Enhancement in Sensitivity and Selectivity of Electrochemical Technique with CuO/g-C3N4 Nanocomposite Combined with Molecularly Imprinted Polymer for Melamine Detection
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
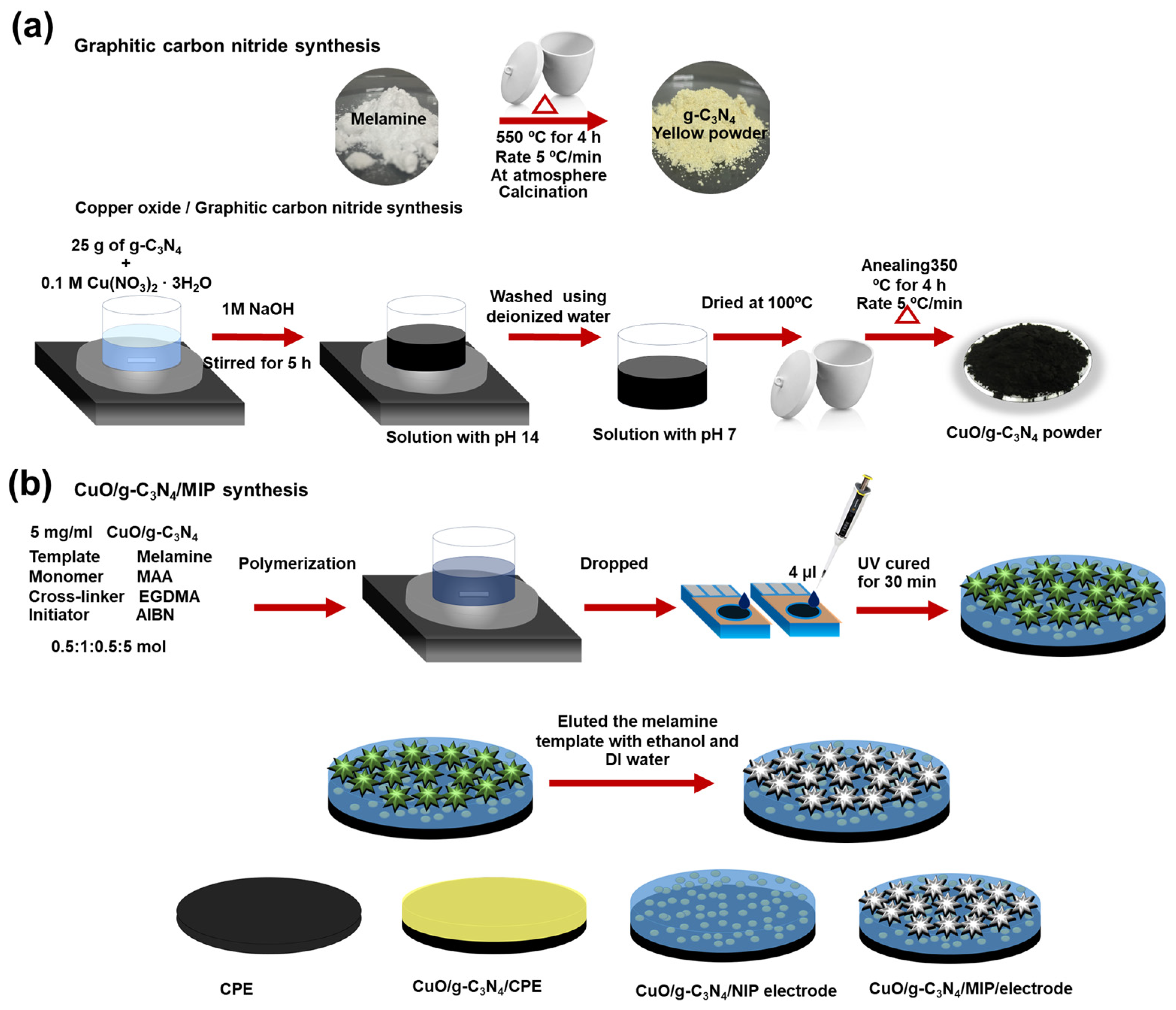
2.2. Preparation of CuO/g-C3N4 Nanocomposite
2.3. Fabrication of CuO/g-C3N4 Nanocomposite Combined with Molecularly Imprinted Polymer
2.4. Material Characterizations
2.5. Electrochemical Measurements
3. Results and Discussions
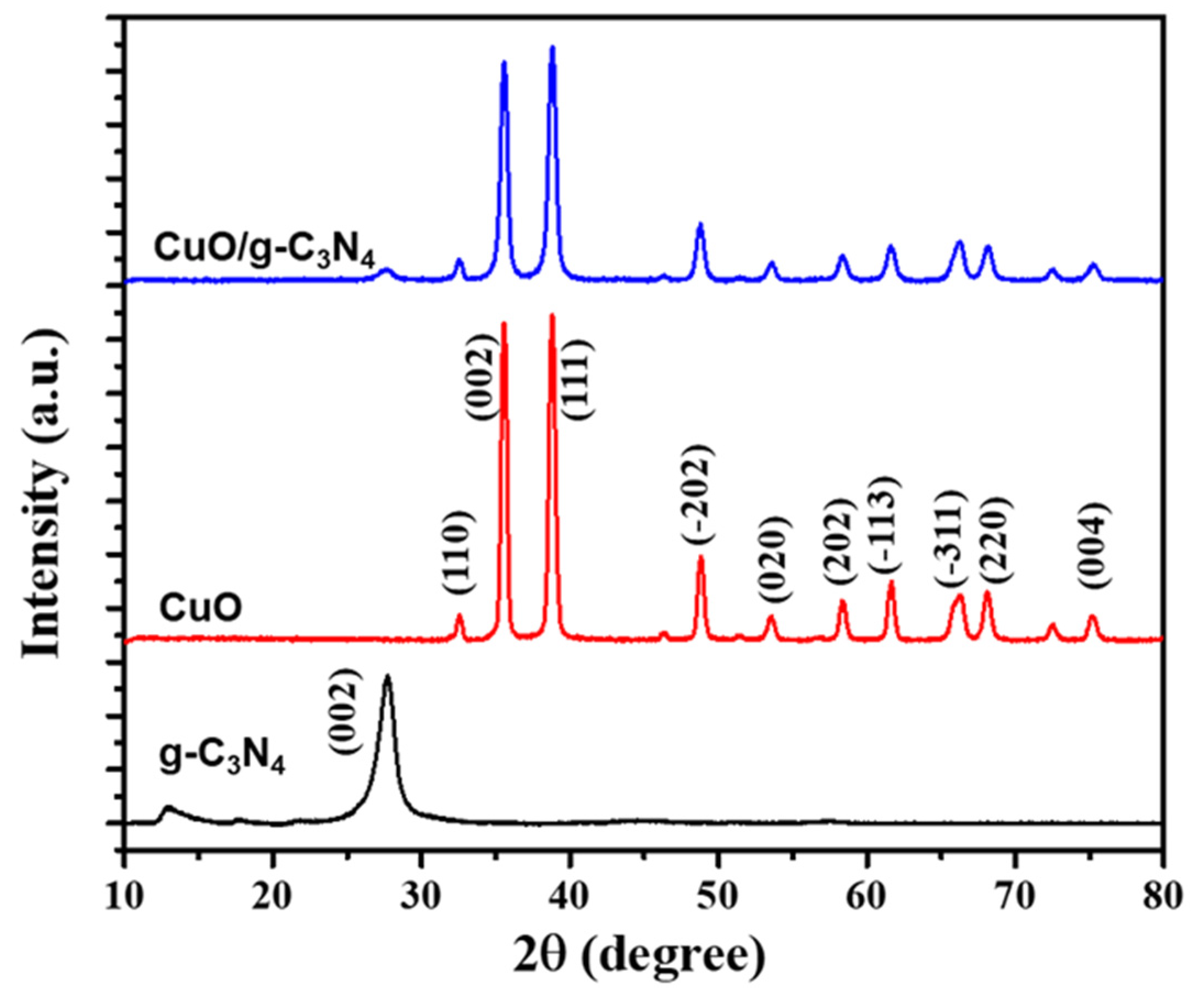
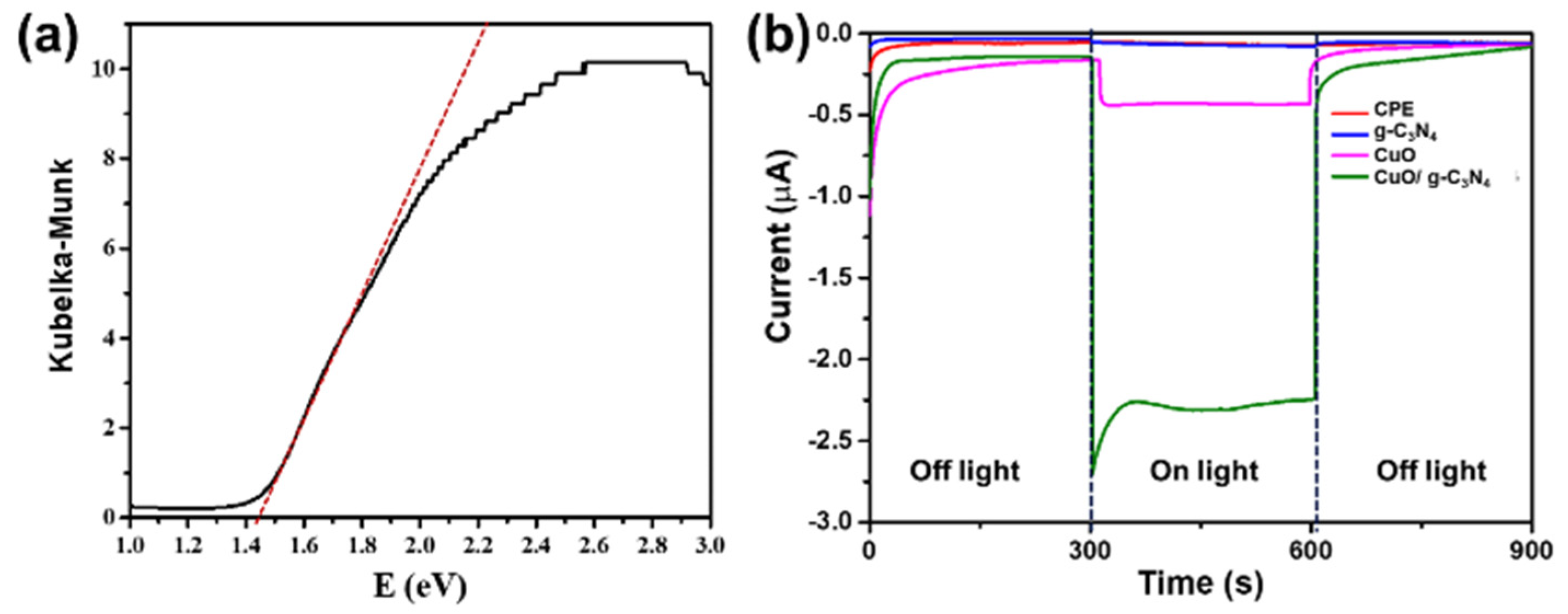
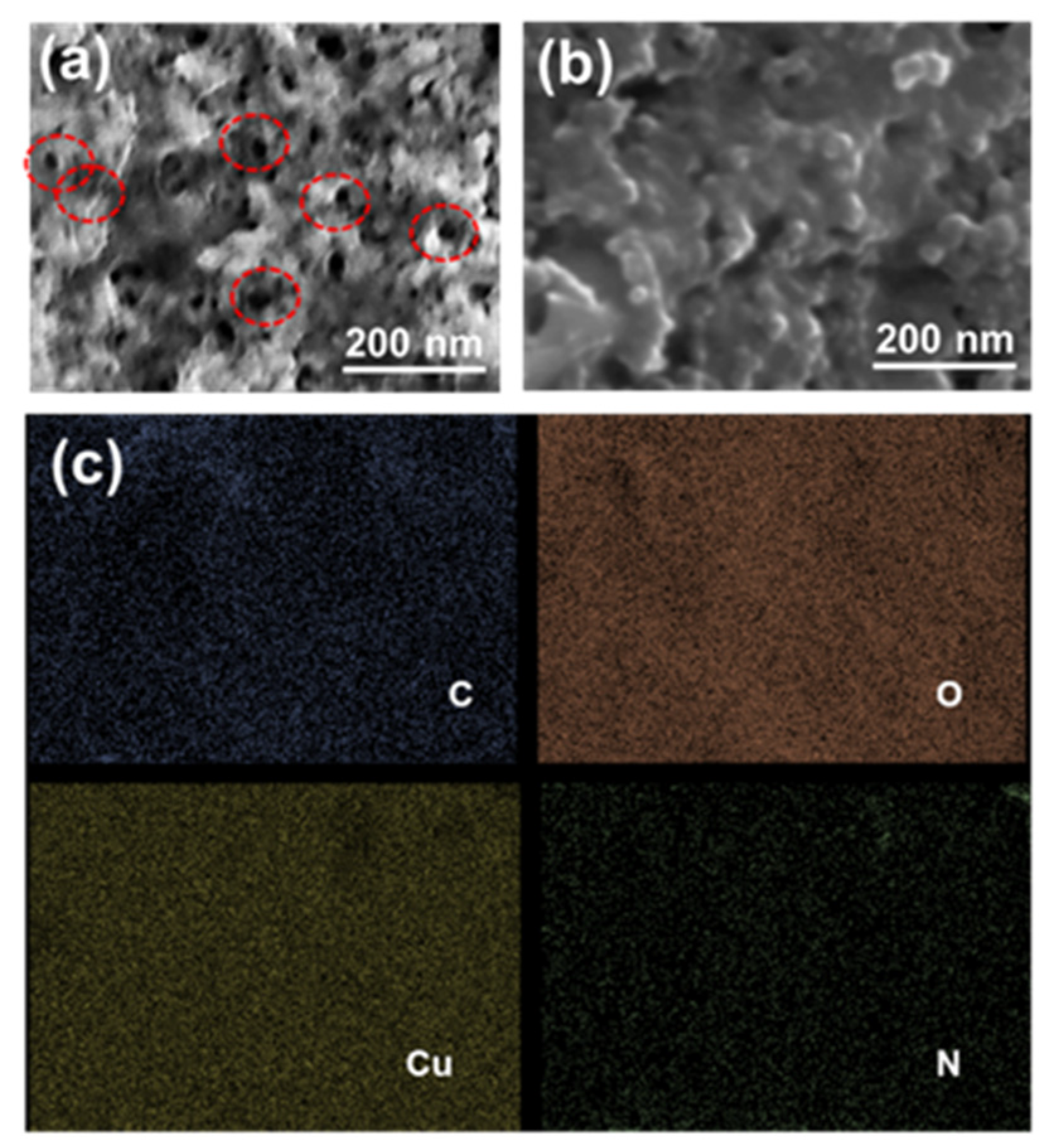
3.1. Characterization of CuO/g-C3N4 Nanocomposite Synthesis
3.2. A Surface-Modified Electrode with a CuO/g-C3N4 Nanocomposite Combined with a Molecularly Imprinted Polymer
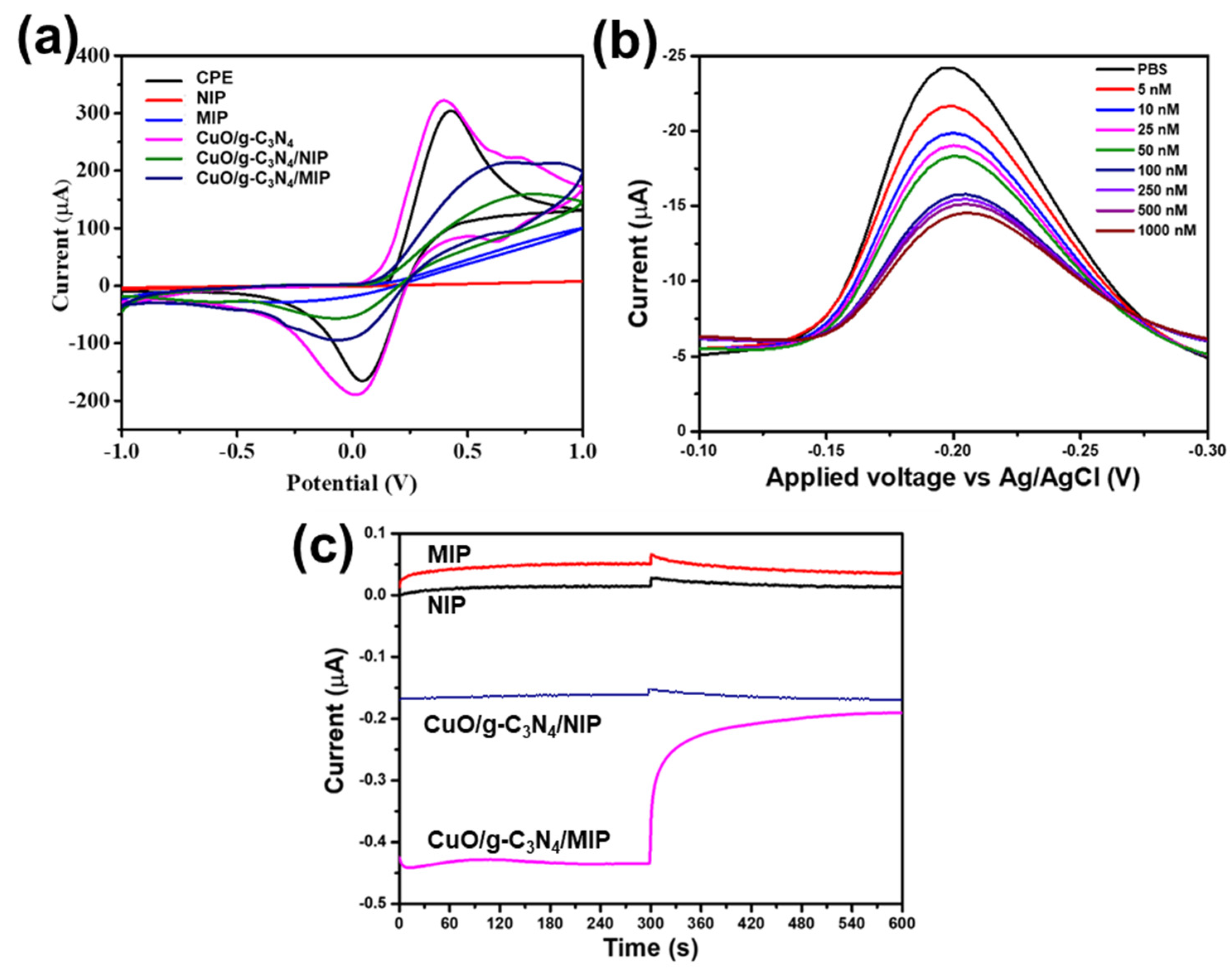
3.3. The Electrochemical Behaviors of Modified Electrodes
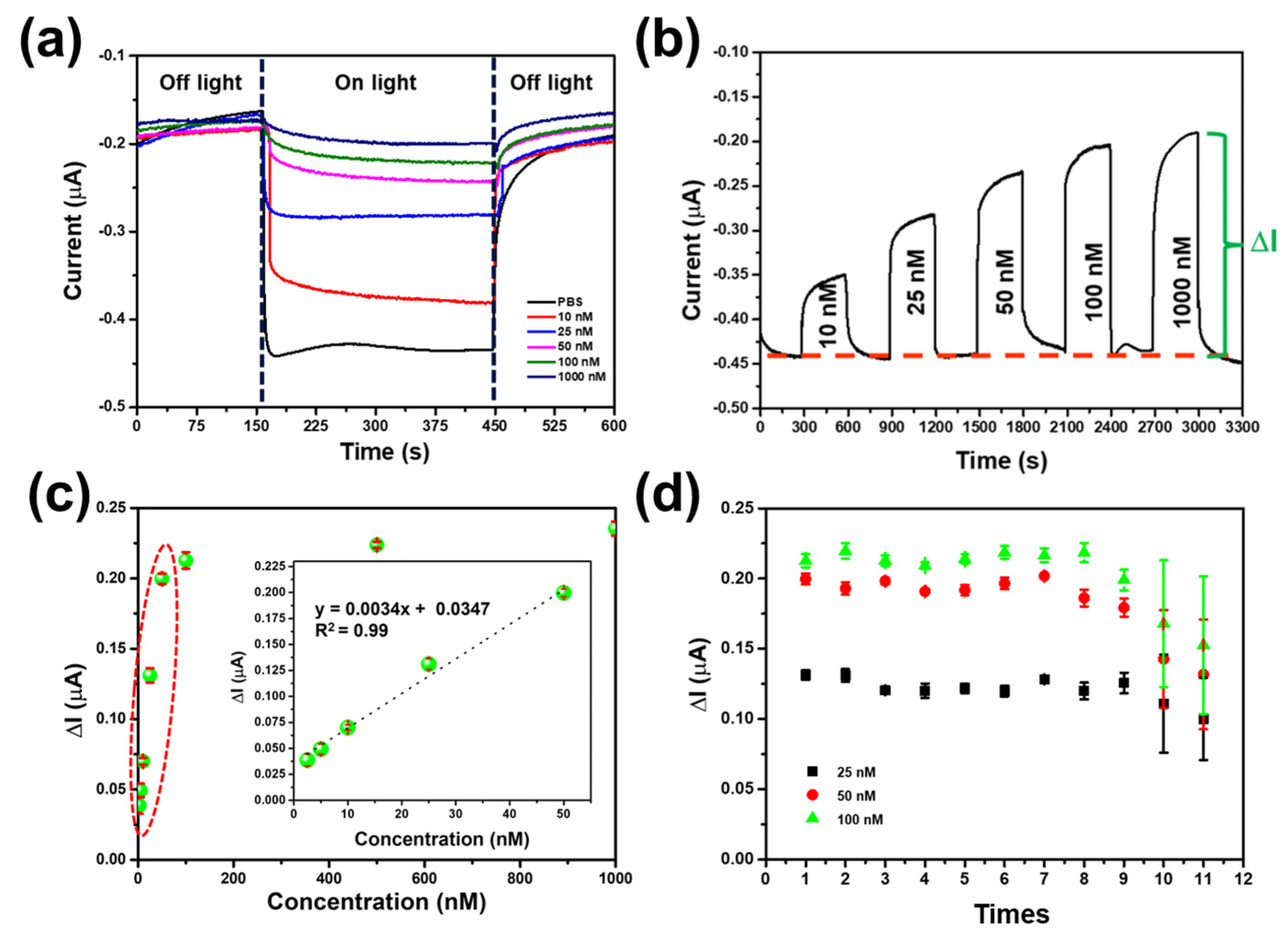
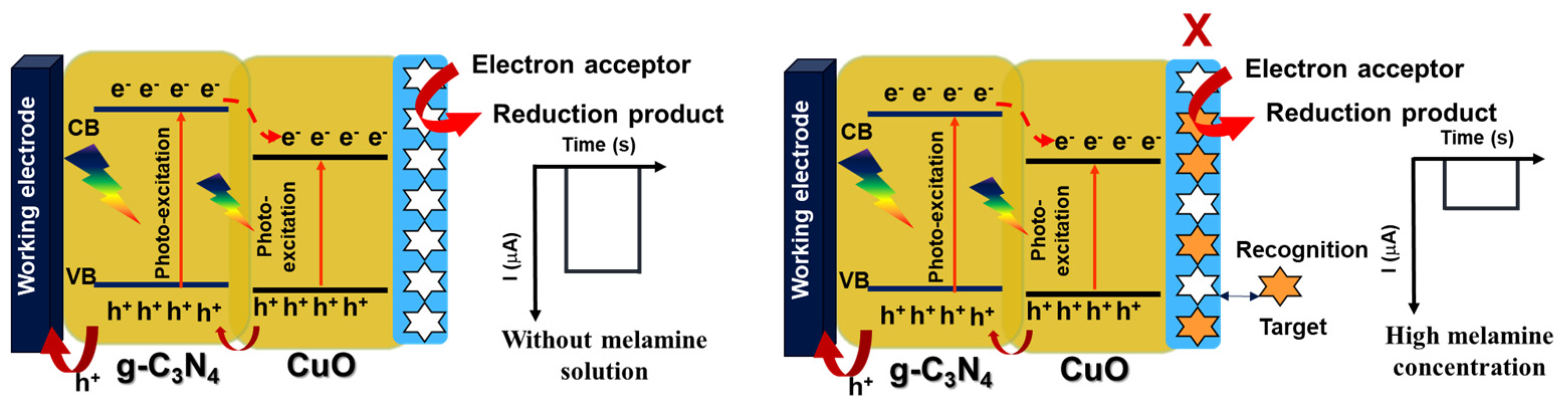
3.4. Effect of Light Irradiation
3.5. Performance of CuO/g-C3N4 /MIP Electrode
3.6. Reusability and Reproducibility
3.7. The Selectivity of the CuO/g-C3N4/MIP Electrode for Melamine Detection
3.8. Real Sample Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rovina, K.; Siddiquee, S. A review of recent advances in melamine detection techniques. J. Food Compos. Anal. 2015, 43, 25–38. [Google Scholar] [CrossRef]
- Skinner, C.G.; Thomas, J.D.; Osterloh, J.D. Melamine toxicity. J. Med. Toxicol. 2010, 6, 50–55. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization; Food and Agriculture Organization of the United Nations. Proceedings of the Toxicological and Health Aspects of Melamine and Cyanuric Acid: Report of a WHO Expert Meeting in Collaboration with FAO, Supported by Health Canada, Ottawa, ON, Canada, 1–4 December 2008; World Health Organization: Geneva, Switzerland, 2009. [Google Scholar]
- Gabriels, G.; Lambert, M.; Smith, P.; Wiesner, L.; Hiss, D. Melamine contamination in nutritional supplements—Is it an alarm bell for the general consumer, athletes, and ‘Weekend Warriors’? Nutr. J. 2015, 14, 69. [Google Scholar] [CrossRef] [PubMed]
- Montesano, D.; Gennari, O.; Festa, C.; Zollo, F.; Seccia, S.; Albrizio, S. A Simple HPLC-DAD Method for the Analysis of Melamine in Protein Supplements: Validation Using the Accuracy Profiles. J. Chem. 2013, 2013, 239342. [Google Scholar] [CrossRef]
- Wu, Y.-T.; Huang, C.-M.; Lin, C.-C.; Ho, W.-A.; Lin, L.-C.; Chiu, T.-F.; Tarng, D.-C.; Lin, C.-H.; Tsai, T.-H. Determination of melamine in rat plasma, liver, kidney, spleen, bladder and brain by liquid chromatography–tandem mass spectrometry. J. Chromatogr. A 2009, 1216, 7595–7601. [Google Scholar] [CrossRef]
