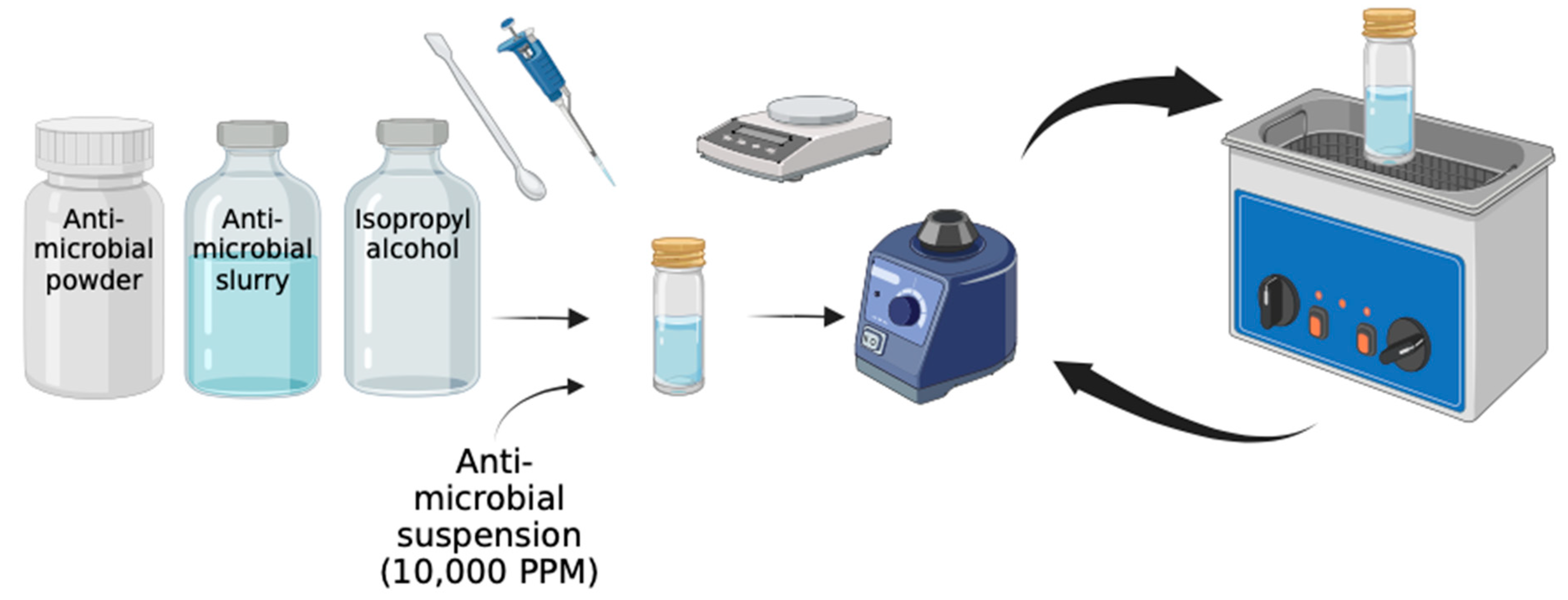
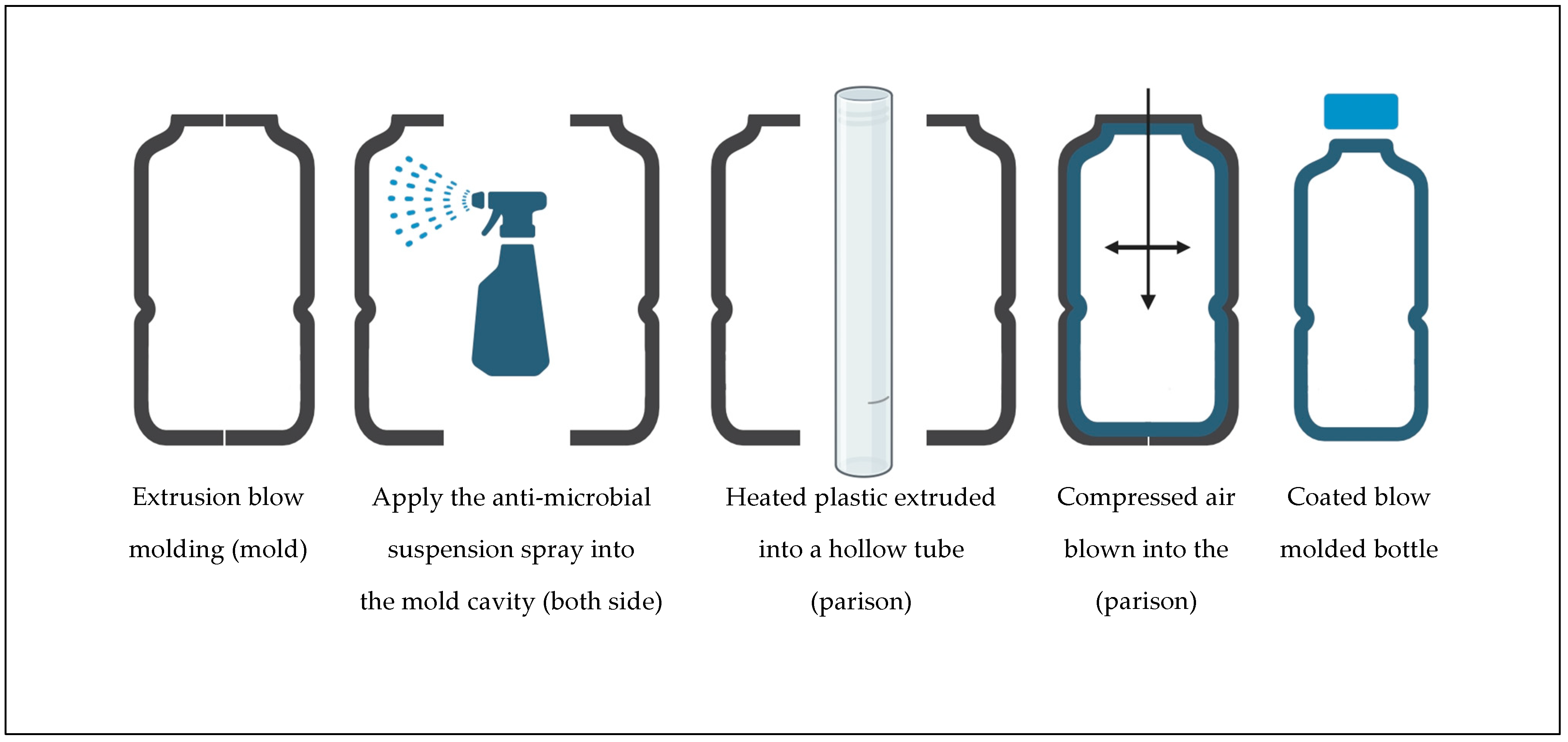
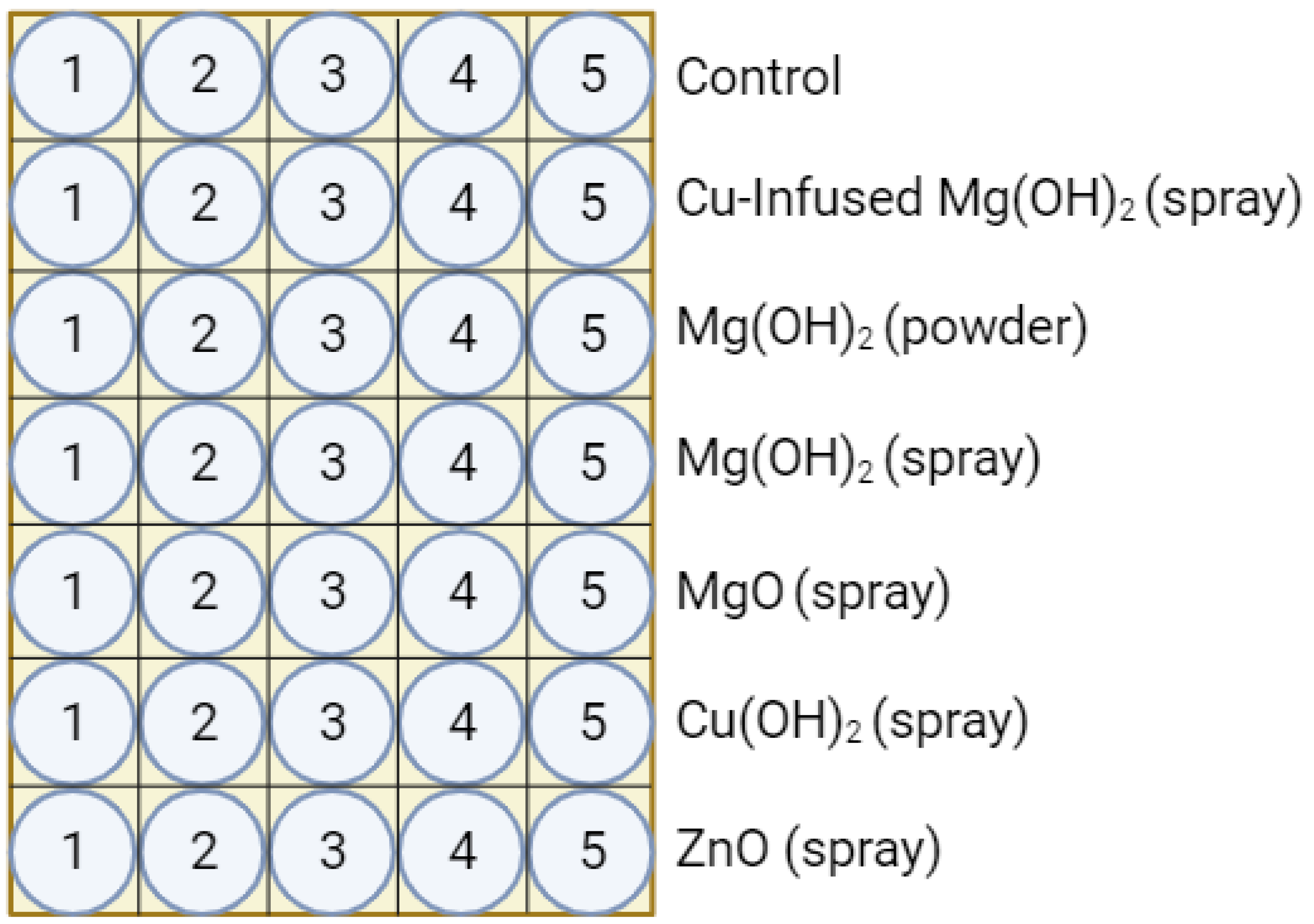
1. Introduction
Extrusion blow molding is a common manufacturing method for generating hollow plastic containers such as bottles and jars. These containers are utilized in many industries, including food and beverage, pharmaceutical, cosmetic, and household product production [
1]. However, microbial contamination can occur on the surfaces of blow-molded bottles, necessitating the development and application of effective anti-microbial treatments to mitigate the hazards associated with microbial growth. For instance, the shelf life of a contained sensitive material stored in blow-molding plastic containers is highly dependent upon the level of sterility of the plastic. Sterilization techniques and aseptic filling are thus commonly employed [
2]. Additionally, microorganisms can be transmitted onto the surfaces of blow-molded bottles through various means [
3]. For instance, an individual may touch contaminated surfaces like door handles, countertops, or packaging materials and then inadvertently transfer microbes onto the bottles through direct contact. Microorganisms can also be disseminated through air movement within a production facility. In environments where microbial aerosols are prevalent, such as hospitals or crowded spaces, airborne microorganisms can also land on the surfaces of blow-molded bottles in their end use location. Airborne fungal spores in a room have been shown to settle on bottles, leading to contamination [
4].
