Assessment of the Biodegradability and Compostability of Finished Leathers: Analysis Using Spectroscopy and Thermal Methods
Abstract
1. Introduction
2. Materials and Methods
2.1. Composting Procedure
2.2. Characterization
2.3. Spectrometry Analysis
2.4. Samples
3. Results and Discussion
3.1. Biodegradation and Composting
3.2. Maturity
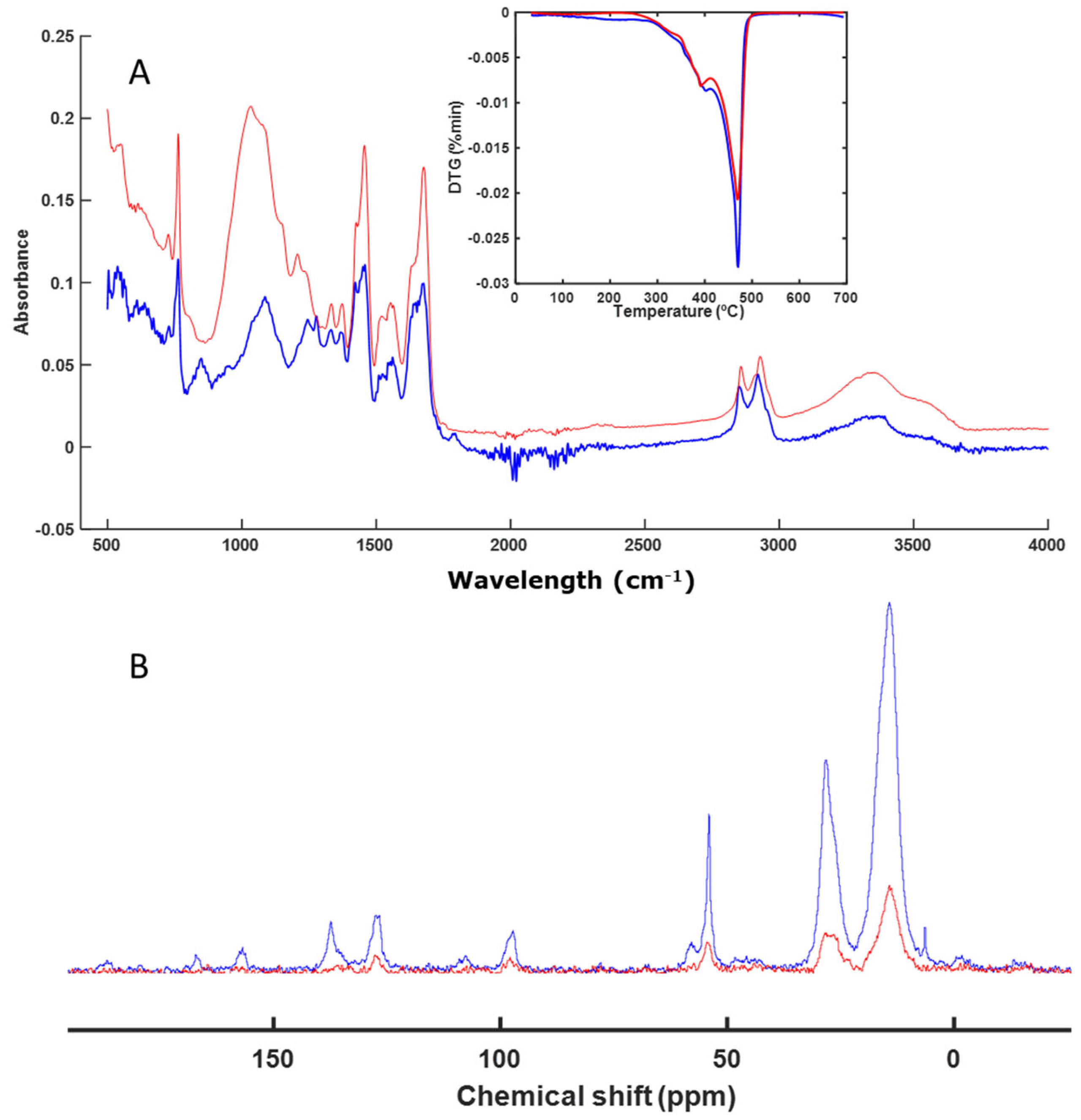
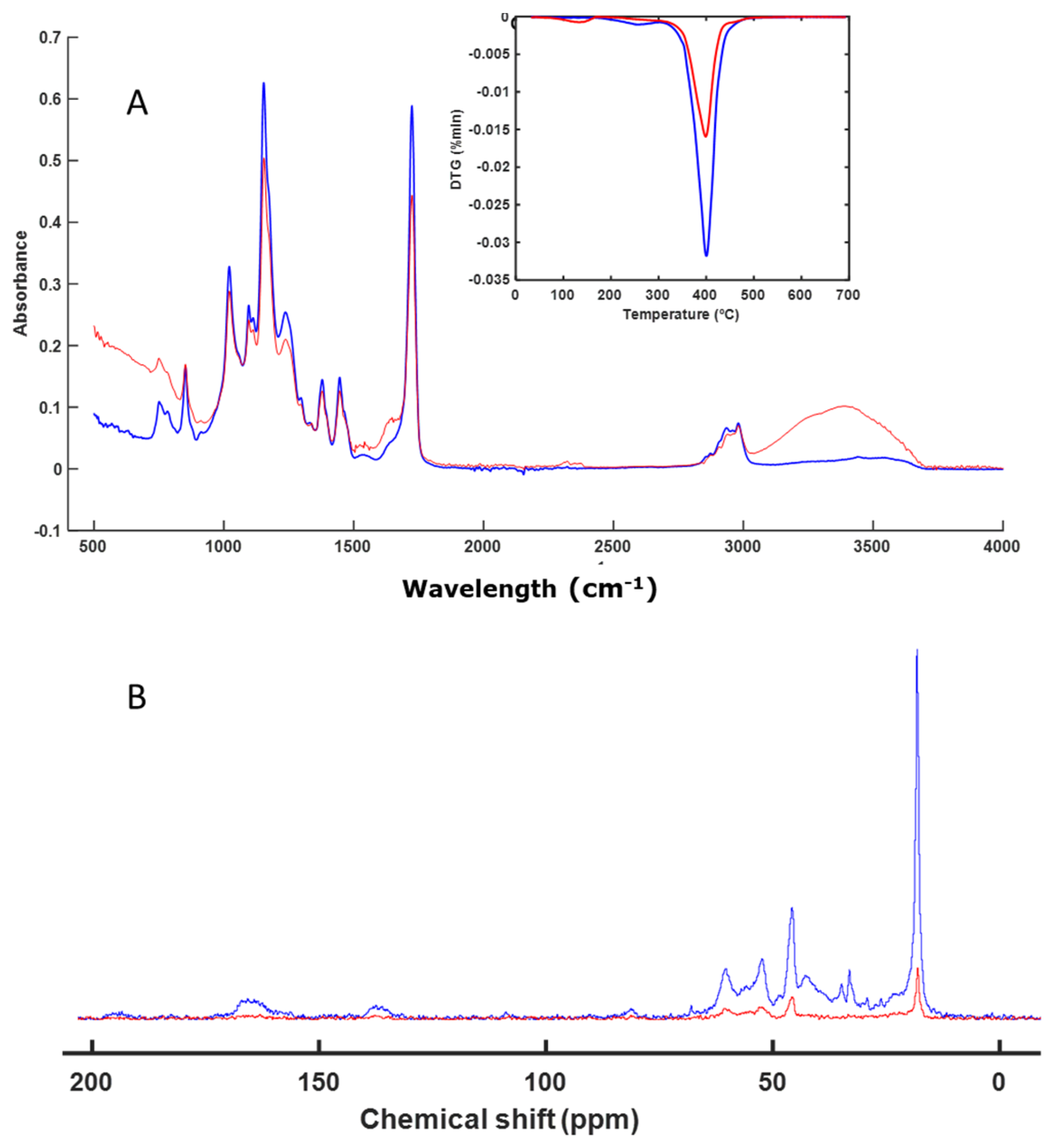
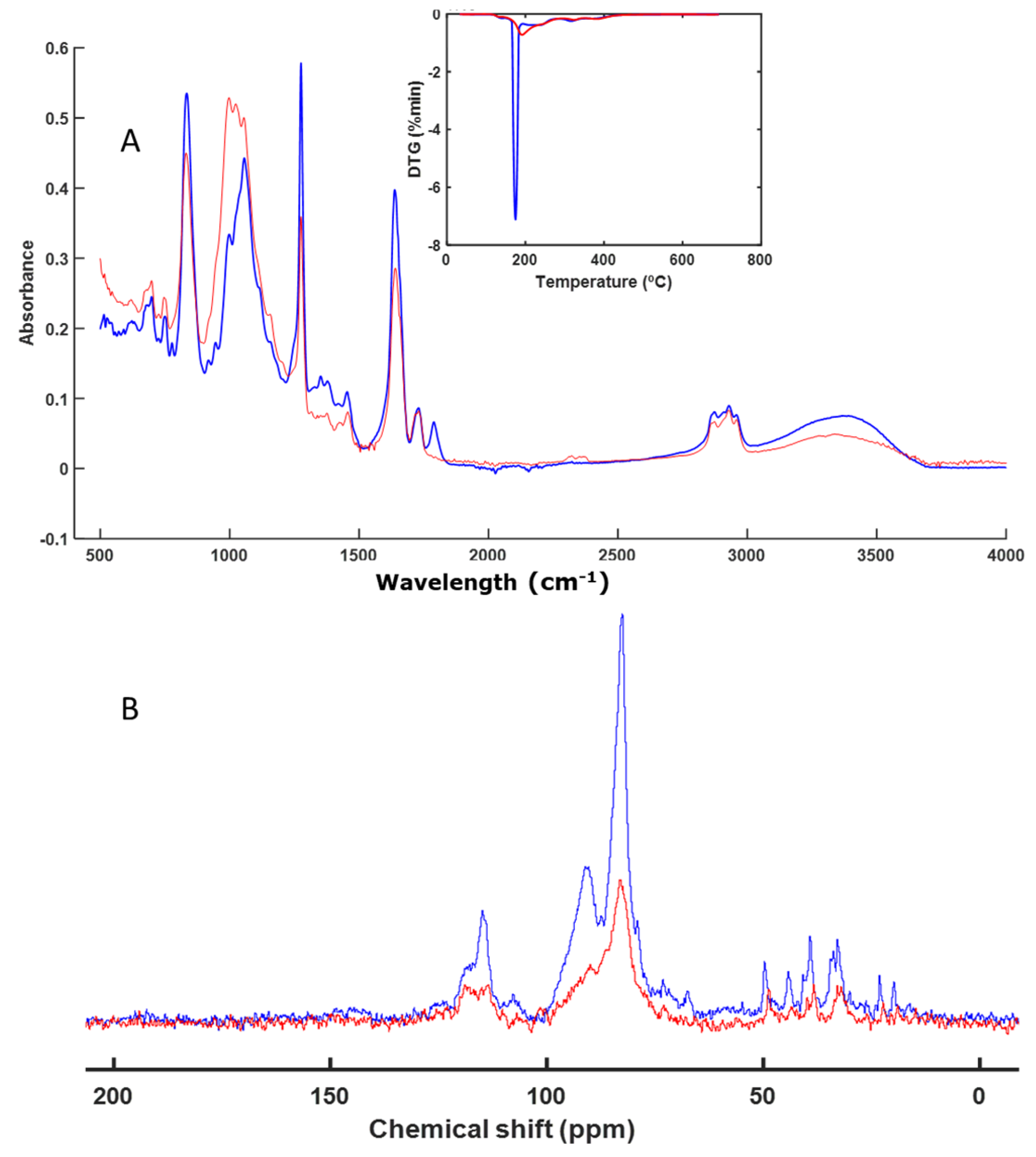
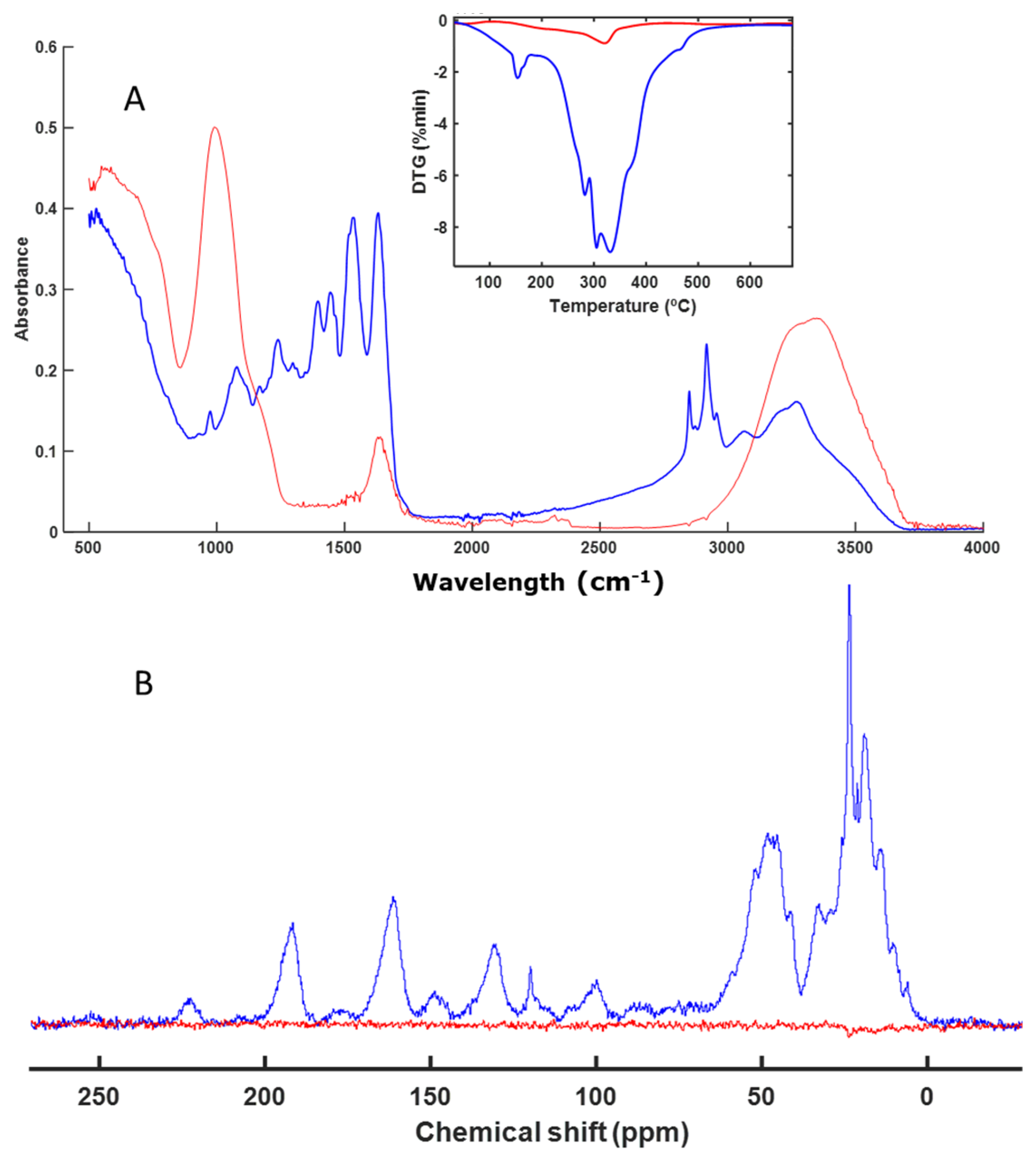
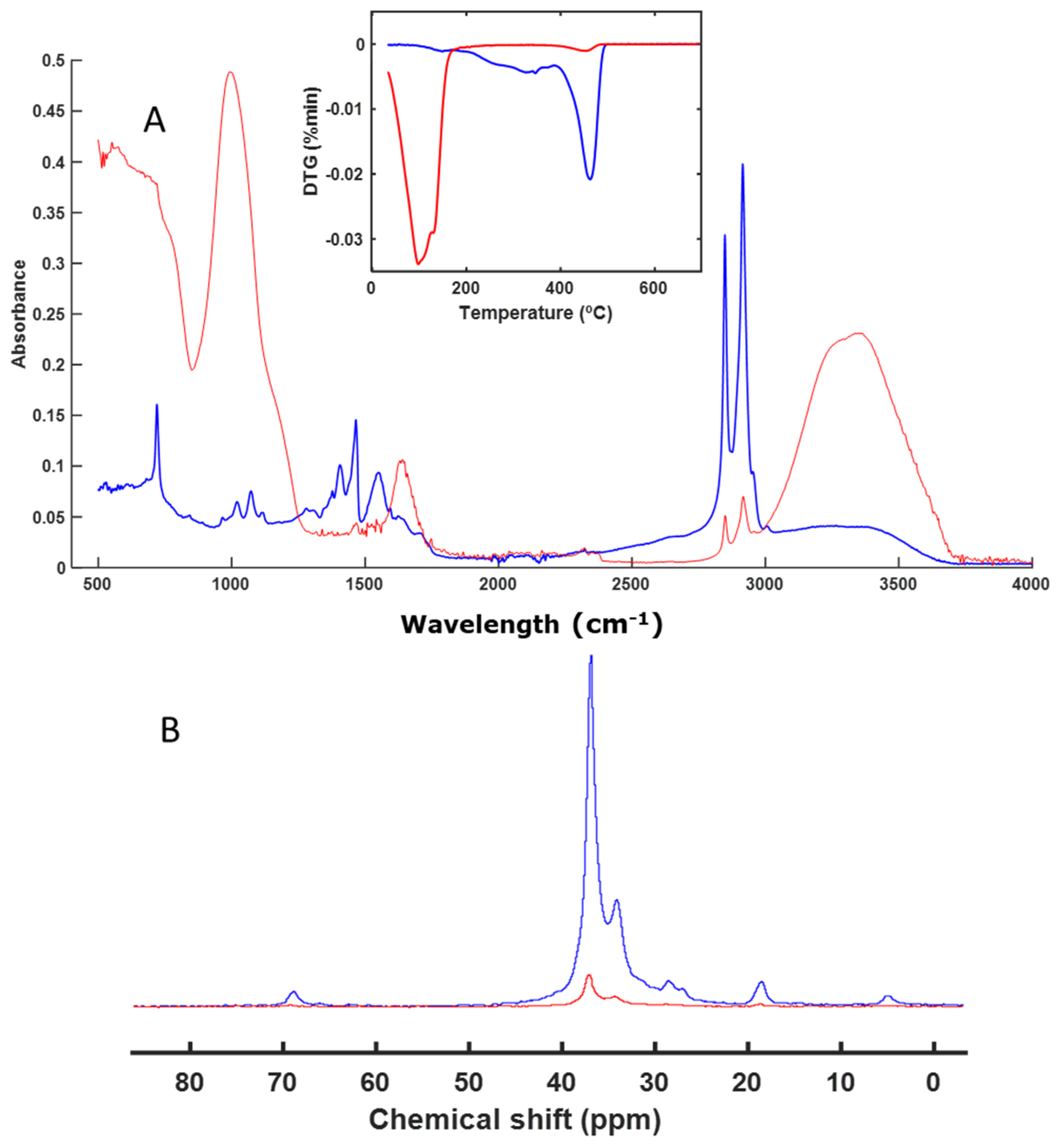
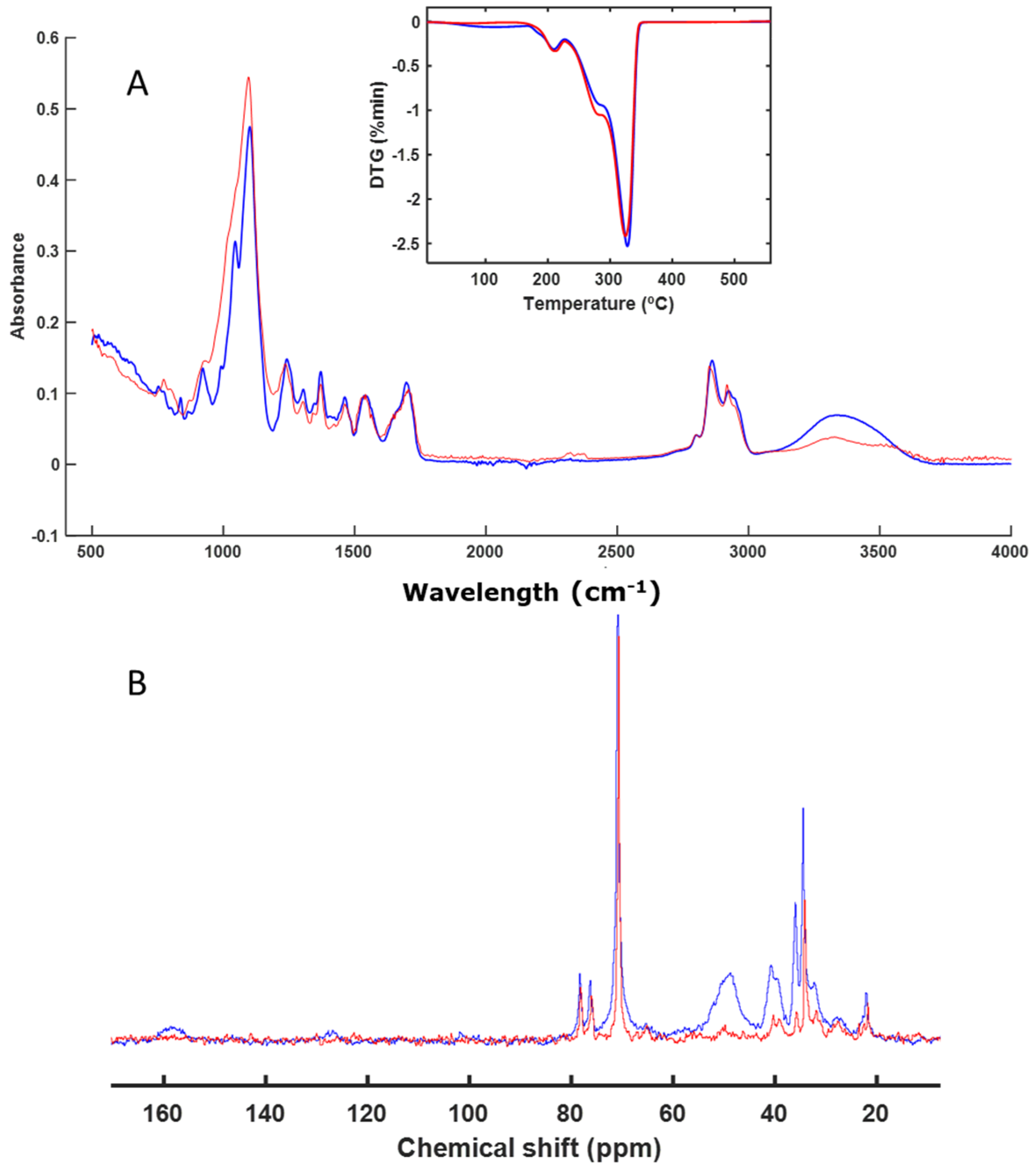
3.3. Spectroscopy and Thermal Analysis
