Clay Tailings Flocculated in Seawater and Industrial Water: Analysis of Aggregates, Sedimentation, and Supernatant Quality
Abstract
1. Introduction
2. Materials and Methods
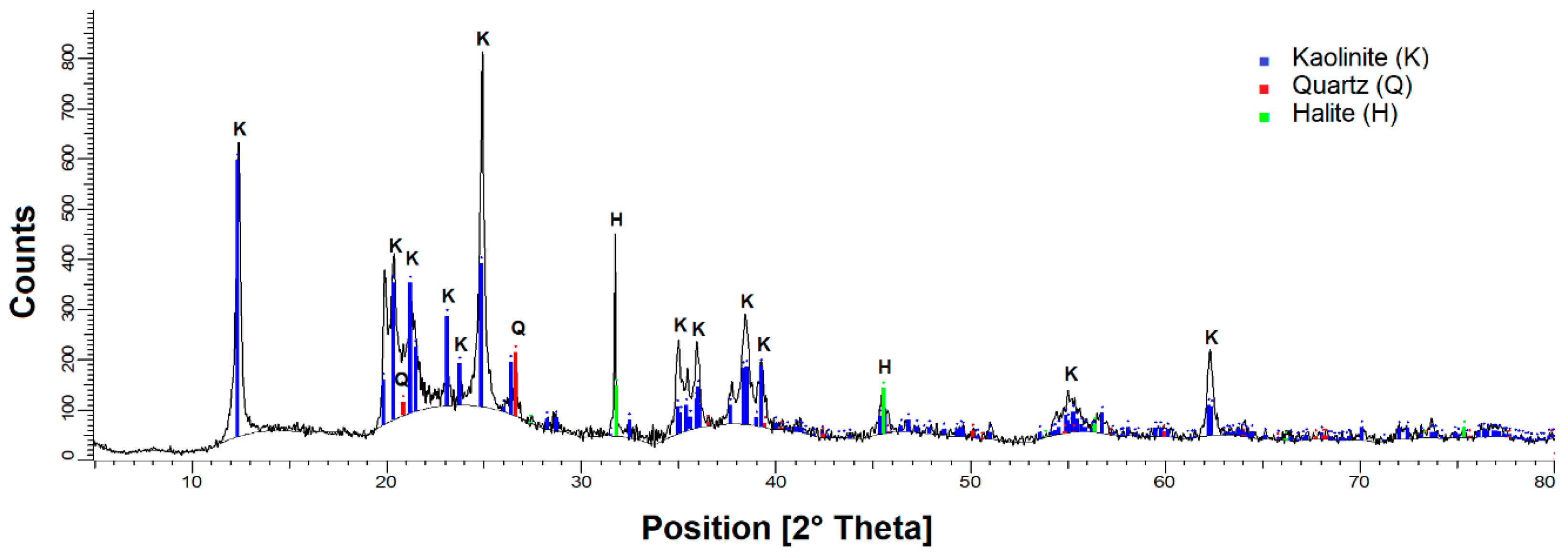
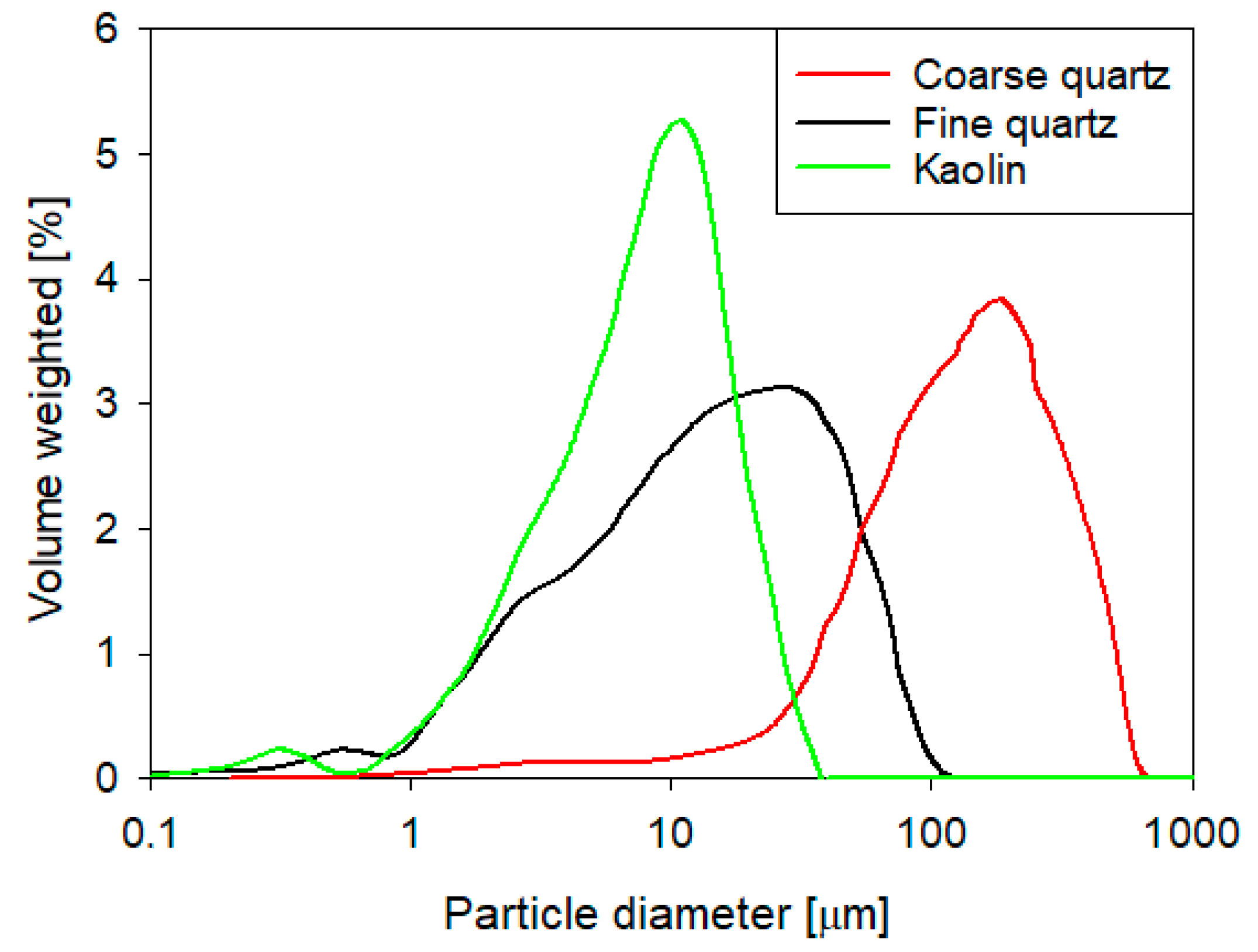
2.1. Materials
2.2. Flocculation Kinetics
2.3. Sedimentation
2.4. Fractal Dimension
- : hindered settling rate, m s−1.
- : aggregate size (floc), m.
- : size of the primary particle (before addition of flocculant), m.
- : densities of the solid and liquid phases, respectively, kg m−3.
- : gravity acceleration (9.81 m s−2).
- : fluid viscosity, N s m−2..
- : volumetric concentration of the solids.
- : mass length fractal dimension.
3. Results and Discussion
3.1. Clay Tailing Flocculation Kinetics
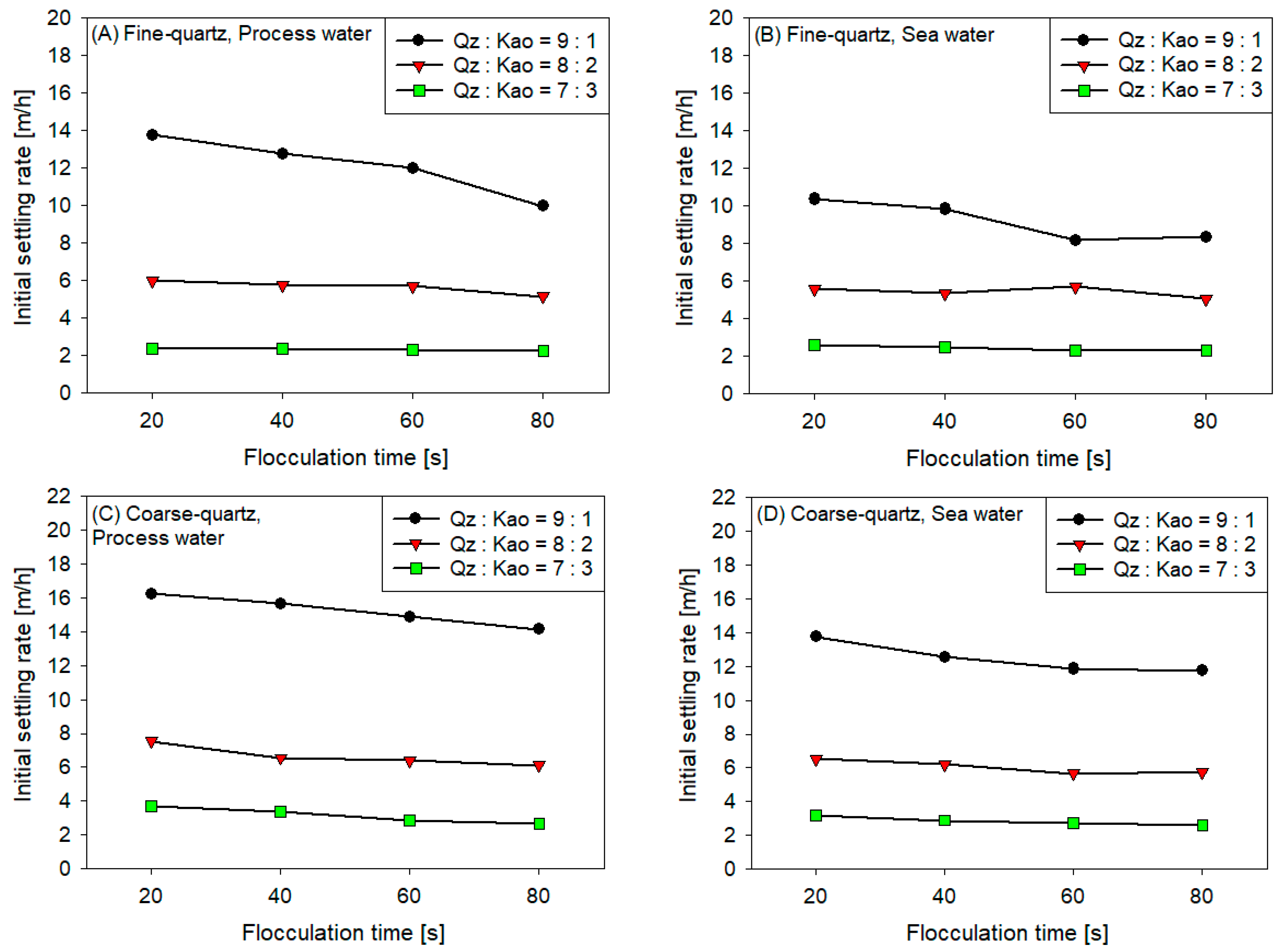
