Oral Films Printed with Green Propolis Ethanolic Extract
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Production of Oral Films (OFs)
2.3. Characterization of Oral Films
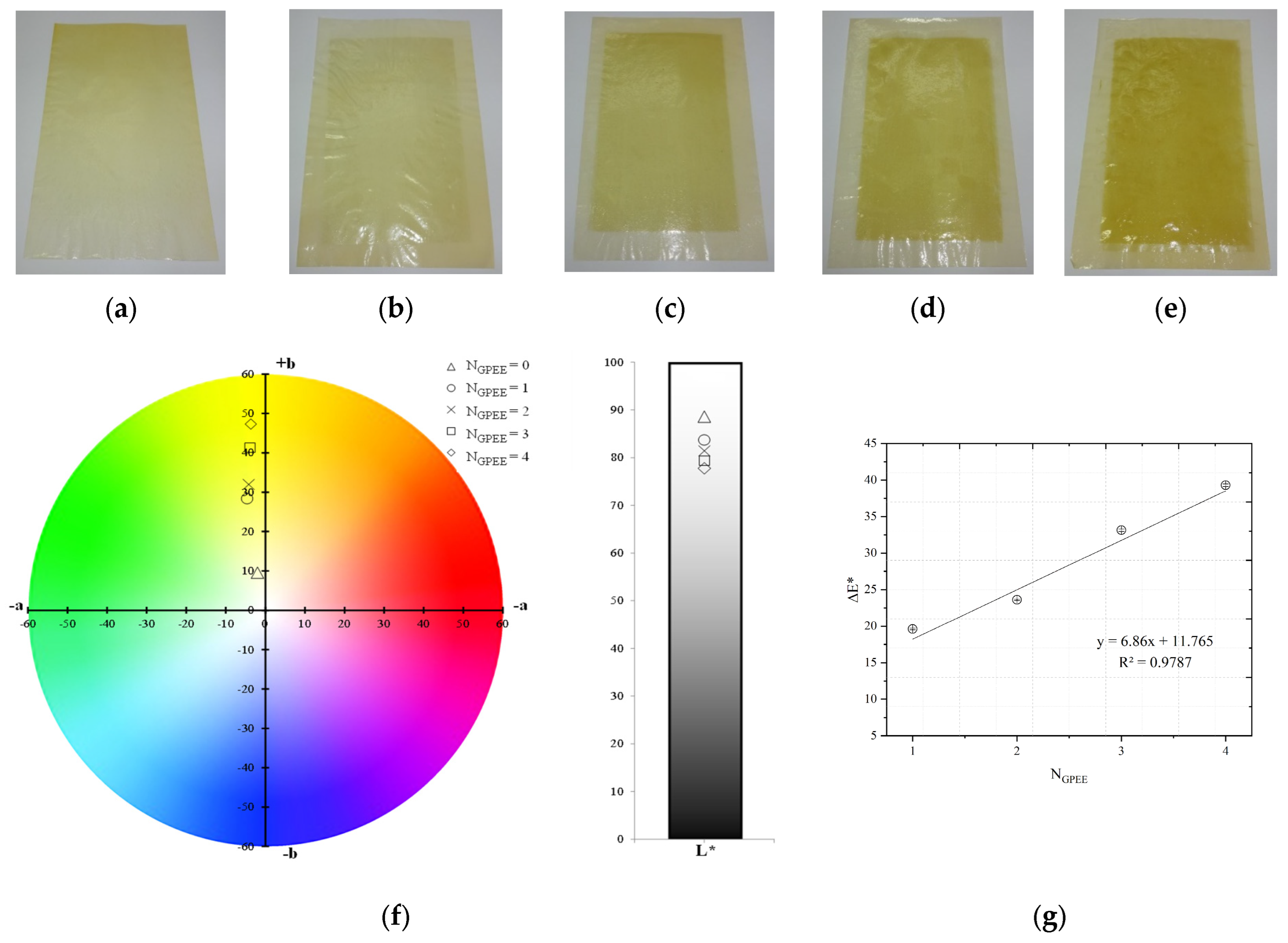
2.3.1. Visual Aspect and Color Parameters
2.3.2. Surface pH
2.3.3. Disintegration Time (DT)
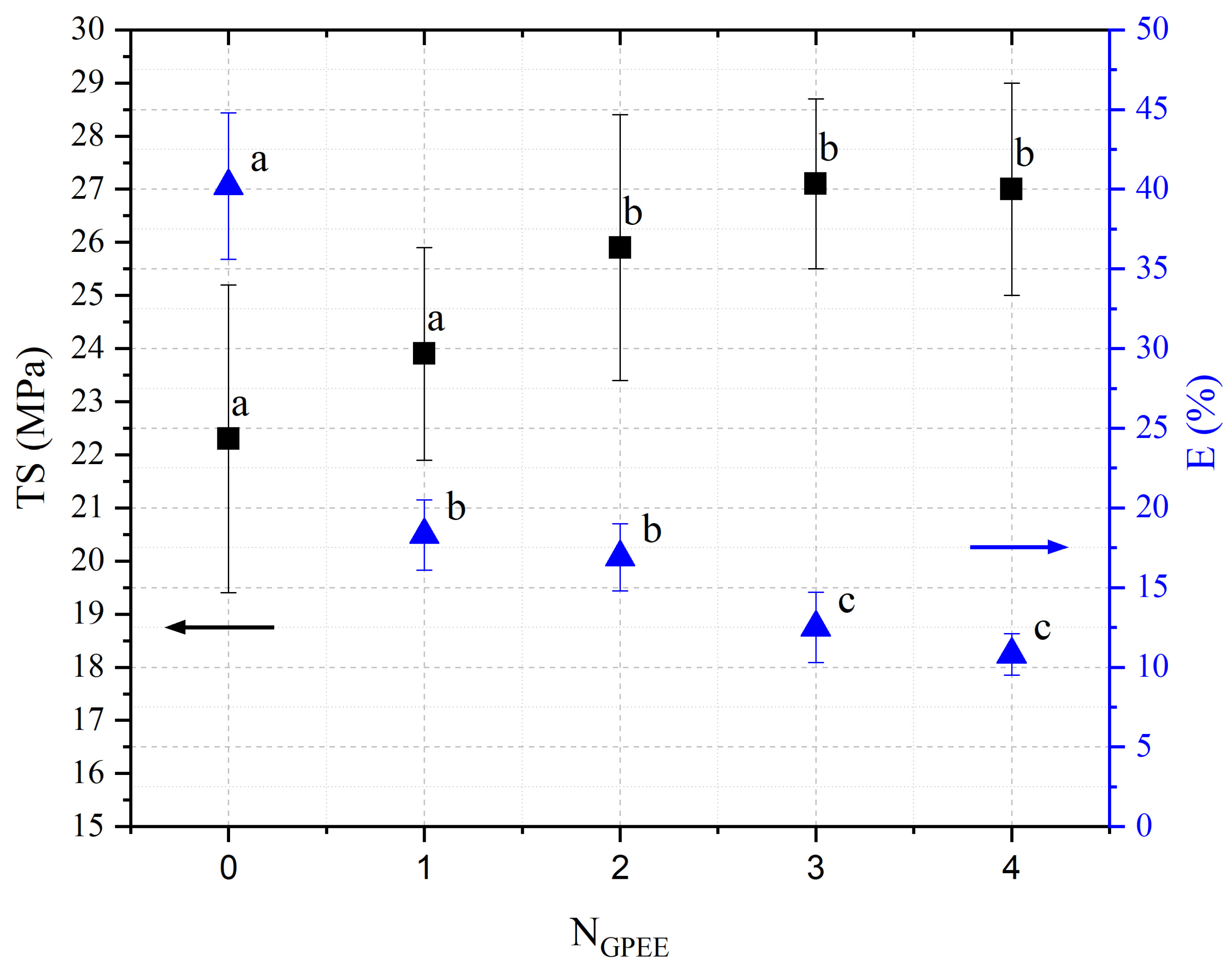
2.3.4. Mechanical Properties
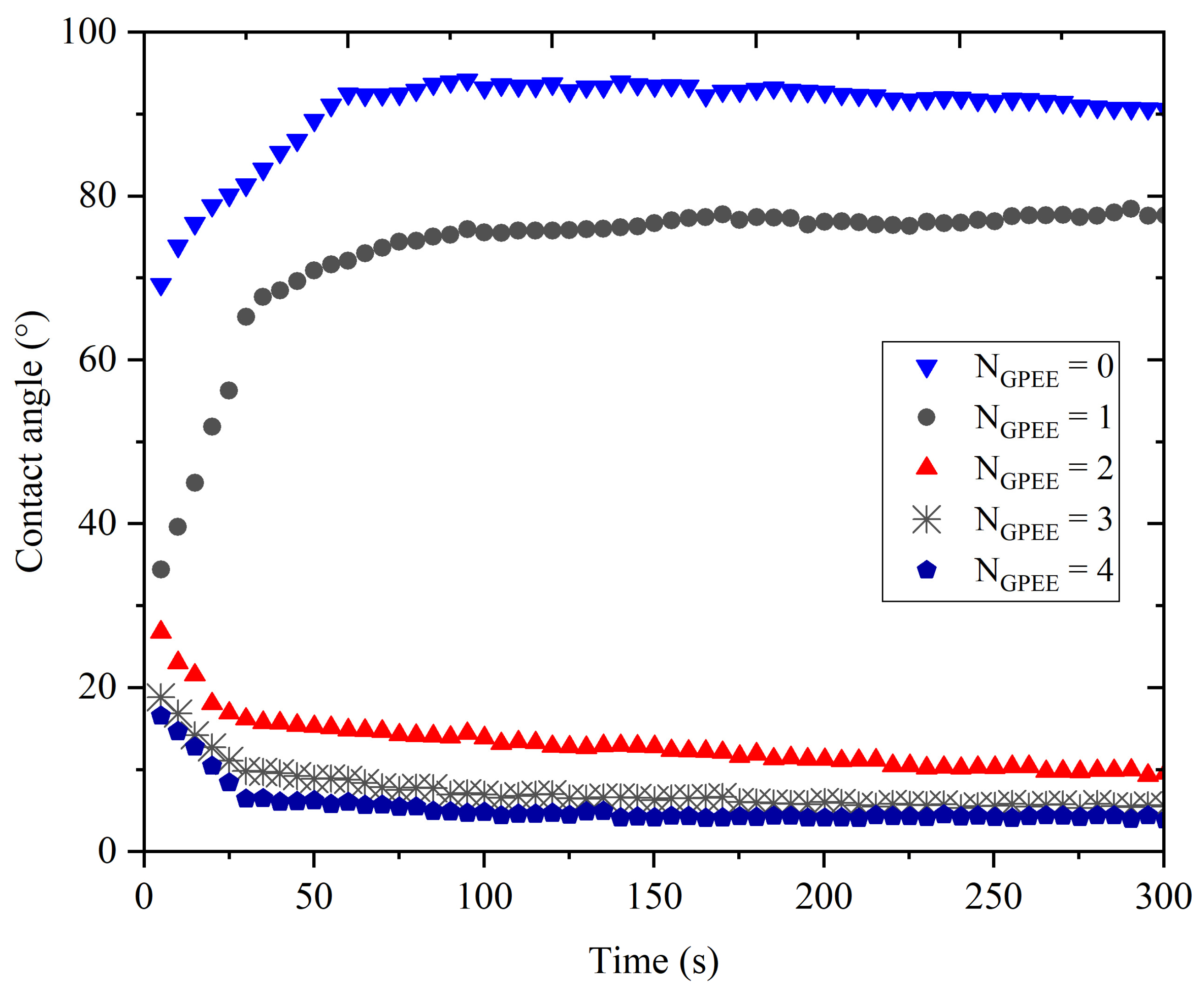
2.3.5. Contact Angle
2.3.6. Scanning Electron Microscopy (SEM) and Atomic Force Microscopy (AFM)
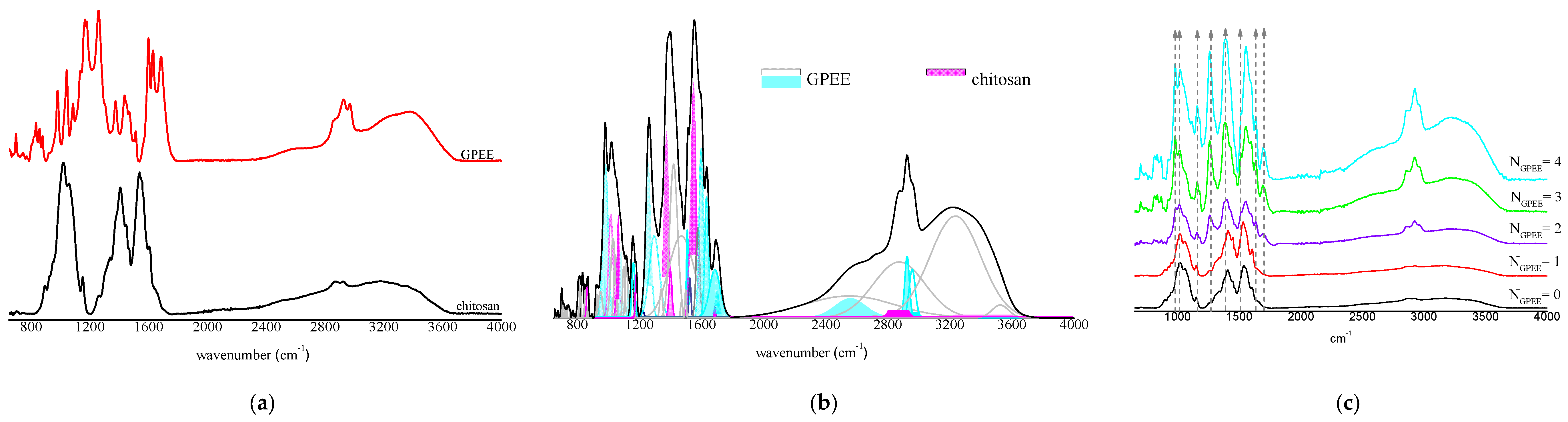
2.3.7. Fourier Transform Infrared Spectroscopy (FTIR)
2.4. Stability
2.5. Statistical Analysis
3. Results and Discussion
3.1. Visual Appearance and Color Parameters
3.2. Surface pH
3.3. Disintegration Time (DT)
3.4. Mechanical Properties
3.5. Contact Angle
3.6. Scanning Electron Microscopy (SEM) and Atomic Force Microscopy (AFM)
3.7. FTIR
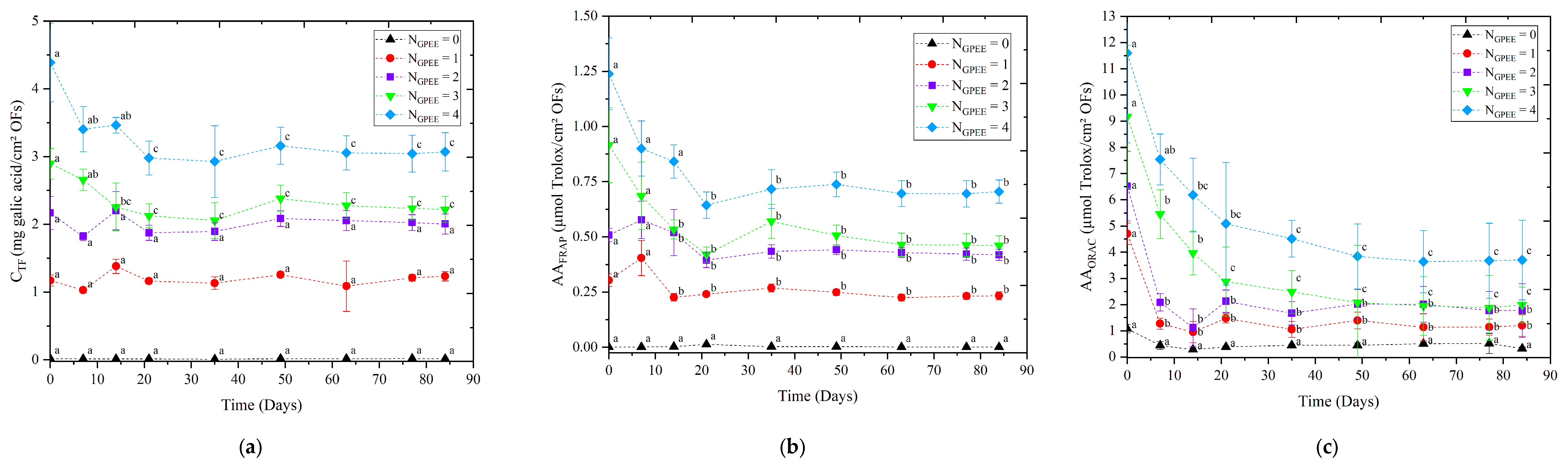
3.8. Stability
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sowjanya, J.N.; Rao, P.R. Development, Optimization, and Invitro Evaluation of Novel Fast Dissolving Oral Films (FDOF’s) of Uncaria Tomentosa Extract to Treat Osteoarthritis. Heliyon 2023, 9, e14292. [Google Scholar] [CrossRef]
- Zaki, R.M.; Alfadhel, M.; DevanathaDesikan Seshadri, V.; Albagami, F.; Alrobaian, M.; Tawati, S.M.; Warsi, M.H.; Almurshedi, A.S. Fabrication and Characterization of Orodispersible Films Loaded with Solid Dispersion to Enhance Rosuvastatin Calcium Bioavailability. Saudi Pharm. J. 2023, 31, 135–146. [Google Scholar] [CrossRef]
- Aravindaraj, N.; Suresh, J.; Krishnaswami, V.; Alagarsamy, S.; Kandasamy, R. Guar Gum Based Oral Films for Hypertensive Urgencies. Nat. Prod. Res. 2022, 36, 6470–6473. [Google Scholar] [CrossRef]
- Tsai, W.; Tsai, H.; Wong, Y.; Hong, J.; Chang, S.; Lee, M. Preparation and Characterization of Gellan Gum/Glucosamine/Clioquinol Film as Oral Cancer Treatment Patch. Mater. Sci. Eng. C 2018, 82, 317–322. [Google Scholar] [CrossRef]
- Remedio, L.N.; Garcia, V.A.d.S.; Rochetti, A.L.; Berretta, A.A.; Yoshida, C.M.P.; Fukumasu, H.; Vanin, F.M.; de Carvalho, R.A. Hydroxypropyl Methylcellulose Orally Disintegration Films Produced by Tape Casting with the Incorporation of Green Propolis Ethanolic Extract Using the Printing Technique. Food Hydrocoll. 2023, 135, 108176. [Google Scholar] [CrossRef]
- Ghisalberti, E.L. Propolis. Bee World 1976, 57, 89–90. [Google Scholar] [CrossRef]
- Salatino, A. Perspectives for Uses of Propolis in Therapy against Infectious Diseases. Molecules 2022, 27, 4594. [Google Scholar] [CrossRef]
- Berretta, A.A.; Arruda, C.; Miguel, F.G.; Baptista, N.; Nascimento, A.P.; Marquele-Oliveira, F.; Hori, J.I.; Barud, H.d.S.; Damaso, B.; Ramos, C.; et al. Functional Properties of Brazilian Propolis: From Chemical Composition Until the Market. In Superfood and Functional Food—An Overview of Their Processing and Utilization; InTech: London, UK, 2017; Volume 11, p. 13. ISBN 0000957720. [Google Scholar]
- Berretta, A.A.; Silveira, M.A.D.; Cóndor Capcha, J.M.; De Jong, D. Propolis and Its Potential against SARS-CoV-2 Infection Mechanisms and COVID-19 Disease. Biomed. Pharmacother. 2020, 131, 110622. [Google Scholar] [CrossRef] [PubMed]
