Hybrid Zinc Phthalocyanine/PVDF-HFP System for Reducing Biofouling in Water Desalination: DFT Theoretical and MolDock Investigations
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Instruments
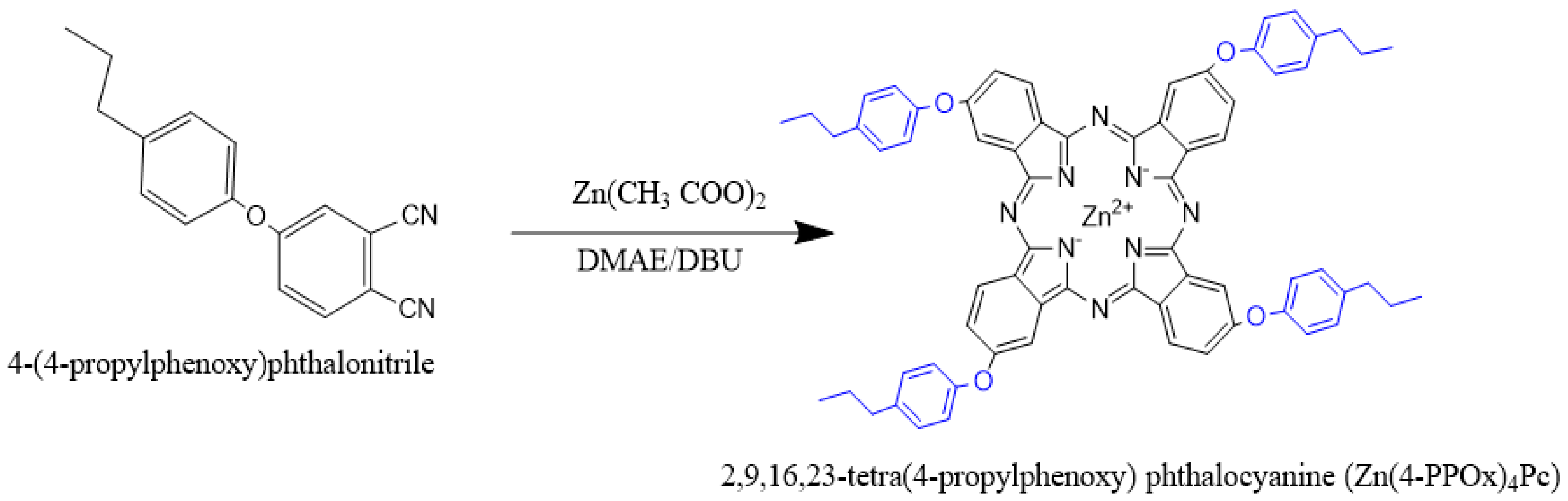
2.3. Synthesis of 4-(4-propylphenoxy)phthalonitrile
2.4. Synthesis of Zinc 2,9,16,23-tetra(4-propylphenoxy) Phthalocyanine (Zn(4-PPOx)4Pc)
2.5. Preparation of Phthalocyanine-PVDF and PVDF-HFP Nanocomposite Membranes by Electrospinning
2.5.1. Process of the Preparation of Polymer Solution
2.5.2. Fabrication of Zn(4-PPOx)4Pc/PVDF-HFP Nanofibers by Electrospinning Technique
2.6. Computational Methodology
Electrostatic Surface Potential Method
3. Results
3.1. Absorption Properties of Zn(4-PPOx)4Pc
3.2. Characterizations of PVDF-HFP/Zn(4-PPOx)4Pc Nanofiber Membranes
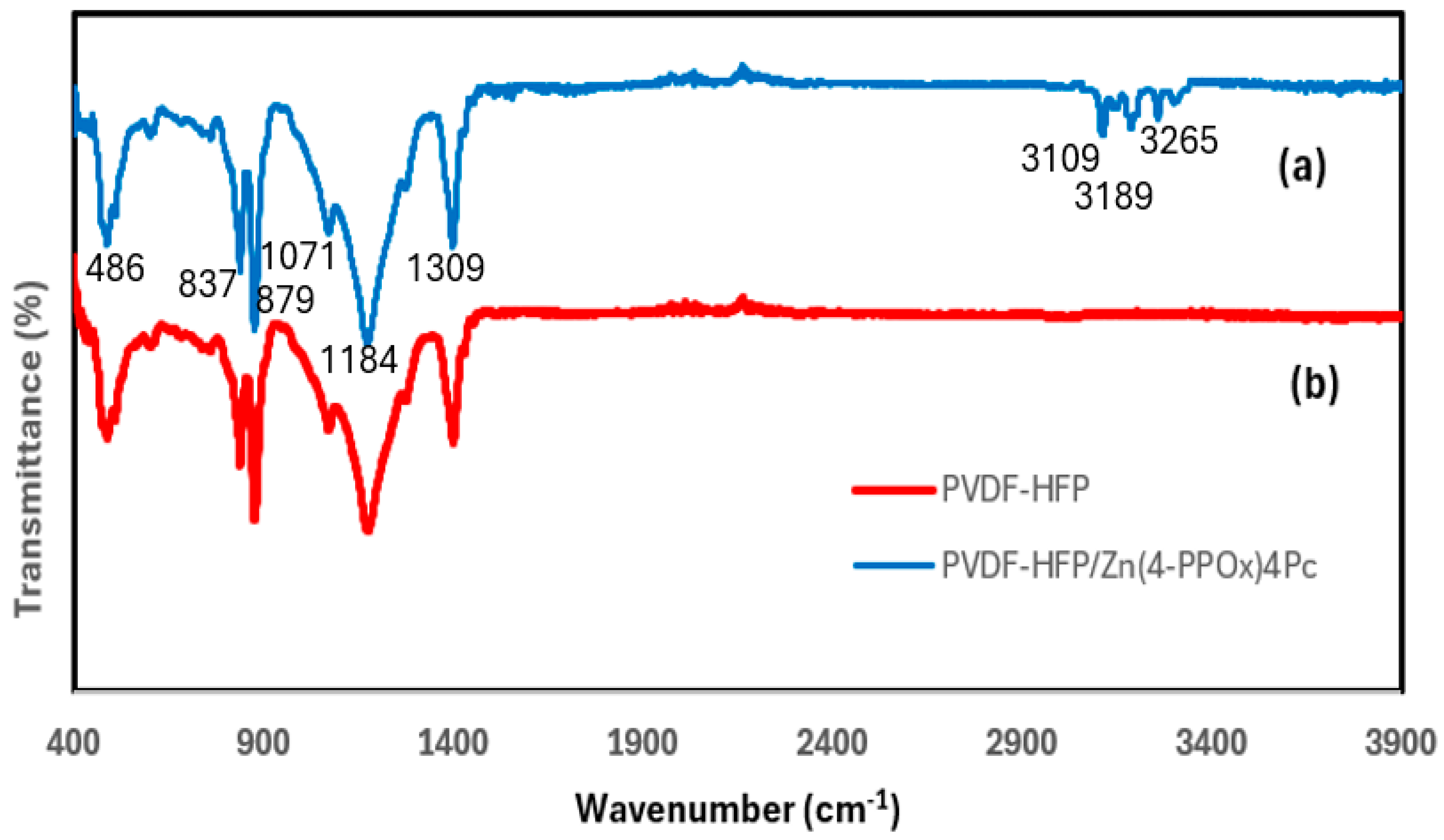
3.2.1. Surface Characterization by FTIR/ATR Spectroscopy
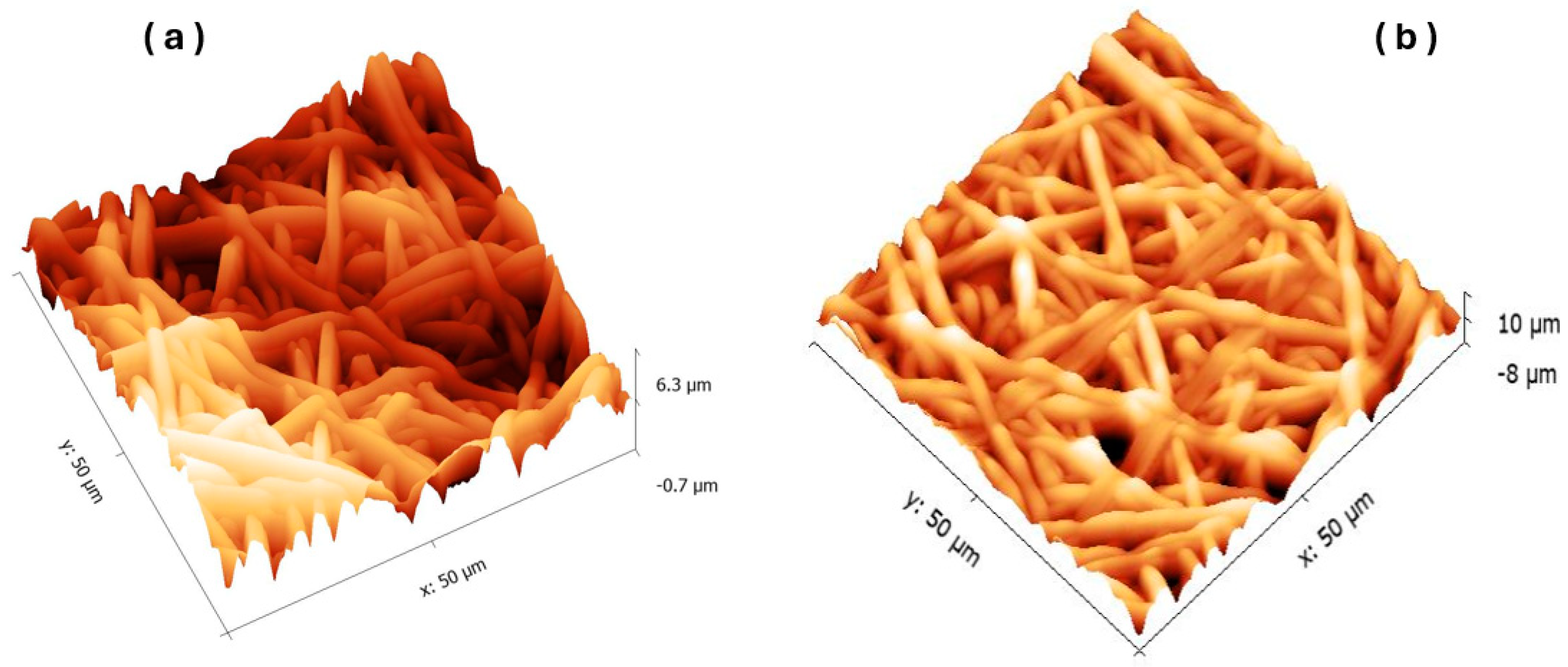
3.2.2. Morphological Studies: AFM
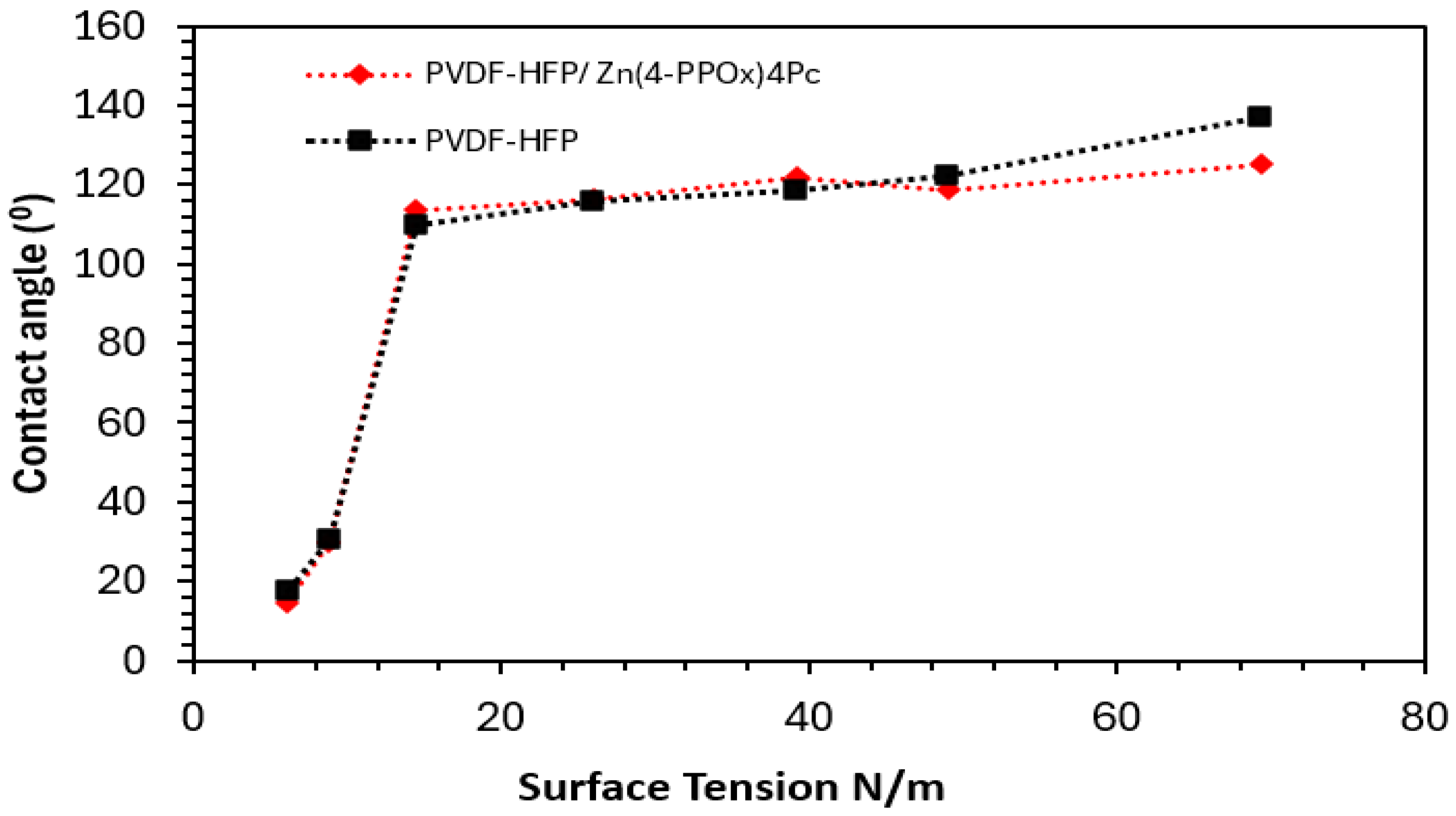
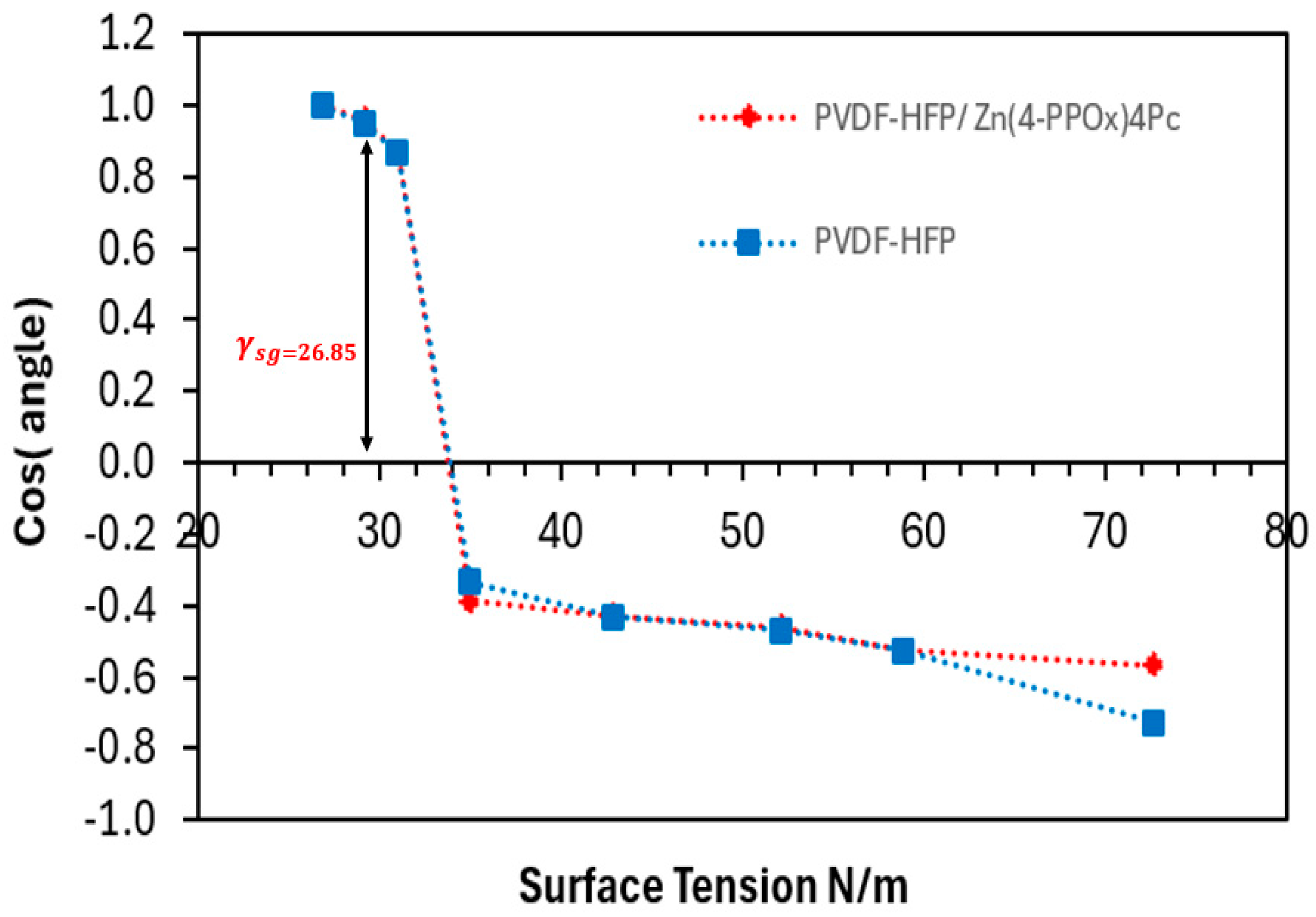
3.2.3. Wettability and Surface Free Energy
3.3. Molecular Modeling Study
3.3.1. Building Model Molecules for PVDF-HFP, Zn(4-PPOx)4Pc, PVDF-HFP/Zn(4-PPOx)4Pc
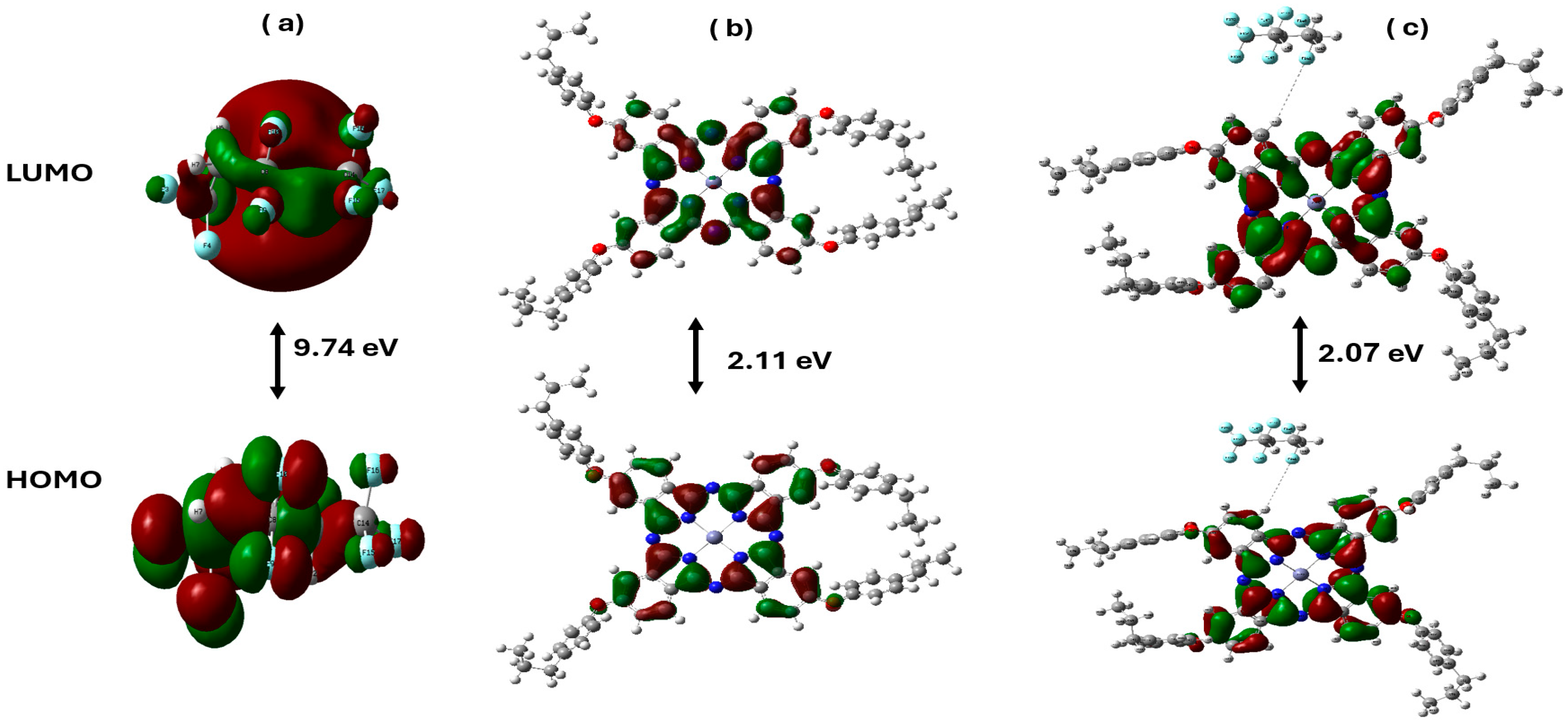
3.3.2. Energy Calculations
3.3.3. Distribution of HOMO/LUMO Molecular Orbitals in Zn(4-PPOx)4Pc Interacting with PVDF-HFP
3.3.4. Molecular Electrostatic Potential (MESP)
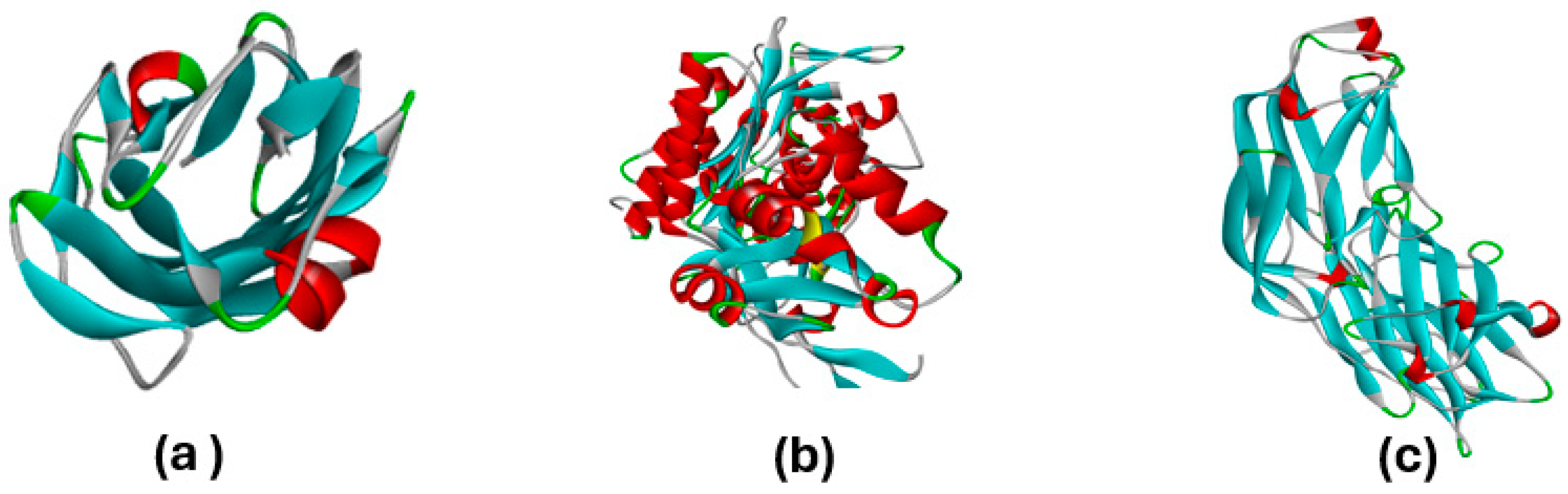
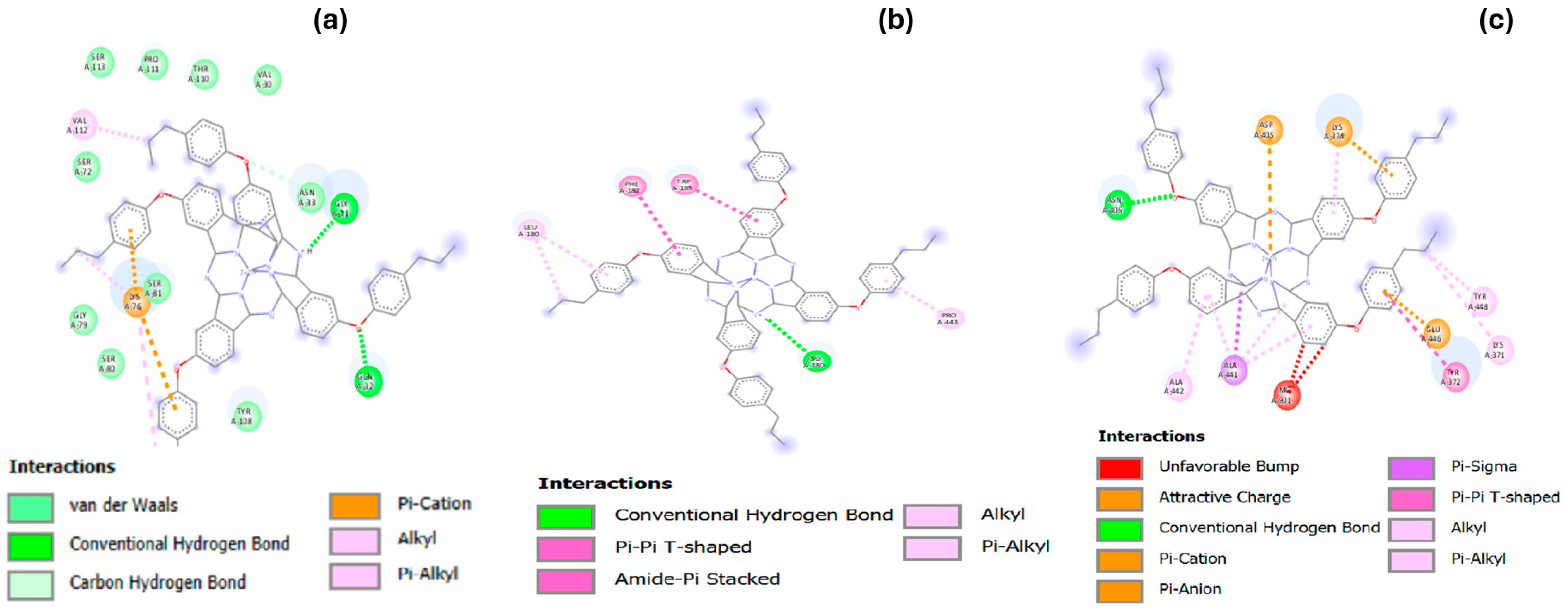
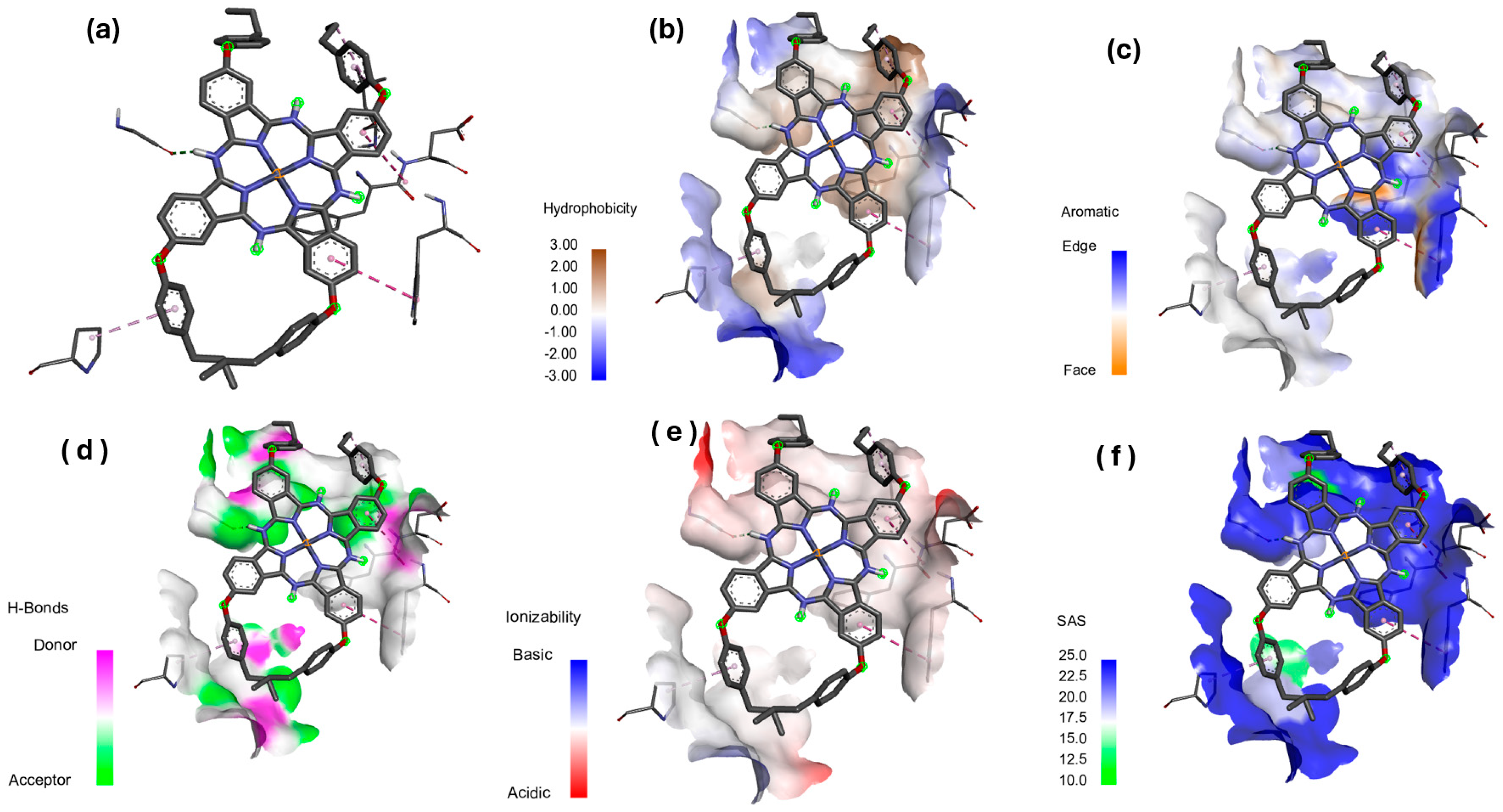
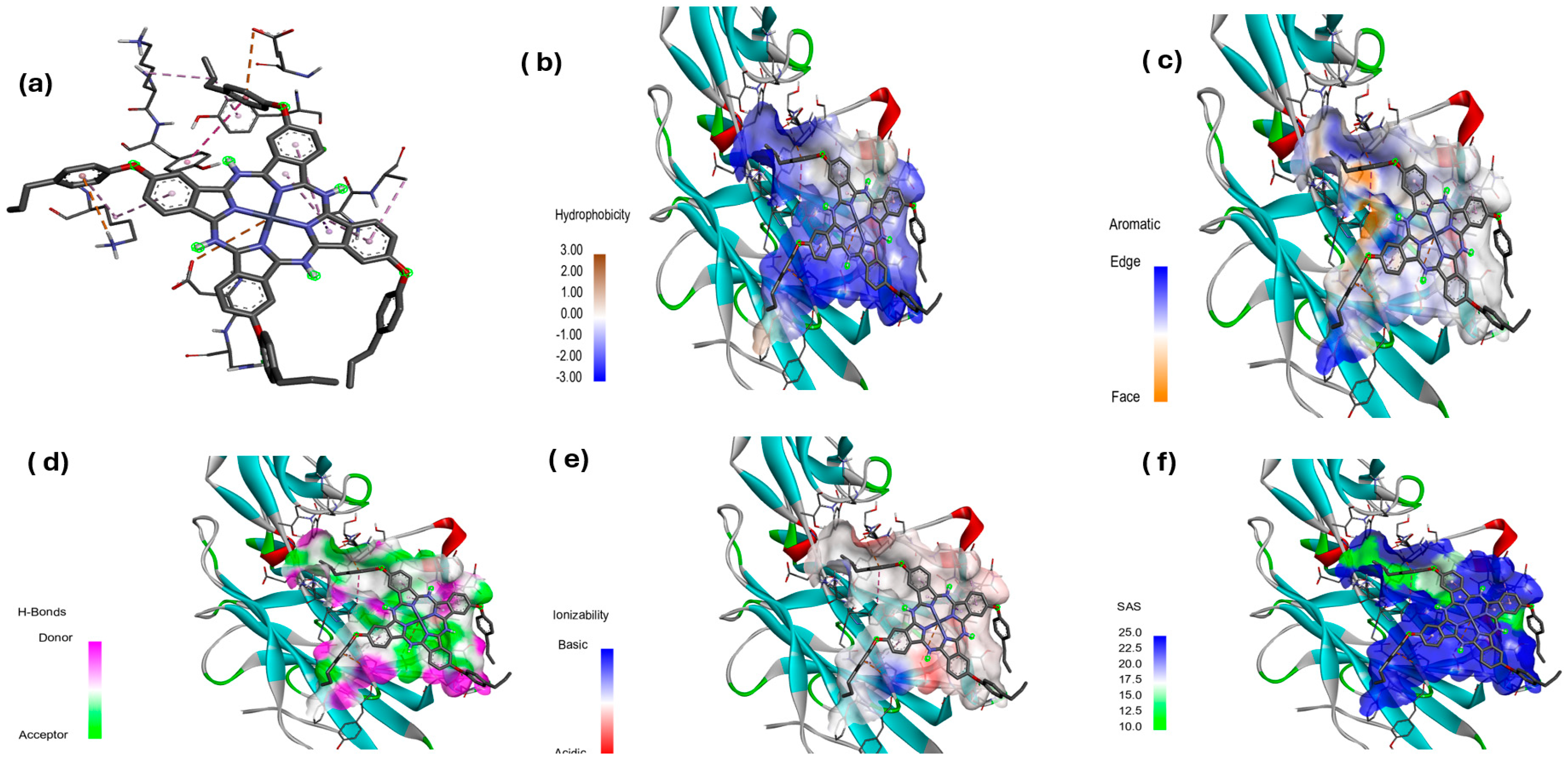
3.4. Molecular Docking Studies
Hydrophobicity, Aromatic Surfaces, Hydrogen Bonds, Ionizability, and Solvent-Accessible Surface
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Almasoudi, S.; Jamoussi, B. Desalination Technologies and Their Environmental Impacts: A Review. Sustain. Chem. One World 2024, 1, 100002. [Google Scholar] [CrossRef]
- Wu, H.; Tang, B.; Wu, P. Preparation and Characterization of Anti-Fouling β-Cyclodextrin/Polyester Thin Film Nanofiltration Composite Membrane. J. Memb. Sci. 2013, 428, 301–308. [Google Scholar] [CrossRef]
- Nejati, S.; Mirbagheri, S.A.; Warsinger, D.M.; Fazeli, M. Biofouling in Seawater Reverse Osmosis (SWRO): Impact of Module Geometry and Mitigation with Ultrafiltration. J. Water Process Eng. 2019, 29, 100782. [Google Scholar] [CrossRef]
