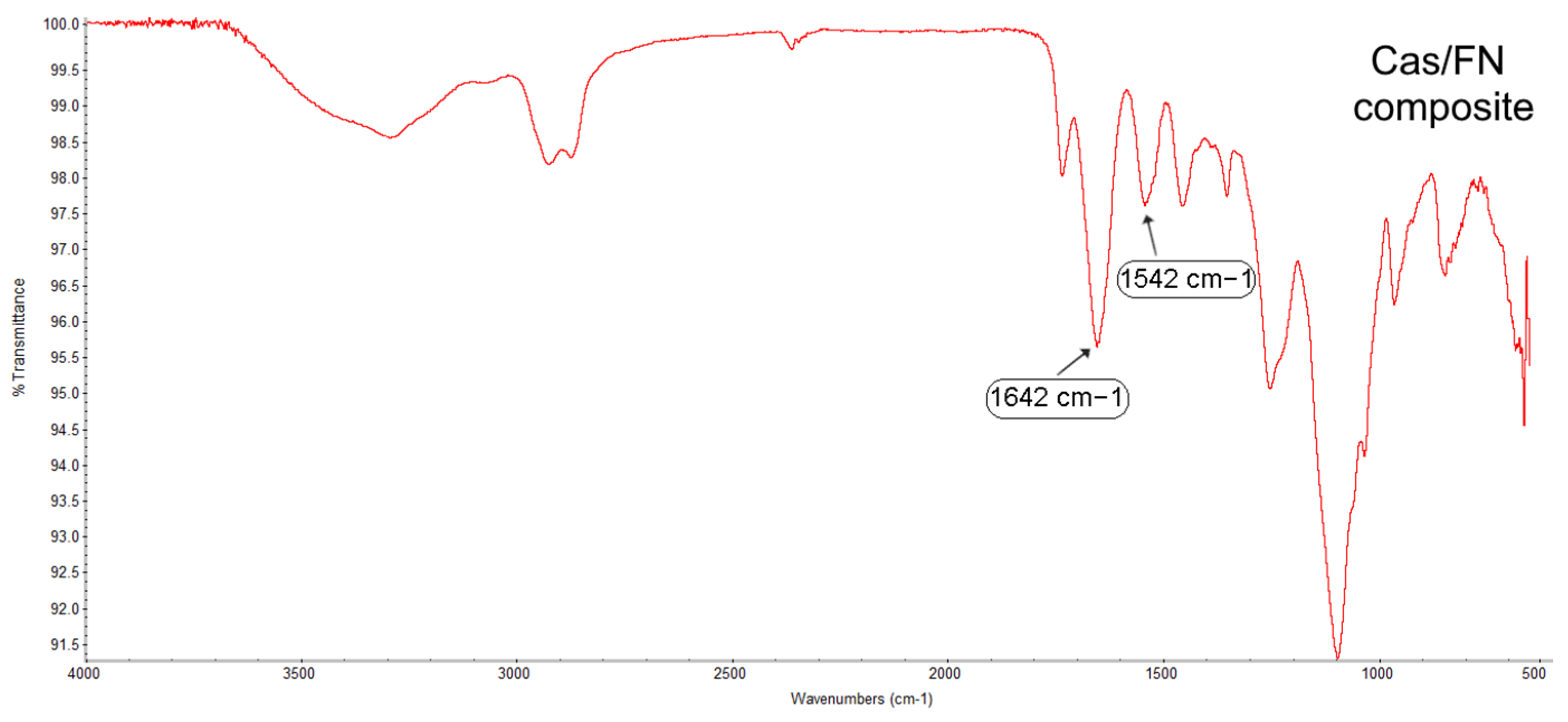
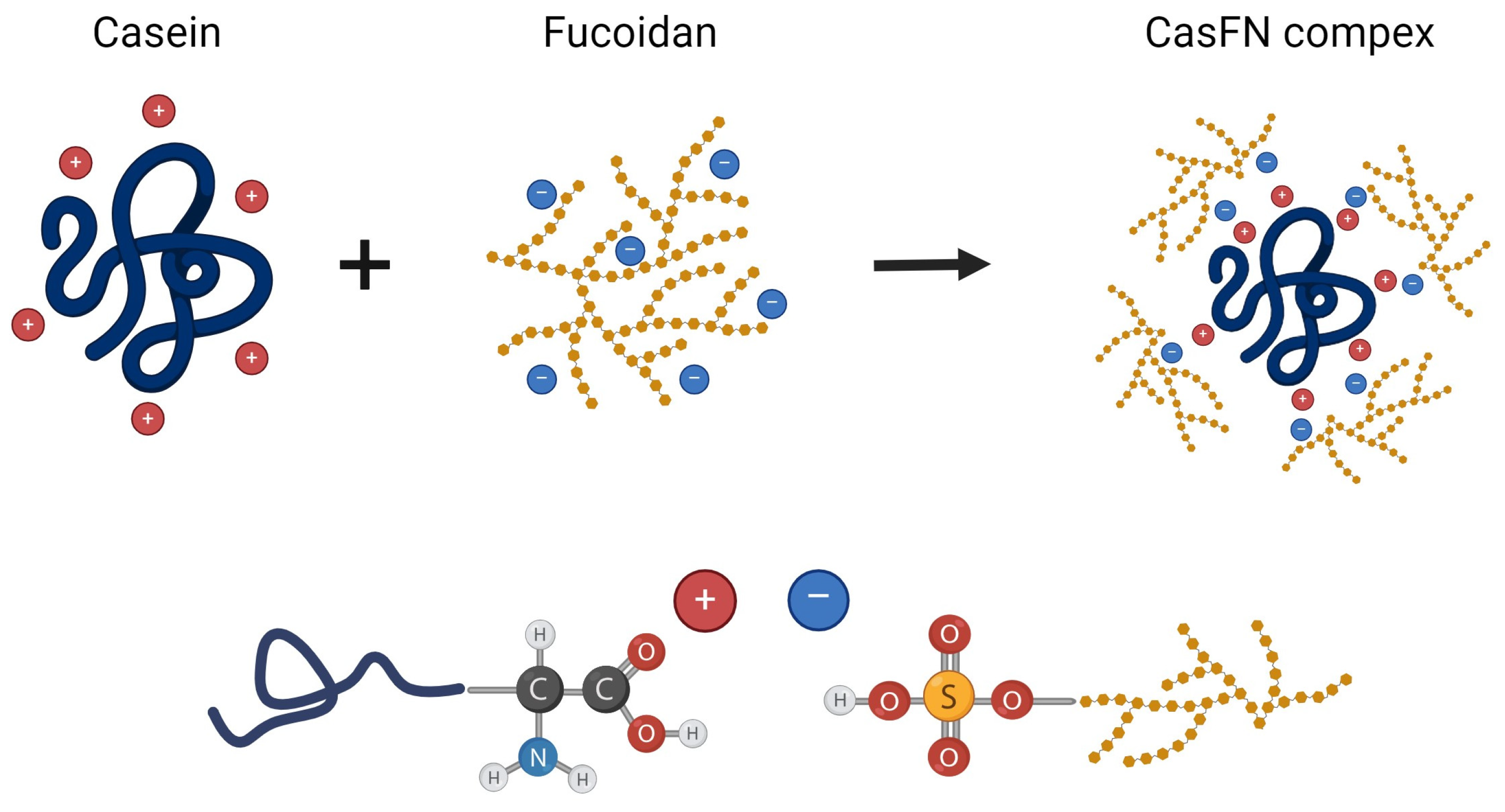
1. Introduction
In recent decades, biopolymers have been the subject of a serious scientific interest for the development of nanosized drug delivery systems due to their specific characteristics and numerous advantages over synthetic polymers, such as low immunogenicity, high biocompatibility, biodegradability, and low toxicity [
1]. Biopolymers such as pep-tides and polysaccharides have been widely used for the preparation of nanoparticulate drug delivery systems that can provide controlled and targeted release, improving biodistribution of the encapsulated drug and minimizing its systemic toxicity [
2]. Compared to polysaccharides, proteins are preferred as structure-forming biopolymers for the production of drug delivery systems due to their ability to form smaller-in-size particles [
3]. Among the proteins used for the formulation of nanosized carriers, casein has shown optimal structural and physicochemical properties [
4]. Casein is a collective term for a family of calcium (phosphate)-binding phosphoproteins commonly found in mammalian milk. Bovine milk casein consists of four peptides, namely αs1, αs2, β, and k, which differ in amino acid, phosphorus, and carbohydrate content, but all of them possess amphiphilic properties [
5]. Cysteine amino acid residues, which are involved in the formation of disulfide bonds, are found only in the polypeptide chains of k-casein. The lack of secondary structures of the casein molecules, due to the proline-rich amino acid residues and the ability to bind to calcium phosphate, leads to the formation of electrostatic, hydrogen, and hydrophobic interactions [
6]. As a result, self-association of the casein peptides occurs, resulting in the formation of stable agglomerates known as casein micelles [
6]. The inner part of the micelle is composed of αs1, αs2, and β caseins, while the outer layer, which stabilizes the micelle, contains glycosylated k-casein [
7]. Casein micelles exhibit pH-dependent behavior. A decrease in the surface negative charge of the casein micelles leads to their contraction, while an increase in the charge leads to an electrostatic repulsion of the casein molecules [
8].