- Berretta, A.A.; Nascimento, A.P.; Bueno, P.C.P.; Vaz, M.M.d.O.L.L.; Marchetti, J.M. Propolis Standardized Extract (EPP-AF®), an Innovative Chemically and Biologically Reproducible Pharmaceutical Compound for Treating Wounds. Int. J. Biol. Sci. 2012, 8, 512–521. [Google Scholar] [CrossRef] [PubMed]
- Hori, J.I.; Zamboni, D.S.; Carrão, D.B.; Goldman, G.H.; Berretta, A.A. The Inhibition of Inflammasome by Brazilian Propolis (EPP-AF). Evid.-Based Complement. Altern. Med. 2013, 2013, 418508. [Google Scholar] [CrossRef]
- Machado, J.L.; Assunção, A.K.M.; da Silva, M.C.P.; Dos Reis, A.S.; Costa, G.C.; Arruda, D.D.S.; Rocha, B.A.; Vaz, M.M.D.O.L.L.; Paes, A.M.D.A.; Guerra, R.N.M.; et al. Brazilian Green Propolis: Anti-Inflammatory Property by an Immunomodulatory Activity. Evid.-Based Complement. Altern. Med. 2012, 2012, 157652. [Google Scholar] [CrossRef] [PubMed]
- Rocha, B.A.; Bueno, P.C.P.; Vaz, M.M.D.O.L.L.; Nascimento, A.P.; Ferreira, N.U.; Moreno, G.D.P.; Rodrigues, M.R.; Costa-Machado, A.R.D.M.; Barizon, E.A.; Campos, J.C.L.; et al. Evaluation of a Propolis Water Extract Using a Reliable RP-HPLC Methodology and In Vitro and In Vivo Efficacy and Safety Characterisation. Evid.-Based Complement. Altern. Med. 2013, 2013, 670451. [Google Scholar] [CrossRef] [PubMed]
- Diniz, D.P.; Lorencini, D.A.; Berretta, A.A.; Cintra, M.A.C.T.; Lia, E.N.; Jordão, A.A.; Coelho, E.B. Antioxidant Effect of Standardized Extract of Propolis (EPP-AF®) in Healthy Volunteers: A “before and after” Clinical Study. Evid.-Based Complement. Altern. Med. 2020, 2020, 7538232. [Google Scholar] [CrossRef] [PubMed]
- Baptista, B.G.; Fanton, S.; Ribeiro, M.; Cardozo, L.F.; Regis, B.; Alvarenga, L.; Ribeiro-Alves, M.; Berretta, A.A.; Shiels, P.G.; Mafra, D. The Effect of Brazilian Green Propolis Extract on Inflammation in Patients with Chronic Kidney Disease on Peritoneal Dialysis: A Randomised Double-Blind Controlled Clinical Trial. Phytomedicine 2023, 114, 154731. [Google Scholar] [CrossRef] [PubMed]
- Chermut, T.R.; Fonseca, L.; Figueiredo, N.; de Oliveira Leal, V.; Borges, N.A.; Cardozo, L.F.; Correa Leite, P.E.; Alvarenga, L.; Regis, B.; Delgado, A.; et al. Effects of Propolis on Inflammation Markers in Patients Undergoing Hemodialysis: A Randomized, Double-Blind Controlled Clinical Trial. Complement. Ther. Clin. Pract. 2023, 51, 101732. [Google Scholar] [CrossRef] [PubMed]
- Silveira, M.A.D.; Teles, F.; Berretta, A.A.; Sanches, T.R.; Rodrigues, C.E.; Seguro, A.C.; Andrade, L. Effects of Brazilian Green Propolis on Proteinuria and Renal Function in Patients with Chronic Kidney Disease: A Randomized, Double-Blind, Placebo-Controlled Trial. BMC Nephrol. 2019, 20, 140. [Google Scholar] [CrossRef] [PubMed]
- Silveira, M.A.D.; Capcha, J.M.C.; Sanches, T.R.; de Sousa Moreira, R.; Garnica, M.S.; Shimizu, M.H.; Berretta, A.; Teles, F.; Noronha, I.L.; Andrade, L. Green Propolis Extract Attenuates Acute Kidney Injury and Lung Injury in a Rat Model of Sepsis. Sci. Rep. 2021, 11, 5925. [Google Scholar] [CrossRef] [PubMed]
- Silveira, M.A.D.; Malta-Santos, H.; Rebouças-Silva, J.; Teles, F.; Batista dos Santos Galvão, E.; Pinto de Souza, S.; Dantas Dutra, F.R.; Dantas Gomes, M.M.; Teixeira, M.B.; Miranda Rebelo da Conceição, L.F.; et al. Effects of Standardized Brazilian Green Propolis Extract (EPP-AF®) on Inflammation in Haemodialysis Patients: A Clinical Trial. Int. J. Nephrol. 2022, 2022, 1035475. [Google Scholar] [CrossRef]
