Mechanochemical Recycling of Flexible Polyurethane Foam Scraps for Quantitative Replacement of Polyol Using Wedge-Block-Reinforced Extruder
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Samples
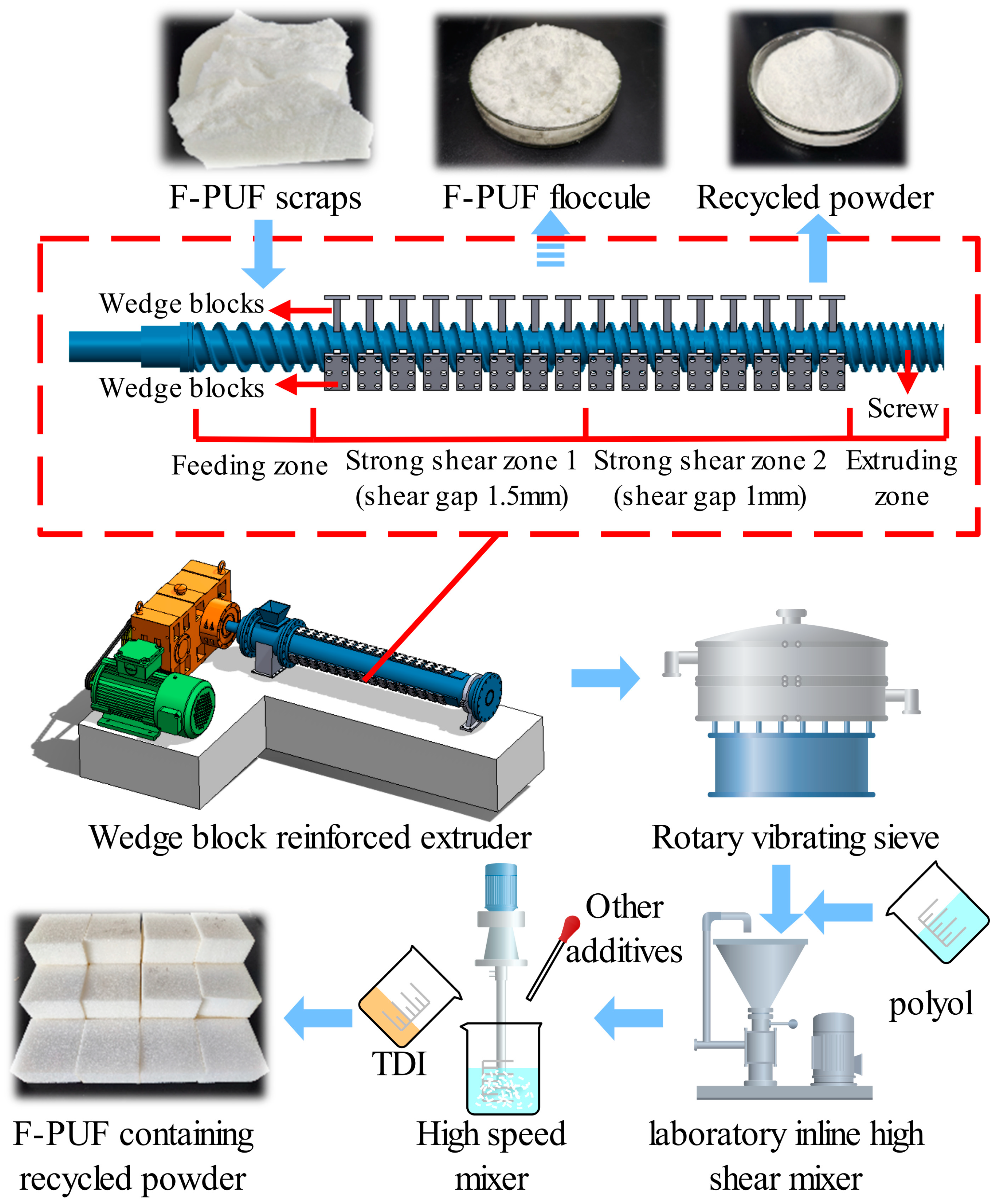
2.2.1. Preparation of Recycled F-PUF Powder
2.2.2. Mixing of Polyol and the Recycled Powder
2.2.3. Preparation of F-PUF Containing Recycled Powder
3. Characterization
3.1. Characterization of Recycled Powder
3.2. Characterization of Powder–Polyol Mixture
3.3. Characterization of F-PUF (Including the F-PUF Containing Recycled Powder and the Original F-PUF)
4. Results and Discussion
4.1. Effects of Room-Temperature Wedge-Block-Reinforced Extrusion Recycling Method on F-PUF
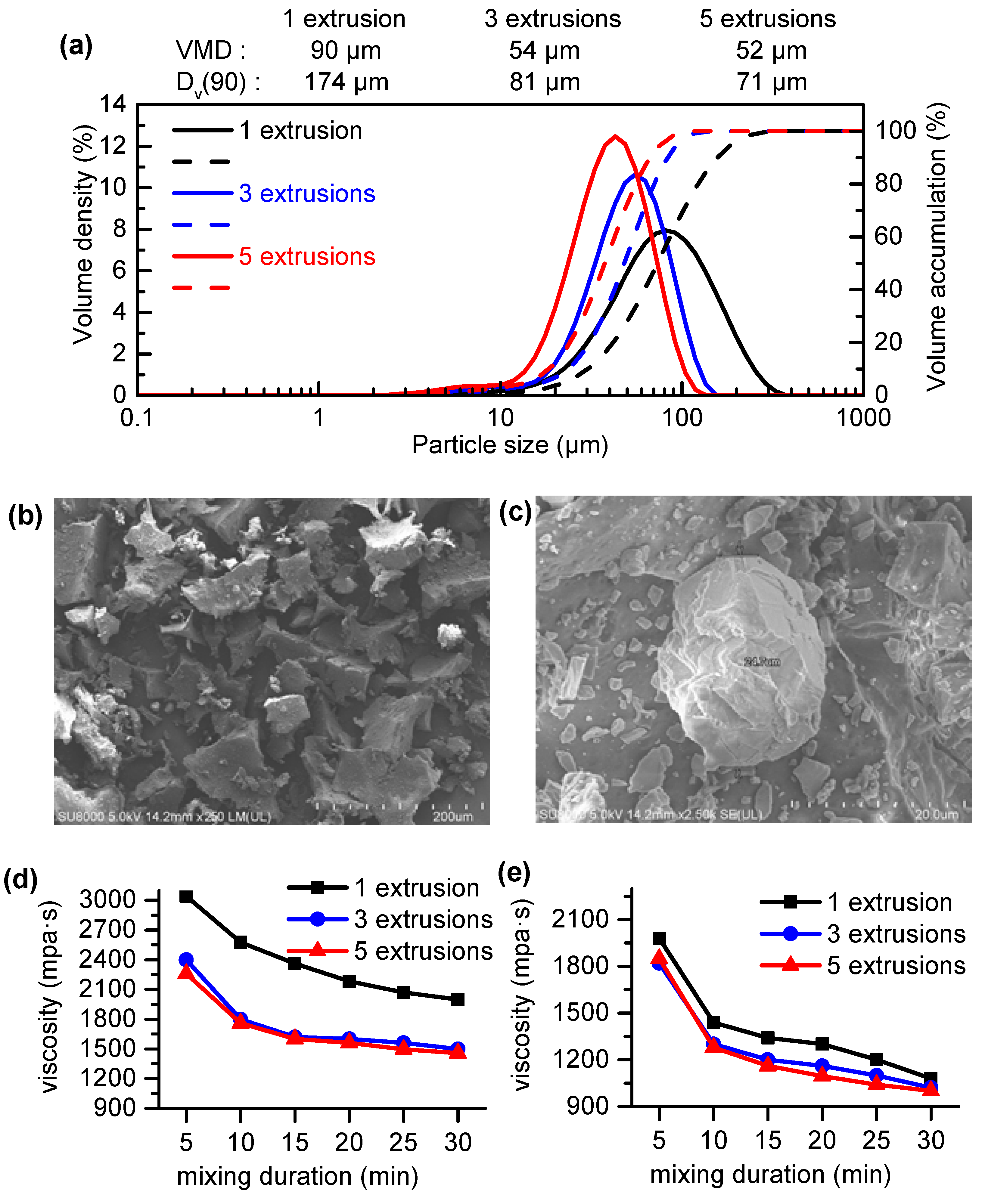
4.1.1. Mechanism and Characterization of Fine Pulverization
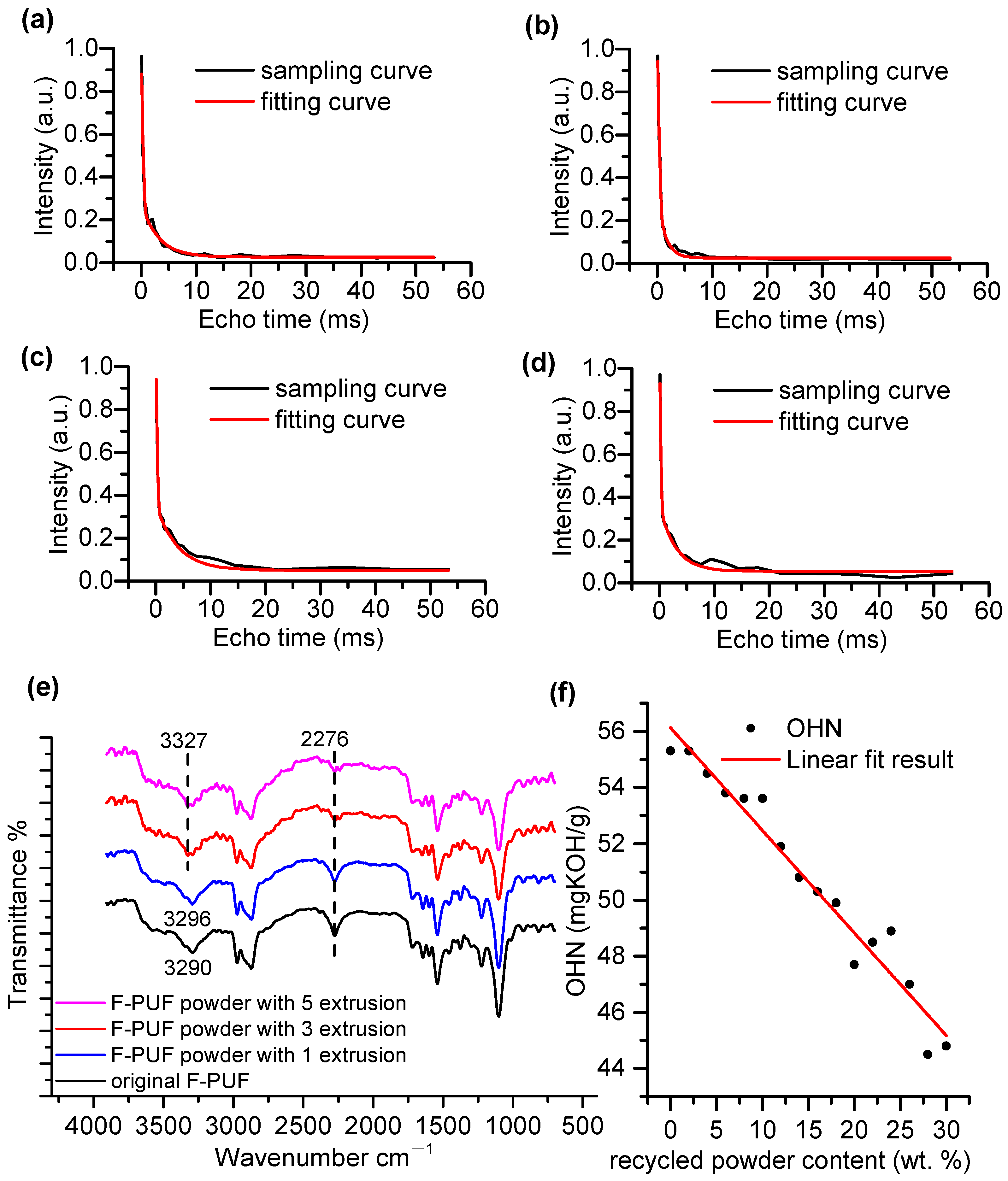
4.1.2. Mechanism and Characterization of Mechanochemical Effect
4.2. Properties of F-PUF Products
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Kemona, A.; Piotrowska, M. Polyurethane Recycling and Disposal: Methods and Prospects. Polymers 2020, 12, 1752. [Google Scholar] [CrossRef]
- Liu, J.; Sun, Z.; Wang, F.; Zhu, D.; Ge, J.; Su, H. Facile solvent-Free preparation of biobased rigid polyurethane foam from raw citric acid fermentation waste. Ind. Eng. Chem. Res. 2020, 59, 10308–10314. [Google Scholar] [CrossRef]
- Husainie, S.M.; Khattak, S.U.; Robinson, J.; Naguib, H.E. A comparative study on the mechanical properties of different natural fiber reinforced free-rise polyurethane foam composites. Ind. Eng. Chem. Res. 2020, 59, 21745–21755. [Google Scholar] [CrossRef]