- Xiao, K.; Wang, X.; Huang, X.; Waite, T.D.; Wen, X. Combined Effect of Membrane and Foulant Hydrophobicity and Surface Charge on Adsorptive Fouling during Microfiltration. J. Memb. Sci. 2011, 373, 140–151. [Google Scholar] [CrossRef]
- Beer, C.; Foldbjerg, R.; Hayashi, Y.; Sutherland, D.S.; Autrup, H. Toxicity of Silver Nanoparticles—Nanoparticle or Silver Ion? Toxicol. Lett. 2012, 208, 286–292. [Google Scholar] [CrossRef]
- Park, N.; Kwon, B.; Kim, I.; Cho, J. Biofouling Potential of Various NF Membranes with Respect to Bacteria and Their Soluble Microbial Products (SMP): Characterizations, Flux Decline, and Transport Parameters. J. Memb. Sci. 2005, 258, 43–54. [Google Scholar] [CrossRef]
- Sadr Ghayeni, S.B.; Beatson, P.J.; Schneider, R.P.; Fane, A.G. Adhesion of Waste Water Bacteria to Reverse Osmosis Membranes. J. Memb. Sci. 1998, 138, 29–42. [Google Scholar] [CrossRef]
- Ridgway, H.F.; Rigby, M.G.; Argo, D.G. Bacterial Adhesion and Fouling of Reverse Osmosis Membranes. J. AWWA 1985, 77, 97–106. [Google Scholar] [CrossRef]
- Nguyen, T.; Roddick, F.; Fan, L. Biofouling of Water Treatment Membranes: A Review of the Underlying Causes, Monitoring Techniques and Control Measures. Membranes 2012, 2, 804–840. [Google Scholar] [CrossRef]
- Ras, M.; Lefebvre, D.; Derlon, N.; Paul, E.; Girbal-Neuhauser, E. Extracellular Polymeric Substances Diversity of Biofilms Grown under Contrasted Environmental Conditions. Water Res. 2011, 45, 1529–1538. [Google Scholar] [CrossRef]
- Songca, S.P.; Adjei, Y. Applications of Antimicrobial photodynamic Therapy against Bacterial Biofilms. Int. J. Mol. Sci. 2022, 23, 3209. [Google Scholar] [CrossRef]
- de Freitas, L.M.; Lorenzón, E.N.; Santos-Filho, N.A.; de Paula Zago, L.H.; Uliana, M.P.; de Oliveira, K.T.; Cilli, E.M.; Fontana, C.R. Antimicrobial Photodynamic Therapy Enhanced by the Peptide Aurein 1.2. Sci. Rep. 2018, 8, 4212. [Google Scholar] [CrossRef]
- Wang, P.; Lv, H.; Cao, X.; Liu, Y.; Yu, D.-G. Recent Progress of the Preparation and Application of Electrospun Porous Nanofibers. Polymers 2023, 15, 921. [Google Scholar] [CrossRef]
- Tang, Y.; Cai, Z.; Sun, X.; Chong, C.; Yan, X.; Li, M.; Xu, J. Electrospun Nanofiber-Based Membranes for Water Treatment. Polymers 2022, 14, 2004. [Google Scholar] [CrossRef]
- Abid, M.B.; Wahab, R.A.; Salam, M.A.; Moujdin, I.A.; Gzara, L. Desalination Technologies, Membrane Distillation, and Electrospinning, an Overview. Heliyon 2023, 9, e12810. [Google Scholar] [CrossRef]
