On the Use of Styrene-Based Nanoparticles to Mitigate the Effect of Montmorillonite in Copper Sulfide Recovery by Flotation
Abstract
1. Introduction
2. Materials and Methods
2.1. Mineral Samples
2.2. Flotation Reagents
2.3. Conditioning Stage
2.4. Contact Angle Measurements
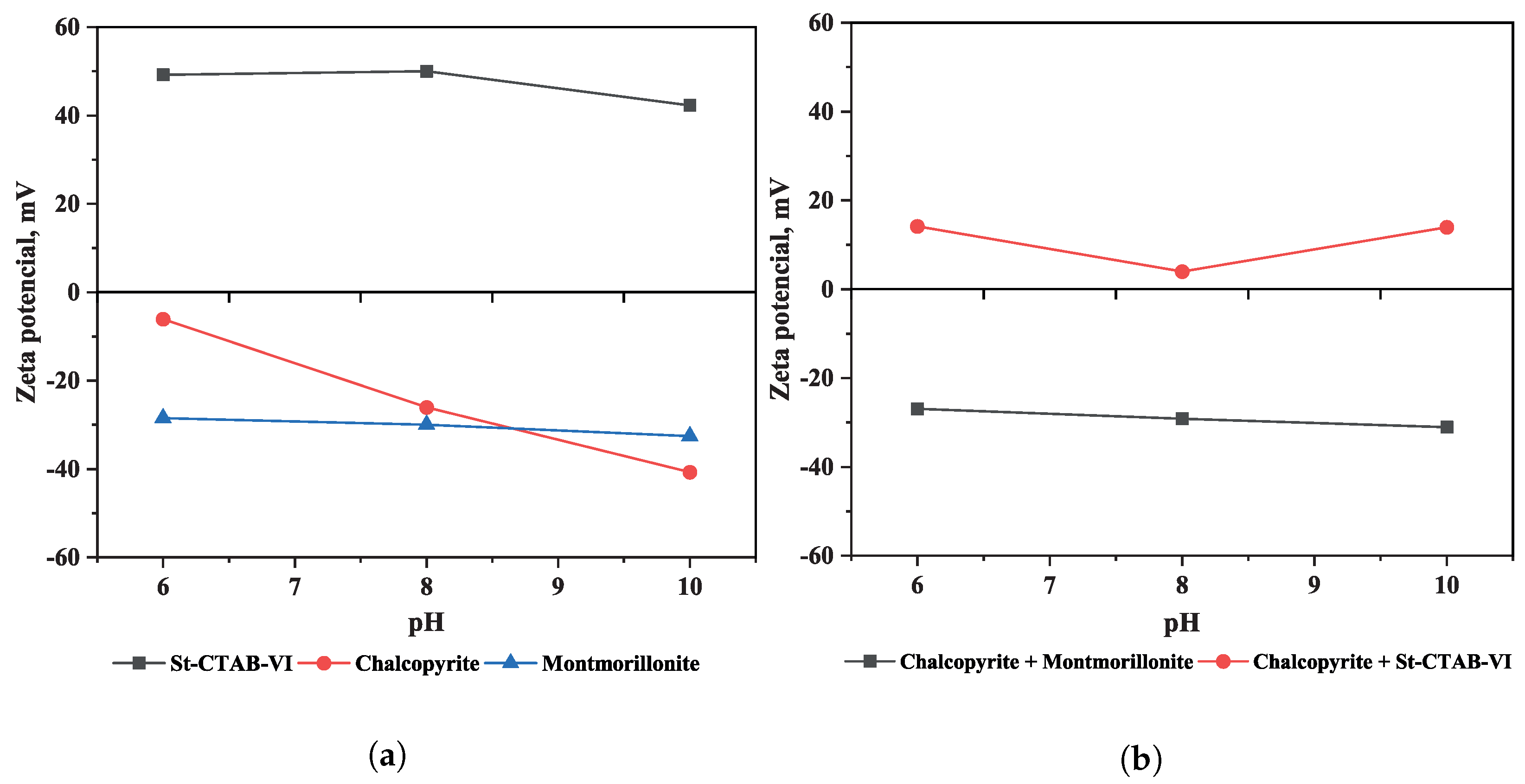
2.5. Zeta Potential Measurements
2.6. Microflotation Tests
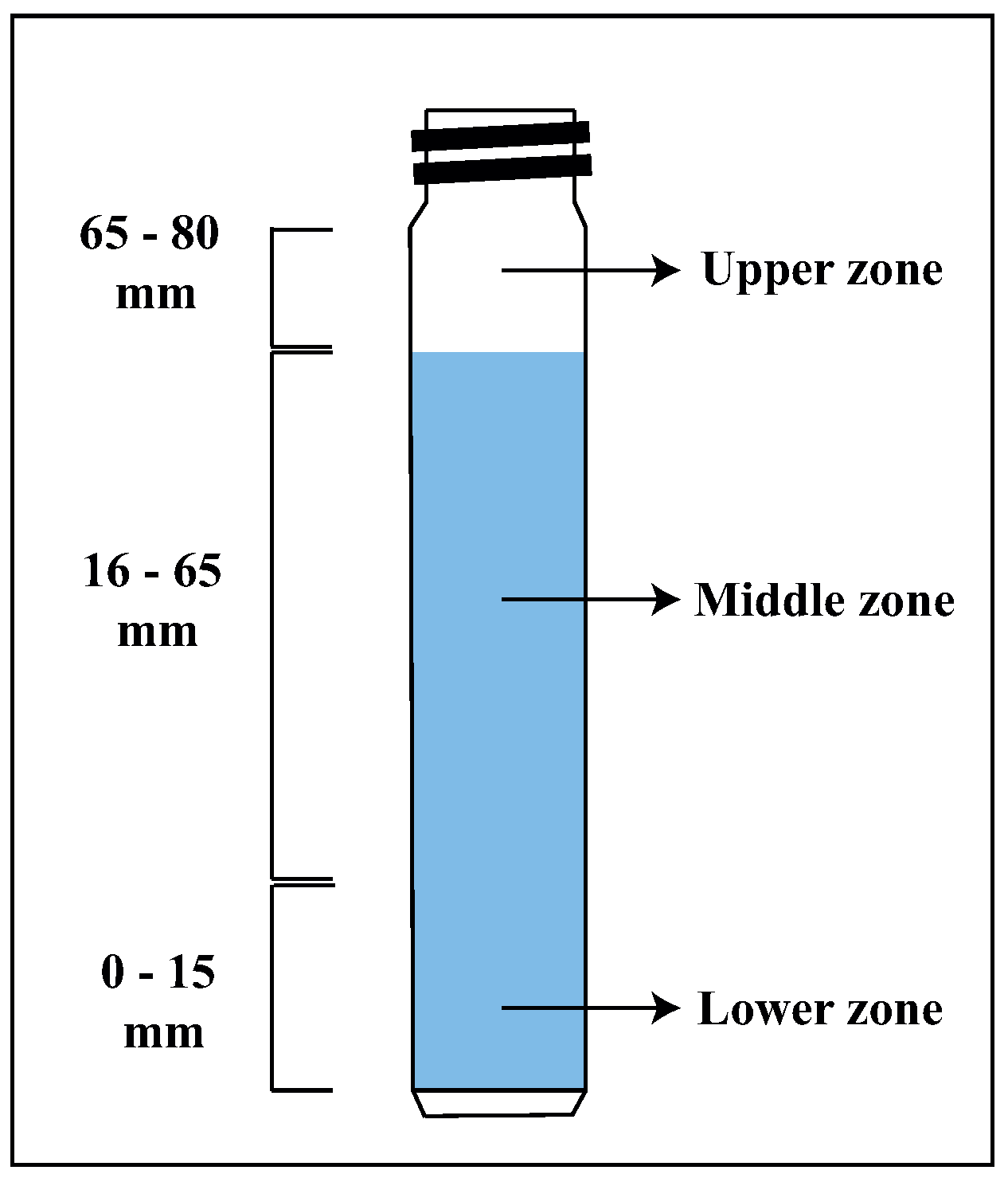
2.7. Turbidity Tests
3. Results and Discussion
3.1. Contact Angle
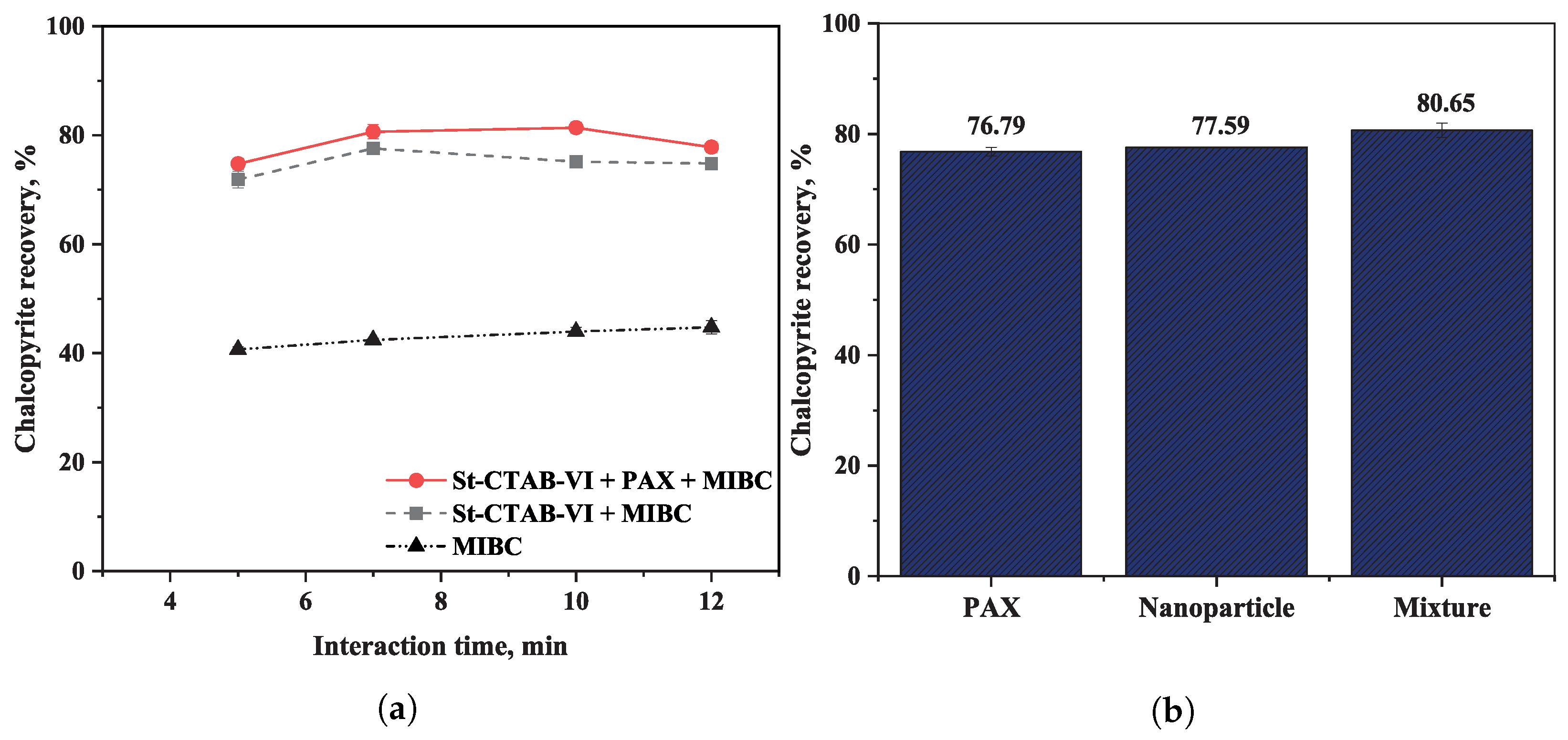
3.2. Microflotation Test
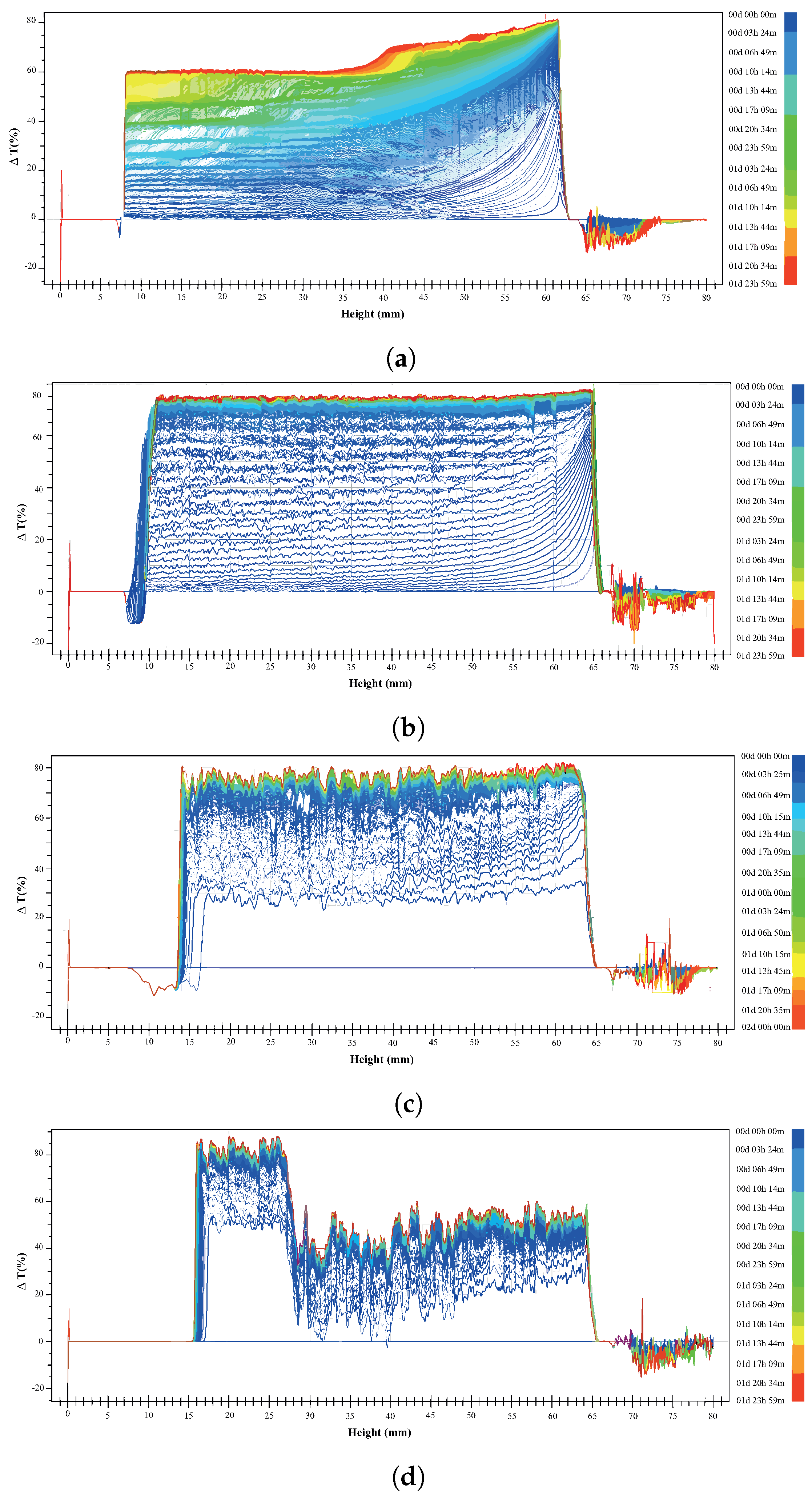
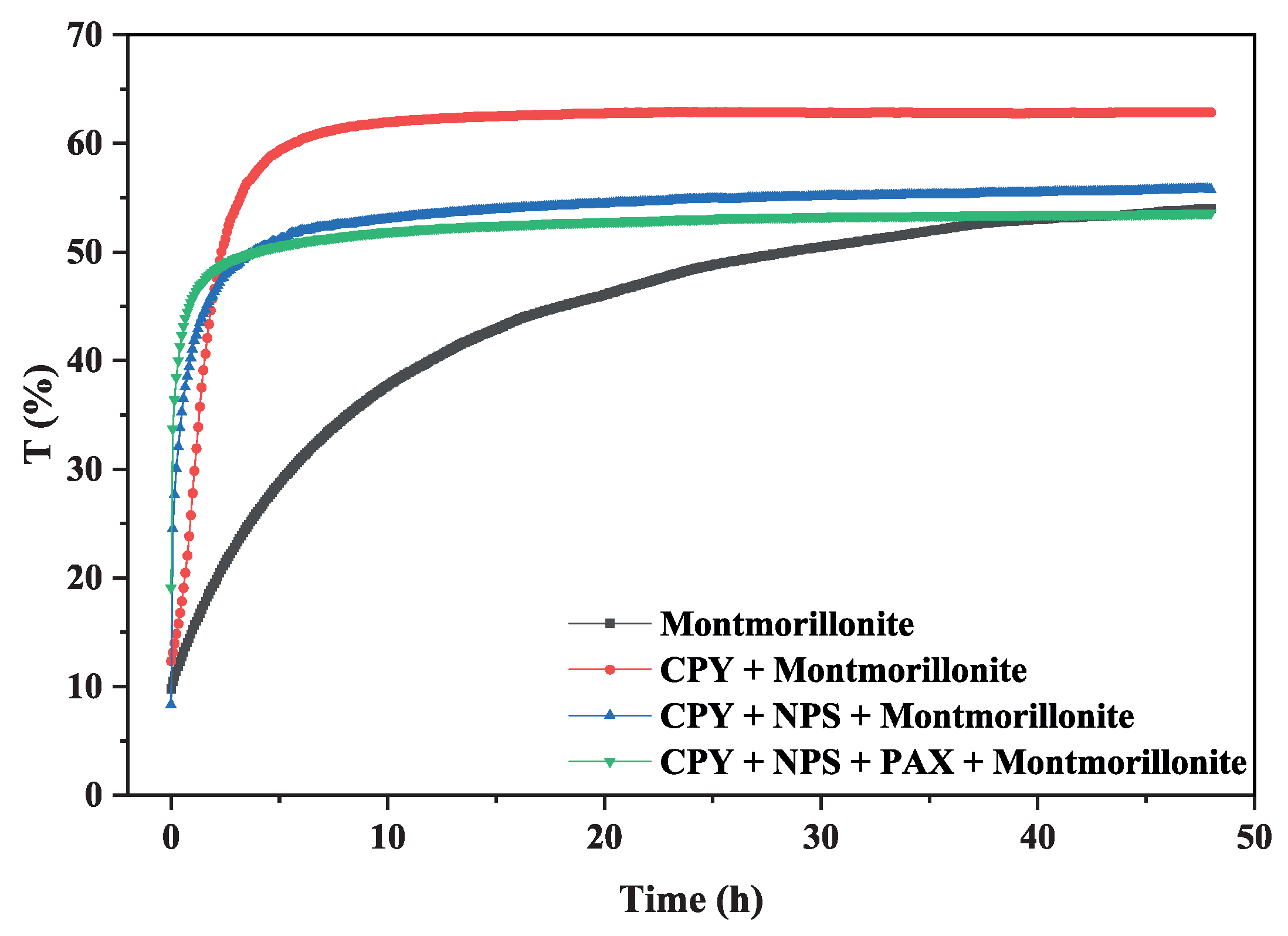
3.3. Turbidity Test
4. Conclusions
- The contact angle measurements and zeta potential results obtained for the chalcopyrite in the presence of montmorillonite provided evidence that the nanoparticle adsorption on the chalcopyrite surface contributed to increasing the hydrophobicity of the chalcopyrite in the presence of this type of clay mineral, reaching a contact angle value similar to that obtained with PAX. According to these findings it is possible to state that the slime coating phenomenon caused by the presence of this type of clay mineral will occur when nanoparticles are used in the process in a similar way as for the conventional collector (PAX).
