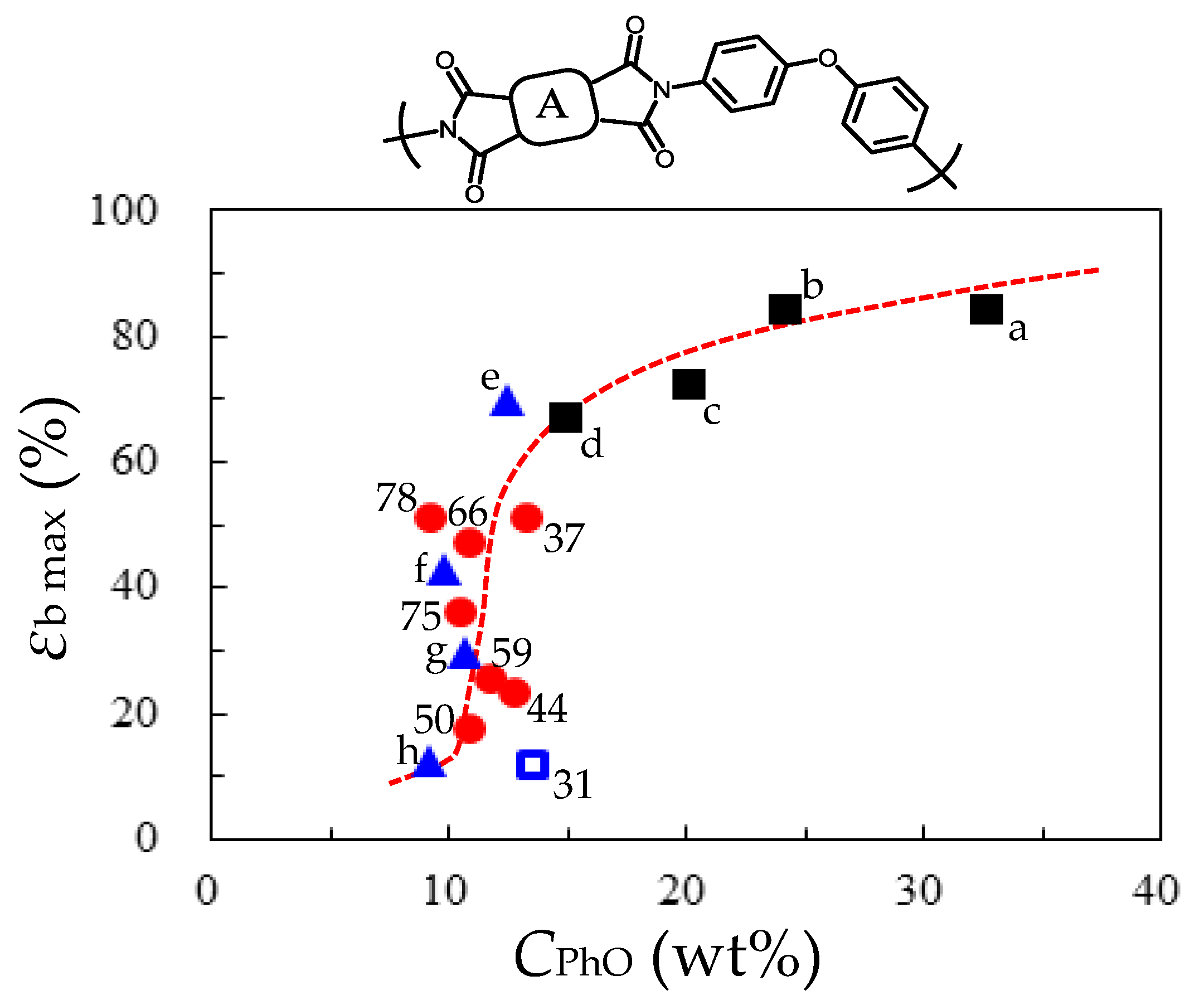
- Comparative Analysis of the Properties of TA-pPh-based PEsIs
- (i)
- Superiority of the Substituents in TA-pPhs
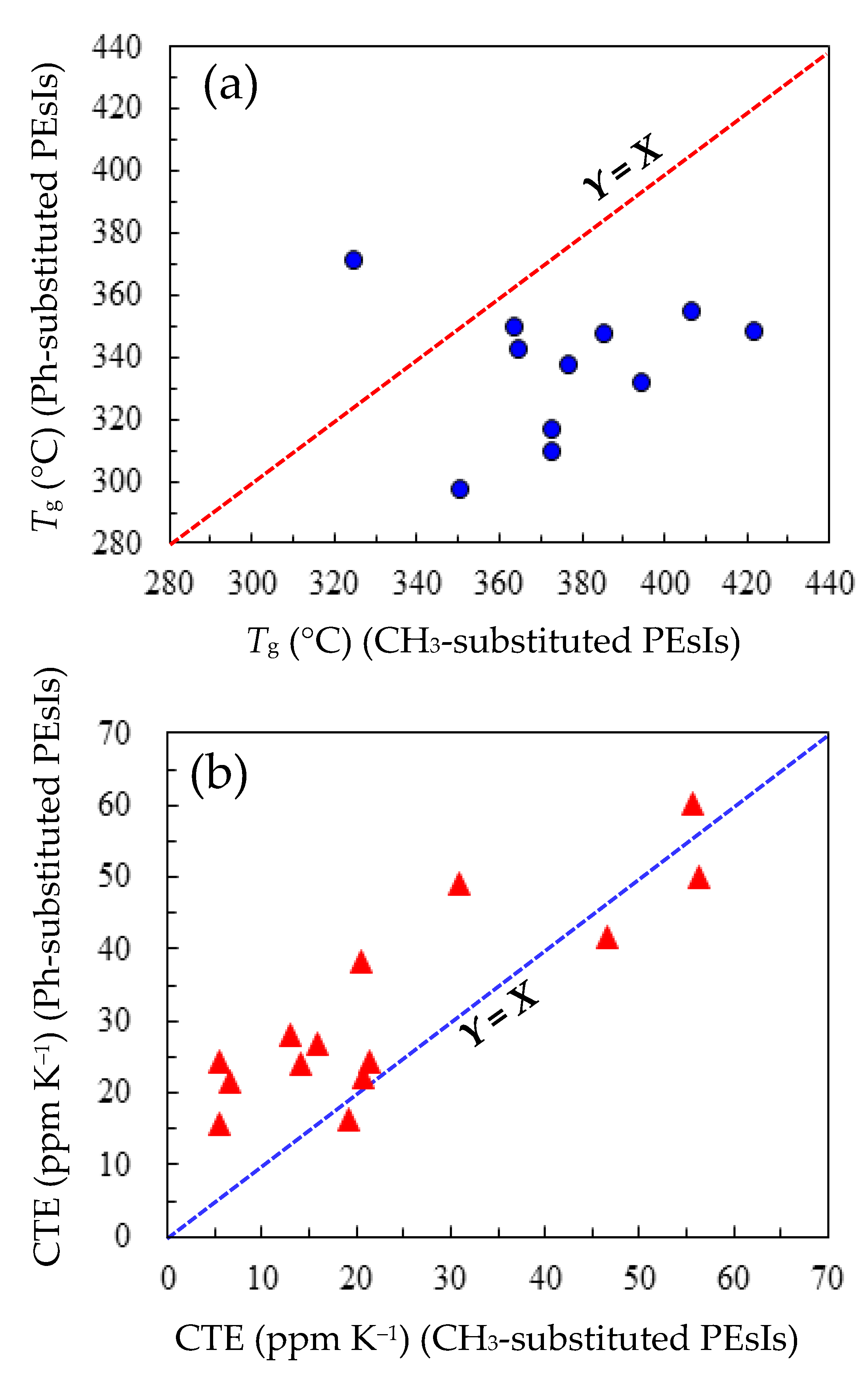
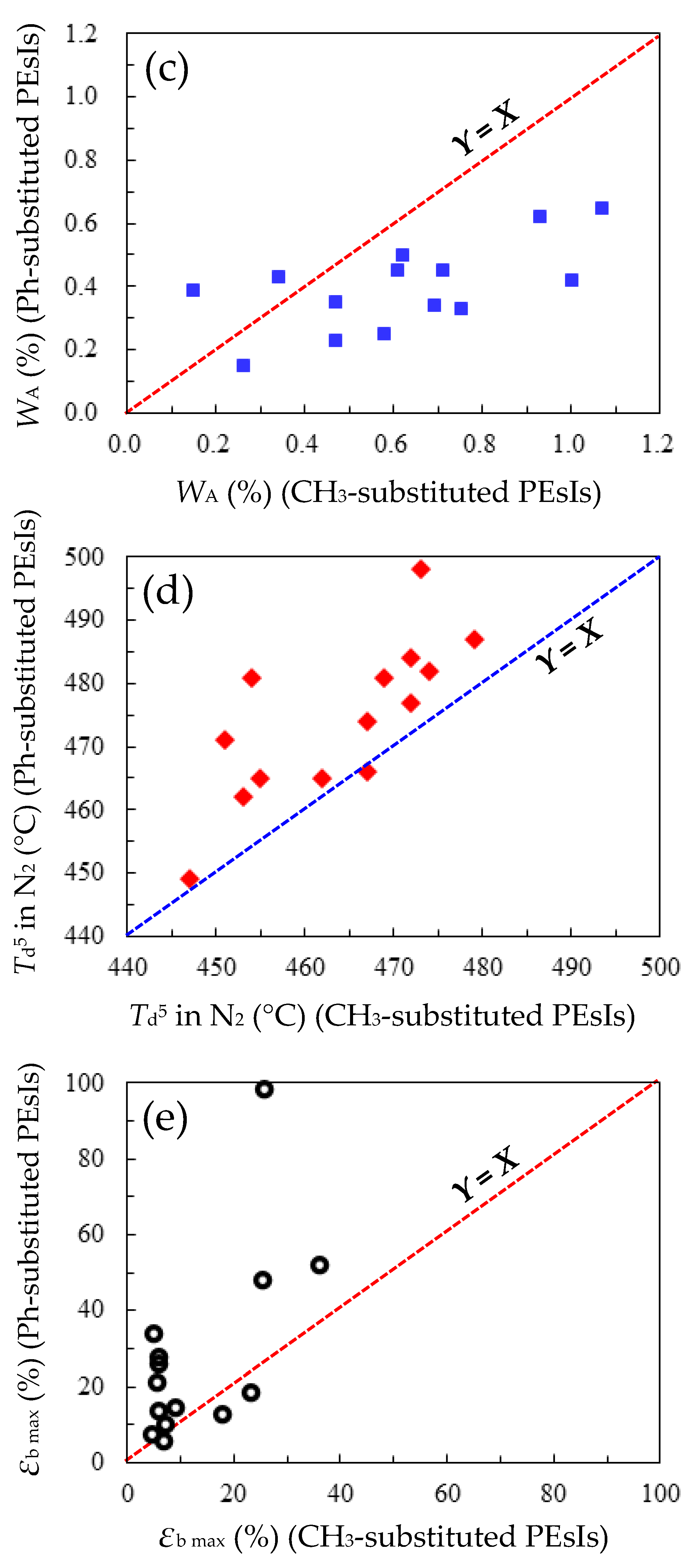
3.5.3. Comprehensive Structure–Property Relationships for TA-NA- and TA-pPh-based PEsIs
- (a)
- WA and CHE
- (b)
- Film Toughness
- (c)
- Flame Retardancy
3.6. Performance Balance of Typical PEsIs Examined Herein
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cassidy, P.E. Thermally Stable Polymers: Syntheses and Properties; Marcel Dekker: New York, NY, USA, 1980. [Google Scholar]
- Mittal, K.L. (Ed.) Polyimides: Synthesis, Characterization, and Applications; Plenum Press: New York, NY, USA, 1984; Volume 1–2. [Google Scholar]
- Bessonov, M.I.; Koton, M.M.; Kudryavtsev, V.V.; Laius, L.A. (Eds.) Polyimides: Thermally Stable Polymers; Plenum: New York, NY, USA, 1987. [Google Scholar]
- Feger, C.; Khojasteh, M.M.; McGrath, J.E. (Eds.) Polyimides: Materials, Chemistry and Characterization; Elsevier Science Publishers: Amsterdam, The Netherlands, 1989. [Google Scholar]
- Sroog, C.E. Polyimide. Prog. Polym. Sci. 1991, 16, 561–694. [Google Scholar] [CrossRef]
- Abadie, M.J.M.; Sillion, B. (Eds.) Polyimides and Other High-Temperature Polymers; Elsevier Science Publishers: Amsterdam, The Netherlands, 1991. [Google Scholar]
- Bessonov, M.I.; Zubkov, V.A. (Eds.) Polyamic Acid and Polyimides: Synthesis, Transformation and Structure; CRC Press: Boca Raton, FL, USA, 1993. [Google Scholar]
- Feger, C.; Khojasteh, M.M.; Htoo, M.S. (Eds.) Advances in Polyimide Science and Technology; Technomic Publishing: Lancaster, UK, 1993. [Google Scholar]
- Ghosh, M.K.; Mittal, K.L. (Eds.) Polyimides: Fundamentals and Applications; Marcel Dekker: New York, NY, USA, 1996. [Google Scholar]