- Lu, Y.; Shu, Y.; Zhao, C. Determination of melamine residue in foods using gas chromatography-tandem mass spectrometry. Se Pu 2008, 26, 749–751. [Google Scholar] [PubMed]
- Squadrone, S.; Ferro, G.L.; Marchis, D.; Mauro, C.; Palmegiano, P.; Amato, G.; Poma Genin, E.; Abete, M.C. Determination of melamine in feed: Validation of a gas chromatography–mass spectrometry method according to 2004/882/CE regulation. Food Control 2010, 21, 714–718. [Google Scholar] [CrossRef]
- Kim, A.; Barcelo, S.J.; Williams, R.S.; Li, Z. Melamine Sensing in Milk Products by Using Surface Enhanced Raman Scattering. Anal. Chem. 2012, 84, 9303–9309. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Wang, J.; Wu, Q.; Wang, Y.; Li, L.; Ding, B. Simple and Label-Free Fluorescent Detection of Melamine Based on Melamine–Thymine Recognition. Sensors 2018, 18, 2968. [Google Scholar] [CrossRef]
- Mao, Y.; Liu, X.; Bao, Y.; Niu, L. Recent Advances in Photoelectrochemical Sensors for Analysis of Toxins and Abused Drugs in the Environment. Chemosensors 2023, 11, 412. [Google Scholar] [CrossRef]
- Ma, J.; Zhang, M.; Su, W.; Wu, B.; Yang, Z.; Wang, X.; Qiao, B.; Pei, H.; Tu, J.; Chen, D.; et al. Photoelectrochemical Enzyme Biosensor Based on TiO2 Nanorod/TiO2 Quantum Dot/Polydopamine/Glucose Oxidase Composites with Strong Visible-Light Response. Langmuir 2022, 38, 751–761. [Google Scholar] [CrossRef] [PubMed]
- Tu, Y.-P.; Wang, H.-H.; Long, D.; Yuan, R.; Chai, Y.-Q. Highly sensitive photoelectrochemical biosensor based on Au nanoparticles sensitized zinc selenide quantum dots for DNA detection. Sens. Actuators B Chem. 2022, 357, 131255. [Google Scholar] [CrossRef]
- Devadoss, A.; Sudhagar, P.; Terashima, C.; Nakata, K.; Fujishima, A. Photoelectrochemical biosensors: New insights into promising photoelectrodes and signal amplification strategies. J. Photochem. Photobiol. C Photochem. Rev. 2015, 24, 43–63. [Google Scholar] [CrossRef]
- Meng, S.; Li, Y.; Dong, N.; Liu, S.; Liu, C.; Gong, Q.; Chen, Z.; Jiang, K.; Li, X.; Liu, D.; et al. Portable Visual Photoelectrochemical Biosensor Based on a MgTi2O5/CdSe Heterojunction and Reversible Electrochromic Supercapacitor for Dual-Modal Cry1Ab Protein Detection. Anal. Chem. 2023, 95, 18224–18232. [Google Scholar] [CrossRef] [PubMed]
- Xing, J.; Wang, N.; Li, X.; Wang, J.; Taiwaikuli, M.; Huang, X.; Wang, T.; Zhou, L.; Hao, H. Synthesis and modifications of g-C3N4-based materials and their applications in wastewater pollutants removal. J. Environ. Chem. Eng. 2022, 10, 108782. [Google Scholar] [CrossRef]