- The mixture of St-CTAB-VI-02-50 and PAX contributed to increasing the contact angle of chalcopyrite in the presence of montmorillonite, which could be due to initial adsorption of the PAX collector contributing to a greater interaction of the NPs with the mineral surface and contributing to reducing the interaction with the clay minerals and generating a higher hydrophobicity on the chalcopyrite particles.
- Microflotation and turbidity test results showed that the nanoparticles could have the same behavior as the PAX collector when montmorillonite is present. However, the mixtures with both NPs and PAX contributed to promoting the generation of nanoparticle aggregates on sulfide mineral surfaces that helped to detach the slime and may facilitate the bubble/mineral attachment step during flotation.
- This study shows that the use of nanoparticles can contribute to achieving an improvement in the recovery of copper and the quality of concentrates obtained by flotation when low-grade minerals and high concentrations of clay minerals are present.
- Further studies are required to improve the performance of NPs in this type of system, evaluate the interactions with other types of minerals such as molybdenite and pyrite, and study their economic and environmental feasibility.
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ramirez, A.; Rojas, A.; Gutierrez, L.; Laskowski, J.S. Sodium hexametaphosphate and sodium silicate as dispersants to reduce the negative effect of kaolinite on the flotation of chalcopyrite in seawater. Miner. Eng. 2018, 125, 10–14. [Google Scholar] [CrossRef]
- Chen, X.; Peng, Y. Managing clay minerals in froth flotation—A critical review. Miner. Process. Extr. Metall. Rev. 2018, 39, 289–307. [Google Scholar] [CrossRef]