- Sachdev, H.S.; Khojasteh, M.M.; Feger, C. (Eds.) Advances in Polyimides and Low Dielectric Polymers; Society of Plastic Engineers: New York, NY, USA, 1997. [Google Scholar]
- Kricheldorf, H.R. (Ed.) Progress in Polyimide Chemistry I & II, Advances in Polymer Science; Springer: New York, NY, USA, 1999; Volume 140–141. [Google Scholar]
- Hergenrother, P.M. The Use, design, synthesis, and properties of high performance/high temperature polymers: An overview. High Perform. Polym. 2003, 15, 3–45. [Google Scholar] [CrossRef]
- Ree, M. High performance polyimides for applications in microelectronics and flat panel displays. Macromol. Res. 2006, 14, 1–33. [Google Scholar] [CrossRef]
- Liaw, D.J.; Wang, K.L.; Huang, Y.C.; Lee, K.R.; Lai, J.Y.; Ha, C.S. Advanced polyimide materials: Syntheses, physical properties and applications. Prog. Polym. Sci. 2012, 37, 907–974. [Google Scholar]
- Tsai, C.L.; Yen, H.J.; Liou, G.S. Highly transparent polyimide hybrids for optoelectronic applications. React. Funct. Polym. 2016, 108, 2–30. [Google Scholar] [CrossRef]
- Yang, S.Y. (Ed.) Advanced Polyimide Materials: Synthesis, Characterization, and Applications; Chemical Industry Press: Amsterdam, The Netherlands; Elsevier: Amsterdam, The Netherlands, 2018. [Google Scholar]
- Diaham, S. (Ed.) Polyimide for Electronic and Electrical Engineering Applications; IntechOpen: London, UK, 2021. [Google Scholar]
- KAPTON® H Data Sheet. Available online: https://www.td-net.co.jp/kapton/data/download/documents/kapton2007.pdf (accessed on 26 June 2024).
- KAPTON® EN Data Sheet. Available online: https://www.td-net.co.jp/kapton/data/download/documents/1207kaptonEN.pdf (accessed on 26 June 2024).
- UPILEX®-S Data Sheet. Available online: https://www.ube.com/upilex/catalog/pdf/upilex_s.pdf (accessed on 26 June 2024).