4. Discussion and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- ISO 14855-1:2012; Determination of the Ultimate Aerobic Biodegradability of Plastic Materials under Controlled Composting Conditions—Method by Analysis of Evolved Carbon Dioxide. ISO–International Organization for Standardization: Geneva, Switzerland, 2012.
- Textiles Strategy—European Commission. Available online: https://environment.ec.europa.eu/strategy/textiles-strategy_en (accessed on 3 April 2024).
- Leather Industry Insight—Pergamena. Available online: https://www.pergamena.net/blog/v3piynz4zg9ysubox7b1r3zszwrfx8 (accessed on 7 March 2024).
- Qiang, T.; Chen, L.; Zhang, Q.; Liu, X. A Sustainable and Cleaner Speedy Tanning System Based on Condensed Tannins Catalyzed by Laccase. J. Clean. Prod. 2018, 197, 1117–1123. [Google Scholar] [CrossRef]
- Wise, W.R.; Davis, S.J.; Hendriksen, W.E.; von Behr, D.J.A.; Prabakar, S.; Zhang, Y. Zeolites as Sustainable Alternatives to Traditional Tanning Chemistries. Green. Chem. 2023, 25, 4260–4270. [Google Scholar] [CrossRef]
- Beghetto, V.; Agostinis, L.; Gatto, V.; Samiolo, R.; Scrivanti, A. Sustainable Use of 4-(4,6-Dimethoxy-1,3,5-Triazin-2-Yl)-4-Methylmorpholinium Chloride as Metal Free Tanning Agent. J. Clean. Prod. 2019, 220, 864–872. [Google Scholar] [CrossRef]
- Lypskyi, T.; Pervaia, N.; Okhmat, O.; Mokrousova, O.; Babych, A. Determining Patterns in the Use of Finishing Formulations for Trimming the Crust Leather. East. Eur. J. Enterp. Technol. 2021, 1, 57–63. [Google Scholar] [CrossRef]
- Ollé, L.; Frías, A.; Sorolla, S.; Cuadros, R.; Bacardit, A. Study of the Impact on Occupational Health of the Use of Polyaziridine in Leather Finishing Compared with a New Epoxy Acrylic Self-Crosslinking Polymer. Prog. Org. Coat. 2021, 154, 106162. [Google Scholar] [CrossRef]
- Available online: https://www.ipcc.ch/site/assets/uploads/sites/4/2019/12/02_Summary-for-Policymakers_SPM.pdf (accessed on 25 June 2024).