- Jeldres, R.I.; Uribe, L.; Cisternas, L.A.; Gutierrez, L.; Leiva, W.H.; Valenzuela, J. The effect of clay minerals on the process of flotation of copper ores-A critical review. Appl. Clay Sci. 2019, 170, 57–69. [Google Scholar] [CrossRef]
- Liu, D.; Peng, Y. Understanding different roles of lignosulfonate in dispersing clay minerals in coal flotation using deionised water and saline water. Fuel 2015, 142, 235–242. [Google Scholar] [CrossRef]
- Yu, Y.; Ma, L.; Cao, M.; Liu, Q. Slime coatings in froth flotation: A review. Miner. Eng. 2017, 114, 26–36. [Google Scholar] [CrossRef]
- Farrokhpay, S.; Ndlovu, B. Effect of phyllosilicate minerals on the rheology, colloidal and flotation behaviour of chalcopyrite mineral. In Chemeca 2013: Challenging Tomorrow; Engineers Australia: Barton, ACT, Australia, 2013; pp. 733–739. [Google Scholar]
- Ndlovu, B.; Forbes, E.; Farrokhpay, S.; Becker, M.; Bradshaw, D.; Deglon, D. A preliminary rheological classification of phyllosilicate group minerals. Miner. Eng. 2014, 55, 190–200. [Google Scholar] [CrossRef]
- Peng, Y.; Zhao, S. The effect of surface oxidation of copper sulfide minerals on clay slime coating in flotation. Miner. Eng. 2011, 24, 1687–1693. [Google Scholar] [CrossRef]
- Zhao, S.; Peng, Y. The oxidation of copper sulfide minerals during grinding and their interactions with clay particles. Powder Technol. 2012, 230, 112–117. [Google Scholar] [CrossRef]
