Characterization of a Delivery System Based on a Hyaluronic Acid 3D Scaffold and Gelatin Microparticles
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Hyaluronic Acid Scaffold
2.2. Preparation of Gelatin Microparticles
2.3. Characterization of the Microparticles: Morphology and Size
2.4. Quantification of Amino Groups in Microparticles: Ninhydrin Test
2.5. Microparticle Degradation Analysis
2.6. Release Kinetics of Bovine Serum Albumin Protein
2.7. Insertion of the Microparticles in the HA Scaffold
2.8. Cytotoxicity
2.9. Analysis of the Cell Distribution in the Scaffold–Microparticle System
2.10. Morphological Characterization of Cell Cultures by SEM
2.11. Statistical Analysis
3. Results and Discussion
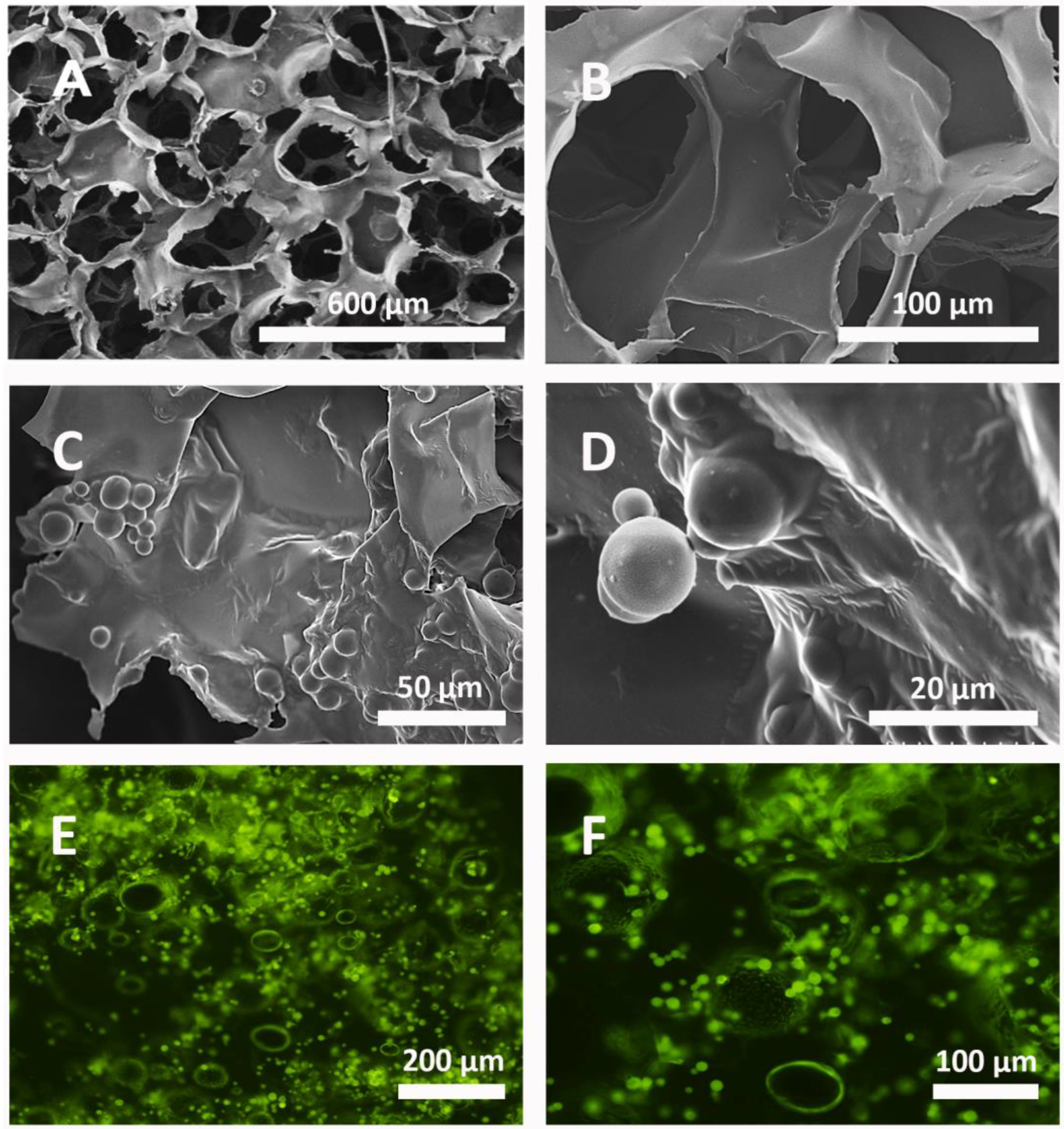
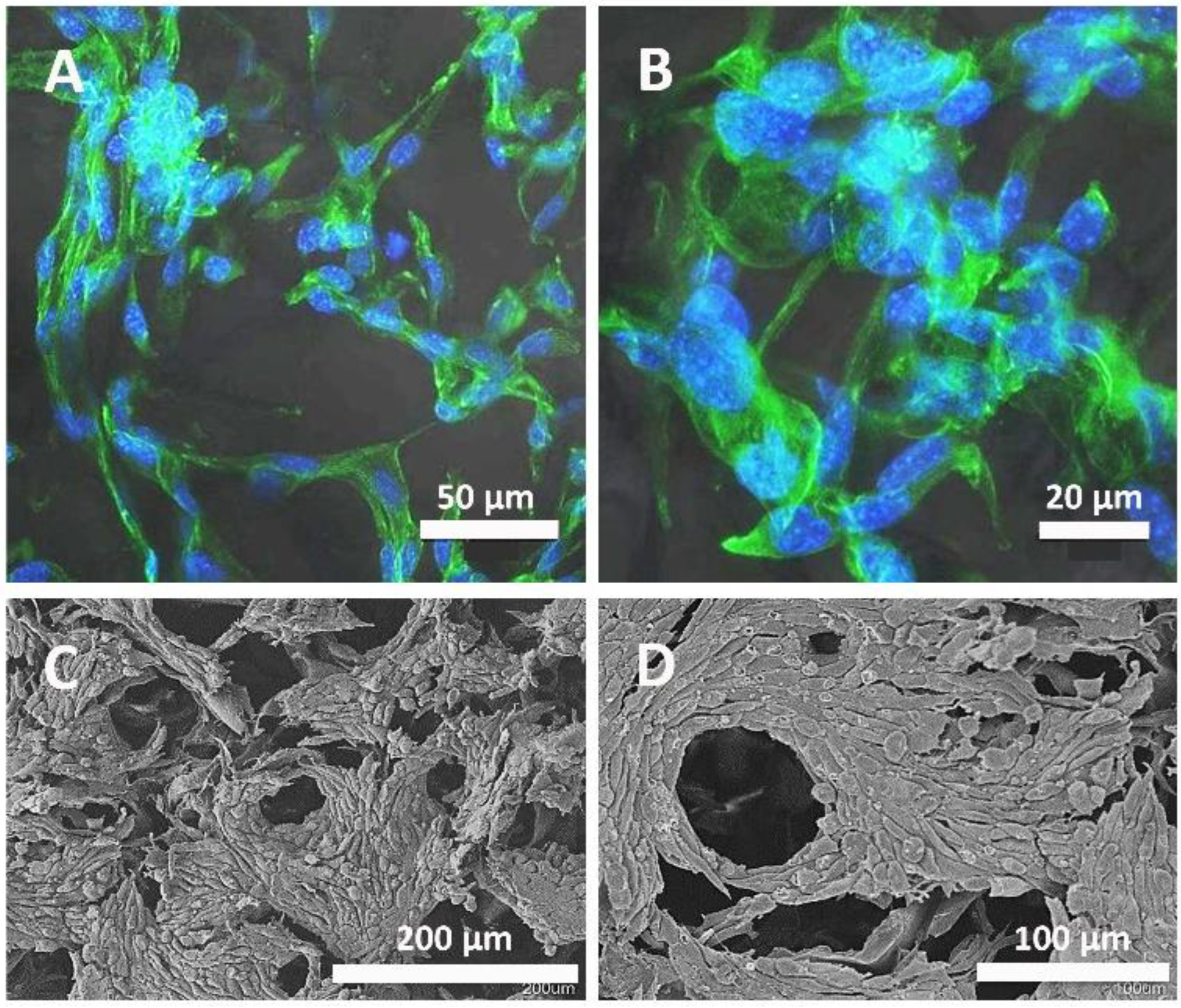
3.1. Morphological Characterization of the System
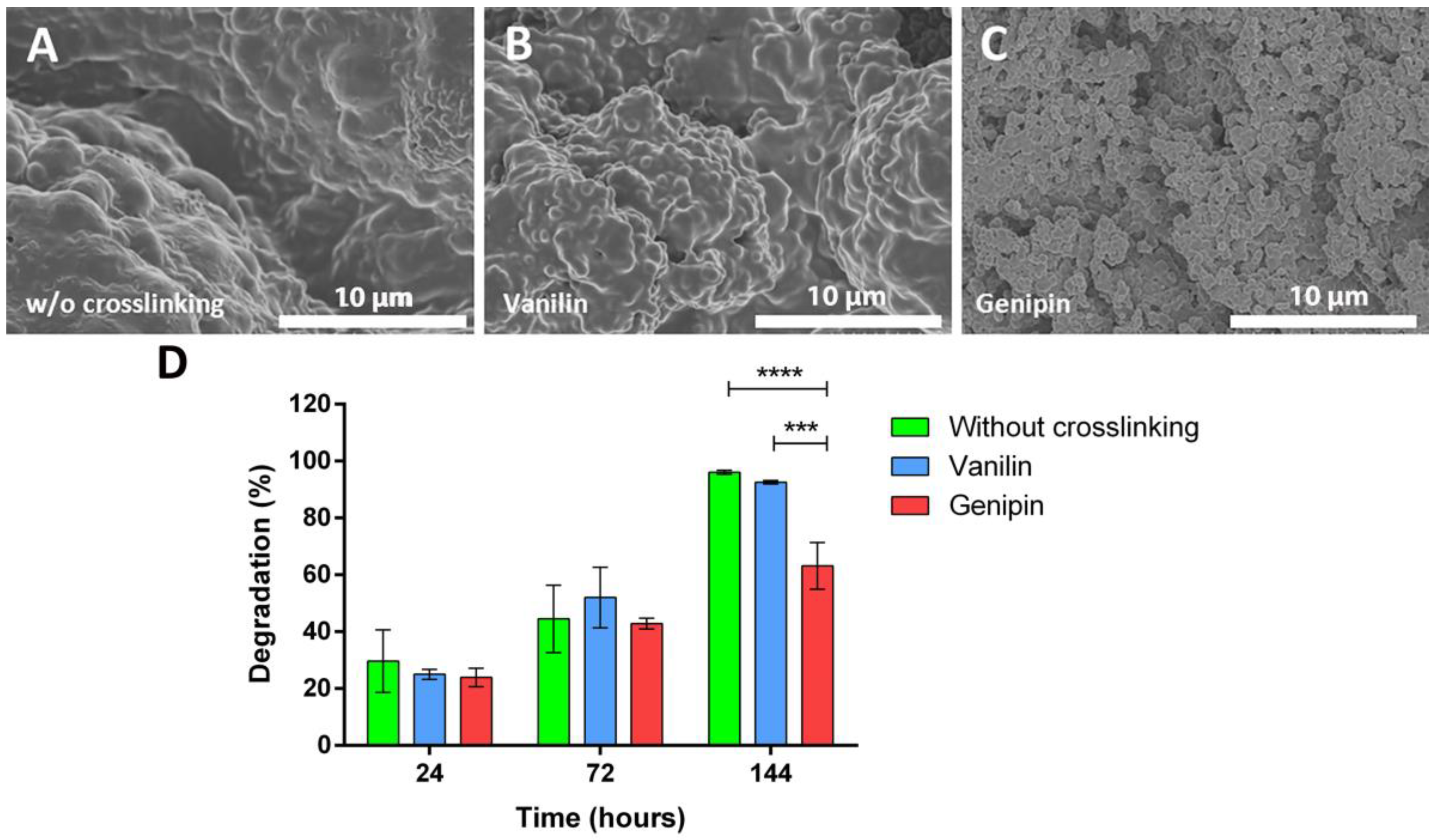
3.2. Morphology, Size, Swelling, and Crosslinking of Microparticles
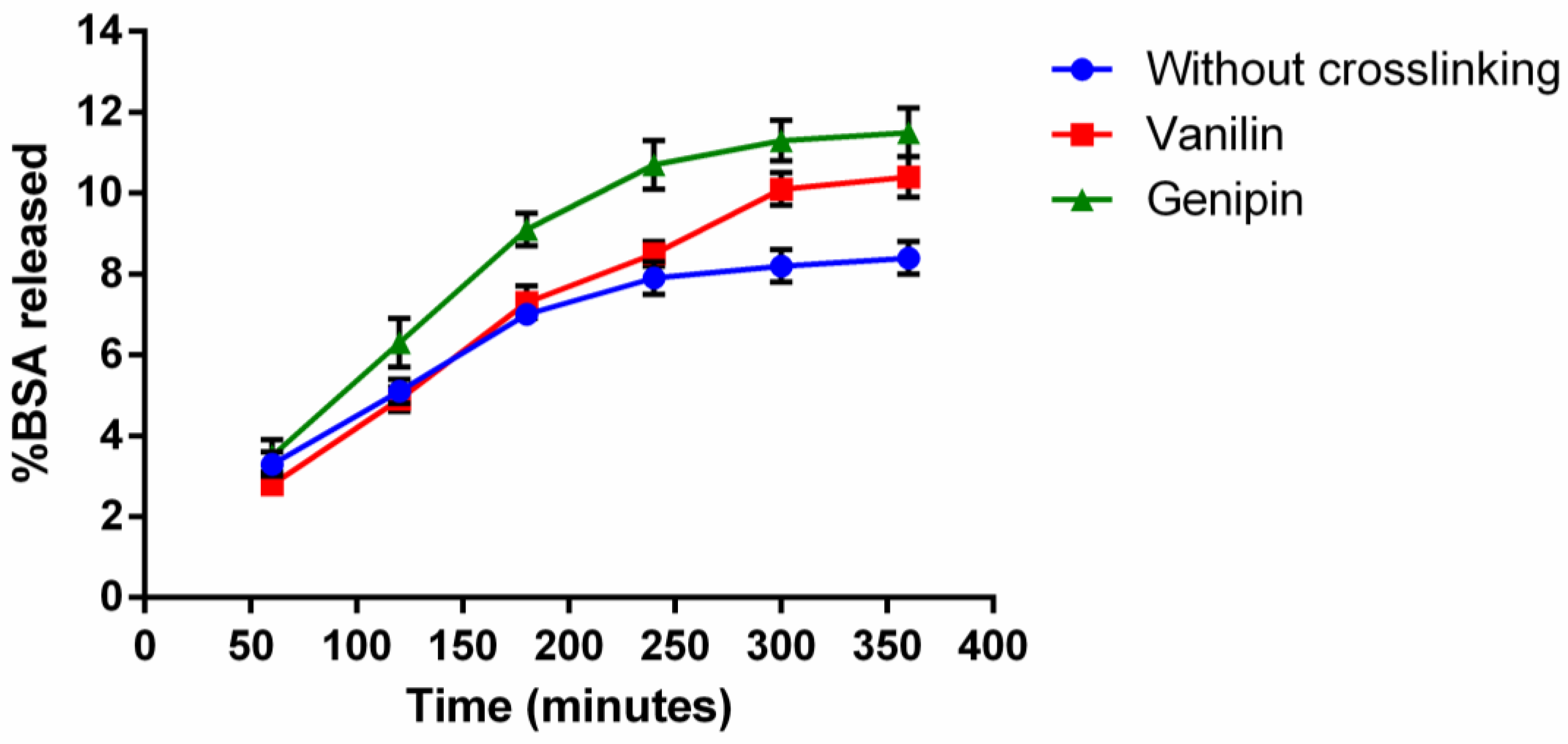
3.3. In Vitro Release Kinetics and Degradation Study of Microparticles
3.4. Method of the Incorporation of the Microparticles into the Scaffolds
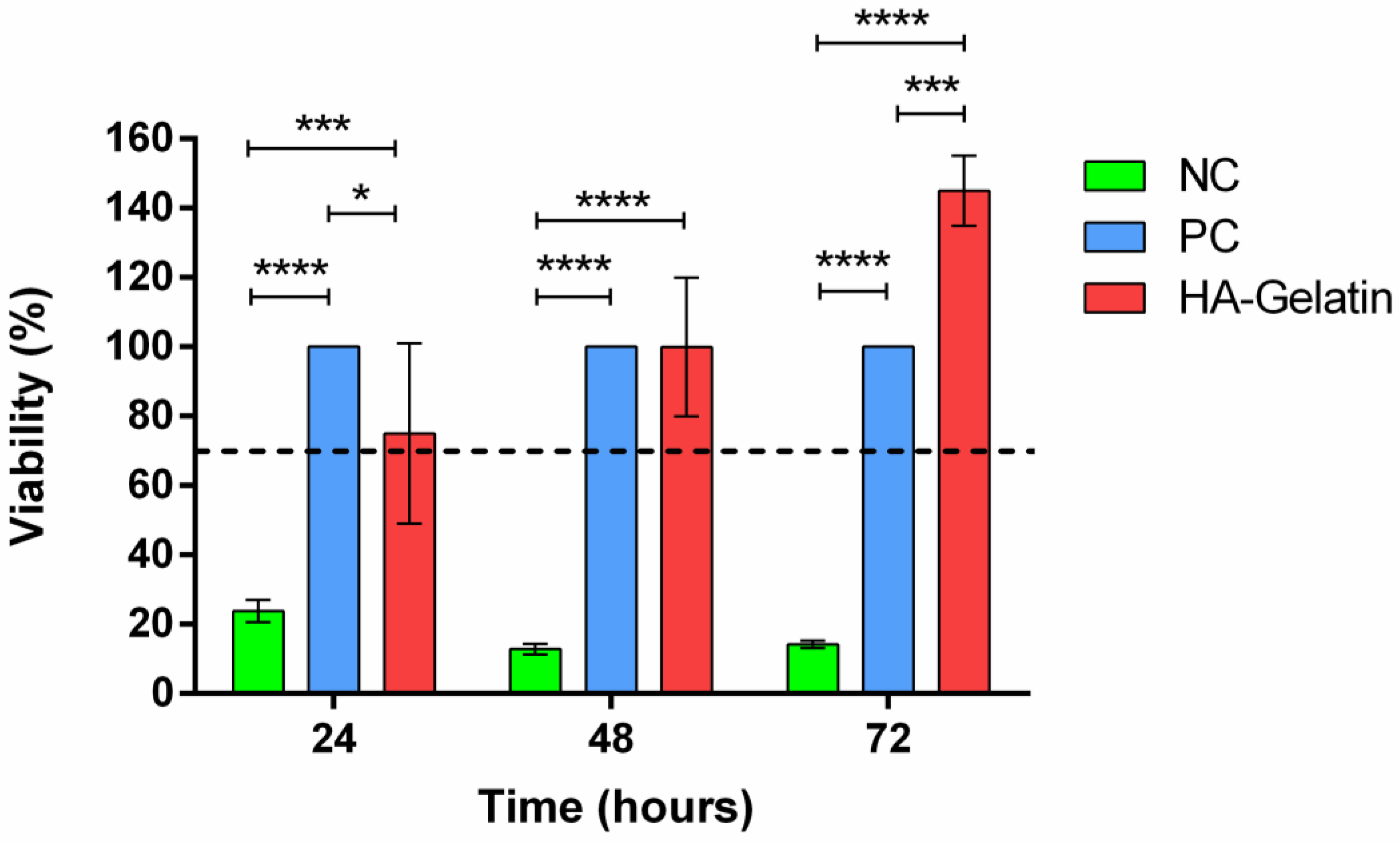
3.5. Cytotoxicity of the System
3.6. Cell Cultures on Scaffolds with Microparticles Inside
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Badylak, S.F.; Freytes, D.O.; Gilbert, T.W. Extracellular Matrix as a Biological Scaffold Material: Structure and Function. Acta Biomater. 2009, 5, 1–13. [Google Scholar] [CrossRef]
- Vach Agocsova, S.; Culenova, M.; Birova, I.; Omanikova, L.; Moncmanova, B.; Danisovic, L.; Ziaran, S.; Bakos, D.; Alexy, P. Resorbable Biomaterials Used for 3D Scaffolds in Tissue Engineering: A Review. Materials 2023, 16, 4267. [Google Scholar] [CrossRef] [PubMed]
- Nikolova, M.P.; Chavali, M.S. Recent Advances in Biomaterials for 3D Scaffolds: A Review. Bioact. Mater. 2019, 4, 271–292. [Google Scholar] [CrossRef] [PubMed]
- Bitar, K.N.; Zakhem, E. Design Strategies of Biodegradable Scaffolds for Tissue Regeneration. Biomed. Eng. Comput. Biol. 2014, 6, 13. [Google Scholar] [CrossRef]
- Mkhabelal, V.J.; Ray, S.S. Poly(ε-Caprolactone) Nanocomposite Scaffolds for Tissue Engineering: A Brief Overview. J. Nanosci. Nanotechnol. 2014, 14, 535–545. [Google Scholar] [CrossRef]