- Silveira, M.A.D.; De Jong, D.; Berretta, A.A.; Galvão, E.B.d.S.; Ribeiro, J.C.; Cerqueira-Silva, T.; Amorim, T.C.; da Conceição, L.F.M.R.; Gomes, M.M.D.; Teixeira, M.B.; et al. Efficacy of Brazilian Green Propolis (EPP-AF®) as an Adjunct Treatment for Hospitalized COVID-19 Patients: A Randomized, Controlled Clinical Trial. Biomed. Pharmacother. 2021, 138, 111526. [Google Scholar] [CrossRef]
- Berretta, A.A.; de Castro, P.A.; Cavalheiro, A.H.; Fortes, V.S.; Bom, V.P.; Nascimento, A.P.; Marquele-Oliveira, F.; Pedrazzi, V.; Ramalho, L.N.Z.; Goldman, G.H. Evaluation of Mucoadhesive Gels with Propolis (EPP-AF) in Preclinical Treatment of Candidiasis Vulvovaginal Infection. Evid.-Based Complement. Altern. Med. 2013, 2013, 641480. [Google Scholar] [CrossRef]
- de Castro, P.A.; Savoldi, M.; Bonatto, D.; Barros, M.H.; Goldman, M.H.S.; Berretta, A.A.; Goldman, G.H. Molecular Characterization of Propolis-Induced Cell Death in Saccharomyces Cerevisiae. Eukaryot. Cell 2011, 10, 398–411. [Google Scholar] [CrossRef] [PubMed]
- de Castro, P.A.; Savoldi, M.; Bonatto, D.; Malavazi, I.; Goldman, M.H.S.; Berretta, A.A.; Goldman, G.H. Transcriptional Profiling of Saccharomyces Cerevisiae Exposed to Propolis. BMC Complement. Altern. Med. 2012, 12, 194. [Google Scholar] [CrossRef] [PubMed]
- de Castro, P.A.; Bom, V.L.P.; Brown, N.A.; de Almeida, R.S.C.; Ramalho, L.N.Z.; Savoldi, M.; Goldman, M.H.S.; Berretta, A.A.; Goldman, G.H. Identification of the Cell Targets Important for Propolis-Induced Cell Death in Candida Albicans. Fungal Genet. Biol. 2013, 60, 74–86. [Google Scholar] [CrossRef] [PubMed]
- Pina, G.d.M.S.; Lia, E.N.; Berretta, A.A.; Nascimento, A.P.; Torres, E.C.; Buszinski, A.F.M.; de Campos, T.A.; Coelho, E.B.; Martins, V.d.P. Efficacy of Propolis on the Denture Stomatitis Treatment in Older Adults: A Multicentric Randomized Trial. Evid.-Based Complement. Altern. Med. 2017, 2017, 8971746. [Google Scholar] [CrossRef] [PubMed]
- Barud, H.D.S.; de Araújo Júnior, A.M.; Saska, S.; Mestieri, L.B.; Campos, J.A.D.B.; de Freitas, R.M.; Ferreira, N.U.; Nascimento, A.P.; Miguel, F.G.; Vaz, M.M.D.O.L.L.; et al. Antimicrobial Brazilian Propolis (EPP-AF) Containing Biocellulose Membranes as Promising Biomaterial for Skin Wound Healing. Evid.-Based Complement. Altern. Med. 2013, 2013, 703024. [Google Scholar] [CrossRef] [PubMed]
- Marquele-Oliveira, F.; da Silva Barud, H.; Torres, E.C.; Machado, R.T.A.; Caetano, G.F.; Leite, M.N.; Frade, M.A.C.; Ribeiro, S.J.L.; Berretta, A.A. Development, Characterization and Pre-Clinical Trials of an Innovative Wound Healing Dressing Based on Propolis (EPP-AF®)-Containing Self-Microemulsifying Formulation Incorporated in Biocellulose Membranes. Int. J. Biol. Macromol. 2019, 136, 570–578. [Google Scholar] [CrossRef] [PubMed]
- Nani, M.; Leone, A.; Bom, V.P.; Buszinski, A.F.; Oliveira de Souza, R.; Pinheiro, V.A.; Danapoulos, P.; Swikidisa, R.; Marquele-Oliveira, F.; Frade, M.A.C.; et al. Evaluation and Comparison of Wound Healing Properties of an Ointment (AlpaWash) Containing Brazilian Micronized Propolis and Peucedanum Ostruthium Leaf Extract in Skin Ulcer in Rats. Int. J. Pharm. Compd. 2018, 22, 154–163. [Google Scholar] [PubMed]
- Zhi, K.; Wang, J.; Zhao, H.; Yang, X. Self-Assembled Small Molecule Natural Product Gel for Drug Delivery: A Breakthrough in New Application of Small Molecule Natural Products. Acta Pharm. Sin. B 2020, 10, 913–927. [Google Scholar] [CrossRef]