3.2. Initial Settling Rates
- The sedimentation rate decreased with the increase in flocculation time for all coarse quartz-based tailings conditions (Figure 4C,D). However, for fine quartz-based tailings, the trend is only clear for tailings of Qz:Kao = 9:1. For tailings with higher kaolin contents, the sedimentation rate tends to be constant with flocculation time. The flocculation kinetics (Figure 3) revealed that the maximum size of the flocs was reached in the first 10–20 s. Subsequently, the destruction of the flocs was superimposed on the aggregation rate, resulting in smaller flocs as the mixing time was prolonged; consequently, smaller flocs produced a lower settling rate.
- The settling rate was clearly affected by the increase in the kaolin content in the tailings. Flocculation kinetics indicated a larger size of the flocs for the tailings with a higher kaolin content, which indicated an open and irregular structure because of the laminated morphology of the clay that, when associated, generates larger but mostly porous aggregates that ultimately prejudice the settling rate.
- The settling rate was slightly faster for tailings with process water than with seawater; the flocculation kinetics revealed that the size of the flocs was slightly larger in process water than in seawater, resulting in a higher sedimentation velocity for tailings with the first type of water.
- The settling rate was higher for tailings with coarse quartz than with fine quartz; with the same explanation as in the previous case, the flocculation kinetics revealed a larger floc size for coarse quartz.