The surfaces of extrusion blow-molded bottles provide an ideal environment for bacterial growth due to their smooth texture and organic residues [
1]. Colonization of surfaces by bacteria such as
Escherichia coli (
E. coli),
Staphylococcus aureus (
S. aureus), and
Pseudomonas aeruginosa (
P. aeruginosa) can result in rapid bacterial growth and the development of biofilms. Kim et al. (2020) state that biofilms may cause food degradation and perhaps spread diseases to customers. Biofilms can also serve as long-term bacterial contamination reservoirs [
5]. To prevent bacterial colonization, creating anti-microbial surfaces for blow-molded bottles is crucial [
6]. Growing demand for plastic containers in various industries has rapidly increased the use of blow molding in recent years [
7,
8]. Chadha et al. (2022) remark that population growth, urbanization, and the increased demand for packaged goods are all factors that affect the extrusion blow molding industry’s market size. The extrusion blow molding market was estimated to be 100 billion USD in 2020, and according to Chadha et al. (2022), it is expected to increase at a CAGR of 5% between 2021 and 2028 [
9]. Polyethylene (PE) and polyethylene terephthalate (PET) are two of the most widely used plastics in blow molding and account for a sizable share of global plastic consumption [
10].
Advancements in the development of anti-microbial agents for plastics application have resulted in the emergence of two distinct categories, leachable and non-leachable agents, according to Gulati, Sharma, and Sharma (2021). Leachable agents can release anti-microbial compounds from the polymer matrix, offering sustained efficacy [
11]. However, concerns about potentially harmful substance release arise. For example, silver nanoparticles embedded in the polymer gradually release silver ions when they come in contact with microorganisms, leading to the detection of silver in good it intends to protect [
12]. In contrast, non-leachable agents remain fixed on the plastic surface, offering immediate and localized anti-microbial effects. Non-leachable agents include Mg(OH)
2 and chitosan, a natural biopolymer that has shown promise in anti-microbial packaging [
12]. While non-leachable agents may be susceptible to wear and degradation, recent research has focused on formulating and evaluating these agents for enhanced performance and safety [
11]. Since non-leachable agents require direct contact with microbes to have an effect, they are better suited as coatings rather than compounded articles, where the additive would be dispersed inside the matrix [
13].
Various anti-microbial substances have been investigated in the field of extrusion blow molding. Incorporating copper (Cu) may be a viable method for doping in Mg(OH)
2, thereby enabling the modulation of the material’s optical bandgap. CuO can modulate electron field emission characteristics owing to its low potential barrier [
14]. However, more research is required to determine its effectiveness.
Anti-microbial agents have been incorporated into the process through different methods. One of these methods is melt-compounding with plastics like PE and PET [
15]. Another method is the use of coating technologies applied to the exterior of the bottles [
16].
In the case of melt-compounding, the anti-microbial agents are mixed with the plastic materials during the manufacturing process, creating a uniform matrix of the polymer and additive [
17]. According to Huang et al., the coating process applies a layer of anti-microbial agent to the bottle surfaces, resulting in a thin layer, with thicknesses typically ranging from a few to tens of micrometers [
16]. The adjustment of concentrations and thicknesses of anti-microbial agents is contingent upon the targeted degree of anti-bacterial efficacy and the particular demands of the application, as noted by Huang et al. [
16]. Notably, the types of anti-microbial agents and the coating techniques employed may exhibit variations across different investigations, contingent upon factors such as the specific microorganisms being targeted and the intended duration of anti-microbial efficacy [
16].
The study conducted by Hutasoit et al. has revealed that Cu-infused Mg(OH)
2 could exhibit robust anti-bacterial characteristics against a wide range of bacteria, including Gram-positive and Gram-negative strains, such as
Salmonella spp.,
E. coli, and
S. aureus because of the
Cu and
Mg contents [
18]. Another study has shown that the alkyd resin nanocomposite derived from palm oil containing Mg(OH)
2/MgO colloidal NPs has displayed catalytic performance and anti-microbial activity. Some bacteria, including methicillin-resistant
S. aureus and
P. aeruginosa, are killed by Mg(OH)
2 and Cu(OH)
2, respectively [
19]. According to Birkett et al., the concentration and thickness of an anti-microbial coating greatly affect its efficiency [
20]. Higher concentrations of anti-microbial compounds are typically associated with increased anti-microbial action. Darvish et al. pointed out that obtaining the optimum concentration is essential to avoid unintended implications, such as altering the polymer’s physical characteristics or making leaching of the agent more likely [
21]. Since the integrity of extrusion blow-molded bottles must be preserved during anti-microbial treatment, it is crucial to determine the concentration of the anti-microbial agent needed to achieve this goal [
22]. Similarly, the thickness of the coating layer influences the anti-microbial performance. Thicker coatings can increase protection against microbial contamination [
23]. However, excessively thick coatings may be prone to cracking or peeling, compromising their effectiveness [
24]. Recent publications have highlighted the importance of optimizing the coating thickness to balance both anti-microbial activity and coating durability [
25,
26].
Among the various anti-bacterial agents mentioned, copper-infused Mg(OH)
2 has exhibited potential efficacy against Gram-positive and Gram-negative bacteria [
27]. The broad-spectrum anti-bacterial activity of copper ions released from copper-infused Mg(OH)
2 targets DNA, proteins, and bacterial cell membranes [
27]. This mechanism makes it effective against a wide range of bacteria, including those with varying cell wall structures [
6,
27].
The effectiveness of anti-microbial substances such as Mg(OH)
2, Cu(OH)
2, MgO, CuCl
2, and ZnO can differ, depending on the type of bacteria [
28]. According to research findings, CuCl
2 exhibits noteworthy inhibitory properties against the proliferation of Gram-negative bacterial strains such as
E. coli and
P. aeruginosa. In contrast, it has been reported that MgO and ZnO exhibit greater efficacy against Gram-positive bacteria [
29]. The observed variation in efficacy underscores the diverse antimicrobial properties of these compounds, as reported by Jakubovskis et al. [
30].
The mechanisms by which anti-microbial particles induce cell death are multifaceted and contingent upon the particular agent utilized. Some examples of anti-microbial modes of action are the disruption of cell membranes, the production of reactive oxygen species (ROS), the suppression of enzymatic activities, or the induction of damage to DNA. According to Imani et al., the anti-microbial effectiveness of Cu-infused Mg(OH)
2, Mg(OH)
2, Cu(OH)
2, MgO, CuCl
2, and ZnO is often attributed to their multi-modal actions [
31]. Imani et al. report that one particular mechanism entails the interference of bacterial cell membranes through the utilization of distinct anti-microbial nanoparticles, namely Mg(OH)
2, Cu(OH)
2, MgO, CuCl
2, and ZnO [
31]. The NPs can interact with the bacterial cell membrane, thereby compromising its structural integrity and the consequent release of its cellular constituents. The disruption of the membrane structure and function results in the disturbance of crucial cellular processes and eventual cell death, as reported by Imani et al. [
31].
An additional mechanism involves the production of ROS. According to Smaoui et al., specific anti-microbial particles, including Mg(OH)
2 and Cu-infused Mg(OH)
2, can produce ROS upon exposure to moisture or light. ROS, such as hydroxyl radicals and superoxide ions, elicit oxidative harm within bacterial cells, thus deactivating them [
32].