- Hou, Y.; Chen, M.; Ruan, H.; Sun, Z.; Wu, H.; Xu, X.; Yang, J.; Ma, G.; Zhou, X. A New Supramolecular Natural Product Gel Based on Self-Assembled Pomolic Acid from Traditional Chinese Medicine. Colloid Interface Sci. Commun. 2022, 46, 100583. [Google Scholar] [CrossRef]
- Farid, A.; Haridyy, H.; Ashraf, S.; Ahmed, S.; Safwat, G. Aloe Vera Gel as a Stimulant for Mesenchymal Stem Cells Differentiation and a Natural Therapy for Radiation Induced Liver Damage. J. Radiat. Res. Appl. Sci. 2022, 15, 270–278. [Google Scholar] [CrossRef]
- Permana, A.D.; Sam, A.; Marzaman, A.N.F.; Rahim, A.; Nainu, F.; Bahar, M.A.; Asri, R.M.; Chabib, L. Solid Lipid Nanoparticles Cyclodextrin-Decorated Incorporated into Gellan Gum-Based Dry Floating in Situ Delivery Systems for Controlled Release of Bioactive Compounds of Safflower (Carthamus tinctorius L): A Proof of Concept Study in Biorelevant Media. Int. J. Biol. Macromol. 2023, 237, 124084. [Google Scholar] [CrossRef]
- Andjić, M.; Draginić, N.; Kočović, A.; Jeremić, J.; Vučićević, K.; Jeremić, N.; Krstonošić, V.; Božin, B.; Kladar, N.; Čapo, I.; et al. Immortelle Essential Oil-Based Ointment Improves Wound Healing in a Diabetic Rat Model. Biomed. Pharmacother. 2022, 150, 112941. [Google Scholar] [CrossRef] [PubMed]
- Kudláček, K.; La Nasa, J.; Ribechini, E.; Colombini, M.P.; Nesměrák, K. Study of the Molecular Compositions of Ointments from the 18th Baroque Pharmacy of the Capuchin Monastery in Hradčany (Prague, Czech Republic). Microchem. J. 2023, 190, 108680. [Google Scholar] [CrossRef]
- Akombaetwa, N.; Muungo, L.T.; Nyirenda, J.; Muwowo, S.; Chichonyi, A.K.; Mukosha, M.; Mwila, C. Formulation and Assessment of the Efficacy and Stability of an Ointment Containing Ocimum americanum L. Extract. Clin. Complement. Med. Pharmacol. 2023, 3, 100078. [Google Scholar] [CrossRef]
- Garcia, V.A.d.S.; Osiro, D.; Vanin, F.M.; Yoshida, C.M.P.; de Carvalho, R.A. Oral Films with Addition Mushroom (Agaricus bisporus) as a Source of Active Compounds. J. Pharm. Sci. 2021, 111, 1739–1748. [Google Scholar] [CrossRef]
- De Carvalho, A.F.F.; Caldeira, V.F.; Oliveira, A.P.; da Cunha Gonsalves, J.K.M.; da Cruz Araújo, E.C. Design and Development of Orally Disintegrating Films: A Platform Based on Hydroxypropyl Methylcellulose and Guar Gum. Carbohydr. Polym. 2023, 299, 120155. [Google Scholar] [CrossRef] [PubMed]
- Kamali, H.; Farzadnia, P.; Movaffagh, J.; Abbaspour, M. Optimization of Curcumin Nanofibers as Fast Dissolving Oral Films Prepared by Emulsion Electrospinning via Central Composite Design. J. Drug Deliv. Sci. Technol. 2022, 75, 103714. [Google Scholar] [CrossRef]
- Lourenço, C.A.M.; Garcia, V.A.; Borges, J.G.; Yoshida, C.M.P.; Vanin, F.M.; Carvalho, R.A. A Novel Phenolic Compounds Delivery System: Oral Films with Extract from Camu-camu Industrial Residue. J. Appl. Polym. Sci. 2022, 139, 52092. [Google Scholar] [CrossRef]
- Qin, Z.Y.; Jia, X.W.; Liu, Q.; Kong, B.H.; Wang, H. Fast Dissolving Oral Films for Drug Delivery Prepared from Chitosan/Pullulan Electrospinning Nanofibers. Int. J. Biol. Macromol. 2019, 137, 224–231. [Google Scholar] [CrossRef]