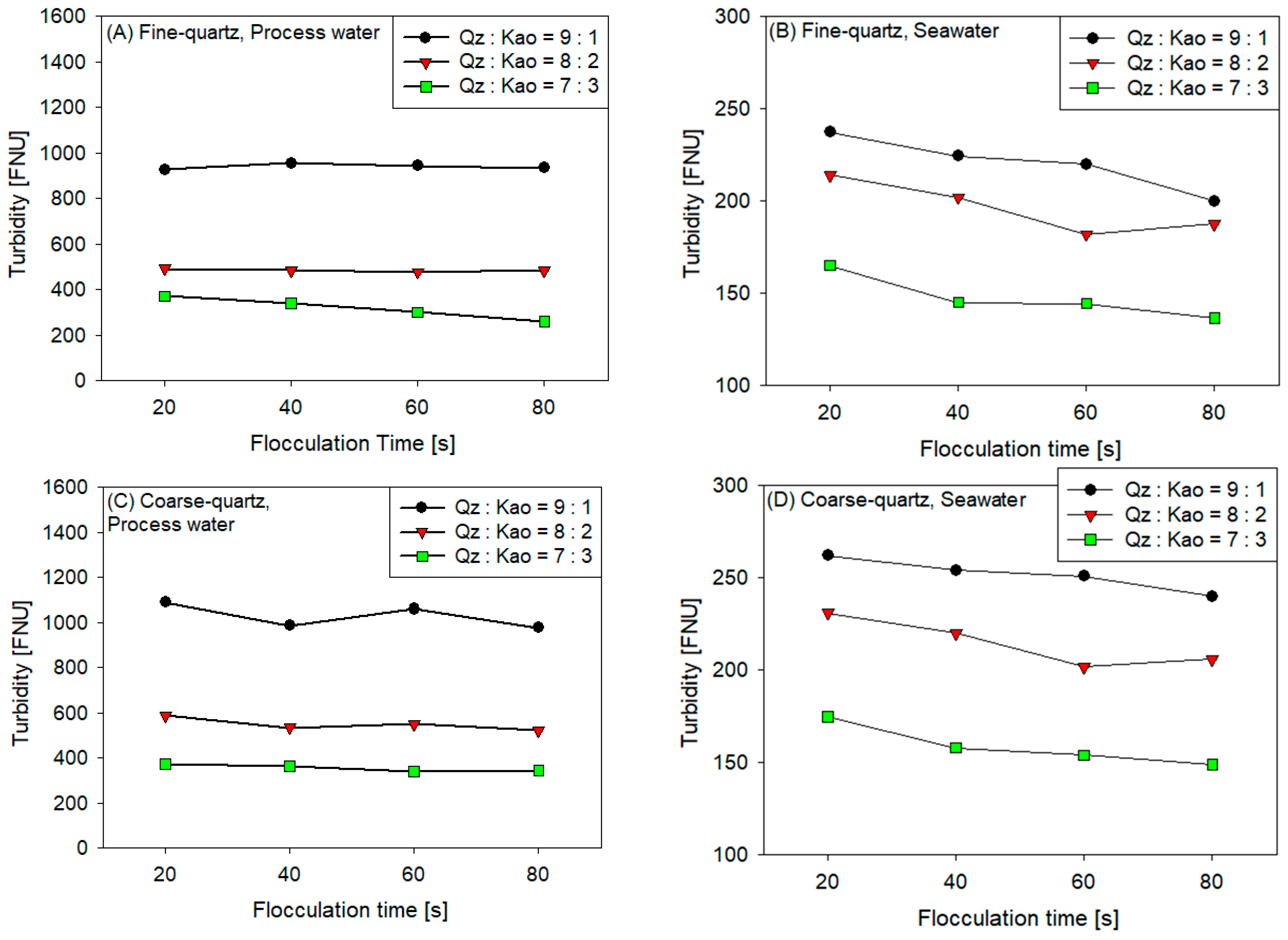
3.3. Turbidity
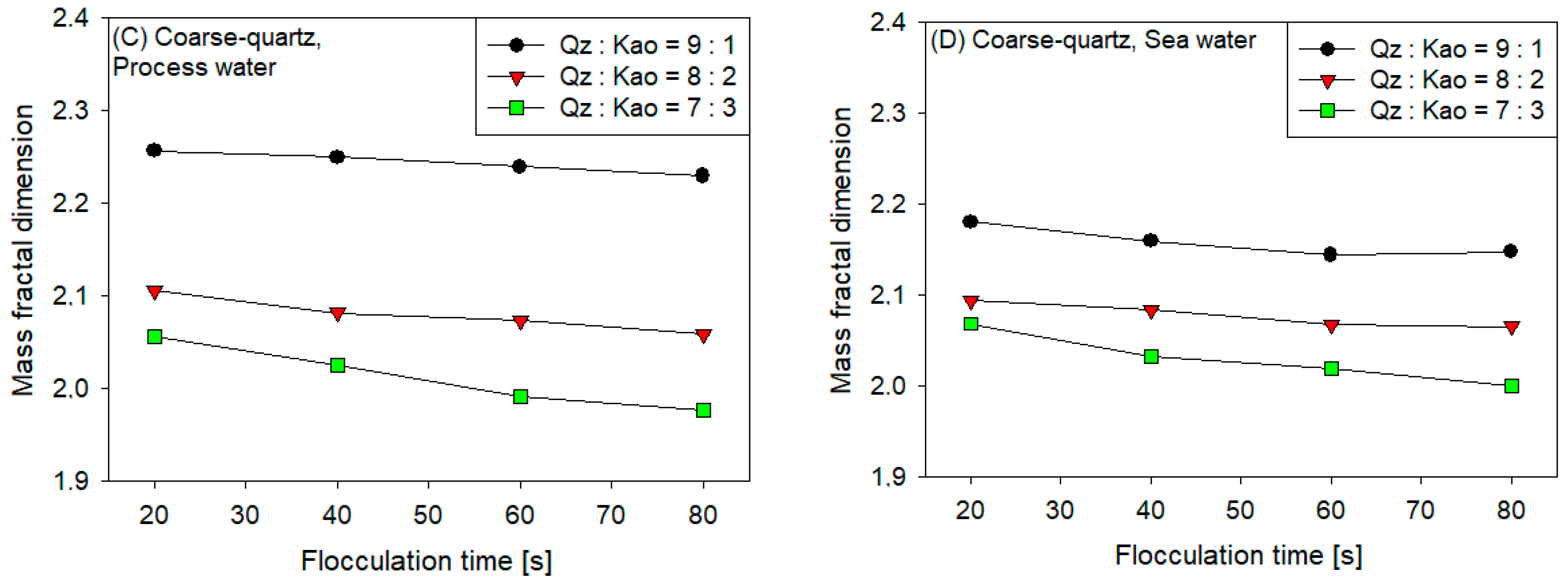
3.4. Fractal Dimension
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Du Toit, T.; Crozie, M. Khumani Iron Ore Mine Paste Disposal and Water Recovery System. J. S. Afr. Inst. Min. Metall. 2012, 112, 243–258. [Google Scholar]
- Deniz, V. Dewatering of barite clay wastewater by inorganic coagulants and co-polymer flocculants. Physicochem. Probl. Miner. Process 2015, 51, 351–364. [Google Scholar] [CrossRef]
- Blanco, A.; Fuente, E.; Negro, C.; Tijero, J. Flocculation Monitoring: Focused Beam Reflectance Measurement as a Measurement Tool. Can. J. Chem. Eng. 2002, 80, 1–7. [Google Scholar] [CrossRef]
- Mpofu, P.; Addai-Mensah, J.; Ralston, J. Influence of hydrolyzable metal ions on the interfacial chemistry, particle interactions, and dewatering behavior of kaolinite dispersions. J. Colloid Interface Sci. 2003, 261, 349–359. [Google Scholar] [CrossRef] [PubMed]
- Penner, D.; Lagaly, G. Influence of Organic and Inorganic Salts on the Coagulation of Montmorillonite Dispersions. Clays Clay Miner. 2000, 48, 246–255. [Google Scholar] [CrossRef]
- Vajihinejad, V.; Gumfekar, S.P.; Bazoubandi, B.; Najafabadi, Z.R.; Soares, J.B.P.; Vajihinejad, V.; Gumfekar, S.P.; Bazoubandi, B.; Rostami, Z.; Soares, N.B.P. Water Soluble Polymer Flocculants: Synthesis, Characterization, and Performance Assessment. Macromol. Mater. Eng. 2019, 304, 1800526. [Google Scholar] [CrossRef]
- Fawell, P. Solid–Liquid Separation of Clay Tailings. In Clays in the Minerals Processing Value Chain; Cambridge University Press: Cambridge, UK, 2017; pp. 327–380. [Google Scholar]
- Bulatovic, S.M.; Wyslouzil, D.M.; Kant, C. Effect of Clay Slimes on Copper, Molybdenum Flotation from Porphyry Ores. In Proceedings of the Copper 99-Cober 99 International Conference, Phoenix, AZ, USA, 10–13 October 1999; pp. 95–111. [Google Scholar]
- Ndlovu, B.; Farrokhpay, S.; Bradshaw, D. The Effect of Phyllosilicate Minerals on Mineral Processing Industry. Int. J. Miner. Process 2013, 125, 149–156. [Google Scholar] [CrossRef]
- De Kretser, R.; Scales, P.J.; Boger, D.V. Improving Clay-Based Tailings Disposal: Case Study on Coal Tailings. AIChE J. 1997, 43, 1894–1903. [Google Scholar] [CrossRef]
- Schofield, R.K.; Samson, H.R. Flocculation of Kaolinite Due to the Attraction of Oppositely Charged Crystal Faces. Discuss. Faraday Soc. 1954, 18, 135–145. [Google Scholar] [CrossRef]
- Zbik, M.S.; Smart, R.S.C.; Morris, G.E. Kaolinite Flocculation Structure. J. Colloid Interface Sci. 2008, 328, 73–80. [Google Scholar] [CrossRef]