The suppression of enzymatic activity represents another pivotal mechanism utilized by certain anti-microbial particles. Peters et al. have demonstrated the effectiveness of Cu-infused Mg(OH)
2 and CuCl
2 in impeding the function of crucial enzymes in bacterial cells [
33]. This interference with enzymatic function leads to the impairment of crucial metabolic processes, ultimately culminating in the demise of the bacteria [
33].
In addition, it has been observed that anti-microbial agents containing copper, such as Cu-infused Mg(OH)
2 and Cu(OH)
2, can potentially induce DNA damage in bacterial cells. According to Rojas et al., the agents interact with bacterial DNA, resulting in structural harm and disruption of its replication and transcription mechanisms [
34]. The amalgamation of physical and chemical mechanisms in these particles effectively contributes to their anti-microbial properties, thereby enabling them to either inhibit bacterial growth or cause bacterial death [
34].
According to Gumienna et al., the regulatory approval status of anti-microbial agents utilized in blow-molding applications may differ, based on the particular agent and its intended application, as determined by the Food and Drug Administration (FDA) [
35]. The authors state that certain anti-microbial agents utilized in blow molding have not obtained approval from the FDA. Some anti-microbial agents, including copper and zinc compounds, have been generally recognized as safe (GRAS) by the FDA for diverse applications [
36]. The substances that have been designated as GRAS have been deemed to meet the safety requirements set forth by the FDA and are therefore suitable for use in contact with pharmaceutical or food items, as per the findings of Mania et al. [
36]. Evaluating anti-microbial agents’ toxicity is critical due to its potential impact on human cells. The toxicity of various NPs, including Cu-infused Mg(OH)
2, Mg(OH)
2, Cu(OH)
2, MgO, CuCl
2, and ZnO, has been investigated in human cells through research conducted by Naz et al. [
37] Their results indicate that nanoparticles typically demonstrate negligible cytotoxicity at the lower concentrations that fall within the anti-microbial range, and that they are well received by human cells [
37]. However, high concentrations or prolonged exposure to specific anti-microbial agents may lead to adverse effects [
38].
Furthermore, the durability and longevity of the anti-microbial effects are important aspects related to anti-microbial techniques in extrusion blow molding applications [
22]. The influence of environmental conditions on the performance of anti-microbial coatings, as well as the development of sustainable and environmentally friendly anti-microbial agents, are also significant. These aspects are critical for the practical implementation and commercial viability of anti-microbial solutions in the extrusion blow molding industry [
39].
Developing effective anti-microbial techniques in blow molding applications is crucial to ensuring product safety and effective protection from microbial contamination. The utilization of anti-microbial agents, including Cu-infused Mg(OH)2, Mg(OH)2, Cu(OH)2, MgO, CuCl2, and ZnO, has shown promising results in inhibiting bacterial growth on blow-molded bottle surfaces. These agents’ concentrations, thicknesses, and mechanisms of action play essential roles in their anti-microbial efficacy. Comprehensive toxicity evaluations are necessary in the future to ensure the safety of these agents for human health.
4. Discussion
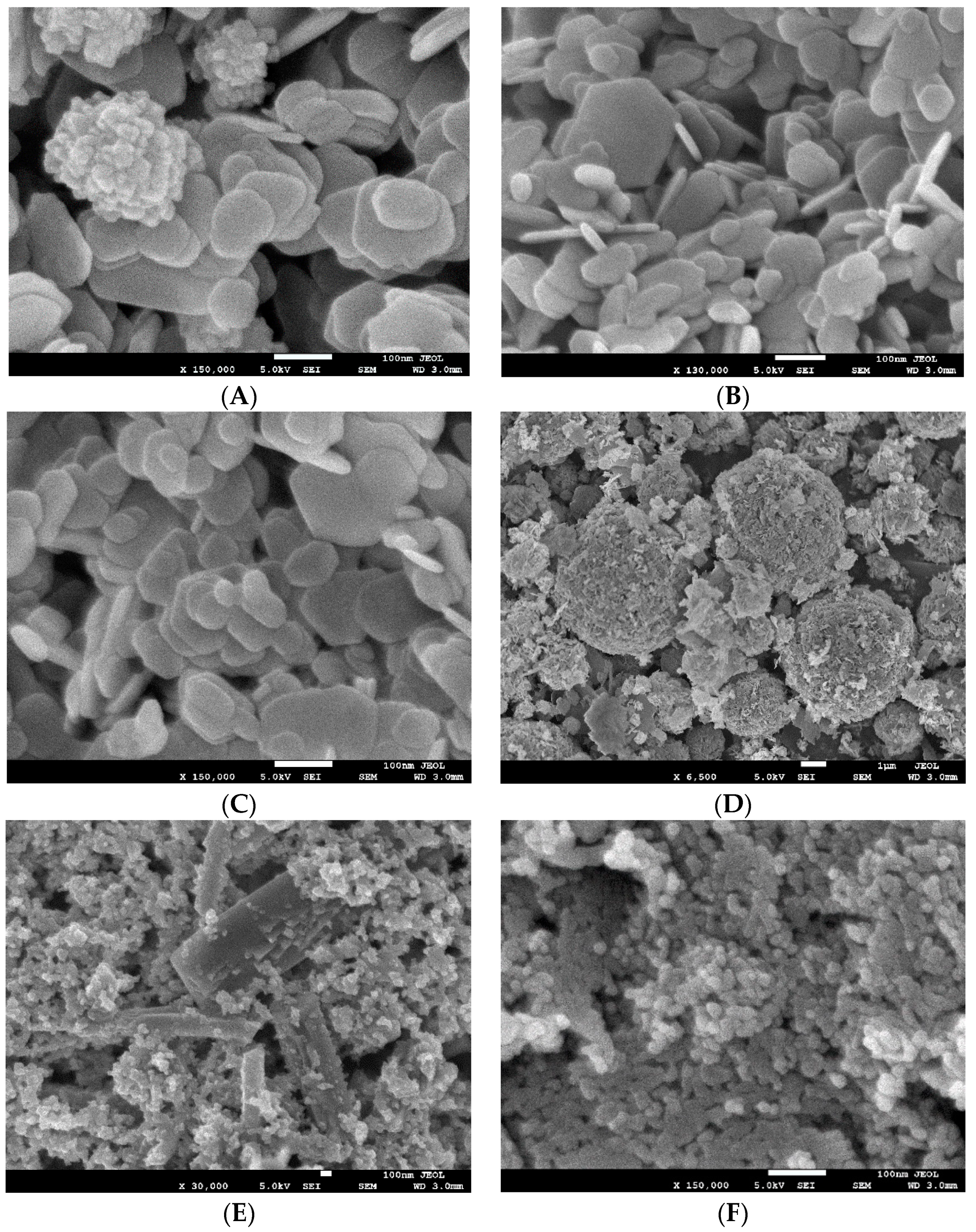
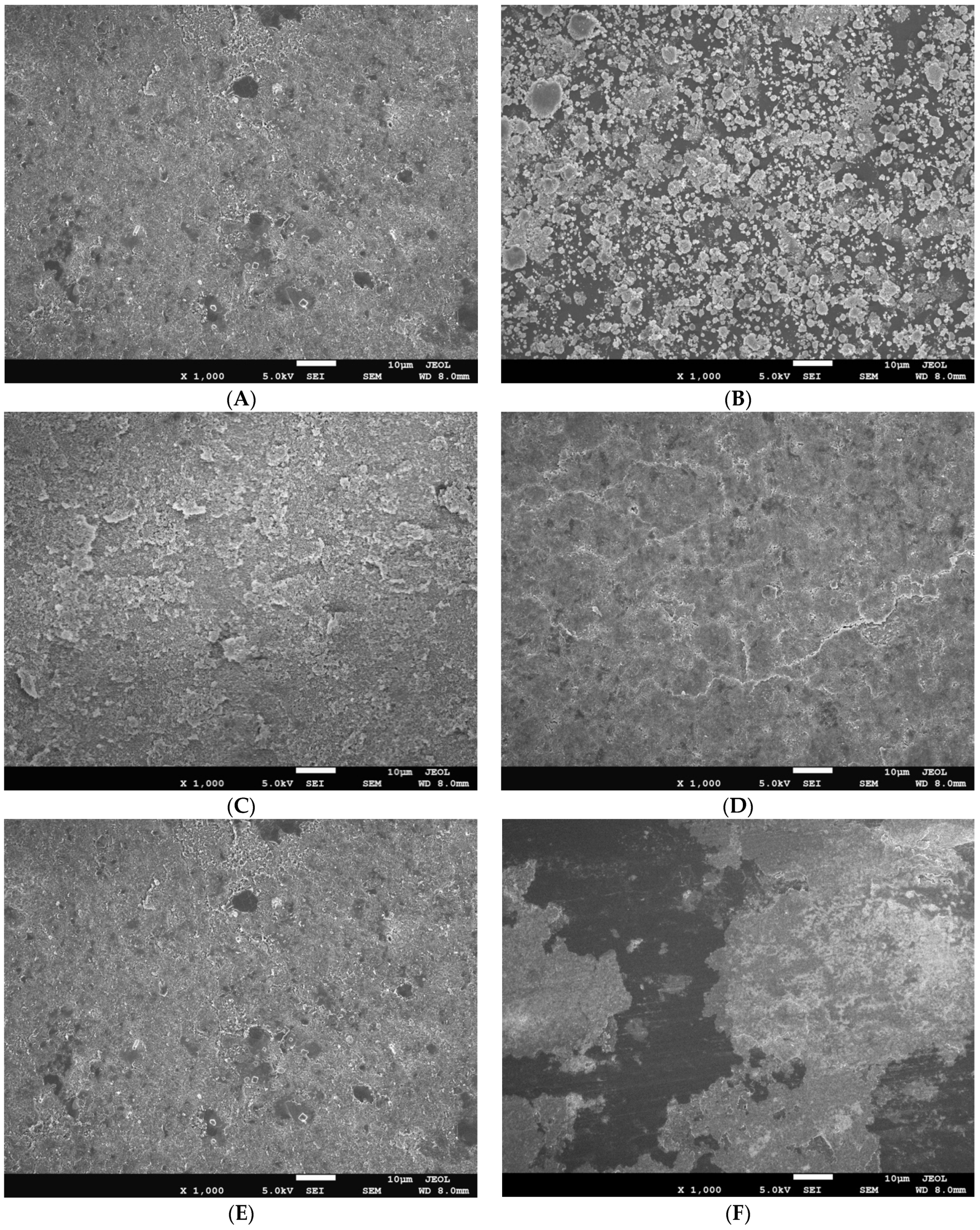
SEM was used to characterize nanoparticle size and morphology, as shown in
Figure 6. Mg(OH)
2 nanoparticles appeared as platelets ranging in size from 50–150 nm. Cu-infused Mg(OH)
2 contained similar platelets of Mg(OH)
2, with the addition of spherical, lobed Cu nanoparticles approximately 150 nm in diameter. MgO nanoparticles formed spherical agglomerates comprised of angular flakes. The flake size ranged from 50–200 nm; spherical agglomerates of MgO flakes measured 1–6 µm in diameter. Cu(OH)
2 appeared in two distinct sizes; large rectangular crystals greater than 500 nm in length were mixed with small rectangular nanoparticles of 20–50 nm in size. ZnO nanoparticles had a rounded shape measuring approximately 10–25 nm.