- Nikje, M.M.A.; Garmarudi, A.B.; Idris, A.B. Polyurethane waste reduction and recycling: From bench to pilot scales. Des. Monomers Polym. 2011, 14, 395–421. [Google Scholar] [CrossRef]
- Mukherjee, M.; Gurusamy-Thangavelu, S.A.; Chelike, D.K.; Alagumalai, A.; Das, B.N.; Jaisankar, S.N.; Mandal, A.B. Biodegradable polyurethane foam as shoe insole to reduce footwear waste: Optimization by morphological physicochemical and mechanical properties. Appl. Surf. Sci. 2020, 499, 143966. [Google Scholar] [CrossRef]
- Szpiłyk, M.; Lubczak, R.; Lubczak, J. The biodegradable cellulose-derived polyol and polyurethane foam. Polym. Test. 2021, 100, 107250. [Google Scholar] [CrossRef]
- Gadhave, R.V.; Srivastava, S.; Mahanwar, P.A.; Gadekar, P.T. Recycling and disposal methods for polyurethane wastes: A review. Open J. Polym. Chem. 2019, 9, 39–51. [Google Scholar] [CrossRef]
- Zia, K.M.; Bhatti, H.N.; Bhatti, I.A. Methods for polyurethane and polyurethane composites, recycling and recovery: A review. React. Funct. Polym. 2007, 67, 675–692. [Google Scholar] [CrossRef]
- Magnin, A.; Entzmann, L.; Pollet, E.; Avérous, L. Breakthrough in polyurethane bio-recycling: An efficient laccase-mediated system for the degradation of different types of polyurethanes. Waste Manag. 2021, 132, 23–30. [Google Scholar] [CrossRef]