- Kotla, N.G.; Mohd Isa, I.L.; Larrañaga, A.; Maddiboyina, B.; Swamy, S.K.; Sivaraman, G.; Vemula, P.K. Hyaluronic Acid-Based Bioconjugate Systems, Scaffolds, and Their Therapeutic Potential. Adv. Healthc. Mater. 2023, 12, 2203104. [Google Scholar] [CrossRef] [PubMed]
- Dicker, K.T.; Gurski, L.A.; Pradhan-Bhatt, S.; Witt, R.L.; Farach-Carson, M.C.; Jia, X. Hyaluronan: A Simple Polysaccharide with Diverse Biological Functions. Acta Biomater. 2014, 10, 1558–1570. [Google Scholar] [CrossRef]
- Lurie, Z.; Offer, T.; Russo, A.; Samuni, A.; Nitzan, D. Do Stable Nitroxide Radicals Catalyze or Inhibit the Degradation of Hyaluronic Acid? Free Radic. Biol. Med. 2003, 35, 169–178. [Google Scholar] [CrossRef]
- Turley, E.A.; Noble, P.W.; Bourguignon, L.Y.W. Signaling Properties of Hyaluronan Receptors. J. Biol. Chem. 2002, 277, 4589–4592. [Google Scholar] [CrossRef]
- Park, J.; Lim, E.; Back, S.; Na, H.; Park, Y.; Sun, K. Nerve Regeneration Following Spinal Cord Injury Using Matrix Metalloproteinase-Sensitive, Hyaluronic Acid-Based Biomimetic Hydrogel Scaffold Containing Brain-Derived Neurotrophic Factor. J. Biomed. Mater. Res. Part A 2010, 93, 1091–1099. [Google Scholar] [CrossRef]
- Zhong, J.; Chan, A.; Morad, L.; Kornblum, H.I.; Fan, G.; Carmichael, S.T. Hydrogel Matrix to Support Stem Cell Survival After Brain Transplantation in Stroke. Neurorehabilit. Neural Repair 2010, 24, 636–644. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, S.; Kang, Q.K.; Ramamurthi, A. The Impact of Hyaluronic Acid Oligomer Content on Physical, Mechanical, and Biologic Properties of Divinyl Sulfone-Crosslinked Hyaluronic Acid Hydrogels. J. Biomed. Mater. Res. Part A 2010, 94, 355–370. [Google Scholar] [CrossRef] [PubMed]
- Collins, M.N.; Birkinshaw, C. Physical Properties of Crosslinked Hyaluronic Acid Hydrogels. J. Mater. Sci. Mater. Med. 2008, 19, 3335–3343. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; De, R.; Cho, D.C.; Kim, S.J.; Choi, S.-i.; Hahn, S.K. Effect of Cross-Linker Chain Length on Biophysical Property of Hyaluronic Acid Hydrogel Dermal Filler. Macromol. Res. 2023, 31, 843–850. [Google Scholar] [CrossRef]