- Hasegawa, M.; Koseki, K. Poly(ester imide)s possessing low CTE and low water absorption. High Perform. Polym. 2006, 18, 697–717. [Google Scholar] [CrossRef]
- Hasegawa, M.; Tsujimura, Y.; Koseki, K.; Miyazaki, T. Poly(ester imide)s Possessing Low CTE and Low Water Absorption (II). Effect of Substituents. Polym. J. 2008, 40, 56–67. [Google Scholar]
- Hasegawa, M.; Sakamoto, Y.; Tanaka, Y.; Kobayashi, Y. Poly(ester imide)s Possessing Low Coefficients of Thermal Expansion (CTE) and Low Water Absorption (III). Use of bis(4-aminophenyl)terephthalate and effect of substituents. Eur. Polym. J. 2010, 46, 1510–1524. [Google Scholar] [CrossRef]
- Hasegawa, M.; Saito, T.; Tsujimura, Y. Poly(ester imide)s possessing low coefficients of thermal expansion and low water Absorption (IV). Effects of ester-linked tetracarboxylic dianhydrides with longitudinally extended structures. Polym. Adv. Technol. 2020, 31, 389–406. [Google Scholar] [CrossRef]
- Hasegawa, M.; Hishiki, T. Poly(ester imide)s possessing low coefficients of thermal expansion and low water absorption (V): Effects of ester-linked diamines with different lengths and substituents. Polymers 2020, 12, 859. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, M.; Hori, A.; Hosaka, C.; Ishii, J. Poly(ester imide)s with low coefficients of thermal expansion (CTEs) and low water absorption (VI). An attempt to reduce the modulus while maintaining low CTEs and other desired properties. Polym. Int. 2022, 71, 1164–1175. [Google Scholar] [CrossRef]
- Hasegawa, M.; Fukuda, T.; Ishii, J. Poly(ester imide)s with low linear coefficients of thermal expansion and low water uptake (VII): A strategy to achieve ultra-low dissipation factors at 10 GHz. Polymers 2024, 16, 653. [Google Scholar] [CrossRef] [PubMed]
- UL-94 Standard. Available online: https://www.ul.com/services/combustion-fire-tests-plastics (accessed on 26 June 2024).
- Mehdipour-Ataei, S.; Keshavarz, S. Use of new diimide-dinaphthols in preparation of novel thermally stable poly(ester-imide)s. J. Appl. Polym. Sci. 2003, 89, 2567–2572. [Google Scholar] [CrossRef]
- Liaw, D.J.; Fan, C.L.; Lin, C.C.; Wang, K.L. Synthesis and characterization of new soluble poly(ester-imide)s containing noncoplanar 2,2′-dimethyl-4,4′-biphenylene unit. J. Appl. Polym. Sci. 2004, 92, 2486–2493. [Google Scholar] [CrossRef]
- Behniafar, H.; Akhlaghinia, B.; Habibian, S. Synthesis and characterization of new soluble and thermally stable poly(ester-imide)s derived from N-[3,5-bis(N-trimellitoyl)phenyl]phthalimide and various bisphenols. Eur. Polym. J. 2005, 41, 1071–1078. [Google Scholar] [CrossRef]
- Butuc, E.; Gherasim, G.M. Ordered heterocyclic copolymers. Polyamide-imides with S-triazine rings. J. Polym. Sci. Polym. Chem. 1984, 22, 503–507. [Google Scholar] [CrossRef]
- Nakayama, J. The infrared absorption spectra of organophosphorus compounds. J. Synth. Org. Chem. Jpn. 1970, 28, 132–143. [Google Scholar] [CrossRef]
- JIS K7209; Plastics-Determination of Water Absorption. Japanese Standards Association: Tokyo, Japan, 2020.
- ASTM D2863; Standard Test Method for Measuring the Minimum Oxygen Concentration to Support Candle-like Combustion of Plastics (Oxygen Index). ASTM: West Conshohocken, PA, USA, 2000. Available online: https://cdn.standards.iteh.ai/samples/4274/5dcc706b840f4ff7847ad13c51f1f8d1/ASTM-D2863-00.pdf (accessed on 26 June 2024).
- Fenimore, C.P.; Martin, F.T. Candle-type test for flammability of polymers. Mod. Plast. 1966, 43, 141. [Google Scholar]
- Fenimore, C.P.; Martin, F.J. Flammability of polymers. Combust. Flame 1966, 10, 135–139. [Google Scholar] [CrossRef]
- van Krevelen, D.W. Some basic aspects of flame resistance of polymeric materials. Polymer 1975, 16, 615–620. [Google Scholar] [CrossRef]
- Hasegawa, M.; Kaneki, T.; Tsukui, M.; Okubo, N.; Ishii, J. High-temperature polymers overcoming the trade-off between excellent thermoplasticity and low thermal expansion properties. Polymer 2016, 99, 292–306. [Google Scholar] [CrossRef]
- Troitzsch, J.; Antonatus, E. (Eds.) Plastics Flammability Handbook: Principles, Regulations, Testing, and Approval, 4th ed.; Hanser Publications: Cincinnati, OH, USA, 2021. [Google Scholar]
- Babu, K.; Rendén, G.; Mensah, R.A.; Kim, N.K.; Jiang, L.; Xu, Q.; Restás, Á.; Neisiany, R.E.; Mikael, S.; Hedenqvist, M.S.; et al. A review on the flammability properties of carbon-based polymeric composites: State-of-the-art and future trends. Polymers 2020, 12, 1518. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Liang, J.; Lin, X.; Lin, H.; Yu, J.; Wang, S. The flame-retardant mechanisms and preparation of polymer composites and their potential application in construction engineering. Polymers 2022, 14, 82. [Google Scholar] [CrossRef] [PubMed]