- Onyuka, A.; Bates, M.; Attenburrow, G.; Covington, A.; Antunes, P. Parameters for Composting Tannery Hair Waste. J. Am. Leather Chem. Assoc. 2012, 107, 159–166. [Google Scholar]
- Siddiqui, S.; Alamri, S.; Al Rumman, S.; Al-Kahtani, M.A.; Meghvansi, M.K.; Jeridi, M.; Shumail, T.; Moustafa, M. Recent Advances in Assessing the Maturity and Stability of Compost. In Biology of Composts; Meghvansi, M.K., Varma, A., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 181–202. ISBN 978-3-030-39173-7. [Google Scholar]
- Rashwan, M.A.; Naser Alkoaik, F.; Abdel-Razzak Saleh, H.; Blanqueza Fulleros, R.; Nagy Ibrahim, M. Maturity and Stability Assessment of Composted Tomato Residues and Chicken Manure Using a Rotary Drum Bioreactor. J. Air Waste Manag. Assoc. 2021, 71, 529–539. [Google Scholar] [CrossRef]
- Lamourou, H.; Karbout, N.; Zriba, Z. Zoghlami, rahma ines Stability and Maturity Indexes of Organic Fraction Compost. Emir. J. Food Agric. 2023, 35, 121–129. [Google Scholar] [CrossRef]
- Pagga, U.; Beimborn, D.B.; Boelens, J.; De Wilde, B. Determination of the Aerobic Biodegradability of Polymeric Material in a Laboratory Controlled Composting Test. Chemosphere 1995, 31, 4475–4487. [Google Scholar] [CrossRef]
- Vico, A.; Pérez-Murcia, M.D.; Bustamante, M.A.; Agulló, E.; Marhuenda-Egea, F.C.; Sáez, J.A.; Paredes, C.; Pérez-Espinosa, A.; Moral, R. Valorization of Date Palm (Phoenix dactylifera L.) Pruning Biomass by Co-Composting with Urban and Agri-Food Sludge. J. Environ. Manag. 2018, 226, 408–415. [Google Scholar] [CrossRef]
- Zucconi, F.; Pera, A.; Forte, M.; Bertoldi, M. Evaluating Toxicity of Immature Compost. Biocycle 1981, 22, 54–57. [Google Scholar]
- OECD. Test No. 208: Terrestrial Plant Test: Seedling Emergence and Seedling Growth Test; Organisation for Economic Co-operation and Development: Paris, Italy, 2006. [Google Scholar]
- The Application of FT-IR Spectroscopy in Waste Management|IntechOpen. Available online: https://www.intechopen.com/chapters/14634 (accessed on 29 May 2024).
- Torres-Climent, A.; Gomis, P.; Martín-Mata, J.; Bustamante, M.A.; Marhuenda-Egea, F.C.; Pérez-Murcia, M.D.; Pérez-Espinosa, A.; Paredes, C.; Moral, R. Chemical, Thermal and Spectroscopic Methods to Assess Biodegradation of Winery-Distillery Wastes during Composting. PLoS ONE 2015, 10, e0138925. [Google Scholar] [CrossRef] [PubMed]
- Smidt, E.; Tintner, J.; Klemm, S.; Scholz, U. FT-IR Spectral and Thermal Characterization of Ancient Charcoals—A Tool to Support Archeological and Historical Data Interpretation. Quat. Int. 2017, 457, 43–49. [Google Scholar] [CrossRef]
- Wilkie, C.A. TGA/FTIR: An Extremely Useful Technique for Studying Polymer Degradation. Polym. Degrad. Stab. 1999, 66, 301–306. [Google Scholar] [CrossRef]
- Almeida, E.; Balmayore, M.; Santos, T. Some Relevant Aspects of the Use of FTIR Associated Techniques in the Study of Surfaces and Coatings. Prog. Org. Coat. 2002, 44, 233–242. [Google Scholar] [CrossRef]
- Bhargava, R.; Wang, S.-Q.; Koenig, J.L. FTIR Microspectroscopy of PolymericSystems. In Liquid Chromatography/FTIR Microspectroscopy/MicrowaveAssisted Synthesis; Springer: Berlin/Heidelberg, Germany, 2003; pp. 137–191. ISBN 978-3-540-36432-0. [Google Scholar]
- Stefanidis, S.D.; Kalogiannis, K.G.; Iliopoulou, E.F.; Michailof, C.M.; Pilavachi, P.A.; Lappas, A.A. A Study of Lignocellulosic Biomass Pyrolysis via the Pyrolysis of Cellulose, Hemicellulose and Lignin. J. Anal. Appl. Pyrolysis 2014, 105, 143–150. [Google Scholar] [CrossRef]
- Narayanan, P.; Janardhanan, S. An Approach towards Identification of Leather from Leather-like Polymeric Material Using FTIR-ATR Technique. Collagen Leather 2024, 6, 1. [Google Scholar] [CrossRef]
- Tavoosi, Y.; Behin, J. Unhairing of Bovine Hide Using Wastewater from Merox Unit of Oil Refinery: Techno-Environmental Aspect. Environ. Sci. Pollut. Res. 2022, 29, 28180–28193. [Google Scholar] [CrossRef] [PubMed]
- Martín-Mata, J.; Lahoz-Ramos, C.; Bustamante, M.A.; Marhuenda-Egea, F.C.; Moral, R.; Santos, A.; Sáez, J.A.; Bernal, M.P. Thermal and Spectroscopic Analysis of Organic Matter Degradation and Humification during Composting of Pig Slurry in Different Scenarios. Environ. Sci. Pollut. Res. 2016, 23, 17357–17369. [Google Scholar] [CrossRef]
- Muñoz, M.; Gomez-rico, M.F.; Font, R. Use of Thermogravimetry for Single Characterisation of Samples of the Composting Process from Sewage Sludge. J. Anal. Appl. Pyrolysis 2013, 103, 261–267. [Google Scholar] [CrossRef]
