Self-Neutralizing Melamine–Urea–Formaldehyde–Citric Acid Resins for Wood Panel Adhesives
Abstract
1. Introduction
2. Materials and Methods
2.1. MUF Resin and Glue Mix Preparation
2.2. Titration and Buffer Action
2.3. Cross-Polarization–Magic Angle Spinning–Nuclear Magnetic Resonance (CP-MAS 13C NMR) Spectra
2.4. MALDI-TOF Analysis
2.5. Particleboard and Plywood Preparation and Testing
3. Results and Discussion
3.1. CP MAS 13C NMR
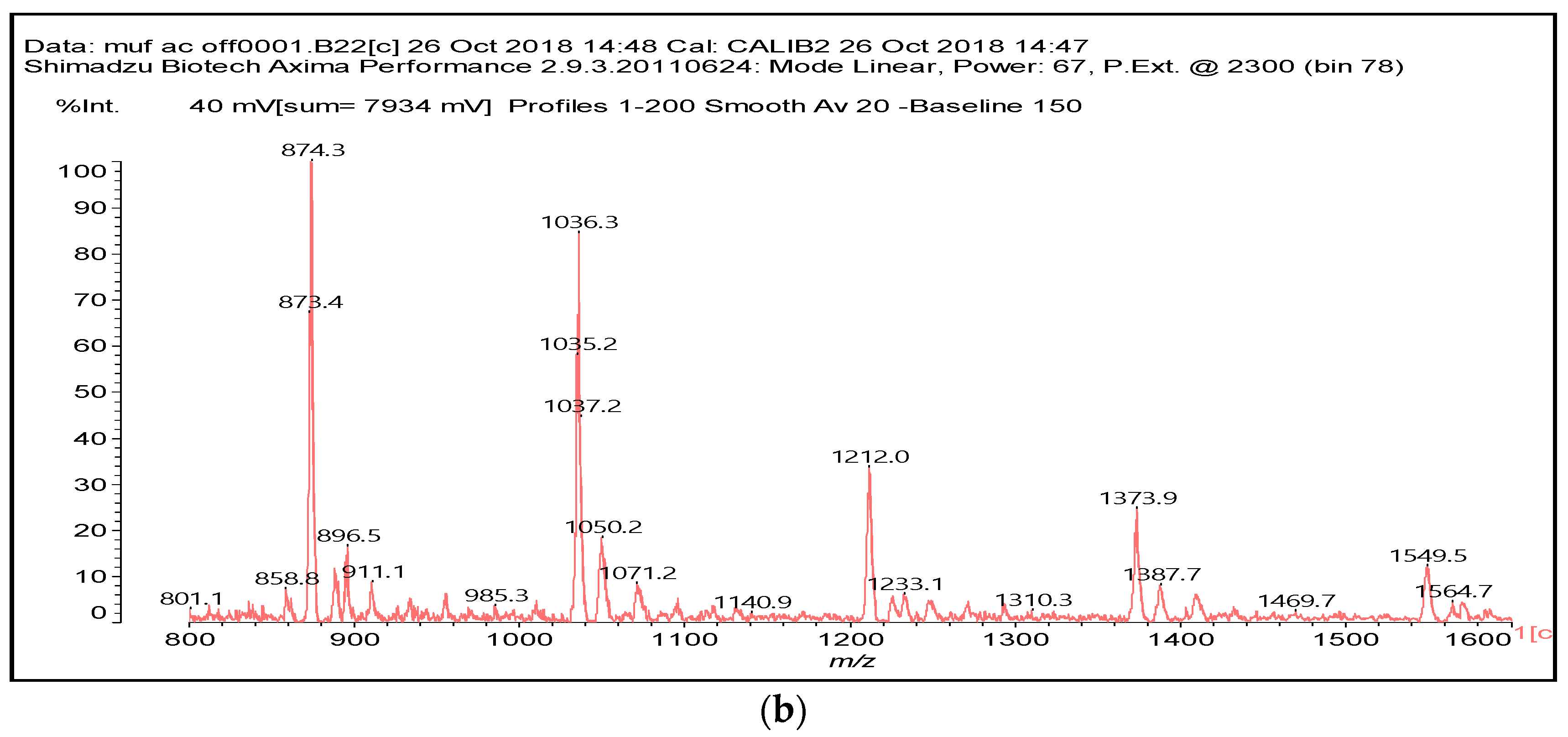
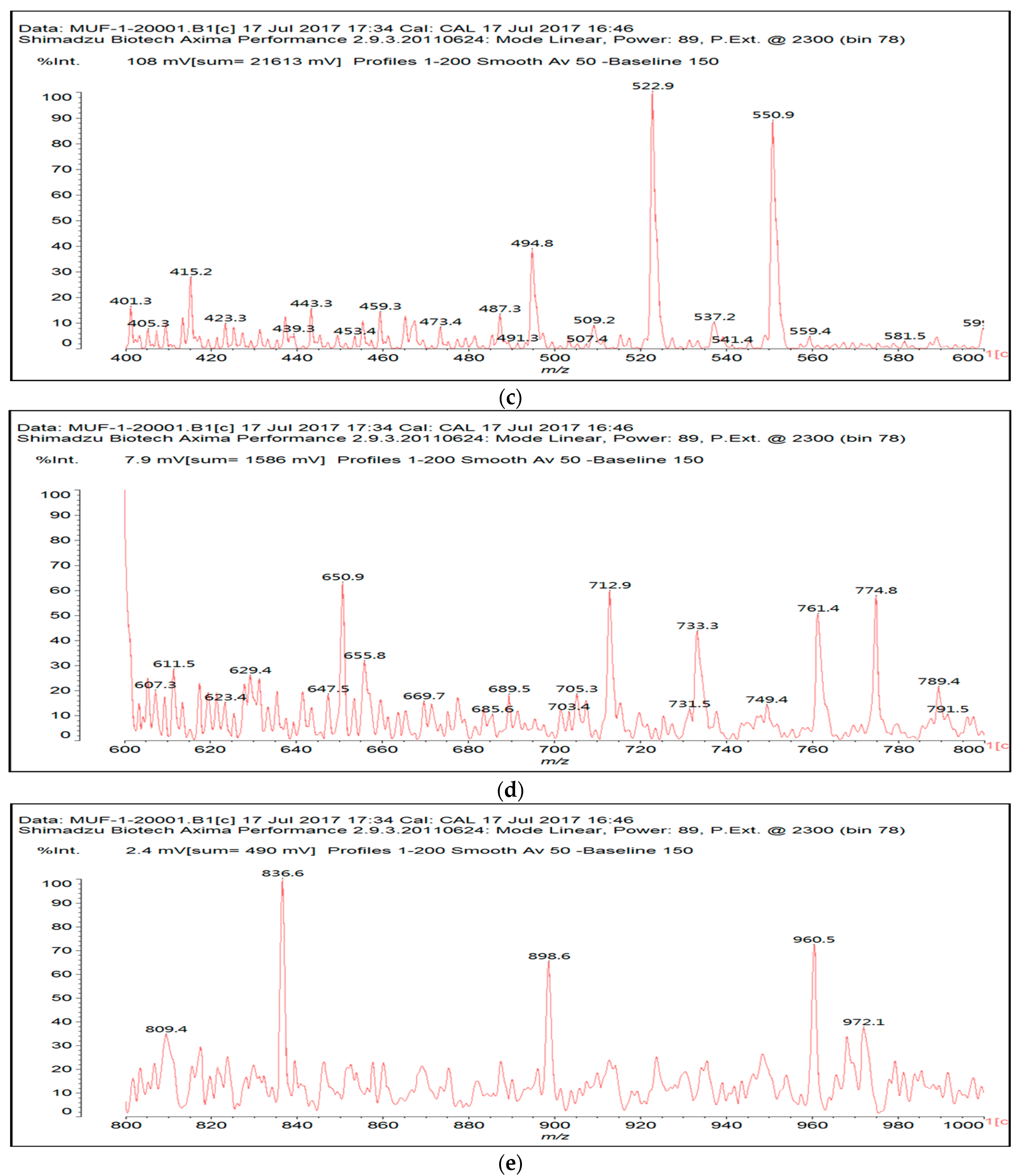
3.2. MALDI ToF

- (1).
- -COOH + H2N- = -CONH- + H2O;
- (2).
- -COOH + HOH2C- = -COOH2C- + H2O;
- (3).
- 2 R-NH2 + HCHO = R-NH-CH2OH = R-NH-CH2-NH-R’.
3.3. Particleboard and Plywood Test Results
- (i).
- The slower cross-linking due to the buffer action certainly plays some role, but it is not the main cause of the improved performance, as has already been ascertained before [29].