Blow-molded bottles thermally embossed with anti-microbial agents were analyzed using
SEM and
EDX to determine the uniformity of the coatings and to confirm the chemical composition of the nanoparticle layer. SEM images show uniform nanoparticle coverage in spray applied coatings of Cu-infused Mg(OH)
2, Mg(OH)
2, MgO, and Cu(OH)
2. However, ZnO spray provided uneven coating, and large areas of exposed bottle surface were visible. Mg(OH)
2 applied in powder form caused significant aggregation of nanoparticles, resulting in a spattered, irregular coating, with the bottle surface exposed throughout the disk, as shown in
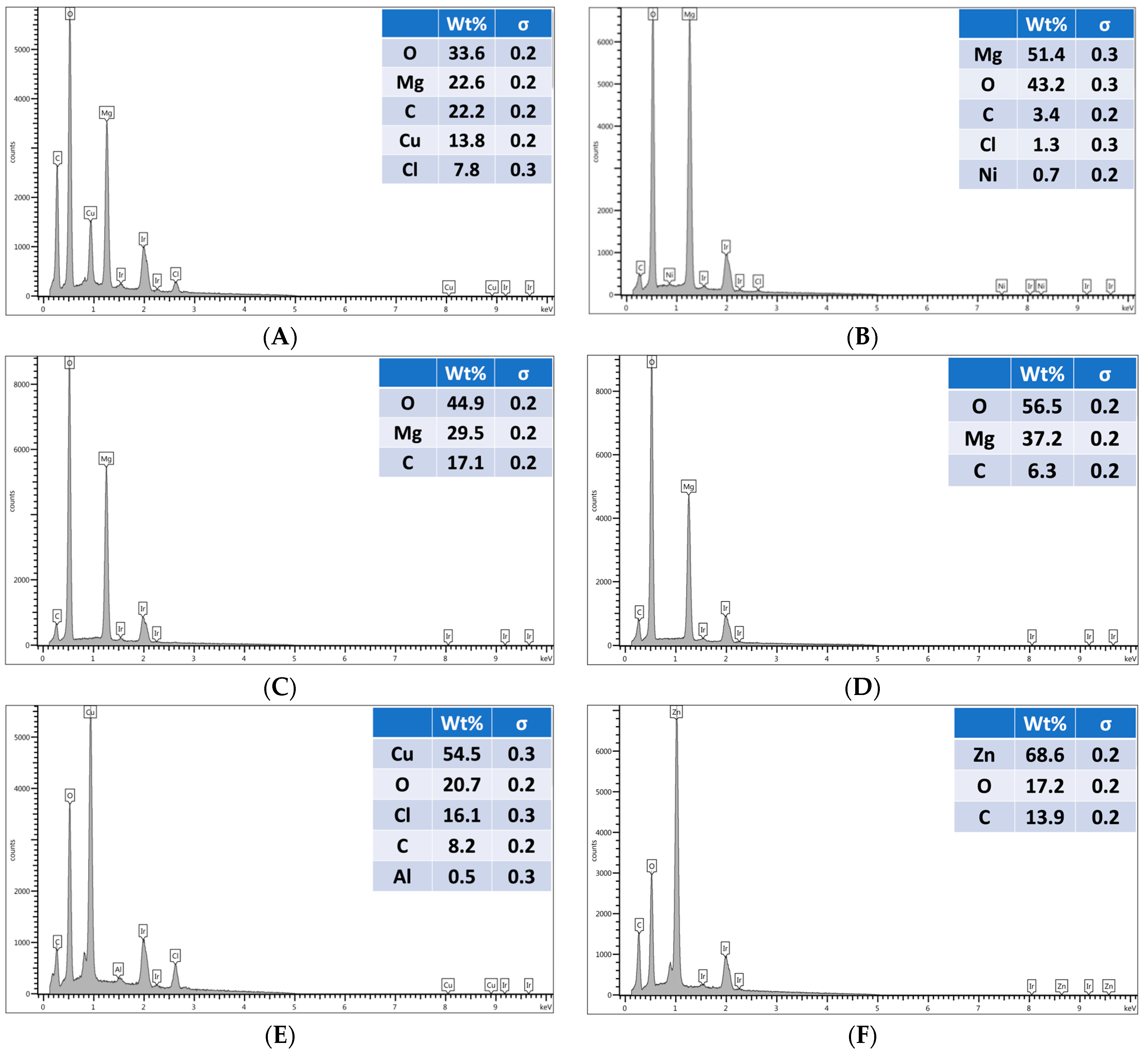
Figure 7. EDX analysis of uniformly coated areas of blow-molded bottles confirmed the presence and composition of the expected nanoparticles on the bottle surface, as shown in
Figure 8. In a previous study, we showed that anti-microbial nanoparticles such as Mg(OH)
2 and CuCl
2 can be affixed to thermoplastic sheets through a thermal embossing process [
42]. The Mg(OH)
2 and CuCl
2 nano crystals were coated on the sheet from a nano crystal suspension, dried, and then the coated sheet was heat pressed. The sheet successfully killed microbes, and the crystals that were thermally fixed on the surface were not affected by wiping or washing the surface [
42]. A limitation of this approach is that such thermal embossing methods can only be applied to sheet substrates, and not to articles with complex shapes, as the heat pressing steps required to fix the crystals to the plastic’s surface are difficult to achieve for non-flat shapes.