- Kanchanapiya, P.; Intaranon, N.; Tantisattayakul, T. Assessment of the economic recycling potential of a glycolysis treatment of rigid polyurethane foam waste: A case study from Thailand. J. Environ. Manag. 2021, 280, 111638. [Google Scholar] [CrossRef]
- Madbouly, S.A. Novel recycling processes for thermoset polyurethane foams. Curr. Opin. Green Sust. Chem. 2023, 42, 100835. [Google Scholar] [CrossRef]
- Motokucho, S.; Nakayama, Y.; Morikawa, H.; Nakatani, H. Environment-friendly chemical recycling of aliphatic polyurethanes by hydrolysis in a CO2-water system. J. Appl. Polym. Sci. 2018, 135, 45897. [Google Scholar] [CrossRef]
- Gewert, B.; Plassmann, M.M.; MacLeod, M. Pathways for degradation of plastic polymers floating in the marine environment. Environ. Sci.-Process. Impacts 2015, 17, 1513–1521. [Google Scholar] [CrossRef]
- Donadini, R.; Boaretti, C.; Lorenzetti, A.; Roso, M.; Penzo, D.; Dal Lago, E.; Modesti, M. Chemical Recycling of Polyurethane Waste via a Microwave-Assisted Glycolysis Process. ACS Omega 2023, 8, 4655–4666. [Google Scholar] [CrossRef] [PubMed]
- Simón, D.; Rodríguez, J.F.; Carmona, M.; Serrano, A.; Borreguero, A.M. Glycolysis of advanced polyurethanes composites containing thermoregulating microcapsules. Chem. Eng. J. 2018, 350, 300–311. [Google Scholar] [CrossRef]
- Miguel-Fernández, R.; Amundarain, I.; Asueta, A.; García-Fernández, S.; Arnaiz, S.; Miazza, N.L.; Montón, E.; Rodríguez-García, B.; Bianca-Benchea, E. Recovery of Green Polyols from Rigid Polyurethane Waste by Catalytic Depolymerization. Polymers 2022, 14, 2936. [Google Scholar] [CrossRef] [PubMed]