- (ii).
- Previous work has determined that the main factor is that the polycondensation–degradation equilibrium is shifted to the left due to the higher pH [31], but it is limited to a narrow buffering pH range. At a much lower pH, degradation predominates, and at a much higher pH, polycondensation becomes too slow to be of use.
- (iii).
- A third contributing factor is the stability of the organic base–acid buffer; hence, the buffer stability is of a long duration under standard heat-induced resin curing [31].
- (iv).
- The additional cross-linking by citric acid of some amine groups of the MUF resin [3] and of some the hydroxymethyl groups formed by formaldehyde on the melamine and/or the urea of the MUF resin.


- (v).
- The direct linking of citric acid with the carbohydrate and lignin of the wood substrate, including cross-linking through its multiplicity of carboxylic moieties of wood constituents belonging to different surfaces [3]. These reactions in which citric acid can cross-link carbohydrates and lignin units through a wood interface have already been shown to occur and reported in previous work [3].

4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Umemura, K.; Ueda, T.; Munawar, S.; Kawai, S. Application of citric acid as natural adhesive for wood. J. Appl. Polym. Sci. 2012, 123, 1991–1996. [Google Scholar] [CrossRef]
- Kusumah, S.S.; Umemura, K.; Oshioka, K.Y.; Miyafuji, H.; Kanajama, K. Utilisation of sweet sorghum bagasse and citric acid for manufacturing particleboard, I: Effects of predrying treatment and citric acid content on the board properties. Ind. Crops Prod. 2016, 84, 34–42. [Google Scholar] [CrossRef]
- Del Menezzi, C.; Amirou, S.; Pizzi, A.; Xi, X.; Delmotte, L. Reactions with Wood Carbohydrates and Lignin of Citric Acid as a Bond Promoter of Wood Veneer Panels. Polymers 2018, 10, 833. [Google Scholar] [CrossRef] [PubMed]
- Liao, R.; Xu, J.; Umemura, K. Low Density Sugarcane Bagasse Particleboard Bonded with Citric Acid and Sucrose: Effect of board density and additive content. Bioresources 2016, 11, 2174–2185. [Google Scholar] [CrossRef]
- Umemura, K.; Sugihara, O.; Kawai, S. Investigation of a new natural adhesive composed of citric acid and sucrose for particleboard. J. Wood Sci. 2023, 59, 203–208. [Google Scholar] [CrossRef]
- Widyorini, R.; Nugraha, P.; Rahman, M.; Prayitno, T. Bonding ability of a new adhesive composed of citric acid-sucrose for particleboard. Bioresources 2016, 11, 4526–4535. [Google Scholar] [CrossRef]
- Amini, M.A.M.; Hashim, R.; Sulaiman, N.S.; Mohamed, M.; Sulaiman, O. Citric acid-modified starch as an environmentally friendly binder for wood composite making. Bioresources 2020, 15, 4234–4248. [Google Scholar] [CrossRef]
- Kusumah, S.S.; Jayadi; Wibowo, D.T.; Pramasari, D.A.; Widyaningrum, B.A.; Darmawan, T.; Ismadi; Dwianto, W.; Umemura, K. Investigation of eco-friendly plywood bonded with citric acid–starch based adhesive. In IOP Conference Series: Earth and Environmental Science; IOP Publishing: Bristol, UK, 2020; Volume 460, p. 01200. [Google Scholar]
- Li, C.; Hou, D.; Lei, H.; Xi, X.; Du, G.; Zhang, H.; Cao, M.; Tondi, G. Effective and eco-friendly safe self-antimildew strategy to simultaneously improve the water resistance and bonding strength of starch-based adhesive. Inter. J. Biol. Macromol. 2023, 248, 125889. [Google Scholar] [CrossRef]
- Cai, L.; Chen, Y.; Lu, Z.; Wei, M.; Zhao, X.; Xie, Y.; Li, J.; Xiao, S. Citric acid/chitosan adhesive with viscosity-controlled for wood bonding through supramolecular self-assembly. Carbohydr. Polym. 2024, 329, 121765. [Google Scholar] [CrossRef] [PubMed]
- Amirou, S.; Pizzi, A.; Delmotte, L. Citric acid as waterproofing additive in butt joints linear wood welding. Eur. J. Wood Prod. 2017, 75, 651–654. [Google Scholar] [CrossRef]