- Abouhussein, D.; El Nabarawi, M.A.; Shalaby, S.H.; El-Bary, A.A. Cetylpyridinium Chloride Chitosan Blended Mucoadhesive Buccal Films for Treatment of Pediatric Oral Diseases. J. Drug Deliv. Sci. Technol. 2020, 57, 101676. [Google Scholar] [CrossRef]
- Ibrahim, Y.H.E.Y.; Regdon, G.; Kristó, K.; Kelemen, A.; Adam, M.E.; Hamedelniel, E.I.; Sovány, T. Design and Characterization of Chitosan/Citrate Films as Carrier for Oral Macromolecule Delivery. Eur. J. Pharm. Sci. 2020, 146, 105270. [Google Scholar] [CrossRef] [PubMed]
- Silvestre, A.L.P.; dos Santos, A.M.; de Oliveira, A.B.; Ferrisse, T.M.; Brighenti, F.L.; Meneguin, A.B.; Chorilli, M. Evaluation of Photodynamic Therapy on Nanoparticles and Films Loaded-Nanoparticles Based on Chitosan/Alginate for Curcumin Delivery in Oral Biofilms. Int. J. Biol. Macromol. 2023, 240, 124489. [Google Scholar] [CrossRef] [PubMed]
- Dana, P.M.; Hallajzadeh, J.; Asemi, Z.; Mansournia, M.A.; Yousefi, B. Chitosan Applications in Studying and Managing Osteosarcoma. Int. J. Biol. Macromol. 2021, 169, 321–329. [Google Scholar] [CrossRef] [PubMed]
- Heras-Mozos, R.; Gavara, R.; Hernández-Muñoz, P. Chitosan Films as PH-Responsive Sustained Release Systems of Naturally Occurring Antifungal Volatile Compounds. Carbohydr. Polym. 2022, 283, 119137. [Google Scholar] [CrossRef] [PubMed]
- Veltman, B.; Harpaz, D.; Cohen, Y.; Poverenov, E.; Eltzov, E. Characterization of the Selective Binding of Modified Chitosan Nanoparticles to Gram-Negative Bacteria Strains. Int. J. Biol. Macromol. 2022, 194, 666–675. [Google Scholar] [CrossRef] [PubMed]
- Adair, P.; Sriprom, P.; Narkrugsa, W.; Phumjan, L.; Manamoongmongkol, K.; Permana, L.; Assawasaengrat, P. Preparation, Characterization, and Antimicrobial Activity of Xyloglucan-Chitosan Film from Tamarind (Tamarind indica L.) Seed Kernel. Prog. Org. Coat. 2023, 179, 107486. [Google Scholar] [CrossRef]
- El Mouzahim, M.; Eddarai, E.M.; Eladaoui, S.; Guenbour, A.; Bellaouchou, A.; Zarrouk, A.; Boussen, R. Food Packaging Composite Film Based on Chitosan, Natural Kaolinite Clay, and Ficus. Carica Leaves Extract for Fresh-Cut Apple Slices Preservation. Int. J. Biol. Macromol. 2023, 233, 123430. [Google Scholar] [CrossRef] [PubMed]
- Turković, E.; Vasiljević, I.; Drašković, M.; Parojčić, J. Orodispersible Films—Pharmaceutical Development for Improved Performance: A Review. J. Drug Deliv. Sci. Technol. 2022, 75, 103708. [Google Scholar] [CrossRef]
- Bashir, S.; Fitaihi, R.; Abdelhakim, H.E. Advances in Formulation and Manufacturing Strategies for the Delivery of Therapeutic Proteins and Peptides in Orally Disintegrating Dosage Forms. Eur. J. Pharm. Sci. 2023, 182, 106374. [Google Scholar] [CrossRef]
- Dodoo, C.C.; Stapleton, P.; Basit, A.W.; Gaisford, S. The Potential of Streptococcus Salivarius Oral Films in the Management of Dental Caries: An Inkjet Printing Approach. Int. J. Pharm. 2020, 591, 119962. [Google Scholar] [CrossRef]
- Panraksa, P.; Rachtanapun, P.; Thipchai, P.; Lesniewska, E.; Brachais, C.-H.; Debeaufort, F.; Chambin, O.; Jantrawut, P. Sustainable 3D Printing of Oral Films with Tunable Characteristics Using CMC-Based Inks from Durian Rind Wastes. Eur. J. Pharm. Biopharm. 2023, 186, 30–42. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, L.M.; dos Santos, J.; de Oliveira, T.V.; Funk, N.L.; Petzhold, C.L.; Benvenutti, E.V.; Deon, M.; Beck, R.C.R. Drug-Loaded Mesoporous Silica on Carboxymethyl Cellulose Hydrogel: Development of Innovative 3D Printed Hydrophilic Films. Int. J. Pharm. 2022, 620, 121750. [Google Scholar] [CrossRef] [PubMed]