- West, J.D.; Stamm, C.E.; Brown, H.A.; Justice, S.L.; Morano, K.A. Enhanced Toxicity of the Protein Cross-Linkers Divinyl Sulfone and Diethyl Acetylenedicarboxylate in Comparison to Related Monofunctional Electrophiles. Chem. Res. Toxicol. 2011, 24, 1457–1459. [Google Scholar] [CrossRef] [PubMed]
- Larsen, N.E.; Dursema, H.D.; Pollak, C.T.; Skrabut, E.M. Clearance Kinetics of a Hylan-Based Viscosupplement after Intra-Articular and Intravenous Administration in Animal Models. J. Biomed. Mater. Res. Part B Appl. Biomater. 2012, 100, 457–462. [Google Scholar] [CrossRef] [PubMed]
- Schanté, C.E.; Zuber, G.; Herlin, C.; Vandamme, T.F. Chemical Modifications of Hyaluronic Acid for the Synthesis of Derivatives for a Broad Range of Biomedical Applications. Carbohydr. Polym. 2011, 85, 469–489. [Google Scholar] [CrossRef]
- Lai, J.Y. Relationship between Structure and Cytocompatibility of Divinyl Sulfone Cross-Linked Hyaluronic Acid. Carbohydr. Polym. 2014, 101, 203–212. [Google Scholar] [CrossRef] [PubMed]
- Conrozier, T.; Mathieu, P.; Schott, A.M.; Laurent, I.; Hajri, T.; Crozes, P.; Grand, P.; Laurent, H.; Marchand, F.; Meignan, F.; et al. Factors Predicting Long-Term Efficacy of Hylan GF-20 Viscosupplementation in Knee Osteoarthritis. Jt. Bone Spine 2003, 70, 128–133. [Google Scholar] [CrossRef]
- Sun, S.F.; Chou, Y.J.; Hsu, C.W.; Chen, W.L. Hyaluronic Acid as a Treatment for Ankle Osteoarthritis. Curr. Rev. Musculoskelet. Med. 2009, 2, 78–82. [Google Scholar] [CrossRef]
- Patel, Z.S.; Yamamoto, M.; Ueda, H.; Tabata, Y.; Mikos, A.G. Biodegradable Gelatin Microparticles as Delivery Systems for the Controlled Release of Bone Morphogenetic Protein-2. Acta Biomater. 2008, 4, 1126–1138. [Google Scholar] [CrossRef] [PubMed]
- Layman, H.; Spiga, M.G.; Brooks, T.; Pham, S.; Webster, K.A.; Andreopoulos, F.M. The Effect of the Controlled Release of Basic Fibroblast Growth Factor from Ionic Gelatin-Based Hydrogels on Angiogenesis in a Murine Critical Limb Ischemic Model. Biomaterials 2007, 28, 2646–2654. [Google Scholar] [CrossRef] [PubMed]
- Nii, T. Strategies Using Gelatin Microparticles for Regenerative Therapy and Drug Screening Applications. Molecules 2021, 26, 6795. [Google Scholar] [CrossRef]