- Li, J.; Lei, H.; Xi, X.; Li, C.; Hou, D.; Song, J.; Du, G. A sustainable tannin-citric acid wood adhesives with favorable bonding properties and water resistance. Ind. Crops Prod. 2023, 201, 116933. [Google Scholar] [CrossRef]
- Del Menezzi, C.; Pizzi, A.; Amirou, S.; Xi, X. MUF Resins Improved by Citric Acid as Adhesives for Wood Veneer Panels. J. Renew. Mater. 2023, 11, 539–553. [Google Scholar] [CrossRef]
- Sakai, S.; Chen, S.; Matsuo-Ueda, M.; Kenji Umemura, K. Curing behavior of sucrose with p-toluenesulfonic acid. Polymers 2023, 15, 4592. [Google Scholar] [CrossRef] [PubMed]
- Müller, A. Kaltleime auf Phenolharzbasis unr die Gefhar der Holzschädigung (Cold-setting phenolic resin adhesives and the risk of damage to wood). Holz Roh-Werkst. 1953, 11, 429–435. [Google Scholar] [CrossRef]
- Plath, E. Die Prüfung von PF Harzen aus Säureschadigungen (testing of PF resins for acid damages). Holz Roh Werkst. 1953, 15, 466–471. [Google Scholar] [CrossRef]
- Sodhi, S. Über die Säure-Schädigung von Holz bei Der Verleimung mit Phenolharzleimen—On the acid corrosion of wood by gluing with phenol resin adhesives. Holz Roh-Werkst. 1957, 15, 261–263. [Google Scholar] [CrossRef]
- Myers, G.E. Use of acid scavengers to improve durability of acid-catalyzed adhesive wood bonds. For. Prod. J. 1982, 33, 49–57. [Google Scholar]
- Cameron, F.A.; Pizzi, A. Acid-setting cold-setting phenolic adhesives for glulam: A controversial issue. Wood Adhes. Present Future 1984, 40, 229–234. [Google Scholar]
- Crisostomo, M.; Del Menezzi, C.; Militz, H.; Kurkowiak, K.; Mayer, A.; Carvalho, L.; Martins, J. Effect of the Citric acid on the Properties of Sapwood of Pinus sylvestris Submitted to Thermomechanical Treatment. Forests 2023, 14, 1839. [Google Scholar] [CrossRef]
- Mihulja, G.; Zivkovic, V.; Poljak, D.; Šefc, B.; Sedlar, T. Influence of Citric Acid on the Bond Strength of Beech Wood. Polymers 2021, 13, 2801. [Google Scholar] [CrossRef]
- Wang, X.-M.; Casilla, R.; Zhang, Y.; Cooper, P.; Huang, Z. Effect of extreme pH on bond durability of selected structural wood adhesives. Wood Fiber Sci. 2016, 48, 245–259. [Google Scholar]
- Xu, D.; Li, C.; Pizzi, A.; Xi, X.; Wang, Z.; Du, G.; Lei, H. Self-neutralizing Citric Acid–Corn Starch Wood Adhesives. Carbohydr. Polym. 2024. submitted. [Google Scholar]
- Pizzi, A.; Vosloo, R.; Cameron, F.A.; Orovan, E. Self-neutralizing acid-set PF wood adhesives. Holz Roh-Werkst. 1986, 44, 229–234. [Google Scholar] [CrossRef]
- Prestifilippo, M.; Pizzi, A.; Norback, H.; Lavisci, P. Low addition of melamine salts for improved UF adhesives water resistance. Holz Roh-Werkst. 1996, 54, 393–398. [Google Scholar] [CrossRef]
- Zanetti, M.; Pizzi, A.; Norback, H.; Lavisci, P. Low addition of melamine salts for improved MUF adhesives water resistance. J. Appl. Polym. Sci. 2003, 88, 287–292. [Google Scholar] [CrossRef]
- Zanetti, M.; Pizzi, A.; Beaujean, M.; Pasch, H.; Rode, K.; Dalet, P. Acetals induced strength increase of MUF polycondensation adhesives, Part 2: Solubility and colloidal state disruption. J. Appl. Polym. Sci. 2002, 86, 1855–1862. [Google Scholar] [CrossRef]
- Pizzi, A.; Beaujean, M.; Zhao, C.; Properzi, M.; Huang, Z. Acetals-induced strength increases and lower resin contents in MUF and other polycondensation adhesives. J. Appl. Polym. Sci. 2002, 84, 2561–2571. [Google Scholar] [CrossRef]
- Zanetti, M.; Pizzi, A.; Kamoun, C. Upgrading of MUF particleboard adhesives and decrease of melamine content by buffer and additives. Holz Roh Werkst. 2003, 61, 55–65. [Google Scholar] [CrossRef]
- Kamoun, C.; Pizzi, A.; Zanetti, M. Upgrading of MUF resins by buffering additives—Part 1: Hexamine sulphate effect and its limits. J. Appl. Polym. Sci. 2003, 90, 203–214. [Google Scholar] [CrossRef]
- Zanetti, M.; Pizzi, A. Upgrading of MUF resins by buffering additives—Part 2: Hexamine sulphate mechanisms and alternate buffers. J. Appl. Polym. Sci. 2003, 90, 215–226. [Google Scholar] [CrossRef]
- Dynea, A.S. (Lillestrom, Norway). Private communication, 2002.