- Elbadawi, M.; Nikjoo, D.; Gustafsson, T.; Gaisford, S.; Basit, A.W. Pressure-Assisted Microsyringe 3D Printing of Oral Films Based on Pullulan and Hydroxypropyl Methylcellulose. Int. J. Pharm. 2021, 595, 120197. [Google Scholar] [CrossRef] [PubMed]
- Borges, J.G.; dos Santos Garcia, V.A.; Osiro, D.; de Carvalho, R.A. Printing Ethanol Pomegranate Extract in Films by Inkjet Technology. Ind. Crops Prod. 2019, 140, 111643. [Google Scholar] [CrossRef]
- Musazzi, U.M.; Selmin, F.; Ortenzi, M.A.; Mohammed, G.K.; Franzé, S.; Minghetti, P.; Cilurzo, F. Personalized Orodispersible Films by Hot Melt Ram Extrusion 3D Printing. Int. J. Pharm. 2018, 551, 52–59. [Google Scholar] [CrossRef] [PubMed]
- Borges, J.G.; De Carvalho, R.A. Orally Disintegrating Films Containing Propolis: Properties and Release Profile. J. Pharm. Sci. 2015, 104, 1431–1439. [Google Scholar] [CrossRef] [PubMed]
- Juliano, C.; Pala, C.L.; Cossu, M. Preparation and Characterisation of Polymeric Films Containing Propolis. J. Drug Deliv. Sci. Technol. 2007, 17, 177–181. [Google Scholar] [CrossRef]
- Santos, J.E.; Soares, J.P.; Dockal, E.R.; Campana Filho, S.P.; Cavalheiro, É.T.G. Caracterização de Quitosanas Comerciais de Diferentes Origens. Polímeros 2003, 13, 242–249. [Google Scholar] [CrossRef]
- Yoshida, C.M.P.; Bastos, C.E.N.; Franco, T.T. Modeling of Potassium Sorbate Diffusion through Chitosan Films. LWT—Food Sci. Technol. 2010, 43, 584–589. [Google Scholar] [CrossRef]
- Föger, F.; Kopf, A.; Loretz, B.; Albrecht, K.; Bernkop-Schnürch, A. Correlation of in Vitro and in Vivo Models for the Oral Absorption of Peptide Drugs. Amino Acids 2008, 35, 233–241. [Google Scholar] [CrossRef]
- Prabhu, P.; Malli, R.; Koland, M.; Vijaynarayana, K.; D’Souza, U.; Harish, N.; Shastry, C.; Charyulu, R. Formulation and Evaluation of Fast Dissolving Films of Levocitirizine Di Hydrochloride. Int. J. Pharm. Investig. 2011, 1, 99. [Google Scholar] [CrossRef] [PubMed]
- Steiner, D.; Finke, J.H.; Kwade, A. Efficient Production of Nanoparticle-Loaded Orodispersible Films by Process Integration in a Stirred Media Mill. Int. J. Pharm. 2016, 511, 804–813. [Google Scholar] [CrossRef] [PubMed]
- ASTM D882; Standard Test Method for Tensile Properties of Thin Plastic Sheeting. ASTM International: West Conshohocken, PA, USA, 2012; Volume 12. [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of Total Phenols and Other Oxidation Substrates and Antioxidants by Means of Folin-Ciocalteu Reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of “Antioxidant Power”: The FRAP Assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Ou, B.; Hampsch-Woodill, M.; Prior, R.L. Development and Validation of an Improved Oxygen Radical Absorbance Capacity Assay Using Fluorescein as the Fluorescent Probe. J. Agric. Food Chem. 2001, 49, 4619–4626. [Google Scholar] [CrossRef] [PubMed]
- Korelc, K.; Larsen, B.S.; Gašperlin, M.; Tho, I. Water-Soluble Chitosan Eases Development of Mucoadhesive Buccal Films and Wafers for Children. Int. J. Pharm. 2023, 631, 122544. [Google Scholar] [CrossRef] [PubMed]
- Genina, N.; Janßen, E.M.; Breitenbach, A.; Breitkreutz, J.; Sandler, N. Evaluation of Different Substrates for Inkjet Printing of Rasagiline Mesylate. Eur. J. Pharm. Biopharm. 2013, 85, 1075–1083. [Google Scholar] [CrossRef] [PubMed]