- Matsuura, T.; Hasuda, Y.; Nishi, S.; Yamada, N. Polyimide derived from 2,2′-bis(trifluoromethyl)-4,4′-diaminobiphenyl.1. Synthesis and characterization of polyimides prepared with 2,2-bis(3,4-dicarboxyphenyl)hexafluoropropane dianhydride or pyromellitic dianhydride. Macromolecules 1991, 24, 5001–5005. [Google Scholar] [CrossRef]
- Hasegawa, M.; Horie, K. Photophysics, photochemistry, and optical properties of polyimides. Prog. Polym. Sci. 2001, 26, 259–335. [Google Scholar] [CrossRef]
- Wu, X.; Jiang, G.; Zhang, Y.; Wu, L.; Jia, Y.; Tan, Y.; Liu, J.; Zhang, X. Enhancement of flame retardancy of colorless and transparent semi-alicyclic polyimide film from hydrogenated-BPDA and 4,4′-oxydianiline via the incorporation of phosphazene oligomer. Polymers 2020, 12, 90. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Wu, X.; Qi, H.; An, Y.; Jia, Y.; Zhang, Y.; Zhi, X.; Liu, J. Colorless and transparent semi-alicyclic polyimide films with intrinsic flame retardancy based on alicyclic dianhydrides and aromatic phosphorous-containing diamine: Preparation and properties. Polym. Adv. Technol. 2021, 32, 1061–1074. [Google Scholar] [CrossRef]
- Hasegawa, M.; Hoshino, Y.; Katsura, N.; Ishii, J. Superheat resistant polymers with low coefficients of thermal expansion. Polymer 2017, 111, 91–102. [Google Scholar] [CrossRef]
- Nishizawa, H. Flame retardant polymeric materials (III). Flame retardants. J. Soc. Rubber. Sci. Technol. Jpn. 2013, 86, 341–347. [Google Scholar] [CrossRef][Green Version]
- Xiao, S.; Akinyi, C.; Longun, J.; Iroh, J.O. Polyimide Copolymers and Nanocomposites: A Review of the synergistic effects of the constituents on the fire-retardancy behavior. Energies 2022, 15, 4014. [Google Scholar] [CrossRef]
- Liu, R.; Wang, X. Synthesis, characterization, thermal properties and flame retardancy of a novel nonflammable phosphazene-based epoxy resin. Polym. Degrad. Stabil. 2009, 94, 617–624. [Google Scholar] [CrossRef]
- Hamciuc, C.; Hamciuc, E.; Serbezeanu, D.; Vlad-Bubulac, T.; Cazacu, M. Phosphorus-containing poly(ester-imide)–polydimethylsiloxane copolymers. Polym. Int. 2011, 60, 312–321. [Google Scholar] [CrossRef]
- Liang, B.; Cao, J.; Hong, X.; Wang, C. Synthesis and properties of a novel phosphorous-containing flame-retardant hardener for epoxy resin. J. Appl. Polym. Sci. 2013, 128, 2759–2765. [Google Scholar] [CrossRef]
- Takahashi, N.; Yoon, D.Y.; Parrish, W. Molecular order in condensed states of semiflexible polyamic acid and polyimide. Macromolecules 1984, 17, 2583–2588. [Google Scholar] [CrossRef]
- Numata, S.; Oohara, S.; Fujisaki, K.; Imaizumi, J.; Kinjo, N. Thermal expansion behavior of various aromatic polyimides. J. Appl. Polym. Sci. 1986, 31, 101–110. [Google Scholar] [CrossRef]
- Numata, S.; Fujisaki, K.; Kinjyo, N. Re-examination of the relationship between packing coefficient and thermal expansion coefficient for aromatic polyimides. Polymer 1987, 28, 2282–2288. [Google Scholar] [CrossRef]
- Numata, S.; Kinjo, N.; Makino, D. Chemical structures and properties of low thermal expansion coefficient polyimides. Polym. Eng. Sci. 1988, 28, 906–911. [Google Scholar] [CrossRef]
- Hasegawa, M.; Matano, T.; Shindo, Y.; Sugimura, T. Spontaneous molecular orientation of polyimides induced by thermal imidization (2). In-plane orientation. Macromolecules 1996, 29, 7897–7909. [Google Scholar] [CrossRef]
- Coburn, J.C.; Pottiger, M.T. Thermal curing in polyimide films and coatings. In Polyimides: Fundamentals and Applications; Ghosh, M.K., Mittal, K.L., Eds.; Marcel Dekker: New York, NY, USA, 1996; pp. 207–247. [Google Scholar]
- Russel, T.P.; Gugger, H.; Swalen, J.D. In-plane orientation of polyimide. J. Polym. Sci. Polym. Phys. 1983, 21, 1745–1756. [Google Scholar] [CrossRef]
- Nakagawa, K. Optical anisotropy of polyimide. J. Appl. Polym. Sci. 1990, 41, 2049–2058. [Google Scholar] [CrossRef]
- Boese, D.; Lee, H.; Yoon, D.Y.; Swalen, J.D.; Rabolt, J.F. Chain orientation and anisotropies in optical and dielectric properties in thin films of stiff polyimides. J. Polym. Sci. Polym. Phys. 1992, 30, 1321–1327. [Google Scholar] [CrossRef]
- Factor, B.J.; Russell, T.P.; Toney, M.F. Grazing incidence X-ray scattering studies of thin films of an aromatic polyimide. Macromolecules 1993, 26, 2847–2859. [Google Scholar] [CrossRef]