- Teoh, M.M.; Chung, T.-S. Membrane Distillation with Hydrophobic Macrovoid-Free PVDF–PTFE Hollow Fiber Membranes. Sep. Purif. Technol. 2009, 66, 229–236. [Google Scholar] [CrossRef]
- Albiladi, A.; Gzara, L.; Organji, H.; Alkayal, N.S.; Figoli, A. Electrospun Poly (Vinylidene Fluoride-Co-Hexafluoropropylene) Nanofiber Membranes for Brine Treatment via Membrane Distillation. Polymers 2023, 15, 2706. [Google Scholar] [CrossRef]
- Khayet, M.; Chowdhury, G.; Matsuura, T. Surface Modification of Polyvinylidene Fluoride Pervaporation Membranes. AIChE J. 2002, 48, 2833–2843. [Google Scholar] [CrossRef]
- Jian, K.; Pintauro, P.N. Integral Asymmetric Poly(Vinylidene Fluoride) (PVDF) Pervaporation Membranes. J. Memb. Sci. 1993, 85, 301–309. [Google Scholar] [CrossRef]
- Li, J.; Ren, L.-F.; Zhou, H.S.; Yang, J.; Shao, J.; He, Y. Fabrication of Superhydrophobic PDTS-ZnO-PVDF Membrane and Its Anti-Wetting Analysis in Direct Contact Membrane Distillation (DCMD) Applications. J. Memb. Sci. 2021, 620, 118924. [Google Scholar] [CrossRef]
- Tian, X.; Jiang, X. Poly(Vinylidene Fluoride-Co-Hexafluoropropene) (PVDF-HFP) Membranes for Ethyl Acetate Removal from Water. J. Hazard. Mater. 2008, 153, 128–135. [Google Scholar] [CrossRef]
- Shi, L.; Wang, R.; Cao, Y. Effect of the Rheology of Poly(Vinylidene Fluoride-Co-Hexafluropropylene) (PVDF–HFP) Dope Solutions on the Formation of Microporous Hollow Fibers Used as Membrane Contactors. J. Memb. Sci. 2009, 344, 112–122. [Google Scholar] [CrossRef]
- Goethals, A.; Mugadza, T.; Arslanoglu, Y.; Zugle, R.; Antunes, E.; Van Hulle, S.W.H.; Nyokong, T.; De Clerck, K. Polyamide Nanofiber Membranes Functionalized with Zinc Phthalocyanines. J. Appl. Polym. Sci. 2014, 131, 40486. [Google Scholar] [CrossRef]
- Bonnett, R.; Krysteva, M.A.; Lalov, I.G.; Artarsky, S.V. Water Disinfection Using Photosensitizers Immobilized on Chitosan. Water Res. 2006, 40, 1269–1275. [Google Scholar] [CrossRef]
- Mikula, P.; Kalhotka, L.; Jancula, D.; Zezulka, S.; Korinkova, R.; Cerny, J.; Marsalek, B.; Toman, P. Evaluation of Antibacterial Properties of Novel Phthalocyanines against Escherichia Coli—Comparison of Analytical Methods. J. Photochem. Photobiol. B 2014, 138, 230–239. [Google Scholar] [CrossRef]