- dos Santos Garcia, V.A.; Borges, J.G.; Vanin, F.M.; de Carvalho, R.A. Orally Disintegrating Films of Biopolymers for Drug Delivery. In Biopolymer Membranes and Films; Elsevier: Amsterdam, The Netherlands, 2020; pp. 289–307. ISBN 9780128181348. [Google Scholar]
- Zaki, D.Y.; Safwat, E.M.; Nagi, S.M.; Salem, H.N.; Hamdy, T.M.; Moharam, L.M.; Hassan, M.L.; Hamzawy, E.M.A. A Novel Dental Re-Mineralizing Blend of Hydroxyethyl-Cellulose and Cellulose Nanofibers Oral Film Loaded with Nepheline Apatite Glass: Preparation, Characterization and in Vitro Evaluation of Re-Mineralizing Effect. Carbohydr. Polym. Technol. Appl. 2021, 2, 100035. [Google Scholar] [CrossRef]
- Khajuria, D.K.; Patil, O.N.; Karasik, D.; Razdan, R. Development and Evaluation of Novel Biodegradable Chitosan Based Metformin Intrapocket Dental Film for the Management of Periodontitis and Alveolar Bone Loss in a Rat Model. Arch. Oral Biol. 2018, 85, 120–129. [Google Scholar] [CrossRef] [PubMed]
- Jyoti, A.; Gurpreet, S.; Seema, S.; Rana, A.C. Fast Dissolving Films: A Novel Approach To Oral Drug Delivery. Int. Res. J. Pharm. 2011, 2, 69–74. [Google Scholar]
- Mi, F.-L.; Sung, H.-W.; Shyu, S.-S.; Su, C.-C.; Peng, C.-K. Synthesis and Characterization of Biodegradable TPP/Genipin Co-Crosslinked Chitosan Gel Beads. Polymer 2003, 44, 6521–6530. [Google Scholar] [CrossRef]
- Batista, P.; Castro, P.; Madureira, A.; Sarmento, B.; Pintado, M. Development and Characterization of Chitosan Microparticles-in-Films for Buccal Delivery of Bioactive Peptides. Pharmaceuticals 2019, 12, 32. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, M.S.; Kamali, B.; Nabid, M.R. Multilayered Mucoadhesive Hydrogel Films Based on Ocimum Basilicum Seed Mucilage/Thiolated Alginate/Dopamine-Modified Hyaluronic Acid and PDA Coating for Sublingual Administration of Nystatin. Int. J. Biol. Macromol. 2022, 203, 93–104. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Ma, W.; Cui, B.; Yuan, C.; Zhao, M. Synergistic Modulation of Chitosan on the Mechanical and Disintegration Properties of Pre-Gelatinized Waxy Corn Starch Orally Disintegrating Film. Food Biosci. 2023, 56, 103317. [Google Scholar] [CrossRef]
- Preis, M.; Knop, K.; Breitkreutz, J. Mechanical Strength Test for Orodispersible and Buccal Films. Int. J. Pharm. 2014, 461, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Farris, S.; Introzzi, L.; Biagioni, P.; Holz, T.; Schiraldi, A.; Piergiovanni, L. Wetting of Biopolymer Coatings: Contact Angle Kinetics and Image Analysis Investigation. Langmuir 2011, 27, 7563–7574. [Google Scholar] [CrossRef] [PubMed]
- César, L.T.; Soares, L.S.; Farias, M.D.P.; Teixeira Sá, D.M.A.; Ayala Valencia, G.; Monteiro, A.R. Chitosan and Acerola (Malpighia emarginata) Fruit Based Active Coating Can Control the Melanosis of Refrigerated Shrimps (Litopenaeus vannamei). J. Food Process. Preserv. 2022, 46, e16983. [Google Scholar] [CrossRef]
- Gümüş, Ö.Y.; Yssaad, I. Immobilization of Propolis Extract on PET Fabric for Biomedical Applications. Politek. Derg. 2022, 25, 1299–1307. [Google Scholar] [CrossRef]
- Queiroz, M.F.; Melo, K.R.T.; Sabry, D.A.; Sassaki, G.L.; Rocha, H.A.O. Does the Use of Chitosan Contribute to Oxalate Kidney Stone Formation? Mar. Drugs 2015, 13, 141–158. [Google Scholar] [CrossRef]