- Baukum, J.; Pranjan, J.; Kaolaor, A.; Chuysinuan, P.; Suwantong, O.; Supaphol, P. The Potential Use of Cross-Linked Alginate/Gelatin Hydrogels Containing Silver Nanoparticles for Wound Dressing Applications. Polym. Bull. 2020, 77, 2679–2695. [Google Scholar] [CrossRef]
- Wen, Y.; Yu, B.; Zhu, Z.; Yang, Z.; Shao, W. Synthesis of Antibacterial Gelatin/Sodium Alginate Sponges and Their Antibacterial Activity. Polymers 2020, 12, 1926. [Google Scholar] [CrossRef] [PubMed]
- Saarai, A.; Kasparkova, V.; Sedlacek, T.; Sáha, P. On the Development and Characterisation of Crosslinked Sodium Alginate/Gelatine Hydrogels. J. Mech. Behav. Biomed. Mater. 2013, 18, 152–166. [Google Scholar] [CrossRef] [PubMed]
- Salahuddin, B.; Wang, S.; Sangian, D.; Aziz, S.; Gu, Q. Hybrid Gelatin Hydrogels in Nanomedicine Applications. ACS Appl. Bio Mater. 2021, 4, 2886–2906. [Google Scholar] [CrossRef] [PubMed]
- Łabowska, M.B.; Cierluk, K.; Jankowska, A.M.; Kulbacka, J.; Detyna, J.; Michalak, I. A Review on the Adaption of Alginate-Gelatin Hydrogels for 3D Cultures and Bioprinting. Materials 2021, 14, 858. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Rodríguez, R.; Espinosa-Andrews, H.; Velasquillo-Martínez, C.; García-Carvajal, Z.Y. Composite Hydrogels Based on Gelatin, Chitosan and Polyvinyl Alcohol to Biomedical Applications: A Review. Int. J. Polym. Mater. Polym. Biomater. 2020, 69, 1–20. [Google Scholar] [CrossRef]
- Young, S.; Wong, M.; Tabata, Y.; Mikos, A.G. Gelatin as a Delivery Vehicle for the Controlled Release of Bioactive Molecules. J. Control. Release 2005, 109, 256–274. [Google Scholar] [CrossRef]
- Solorio, L.; Zwolinski, C.; Lund, A.W.; Farrell, M.J.; Stegemann, J.P. Gelatin Microspheres Crosslinked with Genipin for Local Delivery of Growth Factors. J. Tissue Eng. Regen. Med. 2010, 4, 514–523. [Google Scholar] [CrossRef] [PubMed]
- Prata, A.S.; Grosso, C.R.F. Production of Microparticles with Gelatin and Chitosan. Carbohydr. Polym. 2015, 116, 292–299. [Google Scholar] [CrossRef] [PubMed]
- Tomadoni, B.; Ponce, A.; Pereda, M.; Ansorena, M.R. Vanillin as a Natural Cross-Linking Agent in Chitosan-Based Films: Optimizing Formulation by Response Surface Methodology. Polym. Test. 2019, 78, 105935. [Google Scholar] [CrossRef]