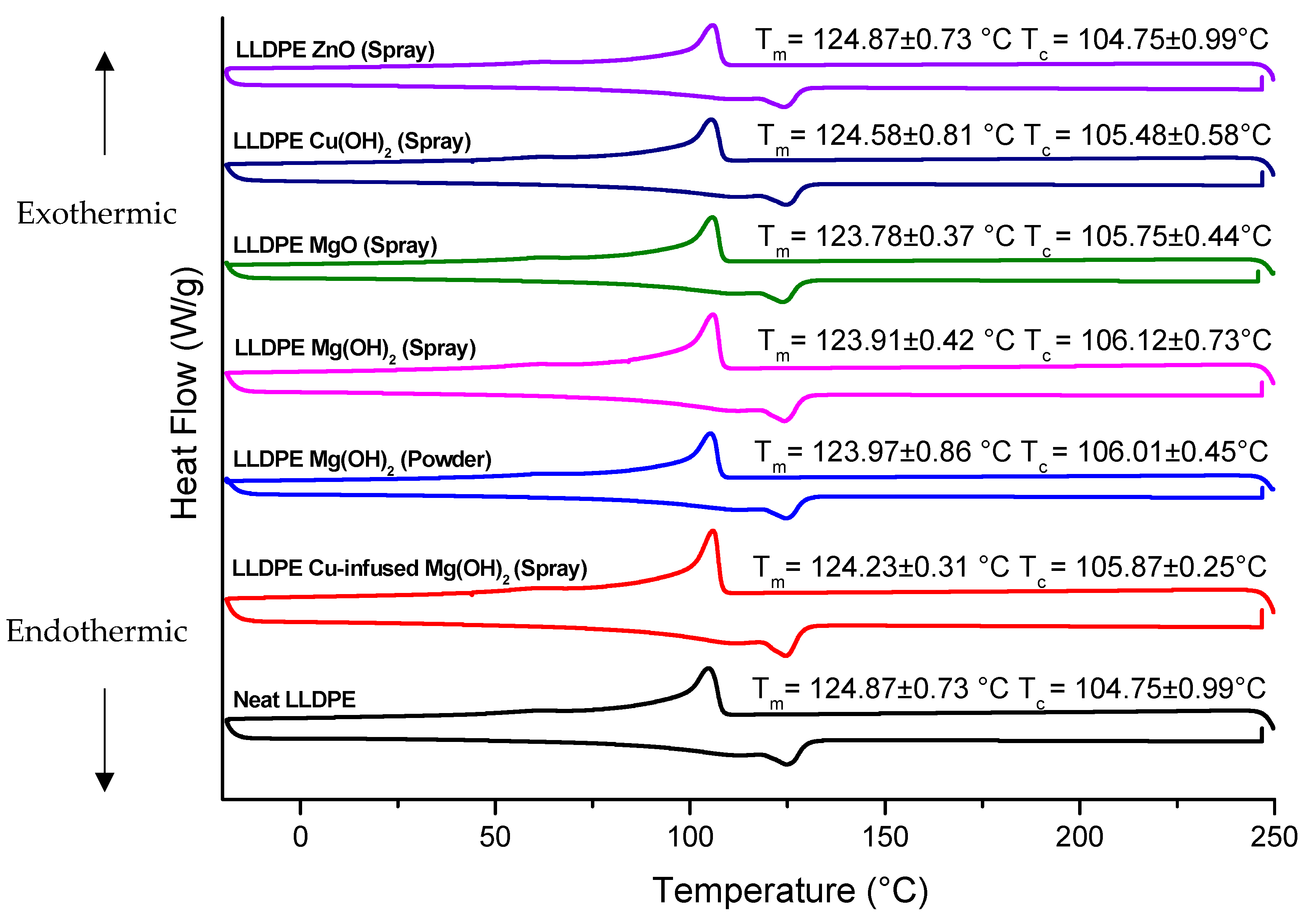
The DSC analysis of the LLDPE extrusion blow-molded bottles thermally embossed with Cu-infused Mg(OH)
2 (spray) showed the following
thermal properties: a
Tm of 124.23 °C ± 0.10, which was 0.51% lower compared to that of the neat LLDPE bottle (124.87 °C ± 0.54), as shown in
Figure 9 and
Table 1; a
Tc of (105.87 °C ± 0.87), which was 1.07% higher compared to that of the neat LLDPE bottle (104.75 °C ± 1.32), as shown in
Figure 9 and
Table 1; a crystallinity (%) of 34.82% ± 1.77, which was 5.92% lower compared to that of the neat LLDPE bottle (37.01% ± 1.25), as shown in
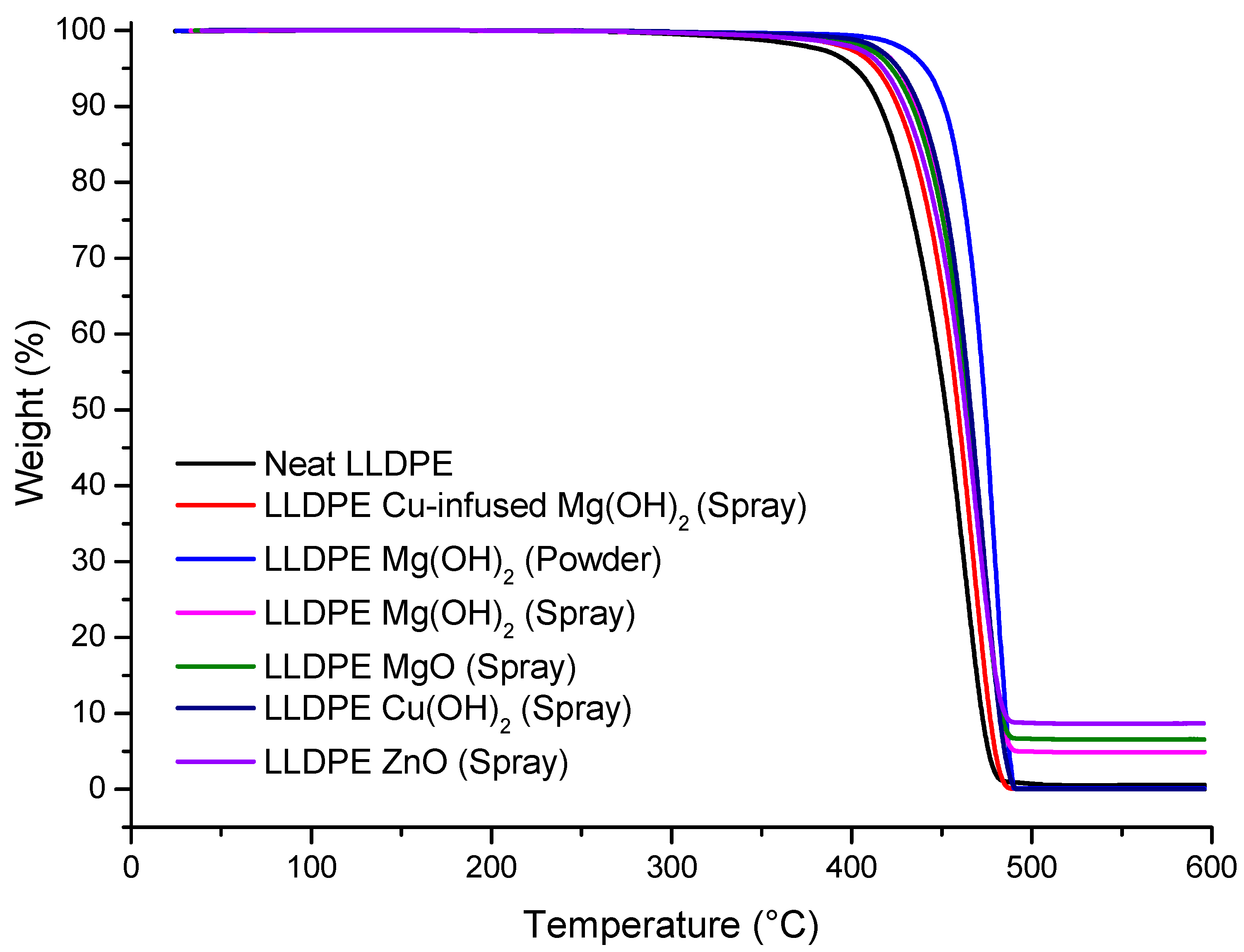
Table 1; the temperature at which 5% weight loss occurred was 413.59 °C ± 0.86, which was 2.88% higher than that of the neat LLDPE (402.03 °C ± 1.62), as shown in
Figure 10 and
Table 2, and the estimated inorganic residual (wt.%) at around 600 (°C) was 0.00 ± 0.43, which was 100% lower than that of the neat LLDPE (0.61 ± 0.11), as shown in
Figure 10 and
Table 2. Overall, the anti-microbial NPs did not significantly impact the thermal properties of the LLDPE extrusion blow-molded bottles. Cu-infused Mg(OH)
2 is a novel anti-microbial agent that had not been previously used for polymer application [
43].
The LLDPE extrusion blow-molded bottles thermally embossed with LLDPE Mg(OH)
2 (powder) showed the following
thermal properties: a
Tm of 123.97 °C ± 1.54, which was 0.72% lower compared to that of the neat LLDPE bottle (124.87 °C ± 0.54), as shown in
Figure 9 and
Table 1; a
Tc of 106.01 °C ± 1.44, which was 1.20% higher compared to that of the neat LLDPE bottle (104.75 °C ± 1.32), as shown in
Figure 9 and
Table 1, a crystallinity (%) of 35.34% ± 0.87, which was 4.51% lower compared to that of the neat LLDPE bottle (37.01% ± 1.25), as shown in
Table 1; the temperature at which 5% weight loss occurred was 441.28 °C ± 1.98, which was 9.76% higher than that of the neat LLDPE (402.03 °C ± 1.62), as shown in
Figure 10 and
Table 2, and the estimated inorganic residual (wt.%) occurred at around 600 (°C) (0.12 ± 0.82), which was 80% lower than that of the neat LLDPE (0.61 ± 0.11), as shown in
Figure 10 and
Table 2. Overall, the anti-microbial NPs did not significantly impact the thermal properties of the LLDPE extrusion blow-molded bottles. The Mg(OH)
2 is a novel anti-microbial agent because of its unique nanoparticle sizes and shapes [
44]. Mg(OH)
2 NPs are broad spectrum anti-microbial agents. Dong et al. demonstrated the anti-microbial activity of Mg(OH)
2 NPs against
E. coli and the plant-associated bacterium
Burkholderia phytofirmans [
45]. Additional plant-associated pathogens,
Xanthomonas alfalfa and
Pseudomonas syringae (Huang et al.), were eliminated by Mg(OH)
2 NPs, as were the oral, caries-associated bacteria
Streptococcus mutans [
46] and
Streptococcus sobrinus (Okamoto et al.) [
47]. Additional work from our laboratories showed the Mg(OH)
2 and Copper Oxide NPs to be similar in their effectiveness against
E. coli (Dong et al.).
The LLDPE extrusion blow-molded bottles thermally embossed with LLDPE Mg(OH)
2 (spray) showed the following
thermal properties: a
Tm of 123.91 °C ± 0.76, which was 0.77% lower compared to that of the neat LLDPE bottle (124.87 °C ± 0.54), as shown in
Figure 9 and
Table 1; a
Tc of 106.12 °C ± 0.45, which was 1.31% higher compared to that of the neat LLDPE bottle (104.75 °C ± 1.32), as shown in
Figure 9 and
Table 1; a crystallinity (%) of 38.41% ± 0.93, which was 3.78% higher compared to that of the neat LLDPE bottle (37.01% ± 1.25), as shown in
Table 1; the temperature at which 5% weight loss occurred was 426.01 °C ± 2.32, which was 5.96% higher than that of the neat LLDPE (402.03 °C ± 1.62), as shown in
Figure 10 and
Table 2; and the estimated inorganic residual (wt.%) at around 600 (°C) was 4.91 ± 0.08, which was 705% higher than that of the neat LLDPE (0.61 ± 0.11), as shown in
Figure 10 and
Table 2. Overall, the anti-microbial NPs did not significantly impact the thermal properties of the LLDPE extrusion blow-molded bottles.