- Holuszko, M.; Franzidis, J.; Manlapig, E.; Hampton, M.; Donose, B.; Nguyen, A. The effect of surface treatment and slime coatings on ZnS hydrophobicity. Miner. Eng. 2008, 21, 958–966. [Google Scholar] [CrossRef]
- Cruz, N.; Peng, Y.; Farrokhpay, S.; Bradshaw, D. Interactions of clay minerals in copper–gold flotation: Part 1—Rheological properties of clay mineral suspensions in the presence of flotation reagents. Miner. Eng. 2013, 50, 30–37. [Google Scholar] [CrossRef]
- WAng, L.; Peng, Y.; Runge, K.; Bradshaw, D. A review of entrainment: Mechanisms, contributing factors and modelling in flotation. Miner. Eng. 2015, 70, 77–91. [Google Scholar] [CrossRef]
- Hajati, A.; Shafaei, S.; Noaparast, M.; Farrokhpay, S.; Aslani, S. Novel application of talc nanoparticles as collector in flotation. RSC Adv. 2016, 6, 98096–98103. [Google Scholar] [CrossRef]
- Lagaly, G.; van Olphen, H. An Introduction to Clay Colloid Chemistry, 2nd Ed. John Wiley & Sons, New York, London, Sydney, Toronto 1977. 318 Seiten. Ber. Bunsenges. Phys. Chem. 1978, 82, 236–237. [Google Scholar] [CrossRef]
- Zhang, M.; Peng, Y. Effect of clay minerals on pulp rheology and the flotation of copper and gold minerals. Miner. Eng. 2015, 70, 8–13. [Google Scholar] [CrossRef]
- Kau, P.; Smith, D.; Binning, P. Experimental sorption of fluoride by kaolinite and bentonite. Geoderma 1998, 84, 89–108. [Google Scholar] [CrossRef]
- Du, J.; Morris, G.; Pushkarova, R.A.; St. C. Smart, R. Effect of surface structure of kaolinite on aggregation, settling rate, and bed density. Langmuir 2010, 26, 13227–13235. [Google Scholar] [CrossRef] [PubMed]