- Beppu, M.M.; Vieira, R.S.; Aimoli, C.G.; Santana, C.C. Crosslinking of Chitosan Membranes Using Glutaraldehyde: Effect on Ion Permeability and Water Absorption. J. Memb. Sci. 2007, 301, 126–130. [Google Scholar] [CrossRef]
- Yang, Q.; Dou, F.; Liang, B.; Shen, Q. Investigations of the Effects of Glyoxal Cross-Linking on the Structure and Properties of Chitosan Fiber. Carbohydr. Polym. 2005, 61, 393–398. [Google Scholar] [CrossRef]
- Xue, J.Q.; Li, J.X.; Wu, M.; Wang, W.; Ma, D.N. Preparation and Characterization of Formaldehyde Crosslinked Chitosan. Adv. Mater. Res. 2011, 239–242, 279–282. [Google Scholar] [CrossRef]
- Zheng, Y.; Yang, W.; Wang, C.; Hu, J.; Fu, S.; Dong, L.; Wu, L.; Shen, X. Nanoparticles Based on the Complex of Chitosan and Polyaspartic Acid Sodium Salt: Preparation, Characterization and the Use for 5-Fluorouracil Delivery. Eur. J. Pharm. Biopharm. 2007, 67, 621–631. [Google Scholar] [CrossRef] [PubMed]
- Woźniak, A.; Biernat, M. Methods for Crosslinking and Stabilization of Chitosan Structures for Potential Medical Applications. J. Bioact. Compat. Polym. 2022, 37, 151–167. [Google Scholar] [CrossRef]
- Singh, B.K.; Dutta, P.K. Chitin and Chitosan for Regenerative Medicine; Springer: Berlin/Heidelberg, Germany, 2016. [Google Scholar] [CrossRef]
- Ramachandran, S.; Nandhakumar, S.; Dhanaraju, M.D. Formulation and Characterization of Glutaraldehyde Cross-Linked Chitosan Biodegradable Microspheres Loaded with Famotidine. Trop. J. Pharm. Res. 2011, 10, 309–316. [Google Scholar] [CrossRef]
- Durán, G.M.; Contento, A.M.; Ríos, Á. β-Cyclodextrin Coated CdSe/ZnS Quantum Dots for Vanillin Sensoring in Food Samples. Talanta 2015, 131, 286–291. [Google Scholar] [CrossRef]
- Zhu, H.B.; Fan, Y.C.; Qian, Y.L.; Tang, H.F.; Ruan, Z.; Liu, D.H.; Wang, H. Determination of Spices in Food Samples by Ionic Liquid Aqueous Solution Extraction and Ion Chromatography. Chin. Chem. Lett. 2014, 25, 465–468. [Google Scholar] [CrossRef]
- Stanzione, J.F.; Sadler, J.M.; La Scala, J.J.; Reno, K.H.; Wool, R.P. Vanillin-Based Resin for Use in Composite Applications. Green Chem. 2012, 14, 2346–2352. [Google Scholar] [CrossRef]