- Murakami, M. Dimensionality and Applications of Various Graphite Carbons. Rev. High Pressure Sci. Technol. 2006, 16, 216–222. [Google Scholar] [CrossRef][Green Version]
- Nishio, S.; Oba, T.; Mase, R.; Matsuzaki, A.; Sato, H. Preparation of semiconducting polymer films by high intensity photo-chemical processes with excimer laser beams. J. Photopolym. Sci. Technol. 1997, 10, 175–180. [Google Scholar] [CrossRef][Green Version]
- Hasegawa, M.; Horii, S. Low-CTE polyimides derived from 2,3,6,7-naphthalenetetracarboxylic dianhydride. Polym. J. 2007, 39, 610–621. [Google Scholar] [CrossRef]
- Volksen, W.; Cotts, P.; Yoon, D.Y. Molecular weight dependence of mechanical properties of poly(p,p′-oxydiphenylene pyromellitimide) films. J. Polym. Sci. Part B Polym. Phys. 1987, 25, 2487–2495. [Google Scholar] [CrossRef]
- Tamaso, K.; Matsumoto, K.; Takeshi Endo, T. Phosphorus-based flame retardant for epoxy resin. J. Netw. Polym. Jpn. 2015, 36, 232–238. [Google Scholar]
- Brandrup, J.; Immergut, E.H.; Grulke, E.A. (Eds.) Polymer Handbook, 4th ed.; John Wiley: New York, NY, USA, 1999. [Google Scholar]
- Takeichi, T.; Zuo, M.; Hasegawa, M. Role of in-plane orientation of polyimide films on graphitization. J. Polym. Sci. Polym. Phys. 2001, 39, 3011–3019. [Google Scholar] [CrossRef]
- Van Krevelen, D.W. (Ed.) Properties of Polymers, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 1991. [Google Scholar]
- Nagano, H. Polyimide Resins, Structure, Properties, High Functionality, Reliability, Thin Films, and Applications; Technical Information Institute: Tokyo, Japan, 1991; pp. 94–102. [Google Scholar]
- Nielsen, L.E. Mechanical Properties of Polymers and Composites; Marcel Dekker: New York, NY, USA, 1975. [Google Scholar]
- Persson, B.N.J. Fracture of polymers. J. Chem. Phys. 1999, 110, 9713–9724. [Google Scholar] [CrossRef]
- Sabouri-Ghomi, M.; Ispolatov, S.; Grant, M. Molecular weight effects on chain pull-out fracture of reinforced polymeric interfaces. Phys. Rev. E 1999, 60, 4460–4464. [Google Scholar] [CrossRef] [PubMed]
- Schnell, R.; Stamm, M.; Creton, C. Mechanical properties of homopolymer interfaces: Transition from simple pullout to crazing with increasing interfacial width. Macromolecules 1999, 32, 3420–3425. [Google Scholar] [CrossRef]
- Baklagina, Y.G.; Milevskaya, I.S.; Efanova, N.V.; Sidorovich, A.V.; Zubkov, V.A. Structure of rigid-chain polyimides from pyromellitic dianhydride. Vysokomol. Soedin. 1976, A18, 1235–1242. [Google Scholar]
- Obata, Y.; Okuyama, K.; Kurihara, S.; Kitano, Y.; Jinda, T. X-ray structure analysis of an aromatic polyimide. Macromolecules 1995, 28, 1547–1551. [Google Scholar] [CrossRef]
- Hasegawa, M.; Fujii, M.; Wada, Y. Approaches to improve the film ductility of colorless cycloaliphatic polyimides. Polym. Adv. Technol. 2018, 29, 921–933. [Google Scholar] [CrossRef]
- Wu, Y.; Zhang, C.; Wang, C.; Rabczuk, T.; Zhu, P.; Zhao, P.; Wang, L.; Zhuang, X.; Zhang, J.; Fang, H. The micro response mechanisms of foamed polymer rehabilitation material under compression: From a closed cell view. Polym. Test. 2023, 124, 108082. [Google Scholar] [CrossRef]
- Bürger, A.; Fitzer, E.; Heym, M.; Terwiesch, B. Polyimides as precursors for artificial carbon. Carbon 1975, 13, 149–157. [Google Scholar] [CrossRef]
- Hasegawa, M.; Sensui, N.; Shindo, Y.; Yokota, R. Improvement of thermoplasticity for s-BPDA/PDA by copolymerization and blend with novel asymmetric BPDA-based polyimides. J. Polym. Sci. Part B Polym. Phys. 1999, 37, 2499–2511. [Google Scholar] [CrossRef]
- Takekoshi, T.; Wirth, J.G.; Heath, D.R.; Kochanowski, J.E.; Manello, J.S.; Webber, M.J. Polymer syntheses via aromatic nitro displacement reaction. J. Polym. Sci. Part A Polym. Chem. 1980, 18, 3069–3080. [Google Scholar] [CrossRef]
- Oishi, J. Transparent polyimide “Neopulim”. In Recent Advances in Polyimides and Aromatic Polymers, Japan 2011; Japan Polyimide & Aromatic Polymer Research Association: Tokyo, Japan, 2011; pp. 23–30. [Google Scholar]
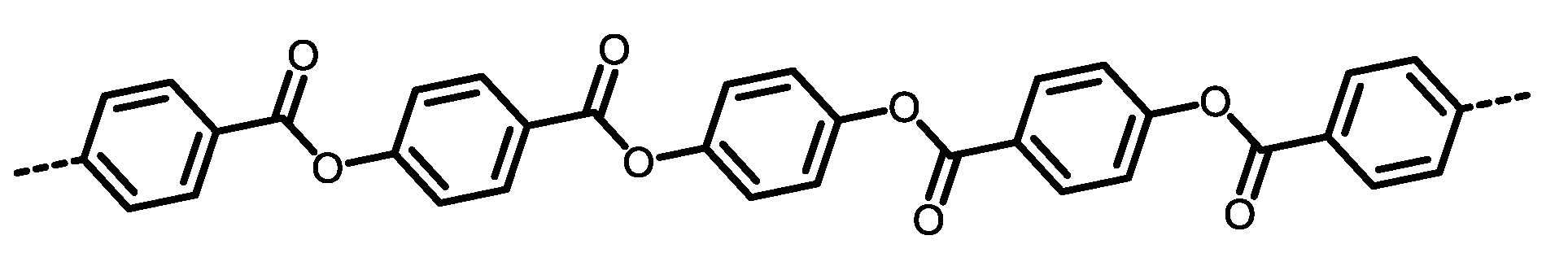

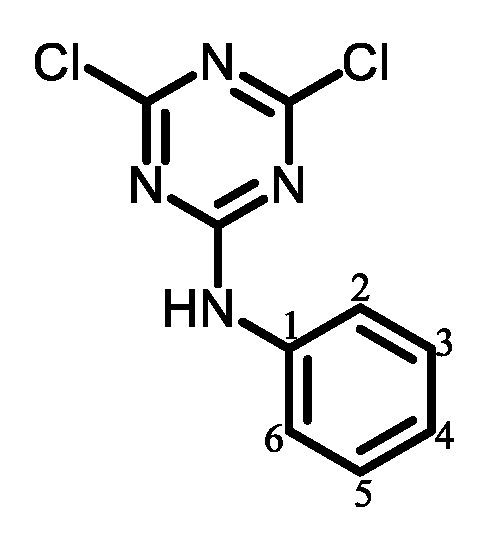

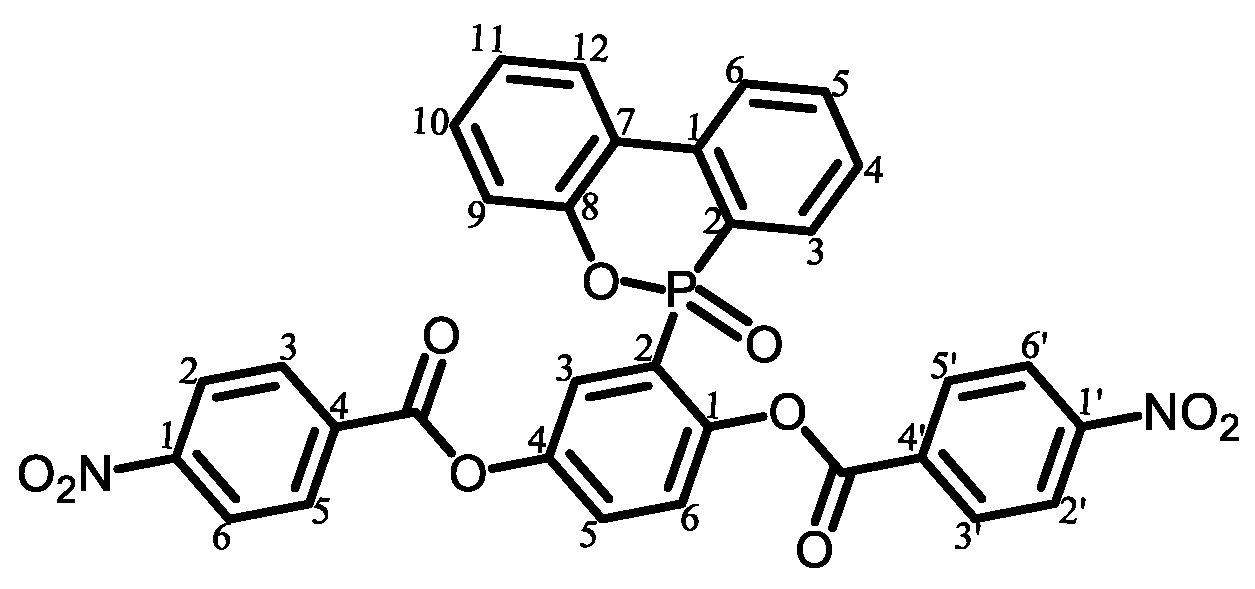
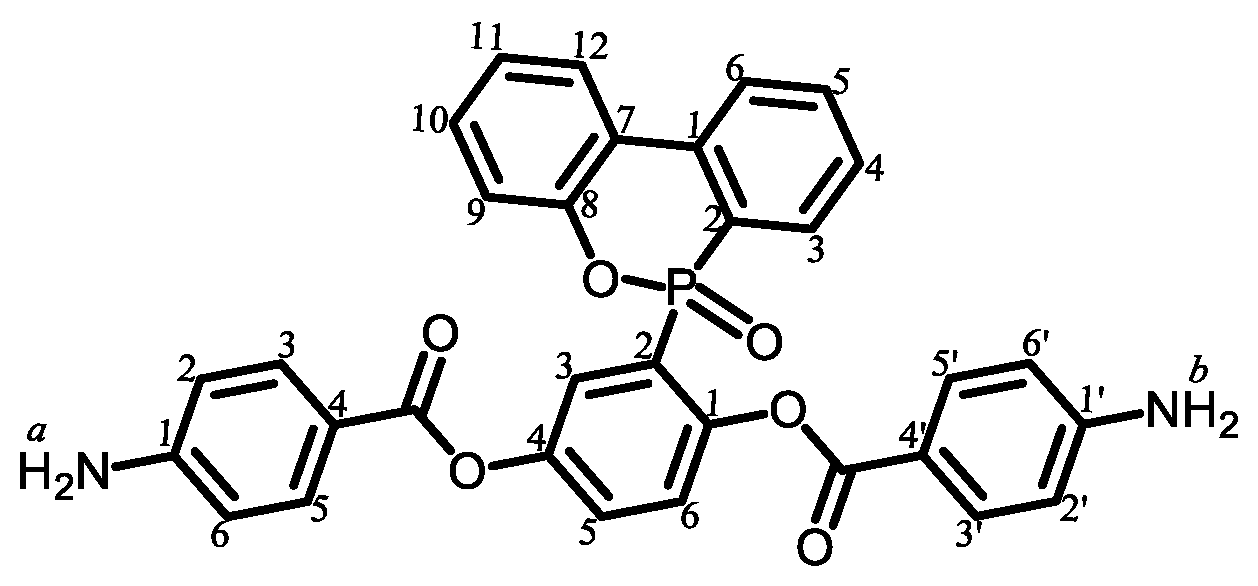
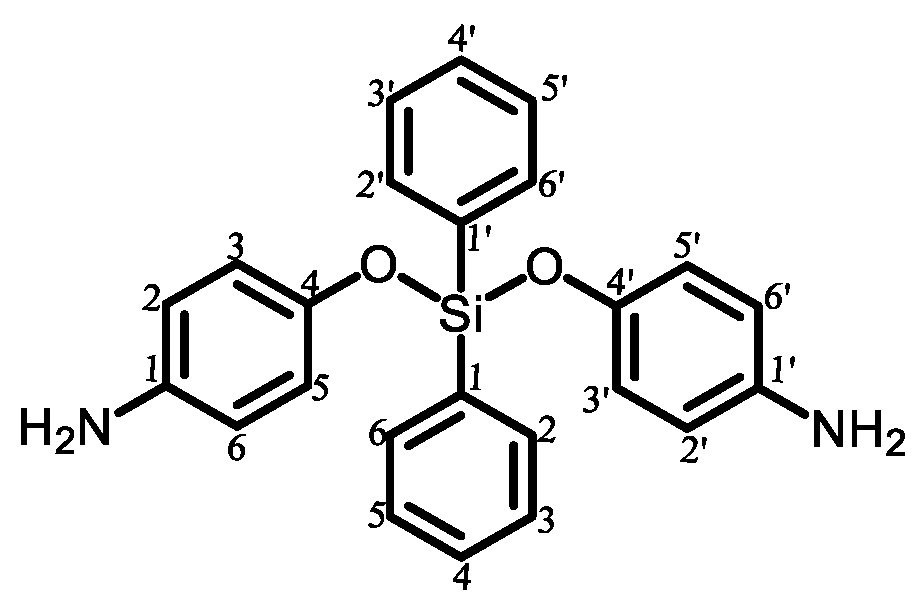







 |
| No. | Chain Structure | UL-94, V-0 (d, μm) |
|---|---|---|
| 1 |  | Passed (30) |
| 2 | 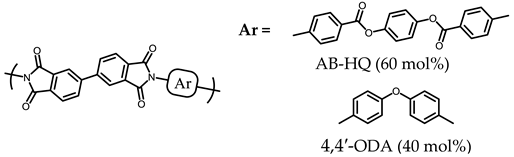 | Failed (24) |
| No. | TCDA (mol%) | Diamine (mol%) | ηred PAA (dL g−1) | Tg (°C) | CTE (ppm K−1) | Δnth | E (GPa) | εb av/max (%) | σb (GPa) | Td5 in N2 (°C) | Td5 in Air (°C) | WA (%) | CHE (ppm/%RH) | UL-94 V-0 (d, μm) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 3 | TA-MHQ | BPTP (60) 4,4′-ODA (40) | 3.76 | 390 a 200 b | 12.3 | 0.137 | 4.32 | 26.8 41.6 | 0.28 | 461 | 444 | 0.52 | 5.0 | Failed (25) |