- Ndlovu, B.; Forbes, E.; Farrokhpay, S.; Becker, M.; Bradshaw, D.; Deglon, D. A Preliminary Rheological Classification of Phyllosilicate Group Minerals. Miner. Eng. 2014, 55, 190–200. [Google Scholar] [CrossRef]
- Nasser, M.S.; James, A.E. Settling and Sediment Bed Behaviour of Kaolinite in Aqueous Media. Sep. Purif. Technol. 2006, 51, 10–17. [Google Scholar] [CrossRef]
- Jeldres, R.I.; Piceros, E.C.; Leiva, W.H.; Toledo, P.G.; Herrera, N. Viscoelasticity and Yielding Properties of Flocculated Kaolinite Sediments in Saline Water. Colloids Surf. A Physicochem. Eng. Asp. 2017, 529, 1009–1015. [Google Scholar] [CrossRef]
- Mierczynska-Vasilev, A.; Kor, M.; Addai-Mensah, J.; Beattie, D.A. The Influence of Polymer Chemistry on Adsorption and Flocculation of Talc Suspensions. Chem. Eng. J. 2013, 220, 375–382. [Google Scholar] [CrossRef]
- Vahedi, A.; Gorczyca, B. Predicting the Settling Velocity of Flocs Formed in Water Treatment Using Multiple Fractal Dimensions. Water Res. 2012, 46, 4188–4194. [Google Scholar] [CrossRef] [PubMed]
- Kuroda, Y.; Nakaishi, K.; Adachi, Y. Settling Velocity and Structure of Kaolinite Floc in Sodium Chloride Solution. Clay Sci. 2003, 12, 103–107. [Google Scholar]
- Gregory, J. Monitoring Particle Aggregation Processes. Adv. Colloid Interface Sci. 2009, 147–148, 109–123. [Google Scholar] [CrossRef] [PubMed]
- He, W.; Nan, J.; Li, H.; Li, S. Characteristic Analysis on Temporal Evolution of Floc Size and Structure in Low-Shear Flow. Water Res. 2012, 46, 509–520. [Google Scholar] [CrossRef]
- Spicer, P.T.; Pratsinis, S.E. Shear-Induced Flocculation: The Evolution of Floc Structure and the Shape of the Size Distribution at Steady State. Water Res. 1996, 30, 1049–1056. [Google Scholar] [CrossRef]
- Logan, B.E.; Kilps, J.R. Fractal Dimensions of Aggregates Formed in Different Fluid Mechanical Environments. Water Res. 1995, 29, 443–453. [Google Scholar] [CrossRef]
- Xu, W.; Gao, B.; Yue, Q.; Wang, Y. Effect of Shear Force and Solution PH on Flocs Breakage and Re-Growth Formed by Nano-Al13 Polymer. Water Res. 2010, 44, 1893–1899. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Gao, B.; Yue, Q.; Bo, X. Influence of PH on Flocs Formation, Breakage and Fractal Properties—The Role of Al 13 Polymer. J. Water Sustain. 2011, 1, 45–57. [Google Scholar]
- Oles, V. Shear-Induced Aggregation and Breakup of Polystyrene Latex Particles. J. Colloid Interface Sci. 1992, 154, 351–358. [Google Scholar] [CrossRef]
- Selomulya, C.; Bushell, G.; Amal, R.; Waite, T.D. Understanding the Role of Restructuring in Flocculation: The Application of a Population Balance Model. Chem. Eng. Sci. 2003, 58, 327–338. [Google Scholar] [CrossRef]
- Bubakova, P.; Pivokonsky, M.; Filip, P. Effect of Shear Rate on Aggregate Size and Structure in the Process of Aggregation and at Steady State. Powder Technol. 2013, 235, 540–549. [Google Scholar] [CrossRef]