- Sapuła, P.; Bialik-Wąs, K.; Malarz, K. Are Natural Compounds a Promising Alternative to Synthetic Cross-Linking Agents in the Preparation of Hydrogels? Pharmaceutics 2023, 15, 253. [Google Scholar] [CrossRef] [PubMed]
- Liang, H.C.; Chang, W.H.; Lin, K.J.; Sung, H.W. Genipin-Crosslinked Gelatin Microspheres as a Drug Carrier for Intramuscular Administration: In Vitro and in Vivo Studies. J. Biomed. Mater. Res. Part A 2003, 65, 271–282. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.J.; Yang, H.H.; Chen, C.H.; Lin, W.W.; Chen, S.C.; Lai, P.H.; Chang, Y.; Sung, H.W. Gelatin Microspheres Encapsulated with a Nonpeptide Angiogenic Agent, Ginsenoside Rg1, for Intramyocardial Injection in a Rat Model with Infarcted Myocardium. J. Control Release 2007, 120, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Xu, S.; Li, S.; Pan, H. Genipin-Cross-Linked Hydrogels Based on Biomaterials for Drug Delivery: A Review. Biomater. Sci. 2021, 9, 1583–1597. [Google Scholar] [CrossRef] [PubMed]
- Nii, T.; Makino, K.; Tabata, Y. Three-Dimensional Culture System of Cancer Cells Combined with Biomaterials for Drug Screening. Cancers 2020, 12, 2754. [Google Scholar] [CrossRef] [PubMed]
- Nii, T.; Makino, K.; Tabata, Y. Influence of Shaking Culture on the Biological Functions of Cell Aggregates Incorporating Gelatin Hydrogel Microspheres. J. Biosci. Bioeng. 2019, 128, 606–612. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Tian, Z.; Jin, X.; Holzman, J.F.; Menard, F.; Kim, K. Visible Light-Based Stereolithography Bioprinting of Cell-Adhesive Gelatin Hydrogels. In Proceedings of the 2017 39th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Jeju, Republic of Korea, 11–15 July 2017. [Google Scholar]
- Flaumenhaft, R.; Rifkin, D.B. Extracellular Matrix Regulation of Growth Factor and Protease Activity. Curr. Opin. Cell Biol. 1991, 3, 817–823. [Google Scholar] [CrossRef]
- Vlodavsky, I.; Ishai-Michaeli, R.; Bashkin, P.; Levi, E.; Korner, G.; Bar-Shavit, R.; Fuks, Z.; Klagsbrun, M. Extracellular Matrix-resident Basic Fibroblast Growth Factor: Implication for the Control of Angiogenesis. J. Cell. Biochem. 1991, 45, 167–176. [Google Scholar] [CrossRef]