- Gomes, D.S.; Rodrigues da Silva, A.N. Electrospun Nanofibers with Copper Phthalocyanine for Detecting Ammonia. In Proceedings of the 2014 IEEE 9th IberoAmerican Congress on Sensors, Bogota, Colombia, 15–18 October 2014; IEEE: New York, NY, USA; pp. 1–3. [Google Scholar]
- Tang, S.; Shao, C.; Liu, Y.; Li, S.; Mu, R. Electrospun Nanofibers of Poly(Ethylene Oxide)/Teraamino-Phthalocyanine Copper(II) Hybrids and Its Photoluminescence Properties. J. Phys. Chem. Solids 2007, 68, 2337–2340. [Google Scholar] [CrossRef]
- Touaiti, S.; Hajri, A.; Kahouech, M.S.; Khiari, J.; Jamoussi, B. Synthesis and Characterization of New Zn-Phtalocyanine-Based Semi-Conducting Materials. Arab. J. Chem. 2017, 10, S1553–S1557. [Google Scholar] [CrossRef]
- Hajri, A.; Touaiti, S.; Jamoussi, B. Preparation of Organic Zn-Phthalocyanine-Based Semiconducting Materials and Their Optical and Electrochemical Characterization. Adv. Optoelectron. 2013, 2013, 1–7. [Google Scholar] [CrossRef]
- Wang, S.H.; Ling, H.L.; Organji, H. Poly (Vinylidene Fluoride-Co-Hexafluoropropylene)/polybenzimidazole blend nanofiber supported Nafion membranes for direct methanol fuel cells. J. Power Sources 2014, 257, 254–263. [Google Scholar] [CrossRef]
- Lu, L.; Hu, H.; Hou, H.; Wang, B. An Improved B3LYP Method in the Calculation of Organic Thermochemistry and Reactivity. Comput. Theor. Chem. 2013, 1015, 64–71. [Google Scholar] [CrossRef]
- Han, S.; Zaniewski, R.P.; Marr, E.S.; Lacey, B.M.; Tomaras, A.P.; Evdokimov, A.; Miller, J.R.; Shanmugasundaram, V. Structural Basis for Effectiveness of Siderophore-Conjugated Monocarbams against Clinically Relevant Strains of Pseudomonas Aeruginosa. Proc. Natl. Acad. Sci. USA 2010, 107, 22002–22007. [Google Scholar] [CrossRef]
- Berman, H.M. The Protein Data Bank. Nucleic Acids Res. 2000, 28, 235–242. [Google Scholar] [CrossRef]
- Sauer, M.M.; Jakob, R.P.; Eras, J.; Baday, S.; Eriş, D.; Navarra, G.; Bernèche, S.; Ernst, B.; Maier, T.; Glockshuber, R. Catch-Bond Mechanism of the Bacterial Adhesin FimH. Nat. Commun. 2016, 7, 10738. [Google Scholar] [CrossRef]
- Morris, G.M.; Huey, R.; Lindstrom, W.; Sanner, M.F.; Belew, R.K.; Goodsell, D.S.; Olson, A.J. AutoDock4 and AutoDockTools4: Automated Docking with Selective Receptor Flexibility. J. Comput. Chem. 2009, 30, 2785–2791. [Google Scholar] [CrossRef]