- Pielichowska, K.; Nowicka, K. Analysis of Nanomaterials and Nanocomposites by Thermoanalytical Methods. Thermochim. Acta 2019, 675, 140–163. [Google Scholar] [CrossRef]
- Conte, P.; Spaccini, R.; Piccolo, A. State of the Art of CPMAS 13C-NMR Spectroscopy Applied to Natural Organic Matter. Prog. Nucl. Magn. Reson. Spectrosc. 2004, 44, 215–223. [Google Scholar] [CrossRef]
- Baldock, J.; Oades, J.; Nelson, P.; Skene, T.; Golchin, A.; Clarke, P. Assessing the Extent of Decomposition of Natural Organic Materials Using Solid-State 13C NMR Spectroscopy. Aust. J. Soil. Res. 1997, 35. [Google Scholar] [CrossRef]
- Yamazawa, A.; Iikura, T.; Shino, A.; Date, Y.; Kikuchi, J. Solid-, Solution-, and Gas-State NMR Monitoring of C-13-Cellulose Degradation in an Anaerobic Microbial Ecosystem. Molecules 2013, 18, 9021–9033. [Google Scholar] [CrossRef] [PubMed]
- Mao, J.; Cao, X.; Olk, D.; Chu, W.; Schmidt-Rohr, K. Advanced Solid-State NMR Spectroscopy of Natural Organic Matter. Prog. Nucl. Magn. Reson. Spectrosc. 2017, 100. [Google Scholar] [CrossRef] [PubMed]
- Spin Dynamics: Basics of Nuclear Magnetic Resonance, 2nd ed.; Wiley: Hoboken, NJ, USA, 2008; Available online: https://www.wiley.com/en-es/Spin+Dynamics%3A+Basics+of+Nuclear+Magnetic+Resonance%2C+2nd+Edition-p-9780470511176 (accessed on 29 May 2024).
- Wilson, M. NMR Techniques and Applications in Geochemistry and Soil Chemistry; Pergamon Press: Oxford, UK, 1987. [Google Scholar]
- Nelson, P.; Baldock, J. Estimating the Molecular Composition of a Diverse Range of Natural Organic Materials from Solid-State 13C NMR and Elemental Analyses. Biogeochemistry 2005, 72, 1–34. [Google Scholar] [CrossRef]
- Huang, Y.; Chen, Y.; Huang, H.; Lin, J.; Yan, M.; Guo, C.; Xiao, X. A Technology to Reduce Ammonia Emission via Controllingproteolytic Bacterial Community and Physicochemical Properties. Res. Sq. 2023. [Google Scholar] [CrossRef]
- Xiang, E.; Moran, C.S.; Ivanovski, S.; Abdal-hay, A. Nanosurface Texturing for Enhancing the Antibacterial Effect of Biodegradable Metal Zinc: Surface Modifications. Nanomaterials 2023, 13, 2022. [Google Scholar] [CrossRef] [PubMed]
- Zucconi, F.; Monaco, A.; Forte, M. Phytotoxins during the Stabilization of Organic Matter. Compost. Agric. Other Wastes 1985, 73–86. [Google Scholar]
- Evaluating Phytotoxicity of Pig Manure from the Pig-on-Litter System. Available online: https://deepblue.lib.umich.edu/handle/2027.42/191304 (accessed on 16 May 2024).
- Emino, E.R.; Warman, P.R. Biological Assay for Compost Quality. Compos. Sci. Util. 2004, 12, 342–348. [Google Scholar] [CrossRef]
- María Teresa Varnero, M.; Claudia Rojas, A.; Roberto Orellana, R. Índices de fitotoxicidad en residuos orgánicos durante el compostaje. Rev. Cienc. Suelo Y Nutr. Veg. 2007, 7, 28–37. [Google Scholar] [CrossRef]
- Bělonožníková, K.; Černý, M.; Hýsková, V.; Synková, H.; Valcke, R.; Hodek, O.; Křížek, T.; Kavan, D.; Vaňková, R.; Dobrev, P.; et al. Casein as Protein and Hydrolysate: Biostimulant or Nitrogen Source for Nicotiana Tabacum Plants Grown In Vitro? Physiol. Plant. 2023, 175, e13973. [Google Scholar] [CrossRef]
- Kaufhold, S.; Reese, A.; Schwiebacher, W.; Dohrmann, R.; Grathoff, G.H.; Warr, L.N.; Halisch, M.; Müller, C.; Schwarz-Schampera, U.; Ufer, K. Porosity and Distribution of Water in Perlite from the Island of Milos, Greece. SpringerPlus 2014, 3, 598. [Google Scholar] [CrossRef] [PubMed]
- Saufi, H.; el Alouani, M.; Alehyen, S.; el Achouri, M.; Jilali, A.; Taibi, M. Photocatalytic Degradation of Methylene Blue from Aqueous Medium onto Perlite-Based Geopolymer. Int. J. Chem. Eng. 2020, 2020, 9498349. [Google Scholar] [CrossRef]
- Bohacz, J. Changes in Mineral Forms of Nitrogen and Sulfur and Enzymatic Activities during Composting of Lignocellulosic Waste and Chicken Feathers. Environ. Sci. Pollut. Res. 2019, 26, 10333–10342. [Google Scholar] [CrossRef] [PubMed]
- Prochoń, M.; Marzec, A.; Szadkowski, B. Preparation and Characterization of New Environmentally Friendly Starch-Cellulose Materials Modified with Casein or Gelatin for Agricultural Applications. Materials 2019, 12, 1684. [Google Scholar] [CrossRef] [PubMed]
- De Gregorio, E.; Miliani, F.M.; Vivaldi, F.M.; Calisi, N.; Poma, N.; Tavanti, A.; Duce, C.; Nardella, F.; Legnaioli, S.; Carota, A.G.; et al. A Casein-Based Biodegradable and Sustainable Capacitive Sensor. Mater. Chem. Phys. 2024, 314, 128888. [Google Scholar] [CrossRef]