- Jones, J.I.; Gockerman, A.; Busby, W.H.; Camacho-Hubner, C.; Clemmons, D.R. Extracellular Matrix Contains Insulin-like Growth Factor Binding Protein-5: Potentiation of the Effects of IGF-I. J. Cell Biol. 1993, 121, 679–687. [Google Scholar] [CrossRef] [PubMed]
- Tabata, Y.; Ikada, Y. Protein Release from Gelatin Matrices. Adv. Drug Deliv. Rev. 1998, 31, 287–301. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez Hernández, J.C.; Serrano Aroca, Á.; Gómez Ribelles, J.L.; Monleón Pradas, M. Three-Dimensional Nanocomposite Scaffolds with Ordered Cylindrical Orthogonal Pores. J. Biomed. Mater. Res. Part B Appl. Biomater. 2008, 84, 541–549. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Pérez, E.; Lloret Compañ, A.; Monleón Pradas, M.; Martínez-Ramos, C. Scaffolds of Hyaluronic Acid–Poly(Ethyl Acrylate) Interpenetrating Networks: Characterization and In Vitro Studies. Macromol. Biosci. 2016, 16, 1147–1157. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Rojas, L.; Gómez-Pinedo, U.; Benito-Martin, M.S.; León-Espinosa, G.; Rascón-Ramirez, F.; Lendinez, C.; Martínez-Ramos, C.; Matías-Guiu, J.; Pradas, M.M.; Barcia, J.A. Biohybrids of Scaffolding Hyaluronic Acid Biomaterials plus Adipose Stem Cells Home Local Neural Stem and Endothelial Cells: Implications for Reconstruction of Brain Lesions after Stroke. J. Biomed. Mater. Res.-Part B Appl. Biomater. 2019, 107, 1598–1606. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez Pérez, E.; Pradas, M.M.; Luís, J.; Ivirico, E. Diseño de Nuevos Biomateriales Basados En Redes Poliméricas Interpenetradas de Ácido Hialurónico y Polímeros Acrílicos. Ph.D. Thesis, Universitat Politècnica de València, Valencia, Spain, 2017. [Google Scholar]
- García Cruz, D.M.; Sardinha, V.; Escobar Ivirico, J.L.; Mano, J.F.; Gómez Ribelles, J.L. Gelatin Microparticles Aggregates as Three-Dimensional Scaffolding System in Cartilage Engineering. J. Mater. Sci. Mater. Med. 2013, 24, 503–513. [Google Scholar] [CrossRef] [PubMed]
- Frisch, H.L. “Diffusion in Polymers” Edited by J. Crank and G. S. Park, Academic Press, London and New York, 1968; 452 Pg. J. Appl. Polym. Sci. 1970, 14, 1657. [Google Scholar] [CrossRef]
- ISO 10993-5:2009; Biological Evaluation of Medical Devices—Part 5: Tests for In Vitro Cytotoxicity (ICS 11.100.20). ISO: Geneva, Switzerland, 2009.