The LLDPE extrusion blow-molded bottles thermally embossed with LLDPE MgO (spray) showed the following
thermal properties: a
Tm of 123.78 °C ± 0.37, which was 0.87% lower compared to that of the neat LLDPE bottle (124.87 °C ± 0.54), as shown in
Figure 9 and
Table 1; a
Tc of 105.75 °C ± 0.44, which was 0.95% higher compared to that of the neat LLDPE bottle (104.75 °C ± 1.32), as shown in
Figure 9 and
Table 1; a crystallinity (%) of 35.64% ± 1.34, which was 3.70% higher compared to that of the neat LLDPE bottle (37.01% ± 1.25), as shown in
Table 1; the temperature at which 5% weight loss occurred was 422.12 °C ± 1.11, which was 5.00% higher than that of the neat LLDPE (402.03 °C ± 1.62), as shown in
Figure 10 and
Table 2; and the estimated inorganic residual (wt.%) at around 600 (°C) was 6.56 ± 0.45, which was 975% higher than that of the neat LLDPE (0.61 ± 0.11), as shown in
Figure 10 and
Table 2. Overall, the anti-microbial NPs did not significantly impact the thermal properties of the LLDPE extrusion blow-molded bottles. Alwaan et al. showed that the crystallinity of the blends of mLLDPE compounded with MgO was continuously increased by the loading of MgO when compared with the neat material [
48].
The LLDPE extrusion blow-molded bottles thermally embossed with LLDPE Cu(OH)
2 (spray) showed the following
thermal properties: a
Tm of 124.58 °C ± 0.37, which was 0.23% lower compared to that of the neat LLDPE bottle (124.87 °C ± 0.54), as shown in
Figure 9 and
Table 1; a
Tc of 105.48 °C ± 1.61, which was 0.70% higher compared to that of the neat LLDPE bottle (104.75 °C ± 1.32), as shown in
Figure 9 and
Table 1; a crystallinity (%) of 40.79% ± 1.21, which was 10.21% higher compared to that of the neat LLDPE bottle (37.01% ± 1.25), as shown in
Table 1; the temperature at which 5% weight loss occurred was 425.96 °C ± 0.95, which was 5.95% higher than that of the neat LLDPE (402.03 °C ± 1.62), as shown in
Figure 10 and
Table 2; and the estimated inorganic residual (wt.%) at around 600 (°C) was 0.03 ± 1.22, which was 95% lower than that of the neat LLDPE (0.61 ± 0.11), as shown in
Figure 10 and
Table 2. Overall, the anti-microbial NPs did not significantly impact the thermal properties of the LLDPE extrusion blow-molded bottles.
The LLDPE extrusion blow-molded bottles thermally embossed with LLDPE ZnO (spray) showed the following
thermal properties: a
Tm of 124.19 °C ± 0.66, which was 0.54% lower compared to that of the neat LLDPE bottle (124.87 °C ± 0.54), as shown in
Figure 9 and
Table 1; a
Tc of 105.84 °C ± 0.93, which was 1.04% higher compared to that of the neat LLDPE bottle (104.75 °C ± 1.32), as shown in
Figure 9 and
Table 1; a crystallinity (%) of 37.05% ± 0.82, which was 0.11% higher compared to that of the neat LLDPE bottle (37.01% ± 1.25), as shown in
Table 1; the temperature at which 5% weight loss occurred was 417.58 °C ± 1.31, which was 3.87% higher than that of the neat LLDPE (417.58 °C ± 1.31), as shown in
Figure 10 and
Table 2; and the estimated inorganic residual (wt.%) at around 600 (°C) was 8.67 ± 0.65, which was 1321% higher than that of the neat LLDPE (0.61 ± 0.11), as shown in
Figure 10 and
Table 2. Overall, the anti-microbial NPs did not significantly impact the thermal properties of the LLDPE extrusion blow-molded bottles.
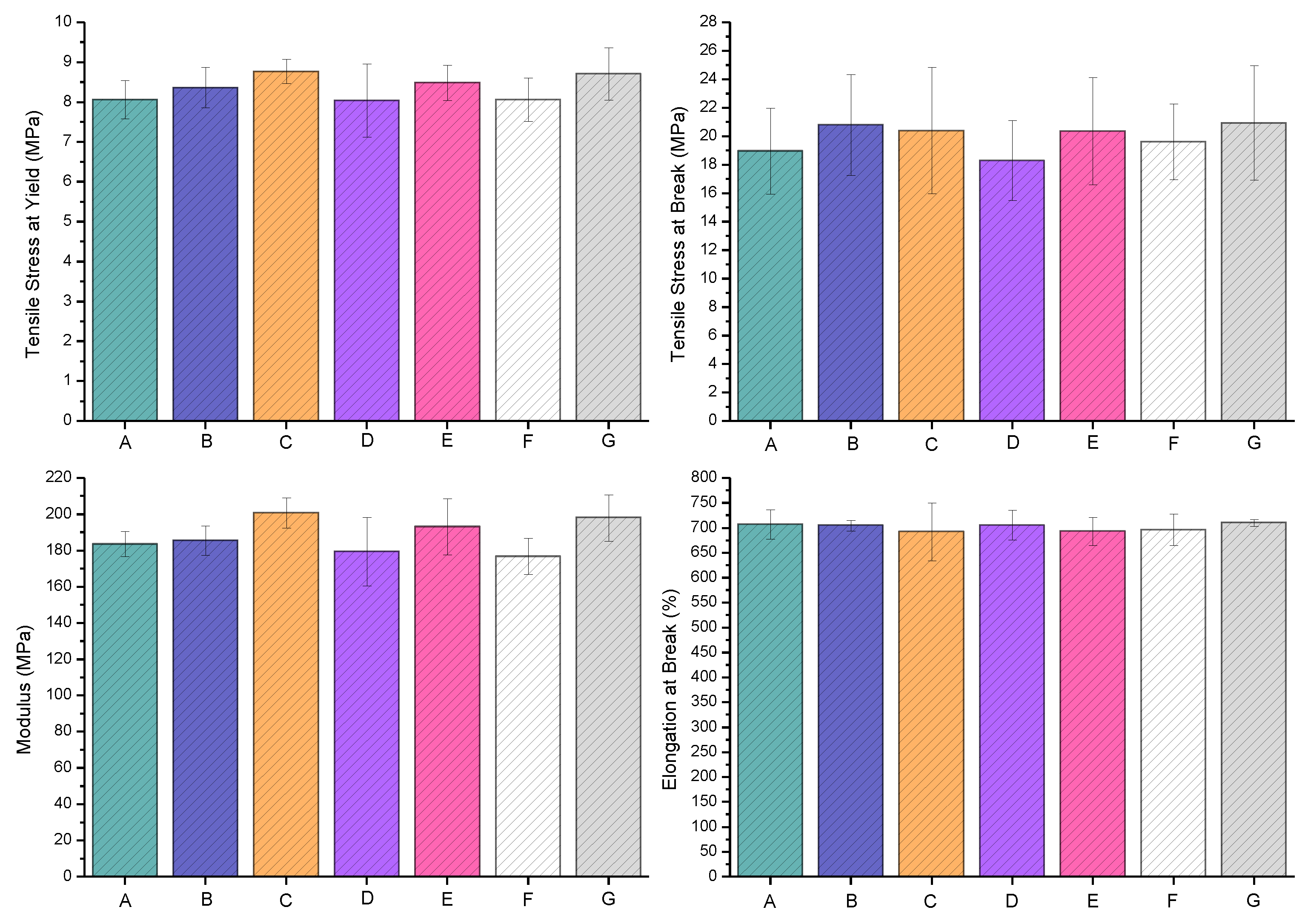
The LLDPE extrusion blow-molded bottles thermally embossed with Cu-infused Mg(OH)
2 (spray) showed the following
tensile properties: the tensile stress at yield (MPa) was 8.37 ± 0.51, which was 3.79% higher compared to that of the neat LLDPE (8.06 ± 0.48); the tensile stress at break (MPa) was 20.81 ± 3.54, which was 9.82% higher compared to that of the neat LLDPE (18.95 ± 3.01); the tensile stress modulus (MPa) was 185.51 ± 8.11, which was 1.09% higher compared to that of the neat LLDPE (183.59 ± 7.05); and the elongation at break (%) was 704.63 ± 10.40, which was 0.33% lower compared to that of the neat LLDPE (706.99 ± 29.79). Overall, the anti-microbial NPs slightly improved the tensile properties, as shown in
Figure 11. In some cases, especially when dealing with epoxy coatings, the incorporation of inorganic NPs can improve the mechanical properties of the polymeric matrices [
49]. Impact strength and stiffness, in particular, were enhanced by the filling of potential pinholes and voids in the matrix.