A major problem in the development of casein-based nanostructures is the low drug encapsulation efficiency and drug loading index [
9]. This is related to the use of large amounts of drug delivery systems to ensure the desired dose of the incorporated therapeutic agent [
9]. Thus, the development of suitable technological approaches to overcome these drawbacks is necessary for the preparation of stable and effective drug delivery systems. One of the main approaches is the formation of casein–polysaccharide complexes.
Due to its specific physicochemical properties, casein can participate in the formation of composite nanostructures because of polyelectrolyte or chemical interactions with one or more polymers. Compared to chemically and physically cross-linked casein nanoparticles, composite nanoparticles are characterized by higher stability and the possibility of modified drug release [
10].
Protein-based carriers for drug delivery, including casein, are characterized by physical instability, resulting in protein aggregation and precipitation at a pH close to the isoelectric point of the protein. The incorporation of polysaccharides into the carrier structure induces steric hindrance, which limits protein aggregation [
11]. As a result, protein–polysaccharide composite nanoparticles have increased stability and more efficiently encapsulate hydrophilic and hydrophobic therapeutic agents [
12]. These complexes also provide protection to the included substance from oxidation and hydrolysis under the influence of environmental factors such as pH, light, and temperature [
13]. In addition, the colloidal network composing the nanoparticles, built of protein–polysaccharide complexes, provides a delayed release of the incorporated therapeutic agent [
14]. Polysaccharide complexation of proteins can be due to both covalent and non-covalent binding of biopolymers. Covalent protein–polysaccharide conjugation is due to enzymatic cross-linking (oxidases and transglutaminases), chemical cross-linking (genipin, glutaraldehyde, and polyethylene glycol), or through the Maillard reaction [
15].
In a study conducted by Pan et al., the Maillard reaction was used to prepare casein–dextran copolymer [
16]. The obtained structures showed pH-dependent behavior, with micelle formation occurring at a pH equal to the isoelectric point of casein, and demonstrated successful incorporation of the fluorescent agent pyrene [
16].
Covalent interaction between proteins and polysaccharides also occurs because of cross-linking with transglutaminase, which catalyzes acyl-transfer reactions between the γ-carboxamide groups of glutamine residues and free amino groups [
17].
Glutaraldehyde is among the most widely used chemical cross-linking agents, and provides a high degree of cross-linking of protein–polysaccharide complexes [
18]. Nevertheless, the use of high concentrations of the cross-linking agent is associated with the occurrence of adverse drug reactions, such as breathing disorders, development of asthma, and dermatitis, as well as irritation of the nasal mucosa and ocular conjunctiva [
19].
Physical mixing of two biopolymers leads to the formation of non-covalent protein–polysaccharide bonds, which largely depends on the nature of the biopolymers, their quantitative ratio, pH of the medium, and ionic strength [
20]. Regarding physical protein–polysaccharide complexes, non-covalent interactions can be due to hydrogen bonding and electrostatic and hydrophobic interactions [
21]. Most polysaccharides, except for chitosan, are negatively charged, and at pH values below the isoelectric point of casein, the formation of a casein–polysaccharide complex occurs [
22].