| 4x | TA-MHQ (95) 1,4,5,8-NTDA (5) | BPTP (60) 4,4′-ODA (40) | 1.81 | 386 c | 10.8 | 0.170 | 3.76 | 25.8 38.2 | 0.24 | 444 | 444 | 0.59 | --- | Failed (25) |
| 4y | TA-MHQ (90) 1,4,5,8-NTDA (10) | BPTP (60) 4,4′-ODA (40) | 1.06 | 382 c | 31.8 | 0.112 | 3.94 | 14.9 27.0 | 0.21 | 445 | 442 | 0.62 | --- | Failed (20) |
| 5x | TA-MHQ (95) 2,3,6,7-NTDA (5) | BPTP (60) 4,4′-ODA (40) | 3.96 | 397 a 381 c | 14.7 | 0.170 | 5.30 | 25.4 45.0 | 0.32 | 447 | 447 | 0.57 | --- | Failed (22) |
| 5y | TA-MHQ (90) 2,3,6,7-NTDA (10) | BPTP (60) 4,4′-ODA (40) | 4.85 | 385 c | 12.5 | 0.173 | 4.30 | 36.0 55.4 | 0.31 | 448 | 452 | 0.56 | --- | Failed (23) |
| 6x | TA-MHQ | BPTP (55) 4,4′-ODA (40) AP-DPSi (5) | 5.40 | 386 c | 11.9 | 0.149 | 3.95 | 30.6 48.4 | 0.27 | 462 | 445 | 0.54 | --- | Failed (24) |
| 6y | TA-MHQ | BPTP (50) 4,4′-ODA (40) AP-DPSi (10) | 4.46 | 388 c | 13.4 | 0.155 | 3.53 | 26.3 39.1 | 0.25 | 463 | 441 | 0.59 | --- | Failed (24) |
| 7x | TA-MHQ | BPTP (55) 4,4′-ODA (40) PDA-TAZ (5) | 5.07 | 381 a | 17.4 | --- | 4.09 | 22.4 47.3 | 0.25 | 460 | 433 | 0.51 | --- | Failed (23) |
| 7y | TA-MHQ | BPTP (50) 4,4′-ODA (40) PDA-TAZ (10) | 6.30 | 373 a | 15.3 | --- | 4.41 | 17.2 27.3 | 0.23 | 455 | 429 | 0.62 | 3.7 | Failed (22) |
| 8x | TA-MHQ | BPTP (55) 4,4′-ODA (40) PDA-PTP (5) | 7.04 | 382 c | 14.8 | 0.166 | 4.41 | 23.7 40.1 | 0.26 | 482 | 450 | 0.73 | 5.2 | Failed (23) |
| 8y | TA-MHQ | BPTP (50) 4,4′-ODA (40) PDA-PTP (10) | 5.19 | 379 c | 15.9 | 0.159 | 4.69 | 35.4 47.4 | 0.32 | 477 | 454 | 0.87 | 6.1 | Failed (22) |
| 9x | TA-MHQ | BPTP (55) 4,4′-ODA (40) AB-HCAHQ (5) | 3.56 | 382 a | 15.7 | 0.139 | 4.08 | 24.6 37.3 | 0.30 | 450 | 439 | 0.54 | 5.5 | Passed (24) |
| 9y | TA-MHQ | BPTP (50) 4,4′-ODA (40) AB-HCAHQ (10) | 2.61 | 378 a | 18.5 | 0.139 | 4.66 | 17.8 32.7 | 0.24 | 452 | 442 | 0.56 | 6.1 | Passed (25) |
 | ||||||||||||||
| No. | Diamine | ηred PAA (dL g−1) | Tg (°C) | CTE (ppm K−1) | E (GPa) | εb av/max (%) | σb (GPa) | Td5 in N2 (°C) | Td5 in Air (°C) | WA (%) | CHE (ppm/%RH) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 10 | 4,4′-ODA | 3.00 | 378 (320) b | 48.2 (51.2) b | --- a | --- a | --- a | 439 | 428 | 1.18 | 10.8 |
| 11 | p-PDA | 3.11 | 423 (385) c,d | 5.0 (3.2) c | 6.67 | 2.5 3.3 | 0.131 | 457 (481) c | 445 (463) c | 1.32 | 4.7 |
| 12 | m-TOL | 4.07 | 354 | 15.6 | 5.46 | 3.3 5.4 | 0.160 | 437 | 426 | 1.06 | 2.5 |
| 13 | o-TOL | 3.07 | 381 | 17.9 | 5.84 | 3.9 4.8 | 0.179 | 446 | 425 | 1.17 | 4.7 |
| 14 | APAB | 1.24 | 384 | 10.0 | --- a | --- a | --- a | 455 | 444 | 0.67 | 1.8 |
| 15 | M-APAB | 1.10 | 359 | 7.0 | 5.66 | 3.1 5.0 | 0.162 | 444 | 428 | 0.82 | --- |
 | |||||||||||
| No. | Diamine | ηred PAA (dL g−1) | Tg (°C) | CTE (ppm K−1) | E (GPa) | εb av/max (%) | σb (GPa) | Td5 in N2 (°C) | Td5 in Air (°C) | WA (%) | CHE (ppm/%RH) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 16 | 4,4′-ODA | 0.83 | 336 | 51.8 | 3.77 | 3.4 5.3 | 0.113 | 447 | 447 | 0.77 | 9.6 |
| 17 | p-PDA | 0.94 | 362 | 21.4 | 4.40 | 3.5 4.6 | 0.117 | 464 | 459 | 1.28 | 6.6 |
| 18 | m-TOL | 1.05 | 376 | 23.8 | 4.13 | 3.0 4.4 | 0.125 | 444 | 451 | 1.31 | 4.7 |
| 19 | o-TOL | 1.36 | 385 | 19.1 | 3.46 | 3.7 5.3 | 0.108 | 444 | 440 | 0.67 | 4.5 |
| 20 | APAB | 0.69 | 383 | 22.5 | 3.86 | 6.1 11.5 | 0.157 | 464 | 458 | 0.68 | 2.3 |
| 21 | M-APAB | 0.83 | 388 | 15.4 | 3.93 | 3.7 5.1 | 0.109 | 447 | 431 | 0.60 | 1.9 |
 | |||||||||||
| No. | Diamine | ηred PAA (dL g−1) | CTE (ppm K−1) | E (GPa) | εb av/max (%) | σb (GPa) | Td5 in N2 (°C) | Td5 in Air (°C) | WA (%) | CHE (ppm/%RH) |
|---|---|---|---|---|---|---|---|---|---|---|
| 22 | 4,4′-ODA | 1.17 | 60.8 | 2.00 | 10.0 14.7 | 0.109 | 458 | 461 | 0.80 | --- |
| 23 | p-PDA | 1.21 | 50.9 | 3.04 | 6.9 10.7 | 0.134 | 462 | 468 | 1.48 | 20.9 |
| 24 | APAB | 1.40 | 52.2 | 2.37 | 9.5 16.5 | --- | 459 | 459 | 0.86 | 15.3 |
| 25 | M-APAB | 0.65 | 52.1 | 2.59 | 6.4 8.7 | 0.108 | 458 | 438 | 1.48 | 13.6 |
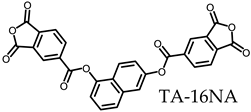 | ||||||||||
| No. | Diamine | ηred PAA (dL g−1) | Tg (°C) | CTE (ppm K−1) | E (GPa) | εb av/max (%) | σb (GPa) | Td5 in N2 (°C) | Td5 in Air (°C) | WA (%) | CHE (ppm/%RH) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 26 | 4,4′-ODA | 1.05 | 381 a 244 b | 50.4 | 3.05 | 4.1 5.3 | 0.096 | 447 | 447 | 0.79 | 4.1 |
| 27 | p-PDA | 3.26 | 404 a | 22.7 | --- c | --- c | --- c | 464 | 453 | 1.32 | 16.1 |
| 28 | o-TOL | 3.34 | 300 a 241 b | 25.6 | 3.61 | 21.9 39.9 | 0.188 | 451 | 441 | 1.08 | 4.1 |