The LLDPE extrusion blow-molded bottles thermally embossed with Mg(OH)
2 (powder) showed the following
tensile properties: the tensile stress at yield (MPa) was 8.77 ± 0.31, which was 8.80% higher compared to that of the neat LLDPE (8.06 ± 0.48); the tensile stress at break (MPa) was 20.40 ± 4.44, which was 7.65% higher compared to that of the neat LLDPE (18.95 ± 3.01); the tensile stress modulus (MPa) was 200.76 ± 8.31, which was 9.35% higher compared to that of the neat LLDPE (183.59 ± 7.05); and the elongation at break (%) was 692.03 ± 57.85, which was 2.12% lower compared to that of the neat LLDPE (706.99 ± 29.79). Overall, the anti-microbial NPs slightly improved the tensile properties, as presented in
Figure 11.
The LLDPE extrusion blow-molded bottles thermally embossed with Mg(OH)
2 (spray) showed the following
tensile properties: the tensile stress at yield (MPa) was 8.04 ± 0.92, which was 0.26% lower compared to that of the neat LLDPE (8.06 ± 0.48); the tensile stress at break (MPa) was 18.30 ± 2.82, which was 3.43% lower compared to that of the neat LLDPE (18.95 ± 3.01); the tensile stress modulus (MPa) was 179.38 ± 18.91, which was 2.29% lower compared to that of the neat LLDPE (183.59 ± 7.05); and the elongation at break (%) was 705.47 ± 30.15, which was 0.21% lower compared to that of the neat LLDPE (706.99 ± 29.79). Overall, the anti-microbial NPs slightly improved the tensile properties, as presented in
Figure 11.
The LLDPE extrusion blow-molded bottles thermally embossed with MgO (spray) showed the following
tensile properties: the tensile stress at yield (MPa) was 8.49 ± 0.44, which was 5.32% higher compared to that of the neat LLDPE (8.06 ± 0.48); the tensile stress at break (MPa) was 20.36 ± 3.76, which was 7.44% higher compared to that of the neat LLDPE (18.95 ± 3.01); the tensile stress modulus (MPa) was 193.21 ± 15.46, which was 5.24% higher compared to that of the neat LLDPE (183.59 ± 7.05); and the elongation at break (%) was 692.45 ± 28.40, which was 2.01% lower compared to that of the neat LLDPE (706.99 ± 29.79). Overall, the anti-microbial NPs slightly improved the tensile properties, as presented in
Figure 11.
The LLDPE extrusion blow-molded bottles thermally embossed with Cu(OH)
2 (spray) showed the following
tensile properties: the tensile stress at yield (MPa) was 8.07 ± 0.54, which was 0.11% higher compared to that of the neat LLDPE (8.06 ± 0.48); the tensile stress at break (MPa) was 19.62 ± 2.66, which was 3.54% higher compared to that of the neat LLDPE (18.95 ± 3.01); the tensile stress modulus (MPa) was 176.74 ± 9.96, which was 3.73% lower compared to that of the neat LLDPE (183.59 ± 7.05); and the elongation at break (%) was 695.96 ± 31.80, which was 1.56% lower compared to that of the neat LLDPE (706.99 ± 29.79). Overall, the anti-microbial NPs slightly improved the tensile properties, as presented in
Figure 11.
The LLDPE extrusion blow-molded bottles thermally embossed with ZnO (spray) showed the following
tensile properties: the tensile stress at yield (MPa) was 8.71 ± 0.66, which was 8.05% higher compared to that of the neat LLDPE (8.06 ± 0.48); the tensile stress at break (MPa) was 20.95 ± 4.01, which was 10.55% higher compared to that of the neat LLDPE (18.95 ± 3.01); the tensile stress modulus (MPa) was 198.10 ± 12.84, which was 7.90% higher compared to that of the neat LLDPE (183.59 ± 7.05); and the elongation at break (%) was 710.27 ± 7.11, which was 0.46% higher compared to that of the neat LLDPE (706.99 ± 29.79). Overall, the anti-microbial NPs slightly improved the tensile properties, as presented in
Figure 11.
The effects of the anti-microbial agents, the variation in tensile bar weight, and the variation in the tensile bar thickness were statistically investigated to study their possible impacts on the tensile properties of the extrusion blow-molded bottles. The introduction of these anti-microbial agents at these loading levels (10,000 ppm and five sprays on each side of the mold cavity) can be achieved without any impact on the tensile properties, while providing a significant anti-microbial property to the bottles. The statistical analysis showed that after adjusting for the variation attributed to tensile bar thickness and bar weight, none of the six types of anti-microbial agents exhibited significantly different results to those of the control, as measured by tensile stress at yield, tensile stress at break, modulus, and elongation at break. A detailed statistical analysis is provided as part of the SI document (please see
Section S4).
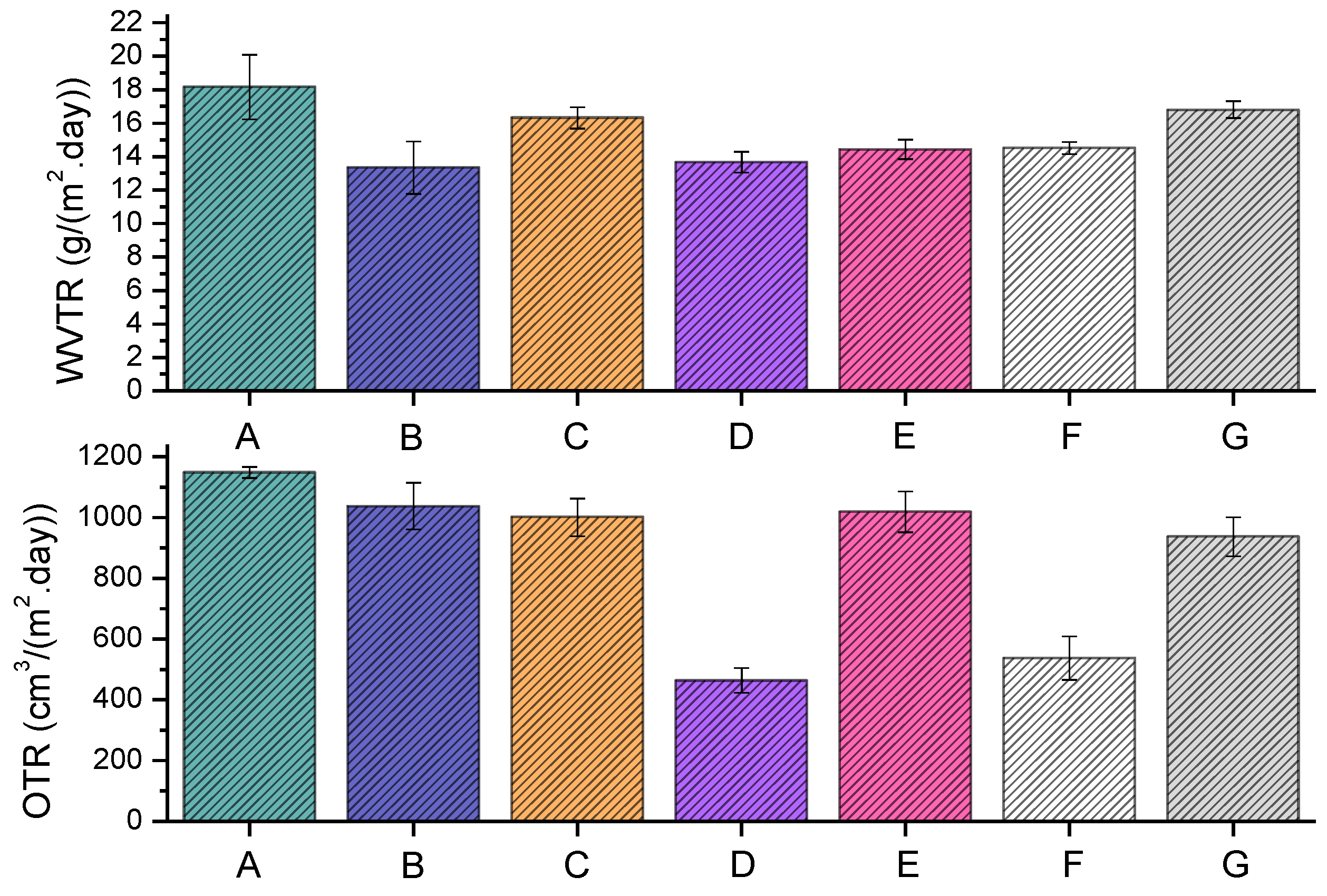
The LLDPE extrusion blow-molded bottles thermally embossed with Cu-infused Mg(OH)
2 (spray) showed the following
barrier properties: the WVTR (g/(m
2·day)) and OTR (cm
3/(m
2·day)) were 13.34 ± 1.56 and 1037.56 ± 76.46, respectively, which were 26.54% and 9.63% lower, respectively, compared to that of the neat LLDPE (18.16 ± 1.93 and 1148.11 ± 18.84). Overall, the anti-microbial NPs improved the barrier properties, as presented in
Figure 12.
The LLDPE extrusion blow-molded bottles thermally embossed with Mg(OH)
2 (powder) showed the following
barrier properties: the WVTR (g/(m
2·day)) and OTR (cm
3/(m
2·day)) were 16.32 ± 0.63 and 1000.95 ± 62.25, respectively, which were 10.13% and 12.82% lower, respectively, compared to that of the neat LLDPE (18.16 ± 1.93 and 1148.11 ± 18.84). Overall, the anti-microbial NPs improved the barrier properties, as presented in
Figure 12.