- Norrish, K. The swelling of montmorillonite. Discuss. Faraday Soc. 1954, 18, 120–134. [Google Scholar] [CrossRef]
- Goh, R.; Leong, Y.K.; Lehane, B. Bentonite slurries—Zeta potential, yield stress, adsorbed additive and time-dependent behaviour. Rheol. Acta 2011, 50, 29–38. [Google Scholar] [CrossRef]
- Liu, J.; Zhou, Z.; Xu, Z.; Masliyah, J. Bitumen–clay interactions in aqueous media studied by zeta potential distribution measurement. J. Colloid Interface Sci. 2002, 252, 409–418. [Google Scholar] [CrossRef]
- Fornasiero, D.; Ralston, J. Cu(II) and Ni(II) activation in the flotation of quartz, lizardite and chlorite. Int. J. Miner. Process. 2005, 76, 75–81. [Google Scholar] [CrossRef]
- Uribe, L.; Gutierrez, L.; Laskowski, J.S.; Castro, S. Role of calcium and magnesium cations in the interactions between kaolinite and chalcopyrite in seawater. Physicochem. Probl. Miner. Process. 2017, 53, 737–749. [Google Scholar] [CrossRef]
- Arnold, B.; Aplan, F. The effect of clay slimes on coal flotation, part I: The nature of the clay. Int. J. Miner. Process. 1986, 17, 225–242. [Google Scholar] [CrossRef]
- Attia, Y.A.; Deason, D. Control of slimes coating in mineral suspensions. Colloids Surf. 1989, 39, 227–238. [Google Scholar] [CrossRef]
- Wei, R.; Peng, Y.; Seaman, D. The interaction of lignosulfonate dispersants and grinding media in copper–gold flotation from a high clay ore. Miner. Eng. 2013, 50, 93–98. [Google Scholar] [CrossRef]