- Ismael, M.; Wu, Y. A mini-review on the synthesis and structural modification of g-C3N4-based materials, and their applications in solar energy conversion and environmental remediation. Sustain. Energy Fuels 2019, 3, 2907–2925. [Google Scholar] [CrossRef]
- Ong, W.-J.; Tan, L.-L.; Ng, Y.H.; Yong, S.-T.; Chai, S.-P. Graphitic Carbon Nitride (g-C3N4)-Based Photocatalysts for Artificial Photosynthesis and Environmental Remediation: Are We a Step Closer To Achieving Sustainability? Chem. Rev. 2016, 116, 7159–7329. [Google Scholar] [CrossRef]
- Wudil, Y.S.; Ahmad, U.F.; Gondal, M.A.; Al-Osta, M.A.; Almohammedi, A.; Sa’id, R.S.; Hrahsheh, F.; Haruna, K.; Mohamed, M.J.S. Tuning of graphitic carbon nitride (g-C3N4) for photocatalysis: A critical review. Arab. J. Chem. 2023, 16, 104542. [Google Scholar] [CrossRef]
- Liang, J.; Yang, X.; Wang, Y.; He, P.; Fu, H.; Zhao, Y.; Zou, Q.; An, X. A review on g-C3N4 incorporated with organics for enhanced photocatalytic water splitting. J. Mater. Chem. A 2021, 9, 12898–12922. [Google Scholar] [CrossRef]
- Molaei, M.J. Graphitic carbon nitride (g-C3N4) synthesis and heterostructures, principles, mechanisms, and recent advances: A critical review. Int. J. Hydrogen Energy 2023, 48, 32708–32728. [Google Scholar] [CrossRef]
- Alaghmandfard, A.; Ghandi, K. A Comprehensive Review of Graphitic Carbon Nitride (g-C3N4)–Metal Oxide-Based Nanocomposites: Potential for Photocatalysis and Sensing. Nanomaterials 2022, 12, 294. [Google Scholar] [CrossRef] [PubMed]
- Gu, J.; Jia, H.; Ma, S.; Ye, Z.; Pan, J.; Dong, R.; Zong, Y.; Xue, J. Fe3O4-Loaded g-C3N4/C-Layered Composite as a Ternary Photocatalyst for Tetracycline Degradation. ACS Omega 2020, 5, 30980–30988. [Google Scholar] [CrossRef] [PubMed]
- Hassannezhad, M.; Hosseini, M.; Ganjali, M.R.; Arvand, M. A graphitic carbon nitride (g-C3N4/Fe3O4) nanocomposite: An efficient electrode material for the electrochemical determination of tramadol in human biological fluids. Anal. Methods 2019, 11, 2064–2071. [Google Scholar] [CrossRef]
- Vu, N.-N.; Kaliaguine, S.; Do, T.-O. Synthesis of the g-C3N4/CdS Nanocomposite with a Chemically Bonded Interface for Enhanced Sunlight-Driven CO2 Photoreduction. ACS Appl. Energy Mater. 2020, 3, 6422–6433. [Google Scholar] [CrossRef]
- Fang, J.; Xie, K.; Kang, Q.; Gou, Y. Facile fabrication of g-C3N4/CdS heterojunctions with enhanced visible-light photocatalytic degradation performances. J. Sci. Adv. Mater. Devices 2022, 7, 100409. [Google Scholar] [CrossRef]
- Garg, R.; Gupta, R.; Bansal, A. Synthesis of g-C3N4/ZnO nanocomposite for photocatalytic degradation of a refractory organic endocrine disrupter. Mater. Today Proc. 2021, 44, 855–859. [Google Scholar] [CrossRef]