- Gama, N.; Godinho, B.; Marques, G.; Silva, R.; Barros-Timmons, A.; Ferreira, A. Recycling of polyurethane scraps via acidolysis. Chem. Eng. J. 2020, 395, 125102. [Google Scholar] [CrossRef]
- Godinho, B.; Gama, N.; Barros-Timmons, A.; Ferreira, A. Recycling of polyurethane wastes using different carboxylic acids via acidolysis to produce wood adhesives. J. Polym. Sci. 2021, 59, 697–705. [Google Scholar] [CrossRef]
- Gomez, J.C.; Zakaria, R.; Aung, M.M.; Mokhtar, M.N.; Yunus, R. Synthesis and characterization of polyurethanes from residual palm oil with high poly-unsaturated fatty acid oils as additive. Polymers 2021, 13, 4214. [Google Scholar] [CrossRef]
- Olazabal, I.; González, A.; Vallejos, S.; Rivilla, I.; Jehanno, C.; Sardon, H. Upgrading polyurethanes into functional ureas through the asymmetric chemical deconstruction of carbamates. ACS Sustain. Chem. Eng. 2023, 11, 332–342. [Google Scholar] [CrossRef]
- Simón, D.; Borreguero, A.M.; De Lucas, A.; Rodríguez, J.F. Recycling of polyurethanes from laboratory to industry, a journey towards the sustainability. Waste Manag. 2018, 76, 147–171. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Hao, K.; Guo, X.; Liu, F.; Xu, Y.; Guo, S.; Bai, L.; Liu, G.; Qu, L.; Liu, M.; et al. Mechano-chemical rubber reclamation using aminolysis products of waste flexible polyurethane foams as the devulcanizing agent. J. Clean. Prod. 2023, 384, 135421. [Google Scholar] [CrossRef]