Several studies on the preparation of casein–polysaccharide complexes have been reported in the literature, but only a few are related to the preparation of composite casein–polysaccharide drug delivery nanocarriers. Markman et al. [
23] reported the preparation of vitamin D2-loaded casein–maltodextrin nano capsules. The obtained conjugates show a high percentage of therapeutic agent incorporation efficiency (up to 90%). Furthermore, the carrier demonstrated high colloidal stability and prevention of vitamin D2 oxidation in acidic pH conditions. Regarding the release, the carrier showed a pH-dependent behavior. Lack of release of the incorporated nutraceutical was observed at an acidic pH (2.5), while at pH 7 up to 90% of the incorporated nutraceutical was released, demonstrating the potential use of the carrier as a drug delivery system for targeted delivery in the small intestine. In another study, a drug delivery system for epigal-locatechin-3-gallate was obtained via polyelectrolyte complexation between casein and chitosan [
24]. The obtained nanostructures demonstrated an average particle size of 150 nm, a high degree of biocompatibility, and improved intestinal permeability of epi-gallocatechin-3-gallate. As a result of polyelectrolyte complexation, the preparation of alginate-coated casein nanoparticles loaded with doxorubicin has also been reported [
25]. The results showed that, after coating the casein particles with alginate, an increase in their average particle size from 264 nm to 294 nm was observed. Furthermore, the drug delivery system showed a high drug loading index and encapsulation efficacy (95%) of the cytostatic agent—doxorubicin. In vitro release was performed in two different media (pH 7.4 and pH 5), simulating systemic circulation and tumor cell pH. The results demonstrated a delayed release in alkaline pH and a more pronounced release in acidic pH [
25].
One of the polysaccharides with potential application for the preparation of casein–polysaccharide complexes is the biopolymer fucoidan. Fucoidan belongs to a family of sulfated, L-fucose-rich polysaccharides found in the cell wall of various species of brown algae (Phaeophyta: Laminariaceae, Fucaceae, Chordariaceae, and Alariaceae) [
26]. It can also be obtained from sea cucumbers (Holothuroidea: Stichopodidae, Holothuri-idae), sea urchin eggs (Echinoidea: Strongylocentrotidae, Arbaciidae), and sea grasses (Cymodoceaceae) [
27]. Over 90% of the total sugar content in fucoidan consists of deoxyhexose—L-fucose [
28]. Along with fucose, other monosaccharides can be found in the fucoidan structure: galactose, glucose, xylose, mannose, rhamnose, arabinose, uronic acid, and acetyl groups [
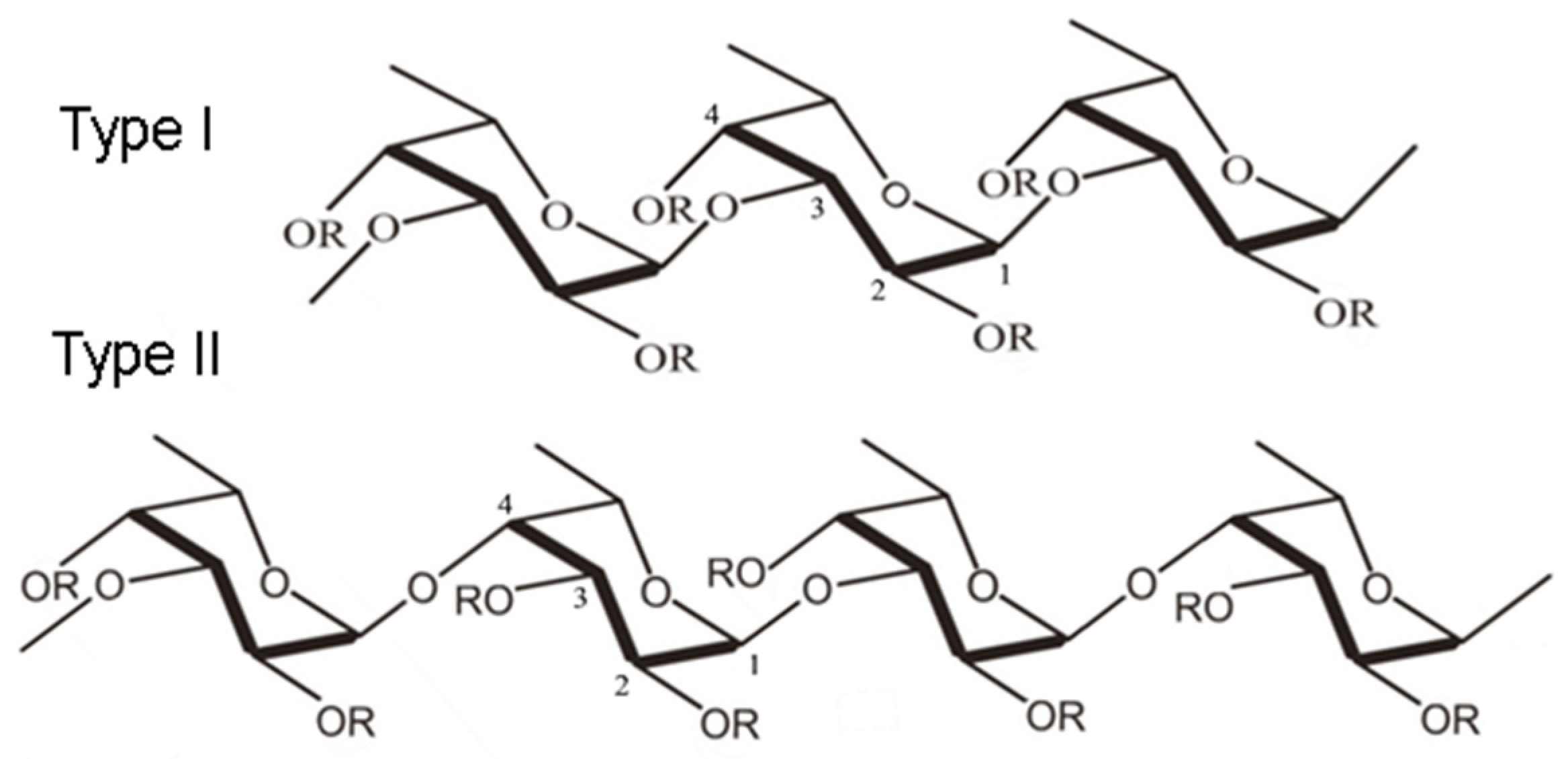
29]. Although the structure and composition of fucoidans differ depending on the origin and extraction method, fucoidans can be categorized into two main types according to their monomeric subunits (