| 29 | APAB | 3.02 | 344 a | 29.1 | 5.31 | 3.3 5.3 | 0.177 | 453 | 448 | 1.05 | 14.5 |
| 30 | M-APAB | 1.80 | 352 a | 28.7 | 4.36 | 4.2 5.7 | 0.130 | 442 | 431 | 1.03 | 8.1 |
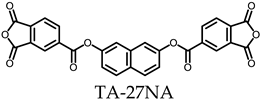 | |||||||||||
| No. | Diamine | ηred PAA (dL g−1) | Tg (°C) | CTE (ppm K−1) | E (GPa) | εb av/max (%) | σb (GPa) | Td5 in N2 (°C) | Td5 in Air (°C) | WA (%) | CHE (ppm/%RH) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 31 | 4,4′-ODA | 2.41 | 361 a 208 b | 41.2 | 3.15 | 8.3 11.5 | 0.142 | 475 | 468 | 0.54 | 2.1 |
| 32 | p-PDA | 2.74 | 418 a | 2.9 | 5.96 | 3.6 5.5 | 0.191 | 499 | 489 | 1.03 | 4.1 |
| 33 | m-TOL | 3.81 | 390 a 212 b | 29.4 | 6.05 | 4.0 5.6 | 0.188 | 450 | 461 | 0.84 | 1.8 |
| 34 | o-TOL | 3.42 | 413 a | 15.6 | 4.68 | 4.5 7.4 | 0.184 | 471 | 445 | 1.23 | 1.0 |
| 35 | APAB | 1.00 | 374 a | 14.6 | 4.71 | 5.5 7.8 | 0.183 | 482 | 480 | 0.89 | 4.7 |
| 36 | M-APAB | 0.89 | 398 a | 13.0 | 5.26 | 4.4 7.5 | 0.175 | 464 | 439 | 0.76 | 2.5 |
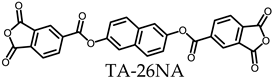 | |||||||||||
| No. | Diamine | ηred PAA (dL g−1) | Tg (°C) | CTE (ppm K−1) | E (GPa) | εb av/max (%) | σb (GPa) | Td5 in N2 (°C) | Td5 in Air (°C) | WA (%) | CHE (ppm/%RH) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 37 | 4,4′-ODA | 5.48 | 376 c 219 d | 47.7 | 2.56 | 32.6 51.5 | 0.172 | 493 | 488 | 0.30 | 0.23 |
| 38 | p-PDA | 13.6 | ND a | 4.3 | 4.85 | 6.9 9.6 | 0.223 | 511 | 501 | 0.91 | 5.5 |
| 39 | m-TOL | 7.90 | 369 b | 22.5 | 4.95 | 5.7 6.2 | 0.194 | 482 | 474 | 1.08 | --- |
| 40 | o-TOL | 7.87 | 405 b | 11.1 | 7.25 | 5.4 6.7 | 0.268 | 472 | 446 | 0.80 | --- |
| 41 | APAB | 1.53 | 409 b | 11.5 | 5.07 | 8.7 9.9 | 0.210 | 497 | 493 | 0.57 | --- |
| 42 | M-APAB | 1.12 | 372 b | 10.8 | 5.83 | 6.5 8.2 | 0.210 | 465 | 440 | 0.57 | --- |
| 43 | TFMB | 1.69 | 405 c | 16.9 | 6.80 | 7.1 11.6 | 0.180 | 508 | 475 | 0.33 | --- |
 | |||||||||||
| No. | Diamine | ηred PAA (dL g−1) | Tg (°C) | CTE (ppm K−1) | E (GPa) | εb av/max (%) | σb (GPa) | Td5 in N2 (°C) | Td5 in Air (°C) | WA (%) | CHE (ppm/%RH) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 44 | 4,4′-ODA | 4.10 | 347 a 188 b | 55.6 | 3.27 | 16.0 23.5 | 0.175 | 451 | 425 | 0.47 | --- |
| 45 | p-PDA | 1.65 | ~420 a | 6.7 | 7.98 | 6.4 9.3 | 0.317 | 454 | 430 | 1.07 | 3.2 |
| 46 | m-TOL | 5.72 | 364 a 185 b | 21.4 | 5.99 | 3.9 4.9 | 0.186 | 447 | 411 | 0.61 | --- |
| 47 | o-TOL | 5.36 | 395 a | 13.0 | 7.62 | 5.5 7.1 | 0.289 | 462 | 441 | 0.15 | --- |
| 48 | APAB | 1.84 | 365 a | 5.4 | 5.52 | 4.8 6.4 | 0.213 | 455 | 436 | 0.34 | |
| 49 | M-APAB | 1.69 | 351 a | 15.9 | 8.24 | 6.0 7.5 | 0.316 | 453 | 420 | 0.62 | --- |
 | |||||||||||
| No. | Diamine | ηred PAA (dL g−1) | Tg (°C) | CTE (ppm K−1) | E (GPa) | εb av/max (%) | σb (GPa) | Td5 in N2 (°C) | Td5 in Air (°C) | WA (%) | CHE (ppm/%RH) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 50 | 4,4′-ODA | 1.62 | 222 a | 60.1 | 2.73 | 11.4 17.8 | 0.140 | 471 | 459 | 0.23 | --- |
| 51 | 3,4′-ODA | 0.59 | 207 a | 64.8 | 2.44 | 26.2 29.8 | 0.110 | 474 | 466 | 0.30 | --- |
| 52 | BAPS | 1.00 | 228 a | 61.3 | 2.47 | 7.5 10.6 | 0.116 | 474 | 472 | 0.39 | --- |
| 53 | p-PDA | 1.57 | 347 b 235 d | 21.7 | 4.43 | 11.6 14.0 | 0.237 | 481 | 470 | 0.65 | 3.9 |
| 54 | m-TOL | 1.78 | 349 b 205 d | 24.4 | 3.83 | 6.1 6.9 | 0.170 | 449 | 468 | 0.45 | --- |
| 55 | o-TOL | 0.63 | 331 b 207 d | 28.0 | 3.94 | 4.6 5.4 | 0.168 | 465 | 456 | 0.39 | --- |
| 56 | APAB | 1.14 | 342 c 213 d | 24.4 | 3.80 | 9.9 13.1 | 0.179 | 465 | 463 | 0.43 | --- |
| 57 | M-APAB | 0.76 | 297 c 199 d | 26.9 e | 4.36 | 6.7 9.8 | 0.172 | 462 | 434 | 0.50 | --- |
| 58 | TFMB | 0.64 | 197 a | 52.5 | 3.46 | 4.3 4.9 | 0.125 | 482 | 471 | --- | --- |
 | |||||||||||
| No. | Diamine | ηred PAA (dL g−1) | Tg (°C) | CTE (ppm K−1) | E (GPa) | εb av/max (%) | σb (GPa) | Td5 in N2 (°C) | Td5 in Air (°C) | WA (%) | CHE (ppm/%RH) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 59 | 4,4′-ODA | 1.26 | 377 a 193 b | 56.2 | 2.72 | 21.8 25.7 | 0.189 | 472 | 466 | 0.71 | --- |
| 60 | p-PDA | 1.49 | 422 a | 5.5 | 7.61 | 5.3 6.3 | 0.299 | 479 | 461 | 0.93 | 3.4 |
| 61 | m-TOL | 1.81 | 325 a | 20.7 | 5.62 | 14.4 18.2 | 0.300 | 467 | 469 | 0.69 | --- |
| 62 | o-TOL | 2.33 | 407 a | 19.1 | 6.51 | 5.4 6.0 | 0.248 | 472 | 462 | 0.58 | 0.8 |
| 63 | APAB | 0.84 | 373 a | 14.1 | 5.87 | 5.3 6.3 | 0.193 | 469 | 460 | 0.75 | --- |
| 64 | M-APAB | 0.73 | 373 a 199 b | 20.5 | 5.26 | 4.7 5.3 | 0.177 | 467 | 447 | 1.00 | --- |
| 65 | TFMB | 1.19 | 350 a | 30.9 | 4.76 | 13.7 26.1 | 0.150 | 473 | 459 | 0.26 | --- |
 | |||||||||||