- Sun, J.-X.; Yuan, Y.-P.; Qiu, L.-G.; Jiang, X.; Xie, A.-J.; Shen, Y.-H.; Zhu, J.-F. Fabrication of composite photocatalyst g-C3N4–ZnO and enhancement of photocatalytic activity under visible light. Dalton Trans. 2012, 41, 6756–6763. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Liu, F.; Wu, H.; Cao, X.; Sun, J.; Lei, W. In situ synthesis of g-C3N4/TiO2 heterostructures with enhanced photocatalytic hydrogen evolution under visible light. RSC Adv. 2017, 7, 40327–40333. [Google Scholar] [CrossRef]
- Ragupathi, V.; Raja, M.A.; Panigrahi, P.; Ganapathi Subramaniam, N. CuO/g-C3N4 nanocomposite as promising photocatalyst for photoelectrochemical water splitting. Optik 2020, 208, 164569. [Google Scholar] [CrossRef]
- Sung, C.-L.; Wang, R.-H.; Shih, Y.-C.; Wu, Z.-Y.; Alvarado, S.R.; Chang, Y.-H.; Lin, C.-C. g-C3N4 Nanosheet Supported CuO Nanocomposites for the Electrochemical Carbon Dioxide Reduction Reaction. ACS Omega 2023, 8, 7368–7377. [Google Scholar] [CrossRef]
- Jiang, X.X.; De Hu, X.; Tarek, M.; Saravanan, P.; Alqadhi, R.; Chin, S.Y.; Rahman Khan, M.M. Tailoring the properties of g-C3N4 with CuO for enhanced photoelectrocatalytic CO2 reduction to methanol. J. CO2 Util. 2020, 40, 101222. [Google Scholar] [CrossRef]
- Bae, H.; Burungale, V.; Na, W.; Rho, H.; Kang, S.H.; Ryu, S.-W.; Ha, J.-S. Nanostructured CuO with a thin g-C3N4 layer as a highly efficient photocathode for solar water splitting. RSC Adv. 2021, 11, 16083–16089. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Jiang, X.; Zhang, Y.; Zhang, B.; Pan, C. A Novel Route to ZnO/TiO2 Heterojunction Composite Fibres. J. Mater. Res. 2013, 28, 507–512. [Google Scholar] [CrossRef]
- Saraf, S.; Giraldo, M.; Paudel, H.P.; Sakthivel, T.S.; Shepard, C.; Gupta, A.; Leuenberger, M.N.; Seal, S. Photoelectrochemical analysis of band gap modulated TiO2 for photocatalytic water splitting. Int. J. Hydrogen Energy 2017, 42, 9938–9944. [Google Scholar] [CrossRef]
- Siavash Moakhar, R.; Hosseini-Hosseinabad, S.M.; Masudy-Panah, S.; Seza, A.; Jalali, M.; Fallah-Arani, H.; Dabir, F.; Gholipour, S.; Abdi, Y.; Bagheri-Hariri, M.; et al. Photoelectrochemical Water-Splitting Using CuO-Based Electrodes for Hydrogen Production: A Review. Adv. Mater. 2021, 33, 2007285. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Jin, X.; Li, R.; Zhao, Y.; Wang, X.; Liu, X.; Jiao, H. Copper oxide nanowires for efficient photoelectrochemical water splitting. Appl. Catal. B Environ. 2019, 240, 1–8. [Google Scholar] [CrossRef]
- Raizada, P.; Sudhaik, A.; Patial, S.; Hasija, V.; Parwaz Khan, A.A.; Singh, P.; Gautam, S.; Kaur, M.; Nguyen, V.-H. Engineering nanostructures of CuO-based photocatalysts for water treatment: Current progress and future challenges. Arab. J. Chem. 2020, 13, 8424–8457. [Google Scholar] [CrossRef]