- Blaya, J.; Marhuenda, F.C.; Pascual, J.A.; Ros, M. Microbiota Characterization of Compost Using Omics Approaches Opens New Perspectives for Phytophthora Root Rot Control. PLoS ONE 2016, 11, e0158048. [Google Scholar] [CrossRef] [PubMed]
- Cerda, A.; Artola, A.; Font, X.; Barrena, R.; Gea, T.; Sánchez, A. Composting of Food Wastes: Status and Challenges. Bioresour. Technol. 2018, 248, 57–67. [Google Scholar] [CrossRef]
- Numata, K.; Asano, A.; Nakazawa, Y. Solid-State and Time Domain NMR to Elucidate Degradation Behavior of Thermally Aged Poly (Urea-Urethane). Polym. Degrad. Stab. 2020, 172, 109052. [Google Scholar] [CrossRef]
- Li, J.; Chen, H. Biodegradation of Whey Protein-Based Edible Films. J. Polym. Environ. 2000, 8, 135–143. [Google Scholar] [CrossRef]
- Auer, N.; Hedger, J.N.; Evans, C.S. Degradation of Nitrocellulose by Fungi. Biodegradation 2005, 16, 229–236. [Google Scholar] [CrossRef] [PubMed]
- Erdal, N.B.; Hakkarainen, M. Degradation of Cellulose Derivatives in Laboratory, Man-Made, and Natural Environments. Biomacromolecules 2022, 23, 2713–2729. [Google Scholar] [CrossRef] [PubMed]
- Roper, M. The Isolation and Characterisation of Bacteria with the Potential to Degrade Waxes That Cause Water Repellency in Sandy Soils. Aust. J. Soil Res. 2004, 42. [Google Scholar] [CrossRef]
- Zafar, U.; Nzeram, P.; Langarica Fuentes, A.; Houlden, A.; Heyworth, A.; Saiani, A.; Robson, G. Biodegradation of Polyester Polyurethane during Commercial Composting and Analysis of Associated Fungal Communities. Bioresour. Technol. 2014, 158. [Google Scholar] [CrossRef]
- Pfohl, P.; Bahl, D.; Rückel, M.; Wagner, M.; Meyer, L.; Bolduan, P.; Battagliarin, G.; Hüffer, T.; Zumstein, M.; Hofmann, T.; et al. Effect of Polymer Properties on the Biodegradation of Polyurethane Microplastics. Environ. Sci. Technol. 2022, 56, 16873–16884. [Google Scholar] [CrossRef]
- Kim, Y.D.; Kim, S.C. Effect of Chemical Structure on the Biodegradation of Polyurethanes under Composting Conditions. Polym. Degrad. Stab. 1998, 62, 343–352. [Google Scholar] [CrossRef]
- Krasowska, K.; Janik, H.; Gradys, A.; Rutkowska, M. Degradation of Polyurethanes in Compost under Natural Conditions. J. Appl. Polym. Sci. 2012, 125, 4252–4260. [Google Scholar] [CrossRef]
- Hernandez, A.-B.; Ferrasse, J.-H.; Akkache, S.; Roche, N. Thermochemical Conversion of Sewage Sludge by TGA-FTIR Analysis: Influence of Mineral Matter Added. Dry Technol. 2015, 33, 1318–1326. [Google Scholar] [CrossRef]
- Tosin, M.; Degli-Innocenti, F.; Bastioli, C. Detection of a Toxic Product Released by a Polyurethane-Containing Film Using a Composting Test Method Based on a Mineral Bed. J. Polym. Environ. 1998, 6, 79–90. [Google Scholar] [CrossRef]
- Vaverková, M.; Adamcová, D.; Hermanová, S.; Voběrková, S. Ecotoxicity of Composts Containing Aliphatic-Aromatic Copolyesters. Pol. J. Environ. Stud. 2015, 24, 1497–1505. [Google Scholar] [CrossRef] [PubMed]
- Aylaj, M.; Adani, F. The Evolution of Compost Phytotoxicity during Municipal Waste and Poultry Manure Composting. J. Ecol. Eng. 2023, 24, 281–293. [Google Scholar] [CrossRef]
- Kapanen, A. Ecotoxicity Assessment of Biodegradable Plastics and Sewage Sludge in Compost and in Soil; University of Helsinki: Helsinki, Finland, 2012. [Google Scholar]
- Zuriaga-Agustí, E.; Galiana-Aleixandre, M.V.; Bes-Piá, A.; Mendoza-Roca, J.A.; Risueño-Puchades, V.; Segarra, V. Pollution Reduction in an Eco-Friendly Chrome-Free Tanning and Evaluation of the Biodegradation by Composting of the Tanned Leather Wastes. J. Clean. Prod. 2015, 87, 874–881. [Google Scholar] [CrossRef]
- Sardroudi, N.P.; Sorolla, S.; Casas, C.; Bacardit, A. A Study of the Composting Capacity of Different Kinds of Leathers, Leatherette and Alternative Materials. Sustainability 2024, 16, 2324. [Google Scholar] [CrossRef]
- Constantinescu, R.R.; Deselnicu, V.; Crudu, M.; Macovescu, G.; Albu, L. Comparative Study Regarding Leather Biodegradability. Leather Footwear J. 2015, 15, 73–84. [Google Scholar] [CrossRef]
- Hashem, M.A.; Hasan, M.; Hasan, M.A.; Sahen, M.S.; Payel, S.; Mizan, A.; Nur-A-Tomal, M.S. Composting of Limed Fleshings Generated in a Tannery: Sustainable Waste Management. Environ. Sci. Pollut. Res. 2023, 30, 39029–39041. [Google Scholar] [CrossRef]





| Groups | Sample | Code | Leather |
|---|---|---|---|
| Polymers and resins | Isocyanate | IS | Crust leather + IS |
| Acrylic | EA | Crust leather + EA | |
| Nitrocellulose lacquer | NL | Crust leather + NL | |
| Bio-based materials | Acrylic BIO | AB | Crust leather + AB |
| Polyurethane top BIO | PTB | Crust leather + PTB + PFB * | |