Figure 1).
Type I fucoidan is composed of an α-(1→3)-α-L-fucopyranose backbone linked to sulfate radicals at the C2 and C4 positions, while type II fucoidan is composed of an α-(1→3)- and α-(1→4)-α-L-fucopyranose backbone, with sulfate radicals attached at the C2, C3, and C4 positions [
28,
29].
Depending on the sulfate content and molecular weight, fucoidans show different biological effects (antioxidant, antibacterial, antiviral, anticoagulant, hypolipidemic, hypoglycemic, antitumor, anti-inflammatory, and immunomodulatory). In addition to its biological activity, fucoidan also possesses suitable physicochemical properties as a drug carrier, which is confirmed by numerous reports in the literature about micro- and nanoparticles developed on this basis, liposomes, films, hydrogels, etc. [
30,
31] The polymer backbone has anionic properties due to the presence of negatively charged sulfate ester groups. Depending on the specific chemical characteristics and charge density, fucoidan can interact with various biomolecules, such as proteins and other polysaccharides [
32]. It is soluble in water and in acidic solutions, and its solubility depends on the molecular weight and the number of sulfate groups. The highly water-soluble fucoidans form solutions with low viscosity and are therefore not generally used as gelling agents. On the other hand, the viscoelastic properties of fucoidan are highly dependent on the origin of the polysaccharide, its concentration, molecular weight, sulfate content, pH, and temperature [
27]. The interaction of fucoidan with oppositely charged biopolymers, such as chitosan [
33], poly (2-hydroxyethyl methacrylate) [
34], soy protein isolate (SPI) [
35], and lactoglobulin [
36], can lead to the formation of polyelectrolyte complexes with improved characteristics for biomedical application. Polyelectrolyte complexation (complex coacervation) is one of the most widely used methods for obtaining fucoidan micro- and nanoparticles. Other common techniques for formulating drug systems from the polysaccharide are simple coacervation, ionotropic gelation, spray drying, emulsification, etc. [
37].
With the possibilities of nanotechnology, the development of innovative carriers capable of providing selective and specific therapy for several diseases has long been the subject of fundamental pharmaceutical science. Polymers of natural origin such as casein and fucoidan enable the development of biodegradable nanocarriers providing modified release and targeted delivery, which significantly improves the distribution of the incorporated drugs in the body and reduces the risk of adverse drug reactions. Their complex biological effects and the ability to form electrostatic interactions provide an opportunity for the development of casein–fucoidan composite nanoscale drug delivery systems.