- Morris, G.M.; Goodsell, D.S.; Halliday, R.S.; Huey, R.; Hart, W.E.; Belew, R.K.; Olson, A.J. Automated Docking Using a Lamarckian Genetic Algorithm and an Empirical Binding Free Energy Function. J. Comput. Chem. 1998, 19, 1639–1662. [Google Scholar] [CrossRef]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the Speed and Accuracy of Docking with a New Scoring Function, Efficient Optimization, and Multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef]
- Wang, J.; Cieplak, P.; Kollman, P.A. How Well Does a Restrained Electrostatic Potential (RESP) Model Perform in Calculating Conformational Energies of Organic and Biological Molecules? J. Comput. Chem. 2000, 21, 1049–1074. [Google Scholar] [CrossRef]
- Mazziotti, D.A. Contracted Schrödinger Equation: Determining Quantum Energies and Two-Particle Density Matrices without Wave Functions. Phys. Rev. A 1998, 57, 4219–4234. [Google Scholar] [CrossRef]
- Sun, W.-B.; Yan, P.-F.; Jiang, S.-D.; Wang, B.-W.; Zhang, Y.-Q.; Li, H.-F.; Chen, P.; Wang, Z.-M.; Gao, S. High Symmetry or Low Symmetry, That Is the Question—High Performance Dy(III) Single-Ion Magnets by Electrostatic Potential Design. Chem. Sci. 2016, 7, 684–691. [Google Scholar] [CrossRef]
- Karimi, A.R.; Khodadadi, A. Synthesis and Solution Properties of New Metal-Free and Metallo-Phthalocyanines Containing Four Bis(Indol-3-Yl)Methane Groups. Tetrahedron Lett. 2012, 53, 5223–5226. [Google Scholar] [CrossRef]
- Jamoussi, B.; Chakroun, R.; Timoumi, A.; Essalah, K. Synthesis and Characterization of New Imidazole Phthalocyanine for Photodegradation of Micro-Organic Pollutants from Sea Water. Catalysts 2020, 10, 906. [Google Scholar] [CrossRef]
- Szewczyk, P.K.; Gradys, A.; Kim, S.K.; Persano, L.; Marzec, M.; Kryshtal, A.; Busolo, T.; Toncelli, A.; Pisignano, D.; Bernasik, A.; et al. Enhanced Piezoelectricity of Electrospun Polyvinylidene Fluoride Fibers for Energy Harvesting. ACS Appl. Mater. Interfaces 2020, 12, 13575–13583. [Google Scholar] [CrossRef]
- He, Z.; Rault, F.; Lewandowski, M.; Mohsenzadeh, E.; Salaün, F. Electrospun PVDF Nanofibers for Piezoelectric Applications: A Review of the Influence of Electrospinning Parameters on the β Phase and Crystallinity Enhancement. Polymers 2021, 13, 174. [Google Scholar] [CrossRef]
- Kozbial, A.; Li, Z.; Conaway, C.; McGinley, R.; Dhingra, S.; Vahdat, V.; Zhou, F.; D’Urso, B.; Liu, H.; Li, L. Study on the Surface Energy of Graphene by Contact Angle Measurements. Langmuir 2014, 30, 8598–8606. [Google Scholar] [CrossRef]