- Liu, D.; Peng, Y. Reducing the entrainment of clay minerals in flotation using tap and saline water. Powder Technol. 2014, 253, 216–222. [Google Scholar] [CrossRef]
- Pawlik, M.; Laskowski, J.; Ansari, A. Effect of carboxymethyl cellulose and ionic strength on stability of mineral suspensions in potash ore flotation systems. J. Colloid Interface Sci. 2003, 260, 251–258. [Google Scholar] [CrossRef] [PubMed]
- Edwards, C.; Kipkie, W.; Agar, G. The effect of slime coatings of the serpentine minerals, chrysotile and lizardite, on pentlandite flotation. Int. J. Miner. Process. 1980, 7, 33–42. [Google Scholar] [CrossRef]
- Seaman, D.; Lauten, R.; Kluck, G.; Stoitis, N. Usage of anionic dispersants to reduce the impact of clay particles in flotation of copper and gold at the Telfer mine. In Proceedings of the 11th Mill Operators’ Conference 2012, Hobart, TAS, Australia, 29–31 October 2012. [Google Scholar]
- Dong, X.; Marway, H.S.; Cranston, E.D.; Pelton, R.H. Relating nanoparticle shape and adhesiveness to performance as flotation collectors. Ind. Eng. Chem. Res. 2016, 55, 9633–9638. [Google Scholar] [CrossRef]
- Yang, S. Nanoparticle Flotation Collectors. Ph.D. Thesis, McMaster University, Hamilton, ON, Canada, 2012. [Google Scholar]
- Yang, S.; Pelton, R.; Raegen, A.; Montgomery, M.; Dalnoki-Veress, K. Nanoparticle Flotation Collectors: Mechanisms Behind a New Technology. Langmuir 2011, 27, 10438–10446. [Google Scholar] [CrossRef] [PubMed]