- Guérin, L.; Frances, C.; Liné, A.; Coufort-Saudejaud, C. Fractal Dimensions and Morphological Characteristics of Aggregates Formed in Different Physico-Chemical and Mechanical Flocculation Environments. Colloids Surf. A Physicochem. Eng. Asp. 2019, 560, 213–222. [Google Scholar] [CrossRef]
- Deng, X.; Davé, R.N. Breakage of Fractal Agglomerates. Chem. Eng. Sci. 2017, 161, 117–126. [Google Scholar] [CrossRef]
- Chakraborti, R.K.; Gardner, K.H.; Atkinson, J.F.; Van Benschoten, J.E. Changes in Fractal Dimension during Aggregation. Water Res. 2003, 37, 873–883. [Google Scholar] [CrossRef]
- Spicer, P.T.; Pratsinis, S.E.; Raper, J.; Amal, R.; Bushell, G.; Meesters, G. Effect of Shear Schedule on Particle Size, Density, and Structure during Flocculation in Stirred Tanks. Powder Technol. 1998, 97, 26–34. [Google Scholar] [CrossRef]
- Lopez-Exposito, P.; Negro, C.; Blanco, A. Direct Estimation of Microalgal Flocs Fractal Dimension through Laser Reflectance and Machine Learning. Algal Res. 2019, 37, 240–247. [Google Scholar] [CrossRef]
- Serra, T.; Casamitjana, X. Structure of the Aggregates during the Process of Aggregation and Breakup Under a Shear Flow. J. Colloid Interface Sci. 1998, 206, 505–511. [Google Scholar] [CrossRef]
- Mobin, M.; Shabnam, H. Corrosion Behavior of Mild Steel and SS 304L in Presence of Dissolved Nickel under Aerated and Deaerated Conditions. Mater. Res. 2011, 14, 524–531. [Google Scholar] [CrossRef]
- Heath, A.R.; Bahri, P.A.; Fawell, P.D.; Farrow, J.B. Polymer Flocculation of Calcite: Relating the Aggregate Size to the Settling Rate. AIChE J. 2006, 52, 1987–1994. [Google Scholar] [CrossRef]
- Leiva, W.H.; Piceros, E.; Robles, P.; Jeldres, R.I. Impact of Hydrodynamic Conditions on the Structure of Clay-Based Tailings Aggregates Flocculated in Freshwater and Seawater. Miner. Eng. 2022, 176, 107313. [Google Scholar] [CrossRef]
- Leiva, W.H.; Fawell, P.D.; Goñi, C.; Toro, N.; Jeldres, R.I. Temporal Evolution of the Structure of Tailings Aggregates Flocculated in Seawater. Miner. Eng. 2021, 160, 106708. [Google Scholar] [CrossRef]
- Ramos, J.J.; Leiva, W.H.; Castillo, C.N.; Ihle, C.F.; Fawell, P.D.; Jeldres, R.I. Seawater Flocculation of Clay-Based Mining Tailings: Impact of Calcium and Magnesium Precipitation. Miner. Eng. 2020, 154, 106417. [Google Scholar] [CrossRef]
- Quezada, G.R.; Ayala, L.; Leiva, W.H.; Toro, N.; Toledo, P.G.; Robles, P.; Jeldres, R.I. Describing Mining Tailing Flocculation in Seawater by Population Balance Models: Effect of Mixing Intensity. Metals 2020, 10, 240. [Google Scholar] [CrossRef]