- GB/T 14074; Testing Methods for Wood Adhesives and Their Resins. China National Standard: Beijing, China, 2006.
- GB/T17657; Test Methods for Evaluating the Properties of Wood-Based Panels and Surface Decorated Wood-Based Panels. China National Standard: Beijing, China, 1999.
- European Norm EN 636:2012; Plywood—Specification. European Committee for Standardization: Brussels, Belgium, 2012.
- Kamoun, C.; Pizzi, A. Mechanism of hexamine as a non-aldehyde polycondensation hardener, Part 1, Hexamine decomposition and reactive intermediates. Holzforsch. Holzverwert. 2000, 52, 16–19. [Google Scholar]
- Kamoun, C.; Pizzi, A. Mechanism of hexamine as a non-aldehyde polycondensation hardener, Part 2: Hexamine recomposition and reactive intermediates. Holzforsch. Holzverwert. 2000, 52, 66–67. [Google Scholar]
- Hultzsch, K. Studien auf dem Gebiet der Phenol-Formaldehyd-Harze, XIV. Mitteil: Über die Ammoniak-Kondensation und die Reaktion von Phenolen mit Hexamethylentetramin. Ber. Dtsch. Chem. Ges. 1949, 82, 16. [Google Scholar] [CrossRef]
- Hultzsch, K. Chemie der Phenolharze; Springer: Berlin, Germany, 1950. [Google Scholar]