| No. | Diamine | ηred PAA (dL g−1) | Tg (°C) | CTE (ppm K−1) | E (GPa) | εb av/max (%) | σb (GPa) | Td5 in N2 (°C) | Td5 in Air (°C) | WA (%) | CHE (ppm/%RH) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 66 | 4,4′-ODA | 1.22 | 337 a 197 c | 50.1 | 3.13 | 40.5 47.5 | 0.195 | 484 | 482 | 0.45 | --- |
| 67 | 3,4′-ODA | 1.09 | 198 b | 28.4 | 3.68 | 37.2 52.4 | 0.161 | 488 | 480 | 0.44 | --- |
| 68 | BAPS | 0.95 | 235 b | 45.2 | 2.53 | 4.7 6.2 | 0.106 | 492 | 478 | 0.58 | --- |
| 69 | p-PDA | 1.84 | 348 a 254 c | 15.7 | 4.22 | 14.3 25.5 | 0.203 | 487 | 484 | 0.62 | 3.1 |
| 70 | m-TOL | 2.81 | 371 a 205 c | 22.3 | 5.65 | 8.4 12.3 | 0.223 | 474 | 472 | 0.34 | --- |
| 71 | o-TOL | 2.52 | 354 a 249 c | 16.2 | 4.68 | 13.4 20.6 | 0.258 | 477 | 473 | 0.25 | 1.6 |
| 72 | APAB | 1.03 | 316 a 181 c | 23.9 | 4.14 | 21.6 27.4 | 0.241 | 481 | 477 | 0.33 | --- |
| 73 | M-APAB | 0.99 | 309 a 196 c | 38.2 | 3.29 | 19.7 33.5 | 0.195 | 466 | 461 | 0.42 | --- |
| 74 | TFMB | 1.32 | 205 b | 49.1 | 3.16 | 64.5 98.0 | 0.162 | 498 | 494 | 0.15 | --- |
 | |||||||||||
| No. | Diamine | ηred PAA (dL g−1) | Tg a (°C) | CTE (ppm K−1) | E (GPa) | εb av/max (%) | σb (GPa) | Td5 in N2 (°C) | Td5 in Air (°C) | WA (%) | CHE (ppm/%RH) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 75 | 4,4′-ODA | 1.52 | 372 | 46.7 | 3.26 | 31.9 36.5 | 0.203 | 474 | 472 | 0.47 | 4.2 |
| 76 | p-PDA (70) 4,4′-ODA (30) | 1.26 | 392 | 10.7 | 5.21 | 8.9 11.5 | 0.207 | 481 | 456 | 0.51 | --- |
| 77 | APAB (70) 4,4′-ODA (30) | 0.66 | 378 | 29.1 | 4.40 | 10.0 17.7 | 0.166 | 483 | 448 | 0.26 | --- |
 | |||||||||||
| No. | TCDA | Diamine | ηred PAA (dL g−1) | Tg (°C) | CTE (ppm K−1) | E (GPa) | εb av/max (%) | σb (GPa) | Td5 in N2 (°C) | Td5 in air (°C) | WA (%) | CHE (ppm/%RH) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 78 | TA-DPQP | 4,4′-ODA | 3.89 | 201 a | 41.6 | 2.56 | 32.6 51.5 | 0.172 | 482 | 477 | 0.35 | --- |
| 79 | ibid | p-PDA | 5.67 | 412 c 220 d | 12.7 | 3.22 | 31.3 36.2 | 0.207 | 501 | 467 | 0.38 | 2.0 |
| 80 | ibid | m-TOL | 5.83 | 311 c 210 d | 28.6 | 5.34 | 13.0 15.3 | 0.258 | 484 | 456 | 0.33 | --- |
| 81 | ibid | o-TOL | 5.72 | 364 b 244 d | 15.2 | 5.40 | 4.7 5.8 | 0.196 | 470 | 444 | --- g | --- |
| 82 | ibid | APAB | 1.40 | 329 c 224 d | 21.8 | 3.76 | 11.6 15.5 | 0.199 | 495 | 480 | 0.46 | --- |
| 83 | ibid | M-APAB | 1.11 | 296 c 200 d | 22.1 | 5.05 | 9.8 11.2 | 0.268 | 473 | 454 | 0.41 | --- |
| 84 e | TA-DPQP (70) TA-44BP (30) | p-PDA | 3.04 | 327 c 224 d | 13.0 | 4.08 | 17.8 22.0 | 0.258 | 494 | 487 | 0.55 | 2.6 |
| 85 f | ibid | ibid | 4.24 | 325 c 221 d | 15.1 | 4.03 | 17.0 29.1 | 0.211 | 495 | 490 | 0.40 | 1.3 |
| 86 e | TA-DPQP (50) TA-44BP (50) | ibid | 3.19 | 417 b 225 d | 11.3 | 4.41 | 12.8 17.7 | 0.227 | 498 | 494 | 0.54 | 1.4 |
| 87 e | ibid | p-PDA (80) 4,4′-ODA (20) | 3.26 | 361 b 223 d | 14.5 | 3.25 | 8.5 13.8 | 0.166 | 495 | 488 | 0.41 | 1.8 |
 | ||||||||||||
| Properties | Parameters | Relative Rank | ||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | ||
| Physical heat resistance | Tg (°C) | ≤210 | 220–250 | 260–290 | 300–330 | ≥360 or ND a |
| Thermal dimensional stability | CTE (ppm K−1) | ≥70 | 60–50 | 45–35 | 30–20 | ≤10 |
| Low water uptake | WA (%) | ≥3.0 | 2.5–2.0 | 1.5–1.0 | 0.6–0.3 | ≤0.1 |
| Hygroscopic dimensional stability | CHE (ppm/%RH) | ≥50 | 30–20 | 15–10 | 8–4 | ≤2 |
| Film toughness | εb max (%) | No film-forming ability or ≤2 | 5–10 | 20–30 | 40–60 | ≥80 |
| Flame retardancy | UL-94V | V-2 failed | V-2 passed | V-1 passed | --- | V-0 passed |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hasegawa, M.; Takeuchi, Y.; Saito, T. Poly(ester imide)s with Low Linear Coefficients of Thermal Expansion and Low Water Uptake (VIII): Structure–Flame Retardancy Relationship. Polymers 2024, 16, 1967. https://doi.org/10.3390/polym16141967
Hasegawa M, Takeuchi Y, Saito T. Poly(ester imide)s with Low Linear Coefficients of Thermal Expansion and Low Water Uptake (VIII): Structure–Flame Retardancy Relationship. Polymers. 2024; 16(14):1967. https://doi.org/10.3390/polym16141967
Chicago/Turabian StyleHasegawa, Masatoshi, Yuta Takeuchi, and Takayuki Saito. 2024. "Poly(ester imide)s with Low Linear Coefficients of Thermal Expansion and Low Water Uptake (VIII): Structure–Flame Retardancy Relationship" Polymers 16, no. 14: 1967. https://doi.org/10.3390/polym16141967
APA StyleHasegawa, M., Takeuchi, Y., & Saito, T. (2024). Poly(ester imide)s with Low Linear Coefficients of Thermal Expansion and Low Water Uptake (VIII): Structure–Flame Retardancy Relationship. Polymers, 16(14), 1967. https://doi.org/10.3390/polym16141967