- Jeldres, M.; Ayala, L.; Robles, P.; Gálvez, E.; Leiva, W.H.; Toledo, P.G.; Jeldres, R.I. A Criterion for Estimating the Strength of Flocculated Aggregates in Salt Solutions. Minerals 2021, 11, 713. [Google Scholar] [CrossRef]
- Jeldres, M.; Piceros, E.C.; Toro, N.; Torres, D.; Robles, P.; Leiva, W.H.; Jeldres, R.I. Copper Tailing Flocculation in Seawater: Relating the Yield Stress with Fractal Aggregates at Varied Mixing Conditions. Metals 2019, 9, 1295. [Google Scholar] [CrossRef]
- Hu, Y.; Wei, S.; Hao, J.; Miller, J.D.; Fa, K. The Anomalous Behavior of Kaolinite Flotation with Dodecyl Amine Collector as Explained from Crystal Structure Considerations. Int. J. Miner. Process 2005, 76, 163–172. [Google Scholar] [CrossRef]
- Johnson, S.B.; Russell, A.S.; Scales, P.J. Volume Fraction Effects in Shear Rheology and Electroacoustic Studies of Concentrated Alumina and Kaolin Suspensions. Colloids Surf. A Physicochem. Eng. Asp. 1998, 141, 119–130. [Google Scholar] [CrossRef]
- Bickmore, B.R.; Nagy, K.L.; Sandlin, P.E.; Crater, T.S. Quantifying Surface Areas of Clays by Atomic Force Microscopy. Am. Mineral. 2002, 87, 780–783. [Google Scholar] [CrossRef]
- Brady, P.V.; Cygan, R.T.; Nagy, K.L. Molecular Controls on Kaolinite Surface Charge. J. Colloid Interface Sci. 1996, 183, 356–364. [Google Scholar] [CrossRef]
- Van Olphen, H. An Introduction to Clay Colloid Chemistry; John Wiley & Sons; Elsevier BV: New York, NY, USA, 1964; Volume 53. [Google Scholar]
- Götze, J. Chemistry, Textures and Physical Properties of Quartz—Geological Interpretation and Technical Application. Miner. Mag. 2009, 73, 645–671. [Google Scholar] [CrossRef]
- Castillo, C.; Ihle, C.F.; Jeldres, R.I. Chemometric Optimisation of a Copper Sulphide Tailings Flocculation Process in the Presence of Clays. Minerals 2019, 9, 582. [Google Scholar] [CrossRef]
- Jeldres, R.; Yang, T.; Costine, A.; Fawell, P.; Bellwood, J. The Impact of High Salinity and Seawater on Aggregate Structures in Clay Tailings Flocculation. In Proceedings of the Paste 2020: 23rd International Conference on Paste, Thickened and Filtered Tailings, Santiago, Chile, 2–6 November 2020. [Google Scholar] [CrossRef]
- Liu, D.; Edraki, M.; Berry, L. Investigating the Settling Behaviour of Saline Tailing Suspensions Using Kaolinite, Bentonite, and Illite Clay Minerals. Powder Technol. 2018, 326, 228–236. [Google Scholar] [CrossRef]
- Hogg, R. Collision Efficiency Factors for Polymer Flocculation. J. Colloid Interface Sci. 1984, 102, 232–236. [Google Scholar] [CrossRef]
- Hogg, R. The Role of Polymer Adsorption Kinetics in Flocculation. Colloids Surf. A Physicochem. Eng. Asp. 1999, 146, 253–263. [Google Scholar] [CrossRef]