- Megson, N.J.L. Phenolic Resin Chemistry; Butterworth: London, UK, 1958. [Google Scholar]



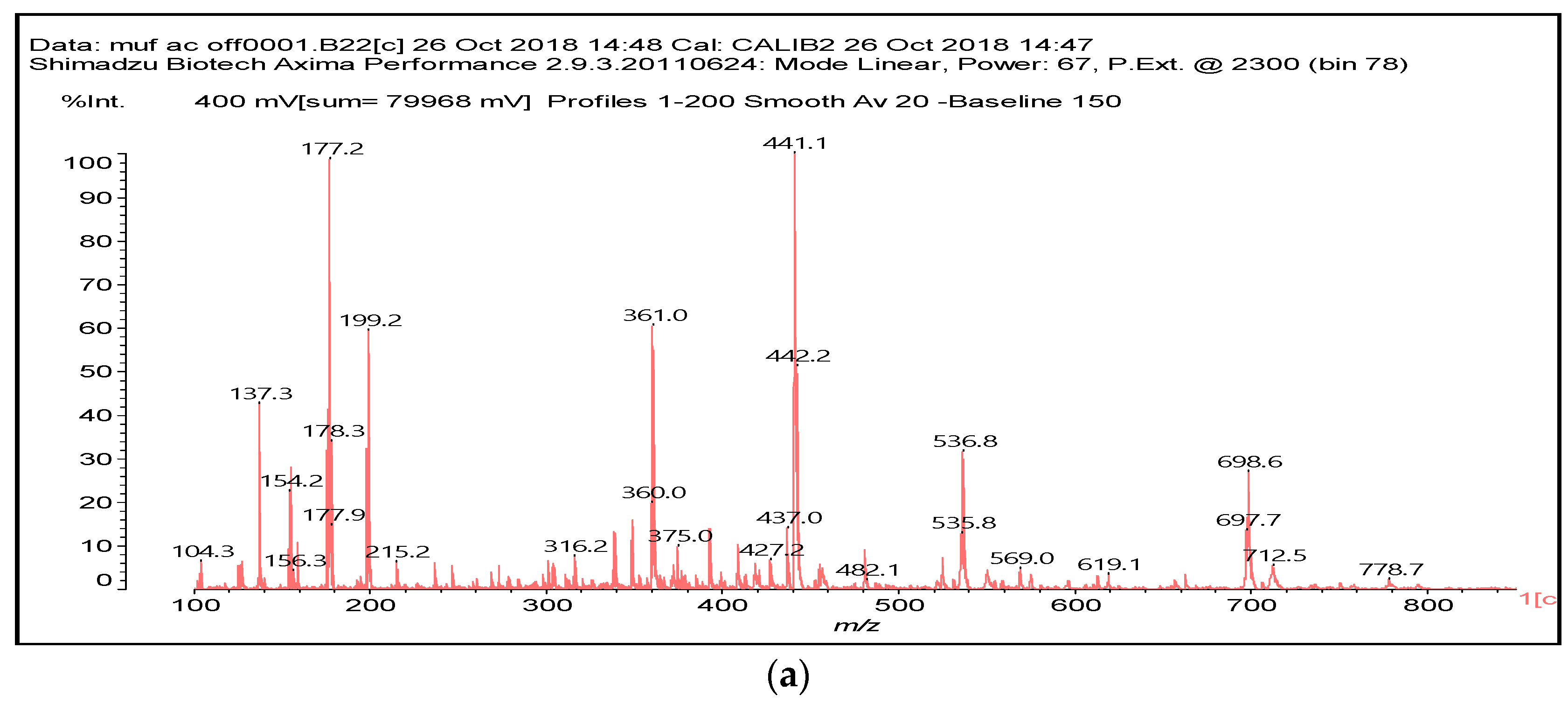

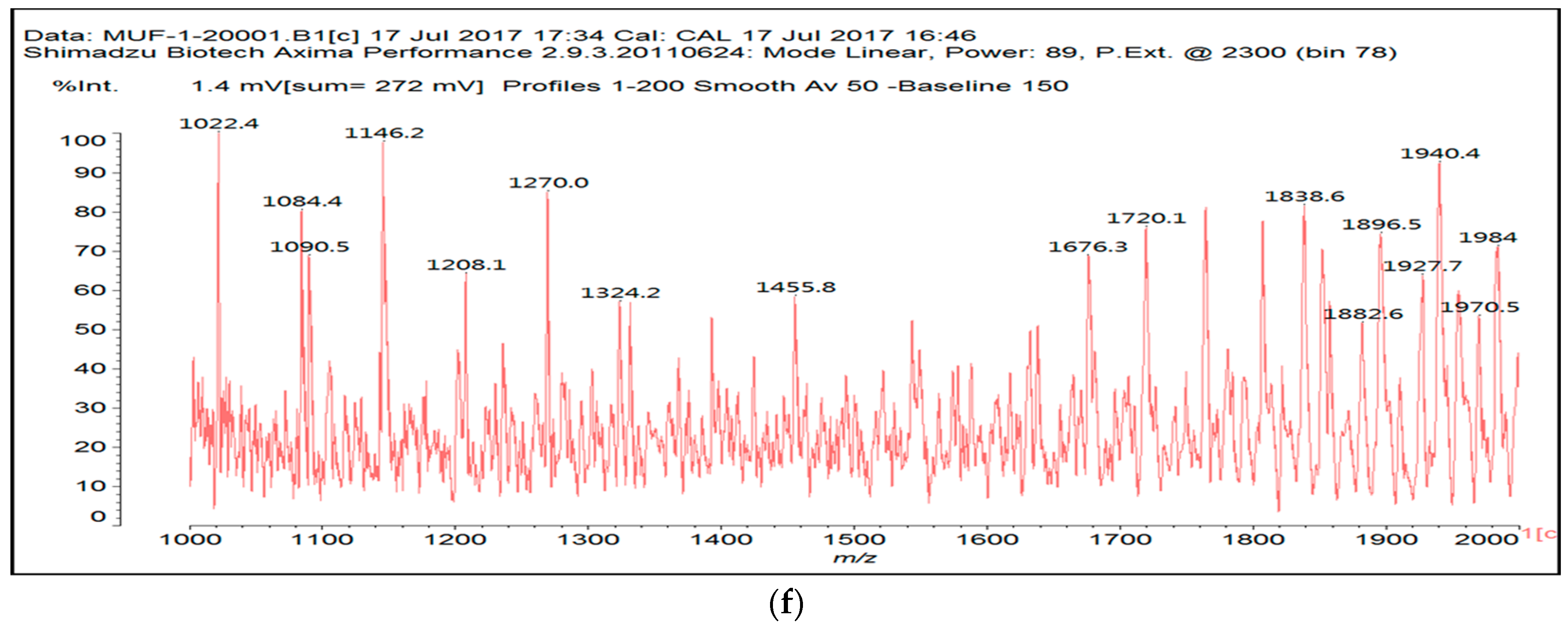
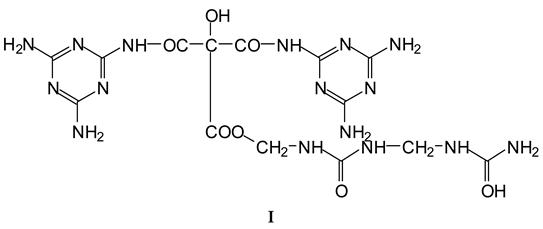
| 154 Da | M-CH2OH without Na+, (exp. 154.2 Da) |
| 177 Da | M-CH2OH with Na+, (exp. 177.2 Da) |
| 215 Da | CH2=N-CH2-OC-C(OH)(COOH)-COOH, no Na+ |
| 305 Da | HOOC-C(OH)(COOH)-CO-NH-M with Na+ (exp.301.2 Da) |
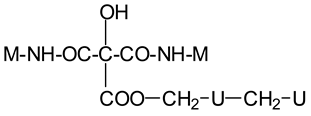
| Thus CITRIC-NH-M | |
| 334 Da | HOCH2-M-CH2-M(CH2+)-CH2OH without Na+ |
| 357 Da | HOCH2-M-CH2-M(CH2+)-CH2OH with Na+ |
| 359 Da | HOOC-C(OH)(COOH)-COO-CH2-U-CH2-U with Na+ (exp. 361 Da) |
| Thus, CITRIC-O-CH2-U-CH2-U | |
| 412 Da | M-NH-OC-C(OH)(COOH)-CO-NH-M with Na+, (exp.409 Da) |
| Thus, M-NH-CITRIC-NH-M | |
| 533 Da | with Na+ (exp. 535–536 Da) |
 | |
| 696 Da | (exp. 697–698 Da) |
 | |
| 874 Da | |
 | |
| Plywood | Shear Strength Dry | Shear Strength 3 h 63 °C | |
|---|---|---|---|
| (MPa) | (MPa) | ||
| MUF control | 0.96 + 0.07 | 0.20 + 0.02 | |
| MUF + hexa/citric | 1.32 + 0.09 | 0.38 + 0.03 | |