- Chen, F.M.; Zhang, M.; Wu, Z.F. Toward Delivery of Multiple Growth Factors in Tissue Engineering. Biomaterials 2010, 31, 6279–6308. [Google Scholar] [CrossRef] [PubMed]
- Park, U.; Kim, K. Multiple Growth Factor Delivery for Skin Tissue Engineering Applications. Biotechnol. Bioprocess Eng. 2017, 22, 659–670. [Google Scholar] [CrossRef]
- Bai, Y.; Moeinzadeh, S.; Kim, S.; Park, Y.; Lui, E.; Tan, H.; Zhao, W.; Zhou, X.; Yang, Y.P. Development of PLGA-PEG-COOH and Gelatin-Based Microparticles Dual Delivery System and E-Beam Sterilization Effects for Controlled Release of BMP-2 and IGF-1. Part. Part. Syst. Charact. 2020, 37, 2000180. [Google Scholar] [CrossRef]
- Habraken, W.J.E.M.; Boerman, O.C.; Wolke, J.G.C.; Mikos, A.G.; Jansen, J.A. In Vitro Growth Factor Release from Injectable Calcium Phosphate Cements Containing Gelatin Microspheres. J. Biomed. Mater. Res. Part A 2009, 91, 614–622. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, A.H.; McKinney, J.; Miller, T.; Bongiorno, T.; McDevitt, T.C. Gelatin Methacrylate Microspheres for Controlled Growth Factor Release. Acta Biomater. 2015, 13, 101–110. [Google Scholar] [CrossRef] [PubMed]
- Tuzlakoglu, K.; Santos, M.I.; Neves, N.; Reis, R.L. Design of Nano- and Microfiber Combined Scaffolds by Electrospinning of Collagen onto Starch-Based Fiber Meshes: A Man-Made Equivalent of Natural Extracellular Matrix. Tissue Eng. Part A 2010, 17, 463–473. [Google Scholar] [CrossRef] [PubMed]
- Pok, S.W.; Wallace, K.N.; Madihally, S.V. In Vitro Characterization of Polycaprolactone Matrices Generated in Aqueous Media. Acta Biomater. 2010, 6, 1061–1068. [Google Scholar] [CrossRef]
- Huang, F.; Cui, L.; Peng, C.H.; Wu, X.B.; Han, B.S.; Dong, Y.D. Preparation of Three-Dimensional Macroporous Chitosan–Gelatin B Microspheres and HepG2-Cell Culture. J. Tissue Eng. Regen. Med. 2016, 10, 1033–1040. [Google Scholar] [CrossRef]

| Diameter in Dry State (µm) | Diameter in Swelling State (µm) | Swelling Ratio | Crosslinking Percentage (%) | |
|---|---|---|---|---|
| Without crosslinking | 12 ± 2 | 32 ± 5 | 20 ± 1 | 0 |
| Vanilin | 11 ± 3 | 15 ± 3 | 2.3 ± 0.1 | 42.2 ± 0.1 |
| Genipin | 13.1 ± 0.7 | 25 ± 2 | 7.0 ± 0.3 | 16.50 ± 0.03 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martínez-Ramos, C.; Rodríguez Ruiz, A.; Monleón Pradas, M.; Gisbert Roca, F. Characterization of a Delivery System Based on a Hyaluronic Acid 3D Scaffold and Gelatin Microparticles. Polymers 2024, 16, 1748. https://doi.org/10.3390/polym16121748
Martínez-Ramos C, Rodríguez Ruiz A, Monleón Pradas M, Gisbert Roca F. Characterization of a Delivery System Based on a Hyaluronic Acid 3D Scaffold and Gelatin Microparticles. Polymers. 2024; 16(12):1748. https://doi.org/10.3390/polym16121748
Chicago/Turabian StyleMartínez-Ramos, Cristina, Alejandro Rodríguez Ruiz, Manuel Monleón Pradas, and Fernando Gisbert Roca. 2024. "Characterization of a Delivery System Based on a Hyaluronic Acid 3D Scaffold and Gelatin Microparticles" Polymers 16, no. 12: 1748. https://doi.org/10.3390/polym16121748
APA StyleMartínez-Ramos, C., Rodríguez Ruiz, A., Monleón Pradas, M., & Gisbert Roca, F. (2024). Characterization of a Delivery System Based on a Hyaluronic Acid 3D Scaffold and Gelatin Microparticles. Polymers, 16(12), 1748. https://doi.org/10.3390/polym16121748