- Villalobos, K.; Rojas, H.; González-Paz, R.; Granados, D.B.; González-Masís, J.; Vega Baudrit, J.; Corrales-Ureña, Y.R. Production of Starch Films Using Propolis Nanoparticles as Novel Bioplasticizer. J. Renew. Mater. 2017, 5, 189–198. [Google Scholar] [CrossRef]
- De Carli, C.; Aylanc, V.; Mouffok, K.M.; Santamaria-Echart, A.; Barreiro, F.; Tomás, A.; Pereira, C.; Rodrigues, P.; Vilas-Boas, M.; Falcão, S.I. Production of Chitosan-Based Biodegradable Active Films Using Bio-Waste Enriched with Polyphenol Propolis Extract Envisaging Food Packaging Applications. Int. J. Biol. Macromol. 2022, 213, 486–497. [Google Scholar] [CrossRef] [PubMed]
- Franca, J.R.; De Luca, M.P.; Ribeiro, T.G.; Castilho, R.O.; Moreira, A.N.; Santos, V.R.; Faraco, A.A.G. Propolis—Based Chitosan Varnish: Drug Delivery, Controlled Release and Antimicrobial Activity against Oral Pathogen Bacteria. BMC Complement. Altern. Med. 2014, 14, 478. [Google Scholar] [CrossRef] [PubMed]
- Borges, J.G.; Silva, A.G.; Cervi-Bitencourt, C.M.; Vanin, F.M.; Carvalho, R.A. Lecithin, Gelatin and Hydrolyzed Collagen Orally Disintegrating Films: Functional Properties. Int. J. Biol. Macromol. 2016, 86, 907–916. [Google Scholar] [CrossRef]
- Yu, J.Y.; Kim, H.W.; Park, H.J. Customized Oral Mucosal Adhesive Film-Based Functional-Substance Delivery System Using Embedded 3D Printing Method. Food Hydrocoll. 2022, 131, 107762. [Google Scholar] [CrossRef]
| Printing Layers | Surface pH | Disintegration Time (s) |
|---|---|---|
| 0 | 6.91 ± 0.02 a | 331 ± 129 a |
| 1 | 6.54 ± 0.21 b | 454 ± 155 a,b |
| 2 | 6.36 ± 0.17 c | 312 ± 243 a |
| 3 | 6.02 ± 0.09 d | 532 ± 166 b |
| 4 | 5.89 ± 0.11 e | 557 ± 22 b |
| Analyses | Printing Layers of Green Propolis Ethanolic Extract | ||||
|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | |
| MEV Surface |  |  |  |  |  |
| MEV Internal |  |  |  |  |  |
| AFM 2D |  |  |  |  |  |
| AFM 3D |  |  |  |  | 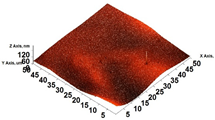 |
| AR | 81.95 ± 12.76 a | 50.18 ± 8.07 b | 37.87 ± 9.38 b | 32.33 ± 8.69 b | 12.37 ± 6.10 c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Remedio, L.N.; Garcia, V.A.d.S.; Rochetti, A.L.; Berretta, A.A.; Ferreira, J.A.; Fukumasu, H.; Vanin, F.M.; Yoshida, C.M.P.; de Carvalho, R.A. Oral Films Printed with Green Propolis Ethanolic Extract. Polymers 2024, 16, 1811. https://doi.org/10.3390/polym16131811
Remedio LN, Garcia VAdS, Rochetti AL, Berretta AA, Ferreira JA, Fukumasu H, Vanin FM, Yoshida CMP, de Carvalho RA. Oral Films Printed with Green Propolis Ethanolic Extract. Polymers. 2024; 16(13):1811. https://doi.org/10.3390/polym16131811
Chicago/Turabian StyleRemedio, Leandro Neodini, Vitor Augusto dos Santos Garcia, Arina Lazaro Rochetti, Andresa Aparecida Berretta, Julieta Adriana Ferreira, Heidge Fukumasu, Fernanda Maria Vanin, Cristiana Maria Pedroso Yoshida, and Rosemary Aparecida de Carvalho. 2024. "Oral Films Printed with Green Propolis Ethanolic Extract" Polymers 16, no. 13: 1811. https://doi.org/10.3390/polym16131811
APA StyleRemedio, L. N., Garcia, V. A. d. S., Rochetti, A. L., Berretta, A. A., Ferreira, J. A., Fukumasu, H., Vanin, F. M., Yoshida, C. M. P., & de Carvalho, R. A. (2024). Oral Films Printed with Green Propolis Ethanolic Extract. Polymers, 16(13), 1811. https://doi.org/10.3390/polym16131811