- Han, B.; Wang, X.; Zheng, J.; Liang, S.; Xiao, K.; Yu, J.; Qian, Z.; Huang, X. Determination of Surface Energy Parameters of Hydrophilic Porous Membranes via a Corrected Contact Angle Approach. Langmuir 2019, 35, 15009–15016. [Google Scholar] [CrossRef]
- Choi, W.; Tuteja, A.; Mabry, J.M.; Cohen, R.E.; McKinley, G.H. A Modified Cassie–Baxter Relationship to Explain Contact Angle Hysteresis and Anisotropy on Non-Wetting Textured Surfaces. J. Colloid. Interface Sci. 2009, 339, 208–216. [Google Scholar] [CrossRef]
- Marmur, A. From Hygrophilic to Superhygrophobic: Theoretical Conditions for Making High-Contact-Angle Surfaces from Low-Contact-Angle Materials. Langmuir 2008, 24, 7573–7579. [Google Scholar] [CrossRef]
- Whyman, G.; Bormashenko, E.; Stein, T. The Rigorous Derivation of Young, Cassie–Baxter and Wenzel Equations and the Analysis of the Contact Angle Hysteresis Phenomenon. Chem. Phys. Lett. 2008, 450, 355–359. [Google Scholar] [CrossRef]
- Pororok, V.; Movrin, D.; Lukic, N.; Propovic, S. New Insights into the Fouling of a Membrane during the Ultrafiltration of cComplex Organic-Inorganic Feed Water. Membranes 2023, 13, 334. [Google Scholar] [CrossRef]
- Wong, P.C.Y.; Kwon, Y.-N.; Criddle, C.S. Use of Atomic Force Microscopy and Fractal Geometry to Characterize the Roughness of Nano-, Micro-, and Ultrafiltration Membranes. J. Memb. Sci. 2009, 340, 117–132. [Google Scholar] [CrossRef]
- Busscher, H.J.; van Pelt, A.W.J.; de Boer, P.; de Jong, H.P.; Arends, J. The Effect of Surface Roughening of Polymers on Measured Contact Angles of Liquids. Colloids Surf. 1984, 9, 319–331. [Google Scholar] [CrossRef]
- Chakroun, R.; Jamoussi, B.; Al-Mur, B.; Timoumi, A.; Essalah, K. Impedance Spectroscopy and Dielectric Relaxation of Imidazole-Substituted Palladium(II) Phthalocyanine (ImPdPc) for Organic Solar Cells. ACS Omega 2021, 6, 10655–10667. [Google Scholar] [CrossRef]
- Felifel, N.T.; Sliem, M.A.; Kamel, Z.; Bojarska, J.; Seadawy, M.G.; Amin, R.M.; Elnagdy, S.M. Antimicrobial Photodynamic Therapy against Escherichia Coli and Staphylococcus Aureus Using Nanoemulsion-Encapsulated Zinc Phthalocyanine. Microorganisms 2023, 11, 1143. [Google Scholar] [CrossRef]
- Unluer, D.; Aktas Kamiloglu, A.; Direkel, S.; Bektas, E.; Kantekin, H.; Sancak, K. Synthesis and Characterization of Metallophthalocyanine with Morpholine Containing Schiff Base and Determination of Their Antimicrobial and Antioxidant Activities. J. Organomet. Chem. 2019, 900, 120936. [Google Scholar] [CrossRef]