| Particleboards | IB strength dry | IB strength 2 h boiling | Density |
| (MPa) | (MPa) | (kg/m3) | |
| MUF + hexa/citric | 1.15 | 0.32 | 701 |
| MUF + hexa/p-TSA | 1.17 | 0.31 | 705 |
| MUF Control | 1.01 | 0.17 | 694 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xi, X.; Pizzi, A.; Lei, H.; Zhou, X.; Du, G. Self-Neutralizing Melamine–Urea–Formaldehyde–Citric Acid Resins for Wood Panel Adhesives. Polymers 2024, 16, 1819. https://doi.org/10.3390/polym16131819
Xi X, Pizzi A, Lei H, Zhou X, Du G. Self-Neutralizing Melamine–Urea–Formaldehyde–Citric Acid Resins for Wood Panel Adhesives. Polymers. 2024; 16(13):1819. https://doi.org/10.3390/polym16131819
Chicago/Turabian StyleXi, Xuedong, Antonio Pizzi, Hong Lei, Xiaojian Zhou, and Guanben Du. 2024. "Self-Neutralizing Melamine–Urea–Formaldehyde–Citric Acid Resins for Wood Panel Adhesives" Polymers 16, no. 13: 1819. https://doi.org/10.3390/polym16131819
APA StyleXi, X., Pizzi, A., Lei, H., Zhou, X., & Du, G. (2024). Self-Neutralizing Melamine–Urea–Formaldehyde–Citric Acid Resins for Wood Panel Adhesives. Polymers, 16(13), 1819. https://doi.org/10.3390/polym16131819