- Murga, R.; Rodriguez, C.; Amalraj, J.; Vega-Garcia, D.; Gutierrez, L.; Uribe, L. Use of Polystyrene Nanoparticles as Collectors in the Flotation of Chalcopyrite. Polymers 2022, 14, 5259. [Google Scholar] [CrossRef]
- Ma, Y.-W.; Han, Y.-X.; Zhu, Y.-M.; Li, Y.-J.; Hao, L. Flotation behaviors and mechanisms of chalcopyrite and galena after cyanide treatment. Trans. Nonferrous Met. Soc. China 2016, 26, 3245–3252. [Google Scholar] [CrossRef]
- Delgado, A.; Gonzalez-Caballero, F.; Bruque, J.M. On the zeta potential and surface charge density of montmorillonite in aqueous electrolyte solutions. J. Colloid Interface Sci. 1986, 113, 203–211. [Google Scholar] [CrossRef]
- Au, P.-I.; Leong, Y.-K. Surface chemistry and rheology of slurries of kaolinite and montmorillonite from different sources. KONA Powder Part. J. 2016, 33, 17–32. [Google Scholar] [CrossRef]
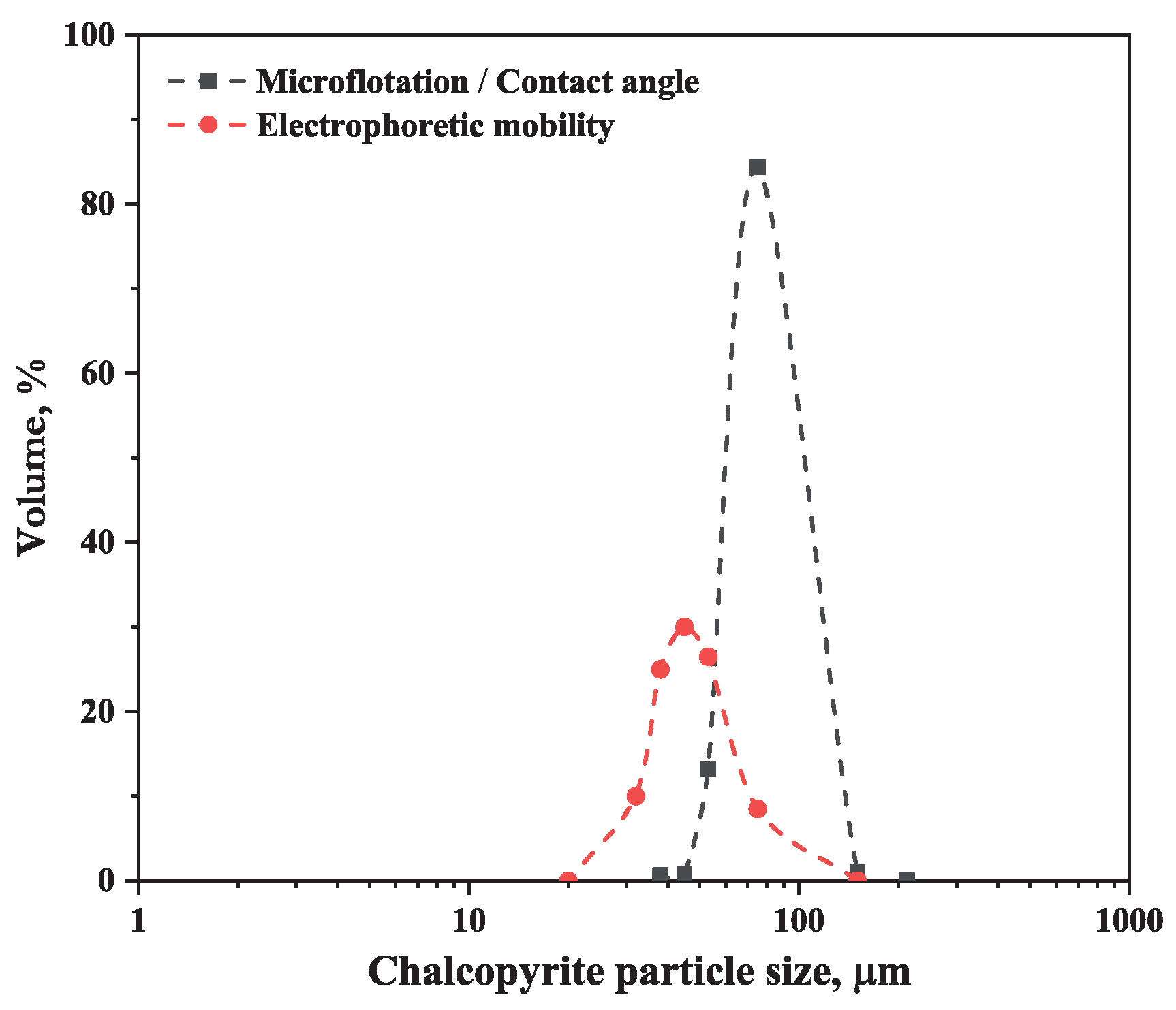
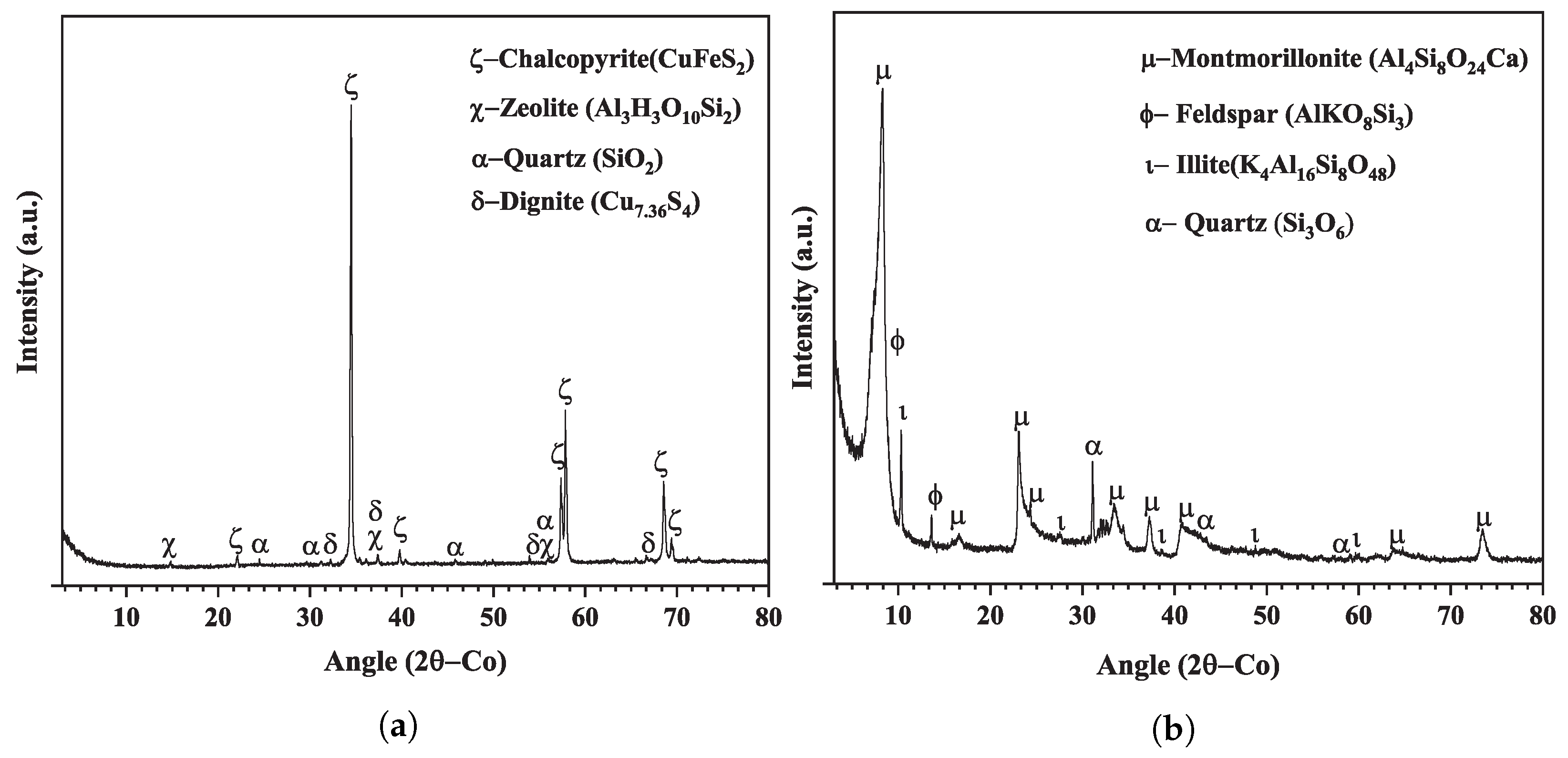
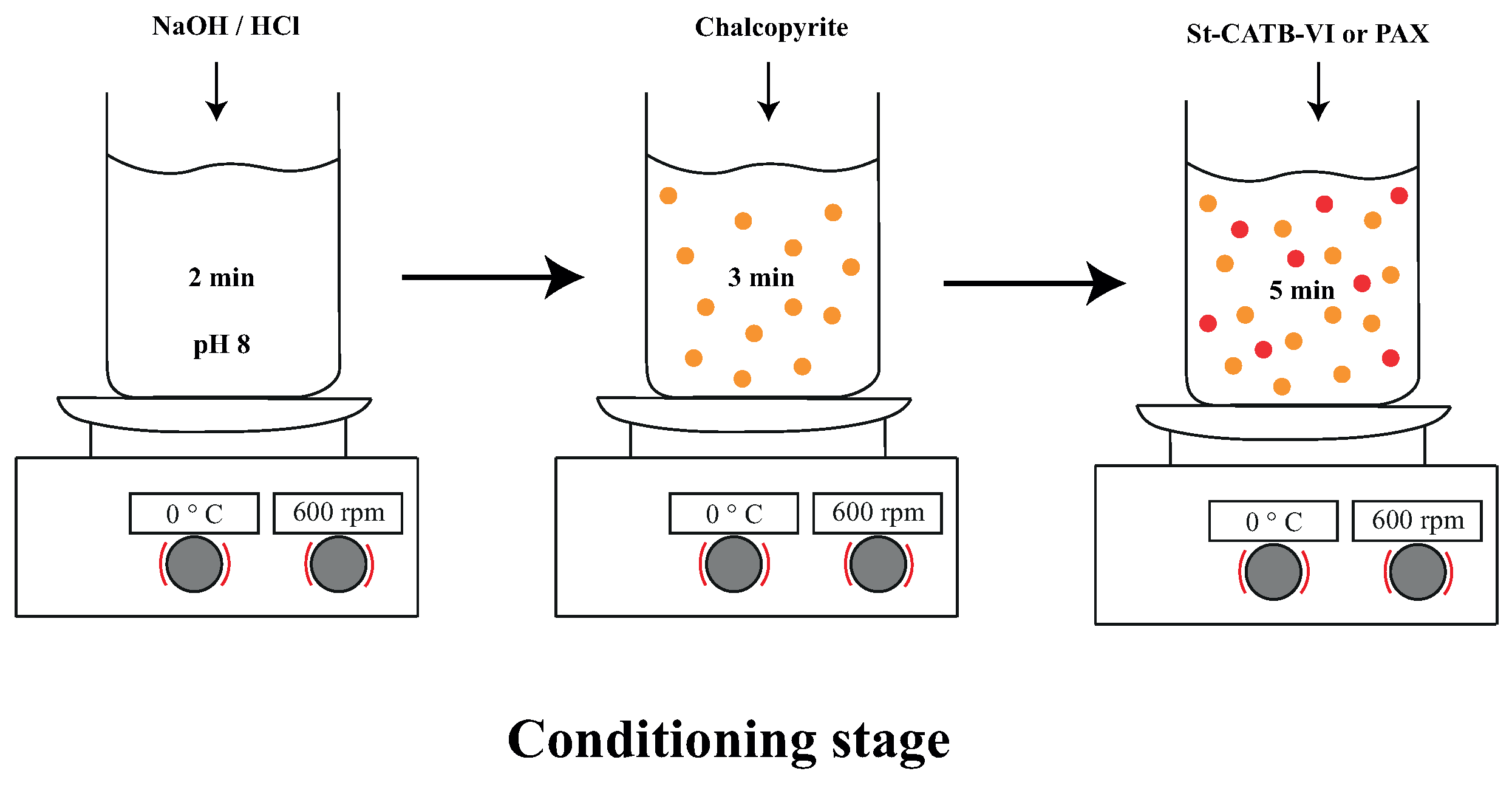
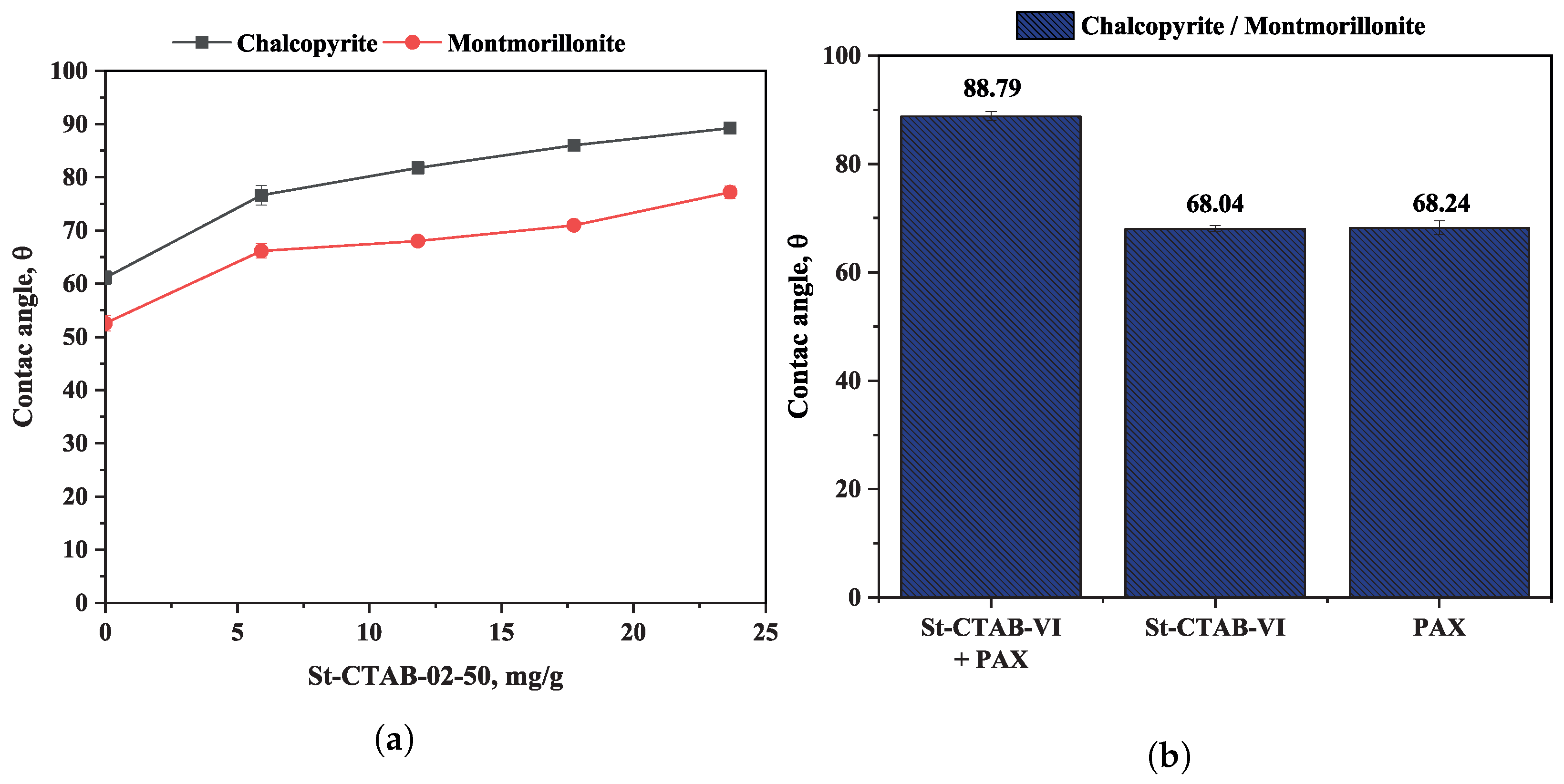
| Test | 150–75 μm, % | 75–45 μm, % | 45–32 μm, % |
|---|---|---|---|
| Microflotation/contact angle | 85.37 | 13.98 | 0.64 |
| Zeta potential | 8.50 | 46.47 | 45.03 |
| Chalcopyrite Sample | Montmorillonite Sample | ||||
|---|---|---|---|---|---|
| Mineral Phase | ICSD or COD | Percentage [%] | Mineral Phase | ICSD or COD | Percentage [%] |
| Chalcopyrite | ICSD-028894 | 93.0 | Montmorillonite | COD-9002779 | 81.0 |
| Zeolite | ICSD-082500 | 4.0 | Quartz | COD-9012600 | 6.0 |