| Polyurethane primer BIO | PFB | Crust leather + PTB + PFB * | |
| Binders and adhesives | Casein | CAS | Crust leather + CAS |
| Protein binder | EB | Crust leather + EB | |
| Finishing and treatment agents | Wax | EX | Crust leather + EX |
| Black pigment | BP | Crust leather + BP | |
| Control | Collagen | C+ | Collagen |
| Crust | CRT | Crust leather |
| Sample | Code | Carbon (%) | CO2 (g) | Nitrogen (%) | C/N |
|---|---|---|---|---|---|
| Polymers and resins | |||||
| Isocyanate | IS | 60.42 | 20.49 | 15.85 | 3.81 |
| Acrylic | EA | 59.08 | 20.58 | 0.83 | 71.35 |
| Nitrocellulose lacquer | NL | 44.09 | 20.21 | 6.83 | 6.45 |
| Bio-based materials | |||||
| Acrylic BIO | AB | 57.59 | 20.59 | 0.72 | 79.92 |
| Polyurethane top BIO | PTB | 61.63 | 20.34 | 5.10 | 12.10 |
| Polyurethane primer BIO | PFB | 98.35 | 20.74 | 0.23 | 433.4 |
| Binders and adhesives | |||||
| Casein | CAS | 51.21 | 20.65 | 12.24 | 4.19 |
| Protein binder | EB | 47.19 | 20.76 | 13.45 | 3.51 |
| Finishing and treatment agents | |||||
| Wax | EX | 79.96 | 20.52 | 1.46 | 54.87 |
| Black pigment | BP | 71.28 | 20.91 | 3.85 | 18.49 |
| Control | |||||
| Collagen | C+ | 45.39 | 20.21 | 16.93 | 2.68 |
| Crust | CRT | 47.93 | -- | 10.49 | 4.57 |
| Sample | Code | Cr | Ni | Cu | Zn | As | Se | Mo | Cd | Pb | Hg |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Batch A | EA | <1 | <1 | <5 | <1 | <1 | <1 | <1 | <1 | 1.2 | <0.15 |
| EX | <1 | <1 | <5 | <1 | <1 | <1 | <1 | <1 | 1.2 | <0.15 | |
| EB | <1 | <1 | <5 | <1 | <1 | <1 | <1 | <1 | 1.2 | <0.15 | |
| Batch B | PTB | <1 | <1 | <5 | <1 | <1 | <1 | <1 | <1 | <1 | <0.15 |
| PFB | <1 | <1 | <5 | <1 | <1 | <1 | <1 | <1 | <1 | <0.15 | |
| Batch C | CAS | <1 | <1 | 5.6 | <1 | <1 | <1 | <1 | <1 | 1 | <0.15 |
| BP | <1 | <1 | 5.6 | <1 | <1 | <1 | <1 | <1 | 1 | <0.15 | |
| AB | <1 | <1 | 5.6 | 1328.6 | <1 | <1 | <1 | <1 | 1 | <0.15 | |
| Batch D | IS | <1 | <1 | <5 | <1 | <1 | <1 | <1 | <1 | <1 | <0.15 |
| NL | <1 | <1 | <5 | <1 | <1 | <1 | <1 | <1 | <1 | <0.15 | |
| Control | C+ | <1 | <1 | <5 | <1 | <1 | <1 | <1 | <1 | <1 | <0.15 |
| CRT | <1 | <1 | <5 | <1 | <1 | <1 | <1 | <1 | <1 | <0.15 |
| Sample | Finishing Product ThCO2 (g) | Biodegradability % | Leather + Finishing Product ThCO2 (g) | Biodegradability % |
|---|---|---|---|---|
| IS | 20.49 | 13.83 | 18.33 | 57.30 |
| EA | 20.58 | 0.00 | 19.19 | 84.20 |
| NL | 20.21 | 5.73 | 18.84 | 52.91 |
| AB | 20.59 | 3.04 | 18.30 | 58.38 |
| PTB | 20.34 | 38.25 | 19.16 * | 79.37 * |
| PFB | 20.74 | 0.00 | 19.16 * | 79.37 * |
| CAS | 20.65 | 87.23 | 18.17 | 62.14 |
| EB | 20.76 | 5.48 | 19.37 | 82.53 |
| EX | 20.52 | 60.1 | 19.42 | 85.09 |
| BP | 20.91 | 26.27 | 19.59 | 88.12 |
| C+ | 20.21 | 93.30 | 19.97 | 97.67 |
| Sample | RGeP a (%) | Biomass R b | RGrP a (%) | Germination Index a (GI) |
|---|---|---|---|---|
| IS | 100.0 | 134.8 | 105.5 | 105.5 |
| EA | 95.0 | 116.9 | 123.1 | 116.9 |
| NL | 102.6 | 88.3 | 100.9 | 103.5 |
| AB | 101.3 | 80.9 | 95.4 | 96.6 |
| PTB * | 63.9 | 90.0 | 84.9 | 54.2 |
| PFB * | 63.9 | 90.0 | 84.9 | 54.2 |
| CAS | 97.4 | 155.7 | 78.0 | 75.9 |
| EB | 81.0 | 115.5 | 83.5 | 67.6 |
| EX | 102.6 | 112.7 | 114.4 | 117.3 |
| BP | 102.6 | 151.5 | 125.9 | 129.1 |
| C+ | 101.3 | 110.6 | 99.8 | 99.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vico, A.; Maestre-Lopez, M.I.; Arán-Ais, F.; Orgilés-Calpena, E.; Bertazzo, M.; Marhuenda-Egea, F.C. Assessment of the Biodegradability and Compostability of Finished Leathers: Analysis Using Spectroscopy and Thermal Methods. Polymers 2024, 16, 1908. https://doi.org/10.3390/polym16131908
Vico A, Maestre-Lopez MI, Arán-Ais F, Orgilés-Calpena E, Bertazzo M, Marhuenda-Egea FC. Assessment of the Biodegradability and Compostability of Finished Leathers: Analysis Using Spectroscopy and Thermal Methods. Polymers. 2024; 16(13):1908. https://doi.org/10.3390/polym16131908
Chicago/Turabian StyleVico, Alberto, Maria I. Maestre-Lopez, Francisca Arán-Ais, Elena Orgilés-Calpena, Marcelo Bertazzo, and Frutos C. Marhuenda-Egea. 2024. "Assessment of the Biodegradability and Compostability of Finished Leathers: Analysis Using Spectroscopy and Thermal Methods" Polymers 16, no. 13: 1908. https://doi.org/10.3390/polym16131908
APA StyleVico, A., Maestre-Lopez, M. I., Arán-Ais, F., Orgilés-Calpena, E., Bertazzo, M., & Marhuenda-Egea, F. C. (2024). Assessment of the Biodegradability and Compostability of Finished Leathers: Analysis Using Spectroscopy and Thermal Methods. Polymers, 16(13), 1908. https://doi.org/10.3390/polym16131908