| Membranes | ||
|---|---|---|
| PVDF-HFP/Zn(4-PPOx)4Pc | PVDF-HFP | |
| Area Roughness | ||
| Area | 2.51 nm2 | 2.52 nm2 |
| Average Roughness (Sa) | 651.58 nm | 763.44 nm |
| Root Mean Square Roughness (Sq) | 817.57 nm | 938.73 nm |
| Maximum Height of Surface (Sy) | 6.2243 µm | 5.6323 µm |
| Maximum Peak Height (Sp) | 3153.8 nm | 2702.7 nm |
| Maximum Valley Depth (Sv) | −3070.5 nm | −2929.6 nm |
| Mean Roughness Depth (Sm) | −18.458 fm | −17.94 fm |
| Line Roughness | ||
| Average Roughness (Ra) | 686.04 nm | 786.6 nm |
| Root Mean Square Roughness (Rq) | 807.47 nm | 980.08 nm |
| Maximum Height (Ry) | 3.1701 µm | 4.4474 µm |
| Maximum Peak Height (Rp) | 1764.5 nm | 2217.9 nm |
| Maximum Valley Depth (Rv) | −1405.6 nm | −2229.5 nm |
| Mean Roughness Depth (Rm) | −18.626 fm | −18.554 fm |
| Structure | E (KeV) | DM (Debye) | Pol α (a.u.) | HyPol β (a.u.) | ΔE (eV) |
|---|---|---|---|---|---|
| PVDF-HFP | −26.9864 | 1.36969 | 65.32976 | 2.567644 | 9.74 |
| Zn(4-PPOx)4Pc | −139.954 | 1.37243 | 1137.99035 | 1804.66747 | 2.11 |
| PVDF-HFP/Zn(4-PPOx)4Pc | −166.945 | 4.55515 | nc | nc | 2.07 |
| Molecules | PVDF-HFP | Zn(4-PPOx)4Pc | PVDF-HFP/Zn(4-PPOx)4Pc |
|---|---|---|---|
| ELUMO | −0.457151654 | −2.732025358 | −3.025364336 |
| EHOMO | −10.19312131 | −4.838188334 | −5.098601508 |
| Ionization potential (IP = −EHOMO) | 10.19312131 | 4.838188334 | 5.098601508 |
| Electron affinity (EA = −ELUMO) | 0.457151654 | 2.732025358 | 3.025364336 |
| Chemical hardness (η = (IP − EA)/2) | 4.867984826 | 1.053081488 | 1.036618586 |
| Chemical softness (s = 1/2η) | 0.102711906 | 0.474797065 | 0.482337483 |
| Chemical potential (μ = (IP − EA)/2) | 4.867984826 | 1.053081488 | 1.036618586 |
| Electronegativity (χ = (1 + EA)/2) | 0.728575827 | 1.866012679 | 2.012682168 |
| Electrophilicity index (ω = μ2/2η) | 2.433992413 | 0.526540744 | 0.518309293 |
| Entry | Protein | Binding Affinity (kcal/mol) | Bond Category | Residues in Contact | Interaction Types | Distance () |
|---|---|---|---|---|---|---|
| 1 | 4XO8 | −3.05 | Hydrogen | GLN32 | H | 2.06173 |
| Hydrogen | GLY31 | CH | 3.53301 | |||
| Electrostatic | LYS76 | PiCa | 4.87757 | |||
| Electrostatic | LYS76 | PiCa | 2.91888 | |||
| Hydrophobic | VAL112 | Pi-A | 5.14891 | |||
| Hydrogen | GLN32 | H | 2.06173 | |||
| Hydrogen | GLY31 | H | 3.53301 | |||
| 2 | 3PBQ | −5.48 | Hydrogen | GLY380 | H | 2.47192 |