| Quartz | ICSD-200722 | 1.0 | Feldspar | ICSD-083532 | 10.0 |
| Dignite | COD-9016668 | 2.0 | Illite | COD-1010318 | 3.0 |
| Value | |
|---|---|
| Hydrodynamic diameter, nm (PI *, %) | 75.81 (20.89) |
| ; ; , nm | 28.98; 43.90; 81.04 |
| Mobility, μm·cm/Vs | 3.90 |
| Zeta potential, mV | 50.00 ± 1.26 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Estrada, D.; Murga, R.; Rubilar, O.; Amalraj, J.; Gutierrez, L.; Uribe, L. On the Use of Styrene-Based Nanoparticles to Mitigate the Effect of Montmorillonite in Copper Sulfide Recovery by Flotation. Polymers 2024, 16, 1682. https://doi.org/10.3390/polym16121682
Estrada D, Murga R, Rubilar O, Amalraj J, Gutierrez L, Uribe L. On the Use of Styrene-Based Nanoparticles to Mitigate the Effect of Montmorillonite in Copper Sulfide Recovery by Flotation. Polymers. 2024; 16(12):1682. https://doi.org/10.3390/polym16121682
Chicago/Turabian StyleEstrada, Darwin, Romina Murga, Olga Rubilar, John Amalraj, Leopoldo Gutierrez, and Lina Uribe. 2024. "On the Use of Styrene-Based Nanoparticles to Mitigate the Effect of Montmorillonite in Copper Sulfide Recovery by Flotation" Polymers 16, no. 12: 1682. https://doi.org/10.3390/polym16121682
APA StyleEstrada, D., Murga, R., Rubilar, O., Amalraj, J., Gutierrez, L., & Uribe, L. (2024). On the Use of Styrene-Based Nanoparticles to Mitigate the Effect of Montmorillonite in Copper Sulfide Recovery by Flotation. Polymers, 16(12), 1682. https://doi.org/10.3390/polym16121682