- Naresh, V.; Lee, N. A Review on Biosensors and Recent Development of Nanostructured Materials-Enabled Biosensors. Sensors 2021, 21, 1109. [Google Scholar] [CrossRef]
- Ribeiro, B.V.; Cordeiro, T.A.R.; Oliveira e Freitas, G.R.; Ferreira, L.F.; Franco, D.L. Biosensors for the detection of respiratory viruses: A review. Talanta Open 2020, 2, 100007. [Google Scholar] [CrossRef]
- Katey, B.; Voiculescu, I.; Penkova, A.N.; Untaroiu, A. A Review of Biosensors and Their Applications. ASME Open J. Eng. 2023, 2, 020201. [Google Scholar] [CrossRef]
- Crapnell, R.D.; Dempsey-Hibbert, N.C.; Peeters, M.; Tridente, A.; Banks, C.E. Molecularly imprinted polymer based electrochemical biosensors: Overcoming the challenges of detecting vital biomarkers and speeding up diagnosis. Talanta Open 2020, 2, 100018. [Google Scholar] [CrossRef]
- BelBruno, J.J. Molecularly Imprinted Polymers. Chem. Rev. 2019, 119, 94–119. [Google Scholar] [CrossRef] [PubMed]
- Vasapollo, G.; Sole, R.D.; Mergola, L.; Lazzoi, M.R.; Scardino, A.; Scorrano, S.; Mele, G. Molecularly imprinted polymers: Present and future prospective. Int. J. Mol. Sci. 2011, 12, 5908–5945. [Google Scholar] [CrossRef]
- Pandey, A.; Singh, S.; Singh, S.P. Chapter 13—Molecularly imprinted polymers: Applications and challenges in biological and environmental sample analysis. In Molecularly Imprinted Polymers (MIPs); Singh, M., Ed.; Elsevier: Amsterdam, The Netherlands, 2023; pp. 321–344. [Google Scholar]
- Limthin, D.; Leepheng, P.; Klamchuen, A.; Phromyothin, D. Enhancement of Electrochemical Detection of Gluten with Surface Modification Based on Molecularly Imprinted Polymers Combined with Superparamagnetic Iron Oxide Nanoparticles. Polymers 2022, 14, 91. [Google Scholar] [CrossRef] [PubMed]
- Limthin, D.; Leepheng, P.; Onlaor, K.; Tunhoo, B.; Klamchuen, A.; Thiwawong, T.; Phromyothin, D. Enhancing the sensitivity and selectivity of salbutamol detection using reduced graphene oxide combined with molecularly imprinted polymers (RGO/MIP). Jpn. J. Appl. Phys. 2022, 61, SD1033. [Google Scholar] [CrossRef]
- Leepheng, P.; Limthin, D.; Onlaor, K.; Tunhoo, B.; Thiwawong, T.; Suramitr, S.; Phromyothin, D. Selective electrochemical determination based on magnetic molecularly imprinted polymers for albumin detection. Jpn. J. Appl. Phys. 2022, 61, SD1009. [Google Scholar] [CrossRef]
- Zaidi, S.A. Molecular imprinting: A useful approach for drug delivery. Mater. Sci. Energy Technol. 2020, 3, 72–77. [Google Scholar] [CrossRef]