| Hydrophobic | TRP185 | PiPiTS | 5.07933 | |||
| Hydrophobic | PHE182 | AmPiS | 5.14985 | |||
| Hydrophobic | LEU180 | A | 4.60392 | |||
| Hydrophobic | LEU180 | PiA | 5.10146 | |||
| Hydrophobic | PRO443 | PiA | 4.38758 | |||
| 3 | 1N67 | −8.56 | Electrostatic | ASP405 | AtCh | 5.35046 |
| Hydrogen | ASN406 | H | 2.27904 | |||
| Electrostatic | LYS374 | PiCa | 4.76248 | |||
| Electrostatic | GLU446 | PiAn | 4.65720 | |||
| Hydrophobic | ALA441 | PiS | 3.55853 | |||
| Hydrophobic | TYR372 | PiPi TS | 5.58462 | |||
| Hydrophobic | LYS371 | Alkyl | 4.70072 | |||
| Hydrophobic | TYR448 | PiA | 4.29719 | |||
| Hydrophobic | LYS374 | PiA | 4.81324 | |||
| Hydrophobic | ALA442 | PiA | 4.36383 | |||
| Hydrophobic | ALA442 | PiA | 4.7961 | |||
| Hydrophobic | ALA442 | PiA | 4.22284 | |||
| Hydrophobic | ALA442 | PiA | 5.2594 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jamoussi, B.; Al-Sharif, M.N.M.; Gzara, L.; Organji, H.; Almeelbi, T.B.; Chakroun, R.; Al-Mur, B.A.; Al Makishah, N.H.M.; Madkour, M.H.F.; Aloufi, F.A.; et al. Hybrid Zinc Phthalocyanine/PVDF-HFP System for Reducing Biofouling in Water Desalination: DFT Theoretical and MolDock Investigations. Polymers 2024, 16, 1738. https://doi.org/10.3390/polym16121738
Jamoussi B, Al-Sharif MNM, Gzara L, Organji H, Almeelbi TB, Chakroun R, Al-Mur BA, Al Makishah NHM, Madkour MHF, Aloufi FA, et al. Hybrid Zinc Phthalocyanine/PVDF-HFP System for Reducing Biofouling in Water Desalination: DFT Theoretical and MolDock Investigations. Polymers. 2024; 16(12):1738. https://doi.org/10.3390/polym16121738
Chicago/Turabian StyleJamoussi, Bassem, Mohhamed Naif M. Al-Sharif, Lassaad Gzara, Hussam Organji, Talal B. Almeelbi, Radhouane Chakroun, Bandar A. Al-Mur, Naief H. M. Al Makishah, Mohamed H. F. Madkour, Fahed A. Aloufi, and et al. 2024. "Hybrid Zinc Phthalocyanine/PVDF-HFP System for Reducing Biofouling in Water Desalination: DFT Theoretical and MolDock Investigations" Polymers 16, no. 12: 1738. https://doi.org/10.3390/polym16121738
APA StyleJamoussi, B., Al-Sharif, M. N. M., Gzara, L., Organji, H., Almeelbi, T. B., Chakroun, R., Al-Mur, B. A., Al Makishah, N. H. M., Madkour, M. H. F., Aloufi, F. A., & Halawani, R. F. (2024). Hybrid Zinc Phthalocyanine/PVDF-HFP System for Reducing Biofouling in Water Desalination: DFT Theoretical and MolDock Investigations. Polymers, 16(12), 1738. https://doi.org/10.3390/polym16121738