The LLDPE extrusion blow-molded bottles thermally embossed with Cu-infused Mg(OH)
2 (spray) showed the following
barrier properties: the WVTR (g/(m
2·day)) and OTR (cm
3/(m
2·day)) were 13.67 ± 0.62 and 463.50 ± 41.29, respectively, which were 24.72% and 59.63% lower, respectively, compared to that of the neat LLDPE (18.16 ± 1.93 and 1148.11 ± 18.84). Overall, the anti-microbial NPs improved the barrier properties, as presented in
Figure 12.
The LLDPE extrusion blow-molded bottles thermally embossed with MgO (spray) showed the following
barrier properties: the WVTR (g/(m
2·day)) and OTR (cm
3/(m
2·day)) were 14.43 ± 0.59 and 1018.70 ± 67.63, respectively, which were 20.54% and 11.27% lower, respectively, compared to that of the neat LLDPE (18.16 ± 1.93 and 1148.11 ± 18.84). Overall, the anti-microbial NPs improved the barrier properties, as presented in
Figure 12.
The LLDPE extrusion blow-molded bottles thermally embossed with Cu(OH)
2 (spray) showed the following
barrier properties: the WVTR (g/(m
2·day)) and OTR (cm
3/(m
2·day)) were 14.52 ± 0.37 and 537.29 ± 70.92, respectively, which were 20.04% and 53.20% lower, respectively, compared to that of the neat LLDPE (18.16 ± 1.93 and 1148.11 ± 18.84). Overall, the anti-microbial NPs improved the barrier properties, as presented in
Figure 12.
The LLDPE extrusion blow-molded bottles thermally embossed with ZnO (spray) showed the following
barrier properties: the WVTR (g/(m
2·day)) and OTR (cm
3/(m
2·day)) were 16.81 ± 0.50 and 937.25 ± 64.30, respectively, which were 7.43% and 18.37% lower, respectively, compared to that of the neat LLDPE (18.16 ± 1.93 and 1148.11 ± 18.84). Overall, the anti-microbial NPs improved the barrier properties, as presented in
Figure 12.
The improved barrier properties were likely due to the fixation of the inorganic crystals over the outer surface of the extrusion blow-molded bottles. Upon the incorporation of these particles, the porosities of the bottles were considerably narrowed. In all cases, the coating improved the gas barrier properties of the bottle. While the improvement of WVTR is only marginal in most cases, the improvement of OTR for sample (
Figure 12D,F) is quite noticeable, with ~50% improvement. It is known that inorganic-based coatings can improve gas barrier performance [
50]. In this case, the coating was not specifically tuned to improve gas barrier performance, hence the marginal improvement.
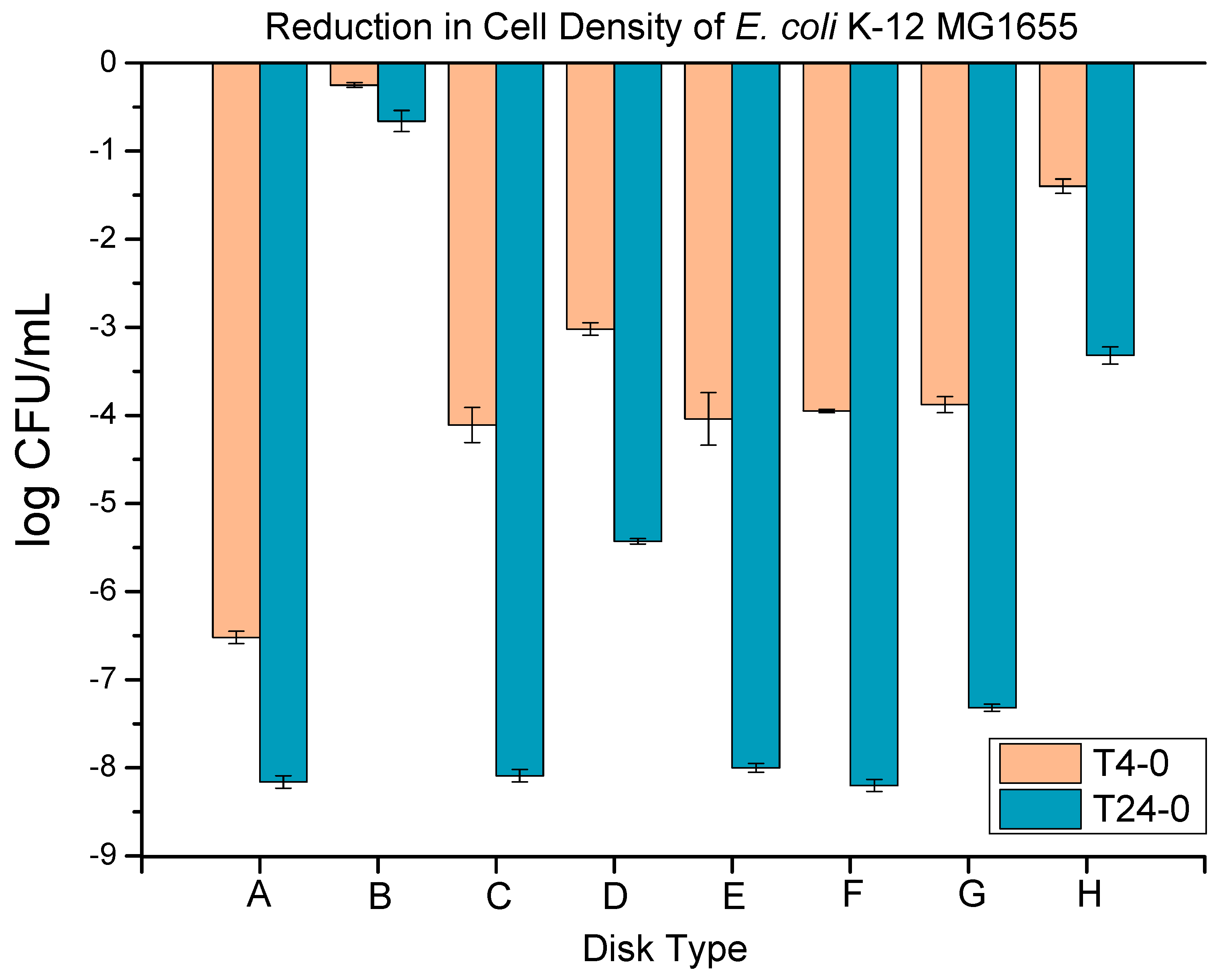
The
anti-microbial performance of extrusion blow-molded bottles was tested against
E. coli K-12 MG1655 (8.16 ± 0.10 log), as presented in
Figure 13. The metallic copper disks (positive control) showed a 6.52 ± 0.07 and 8.16 ± 0.07 log reduction at 4 h and 24 h, respectively. The neat LLDPE disks (negative control) showed a 0.25 ± 0.03 and 0.66 ± 0.12 log reduction at 4 h and 24 h, respectively. The negative control’s performance shows a bacterial reduction too low to be considered antibacterial and in combination with the positive control’s result, this constitutes proof of the validity of the anti-bacterial test. The extrusion blow-molded bottles thermally embossed with Cu-infused Mg(OH)
2 particles (spray) showed a 4.11 ± 0.20 and 8.09 ± 0.07 log reduction at 4 h and 24 h, respectively, which presented a 99.999996 and 99.999996% reduction from the negative control, respectively. The extrusion blow-molded bottles thermally embossed with Mg(OH)
2 particles (powder) showed a 3.02 ± 0.07 and 5.43 ± 0.03 log reduction at 4 h and 24 h, respectively, which exhibited a 99.8333336 and 99.999996% reduction from the negative control, respectively. The extrusion blow-molded bottles thermally embossed with Mg(OH)
2 particles (spray) showed a 4.04 ± 0.30 and 8.00 ± 0.05 log reduction at 4 h and 24 h, respectively, which presented a 99.988886 and 99.999996% reduction from the negative control, respectively. The extrusion blow-molded bottles thermally embossed with MgO particles (spray) showed a 3.95 ± 0.02 and 8.20 ± 0.07 log reduction at 4 h and 24 h, respectively, which was a 99.988886 and 99.999996% reduction from the negative control, respectively. The extrusion blow-molded bottles thermally embossed with Cu(OH)
2 particles (spray) showed a 3.88 ± 0.09 and 7.32 ± 0.04 log reduction at 4 h and 24 h, respectively, which showed a 99.988886 and 99.999996% reduction from the negative control, respectively. The extrusion blow-molded bottles thermally embossed with ZnO particles (spray) showed a 1.40 ± 0.08 and 3.32 ± 0.10 log reduction at 4 h and 24 h, respectively, which was a 92.9222226 and 99.7888886% reduction from the negative control, respectively.
In all cases, the bacterial reduction after 24 h is highly increased compared to that at 4 h, showing that these coatings require several hours to a day to fully eradicate the initial incubated bacterial colonies. The ZnO reflects the lowest performance, with only a log 3 reduction after 24 h. ZnO NPs are known to be effective anti-bacterial agents, but superior additives, such as Ag NPs, are available [
51]. For instance, Mg(OH)
2 powder provided better performance, reaching a log 5.5 bacterial reduction after 24 h. The performance was further enhanced when applying Cu- or Mg-based additives via the spray coating (
Figure 13C,E–G); all have a bacterial reduction > to log 7 after 24 h. This level of sterility is far superior to that provided by disinfection and can only be achieved through sterilization techniques such as gamma radiation [
52].