- Mustafa, Y.L.; Keirouz, A.; Leese, H.S. Molecularly imprinted polymers in diagnostics: Accessing analytes in biofluids. J. Mater. Chem. B 2022, 10, 7418–7449. [Google Scholar] [CrossRef]
- El-Schich, Z.; Zhang, Y.; Feith, M.; Beyer, S.; Sternbæk, L.; Ohlsson, L.; Stollenwerk, M.; Wingren, A.G. Molecularly imprinted polymers in biological applications. BioTechniques 2020, 69, 406–419. [Google Scholar] [CrossRef]
- Leepheng, P.; Phromyothin, D.; Thanachayanont, C.; Inpor, K.; Olarnwanich, A.; Jiraseree-Amornkun, A. Molecularly Imprinted Polymers Near Field Communication sensors for pesticide detection. In Proceedings of the 2022 19th International Conference on Electrical Engineering/Electronics, Computer, Telecommunications and Information Technology (ECTI-CON), Prachuap Khiri Khan, Thailand, 24–27 May 2022; pp. 1–4. [Google Scholar]
- Farooq, S.; Nie, J.; Cheng, Y.; Yan, Z.; Li, J.; Bacha, S.A.S.; Mushtaq, A.; Zhang, H. Molecularly imprinted polymers’ application in pesticide residue detection. Analyst 2018, 143, 3971–3989. [Google Scholar] [CrossRef]
- Yang, C.; Xiao, F.; Wang, J.; Su, X. 3D flower- and 2D sheet-like CuO nanostructures: Microwave-assisted synthesis and application in gas sensors. Sens. Actuators B Chem. 2015, 207, 177–185. [Google Scholar] [CrossRef]
- Qiu, P.; Chen, H.; Xu, C.; Zhou, N.; Jiang, F.; Wang, X.; Fu, Y. Fabrication of an exfoliated graphitic carbon nitride as highly active visible light photocatalyst. J. Mater. Chem. A 2015, 3, 24237–24244. [Google Scholar] [CrossRef]
- Suresh, S.; Karthikeyan, S.; Jayamoorthy, K. FTIR and multivariate analysis to study the effect of bulk and nano copper oxide on peanut plant leaves. J. Sci. Adv. Mater. Devices 2016, 1, 343–350. [Google Scholar] [CrossRef]
- Yuan, J.; Zhang, J.-J.; Yang, M.-P.; Meng, W.-J.; Wang, H.; Lu, J.-X. CuO Nanoparticles Supported on TiO2 with High Efficiency for CO2 Electrochemical Reduction to Ethanol. Catalysts 2018, 8, 171. [Google Scholar] [CrossRef]
- Zhou, Q.; Li, T.-T.; Xu, W.; Zhu, H.-L.; Zheng, Y.-Q. Ultrathin nanosheets-assembled CuO flowers for highly efficient electrocatalytic water oxidation. J. Mater. Sci. 2018, 53, 8141–8150. [Google Scholar] [CrossRef]
- Wang, Y.; Lü, Y.; Zhan, W.; Xie, Z.; Kuang, Q.; Zheng, L. Synthesis of Porous Cu2O/CuO Cages using Cu-based Metal-Organic-Framework as Templates and their Gas-sensing Properties. J. Mater. Chem. A 2015, 3, 12796–12803. [Google Scholar] [CrossRef]
- Tan, L.; Xu, J.; Zhang, X.; Hang, Z.; Jia, Y.; Wang, S. Synthesis of g-C3N4/CeO2 nanocomposites with improved catalytic activity on the thermal decomposition of ammonium perchlorate. Appl. Surf. Sci. 2015, 356, 447–453. [Google Scholar] [CrossRef]