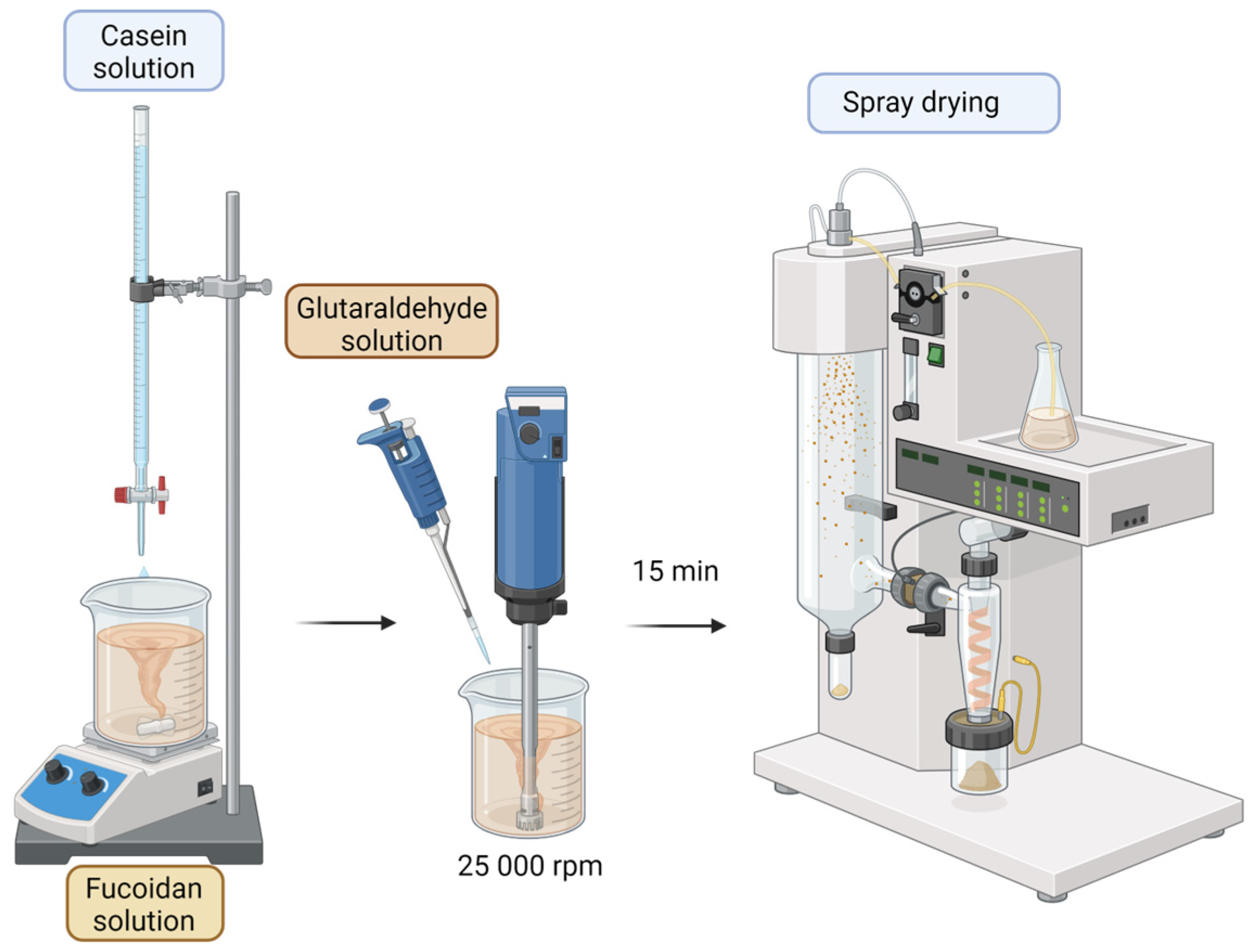
The aim of the present work is to develop casein–fucoidan composite nanostructures by polyelectrolyte complexation and cross-linking of the complex with glutaraldehyde. By further applying 3(k-p) fractional factorial design, the work aims to evaluate the effect of dependent variables on independent variables and outline optimal technological and process parameters for the development of effective drug delivery systems.