- Grabsch, A.F.; Yahyaei, M.; Fawell, P.D. Number-Sensitive Particle Size Measurements for Monitoring Flocculation Responses to Different Grinding Conditions. Miner. Eng. 2020, 145, 106088. [Google Scholar] [CrossRef]
- Rulyov, N.N.; Korolyov, V.J.; Kovalchuk, N.M. Ultra Flocculation of Quartz Suspensions: Effects of Shear Rate, Particle Size Distribution and Solids Content. Miner. Process. Extr. Metall. 2009, 118, 175–181. [Google Scholar] [CrossRef]
- Nasser, M.S.; James, A.E. The Effect of Polyacrylamide Charge Density and Molecular Weight on the Flocculation and Sedimentation Behaviour of Kaolinite Suspensions. Sep. Purif. Technol. 2006, 52, 241–252. [Google Scholar] [CrossRef]
- Ofori, P.; Nguyen, A.V.; Firth, B.; McNally, C.; Ozdemir, O. Shear-Induced Floc Structure Changes for Enhanced Dewatering of Coal Preparation Plant Tailings. Chem. Eng. J. 2011, 172, 914–923. [Google Scholar] [CrossRef]
- Moruzzi, R.B.; de Oliveira, A.L.; da Conceição, F.T.; Gregory, J.; Campos, L.C. Fractal Dimension of Large Aggregates under Different Flocculation Conditions. Sci. Total Environ. 2017, 609, 807–814. [Google Scholar] [CrossRef] [PubMed]
- Li, X.Y.; Logan, B.E. Permeability of Fractal Aggregates. Water Res. 2001, 35, 3373–3380. [Google Scholar] [CrossRef] [PubMed]



| Component | Concentration (g/L) |
|---|---|
| NaCl | 24.53 |
| MgCl2 × 6H2O | 11.10 |
| Na2SO4 | 4.09 |
| CaCl2 | 1.16 |
| KCl | 0.69 |
| NaHCO3 | 0.20 |
| KBr | 0.10 |
| H3BO3 | 0.03 |
| Parameter | Magnitude |
|---|---|
| 9.81 | |
| 0.001021 | |
| 0.001077 | |
| 0.0413 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leiva, W.H.; Toro, N.; Robles, P.; Quezada, G.R.; Salazar, I.; Jeldres, R. Clay Tailings Flocculated in Seawater and Industrial Water: Analysis of Aggregates, Sedimentation, and Supernatant Quality. Polymers 2024, 16, 1441. https://doi.org/10.3390/polym16101441
Leiva WH, Toro N, Robles P, Quezada GR, Salazar I, Jeldres R. Clay Tailings Flocculated in Seawater and Industrial Water: Analysis of Aggregates, Sedimentation, and Supernatant Quality. Polymers. 2024; 16(10):1441. https://doi.org/10.3390/polym16101441
Chicago/Turabian StyleLeiva, Williams H., Norman Toro, Pedro Robles, Gonzalo R. Quezada, Iván Salazar, and Ricardo Jeldres. 2024. "Clay Tailings Flocculated in Seawater and Industrial Water: Analysis of Aggregates, Sedimentation, and Supernatant Quality" Polymers 16, no. 10: 1441. https://doi.org/10.3390/polym16101441
APA StyleLeiva, W. H., Toro, N., Robles, P., Quezada, G. R., Salazar, I., & Jeldres, R. (2024). Clay Tailings Flocculated in Seawater and Industrial Water: Analysis of Aggregates, Sedimentation, and Supernatant Quality. Polymers, 16(10), 1441. https://doi.org/10.3390/polym16101441