- Qiao, F.; Wang, J.; Ai, S.; Li, L. As a new peroxidase mimetics: The synthesis of selenium doped graphitic carbon nitride nanosheets and applications on colorimetric detection of H2O2 and xanthine. Sens. Actuators B Chem. 2015, 216, 418–427. [Google Scholar] [CrossRef]
- Makuła, P.; Pacia, M.; Macyk, W. How To Correctly Determine the Band Gap Energy of Modified Semiconductor Photocatalysts Based on UV–Vis Spectra. J. Phys. Chem. Lett. 2018, 9, 6814–6817. [Google Scholar] [CrossRef]
- Allaham, M.; Dallaev, R.; Burda, D.; Sobola, D.; Nebojsa, A.; Knápek, A.; Mousa, M.S.; Kolařík, V. Energy gap measurements based on enhanced absorption coefficient calculation from transmittance and reflectance raw data. Phys. Scr. 2024, 99, 025952. [Google Scholar] [CrossRef]
- Song, T.-s.; Li, T.; Tao, R.; Huang, H.F.; Xie, J. CuO/g-C3N4 heterojunction photocathode enhances the microbial electrosynthesis of acetate through CO2 reduction. Sci. Total Environ. 2022, 818, 151820. [Google Scholar] [CrossRef] [PubMed]
- Kai, Y.; Karthick Kannan, P.; Doonyapisut, D.; Wu, K.; Chung, C.-H.; Zhang, J. Advanced Functional Electroactive and Photoactive Materials for Monitoring the Environmental Pollutants. Adv. Funct. Mater. 2020, 31, 2008227. [Google Scholar] [CrossRef]
- Jia, B.; Feng, F.; Wang, X.; Song, Y.; Zhang, F. Recent advances in magnetic molecularly imprinted polymers and their application in the food safety analysis. J. Future Foods 2024, 4, 1–20. [Google Scholar] [CrossRef]
- Zeilinger, M.; Sussitz, H.; Cuypers, W.; Jungmann, C.; Lieberzeit, P. Mass-Sensitive Sensing of Melamine in Dairy Products with Molecularly Imprinted Polymers: Matrix Challenges. Sensors 2019, 19, 2366. [Google Scholar] [CrossRef] [PubMed]
- Ge, X.; Wu, X.; Wang, J.; Liang, S.; Sun, H. Highly sensitive determination of cyromazine, melamine, and their metabolites in milk by molecularly imprinted solid-phase extraction combined with ultra-performance liquid chromatography. J. Dairy Sci. 2015, 98, 2161–2171. [Google Scholar] [CrossRef]
- Liu, Y.T.; Deng, J.; Xiao, X.L.; Ding, L.; Yuan, Y.L.; Li, H.; Li, X.T.; Yan, X.N.; Wang, L.L. Electrochemical sensor based on a poly(para-aminobenzoic acid) film modified glassy carbon electrode for the determination of melamine in milk. Electrochim. Acta 2011, 56, 4595–4602. [Google Scholar] [CrossRef]
- Daizy, M.; Tarafder, C.; Al-Mamun, M.R.; Liu, X.; Aly Saad Aly, M.; Khan, M.Z.H. Electrochemical Detection of Melamine by Using Reduced Graphene Oxide–Copper Nanoflowers Modified Glassy Carbon Electrode. ACS Omega 2019, 4, 20324–20329. [Google Scholar] [CrossRef] [PubMed]
- Limthin, D.; Leepheng, P.; Tunhoo, B.; Onlaor, K.; Klamchuen, A.; Phromyothin, D.; Thiwawong, T. Preparation of surface-modified electrode of copper(ii) oxide mixed with the molecularly imprinted polymer for enhancement of melamine detection with photoelectrochemical technique. RSC Adv. 2023, 13, 14729–14736. [Google Scholar] [CrossRef] [PubMed]




| Electrode | Linear Equation | R2 | Sensitivity (nA/nM) | LOD (nM) | LOQ (nM) |
|---|---|---|---|---|---|
| 1 | y = 0.0036x + 0.034 | 0.990 | 4.17 | 0.42 | 1.42 |
| 2 | y = 0.0032x + 0.032 | 0.991 | 4.29 | 0.30 | 1.02 |
| 3 | y = 0.0033x + 0.039 | 0.995 | 4.69 | 0.29 | 0.97 |
| 4 | y = 0.0033x + 0.031 | 0.992 | 3.81 | 0.56 | 1.89 |
| 5 | y = 0.0033x + 0.027 | 0.992 | 3.45 | 0.42 | 1.40 |
| 6 | y = 0.0049x + 0.023 | 0.992 | 4.37 | 0.24 | 0.80 |
| Methods | Materials | Linear Range (nM) | LOD (nM) | Ref. |
|---|---|---|---|---|
| HPLC–MS/MS | - | 200–5000 | 50 | [6] |
| Quartz crystal microbalances | MIP | 1 × 103–3 × 105 | 8 | [67] |
| Fluorescence | Thymine/SYBR Green I | 1 × 104–2 × 106 | 158 | [10] |
| Electrochemistry | poly(para-aminobenzoic acid)/GCE | 4.0 × 103–450 × 103 | 36 | [69] |
| Electrochemistry | GCE/P-Arg/ErGO–CuNFs Electrode | 10–90 | 5.0 | [70] |
| PEC | CuO/MIP | 5–50 | 2.45 | [71] |
| PEC | CuO/g-C3N4/MIP | 2.5–50 | 0.42 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Limthin, D.; Leepheng, P.; Tunhoo, B.; Klamchuen, A.; Suramitr, S.; Thiwawong, T.; Phromyothin, D. Enhancement in Sensitivity and Selectivity of Electrochemical Technique with CuO/g-C3N4 Nanocomposite Combined with Molecularly Imprinted Polymer for Melamine Detection. Polymers 2024, 16, 1800. https://doi.org/10.3390/polym16131800
Limthin D, Leepheng P, Tunhoo B, Klamchuen A, Suramitr S, Thiwawong T, Phromyothin D. Enhancement in Sensitivity and Selectivity of Electrochemical Technique with CuO/g-C3N4 Nanocomposite Combined with Molecularly Imprinted Polymer for Melamine Detection. Polymers. 2024; 16(13):1800. https://doi.org/10.3390/polym16131800
Chicago/Turabian StyleLimthin, Dalawan, Piyawan Leepheng, Benchapol Tunhoo, Annop Klamchuen, Songwut Suramitr, Thutiyaporn Thiwawong, and Darinee Phromyothin. 2024. "Enhancement in Sensitivity and Selectivity of Electrochemical Technique with CuO/g-C3N4 Nanocomposite Combined with Molecularly Imprinted Polymer for Melamine Detection" Polymers 16, no. 13: 1800. https://doi.org/10.3390/polym16131800
APA StyleLimthin, D., Leepheng, P., Tunhoo, B., Klamchuen, A., Suramitr, S., Thiwawong, T., & Phromyothin, D. (2024). Enhancement in Sensitivity and Selectivity of Electrochemical Technique with CuO/g-C3N4 Nanocomposite Combined with Molecularly Imprinted Polymer for Melamine Detection. Polymers, 16(13), 1800. https://doi.org/10.3390/polym16131800