- Heiran, R.; Ghaderian, A.; Reghunadhan, A.; Sedaghati, F.; Thomas, S.; Haghighi, A.H. Glycolysis: An efficient route for recycling of end of life polyurethane foams. J. Polym. Res. 2021, 28, 22. [Google Scholar] [CrossRef]
- Grdadolnik, M.; Drinčić, A.; Oreški, A.; Onder, O.C.; Utroša, P.; Pahovnik, D.; Žagar, E. Insight into chemical recycling of flexible polyurethane foams by acidolysis. ACS Sustain. Chem. Eng. 2022, 10, 1323–1332. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Westman, Z.; Richardson, K.; Lim, D.; Stottlemyer, A.L.; Farmer, T.; Gillis, P.; Vlcek, V.; Christopher, P.; Christopher, P.; et al. Opportunities in Closed-Loop Molecular Recycling of End-of-Life Polyurethane. ACS Sustain. Chem. Eng. 2023, 11, 6114–6128. [Google Scholar] [CrossRef]
- Eling, B.; Tomović, Ž.; Schädler, V. Current and future trends in polyurethanes: An industrial perspective. Macromol. Chem. Phys. 2020, 221, 2000114. [Google Scholar] [CrossRef]
- Oenema, J.; Liu, H.; De Coensel, N.; Eschenbacher, A.; Van de Vijver, R.; Weng, J.; Li, L.; Wang, C.; Van Geem, K.M. Review on the pyrolysis products and thermal decomposition mechanisms of polyurethanes. J. Anal. Appl. Pyrol. 2022, 168, 105723. [Google Scholar] [CrossRef]
- Mao, A.; Shmulsky, R.; Li, Q.; Wan, H. Recycling polyurethane materials: A comparison of polyol from glycolysis with micronized polyurethane powder in particleboard applications. BioResources 2014, 9, 4253–4265. [Google Scholar] [CrossRef]
- Mitrevska, M.J.; Mickovski, V.; Samardzioska, T.; Iannace, G. Experimental and Numerical Investigation of Sound Absorption Characteristics of Rebonded Polyurethane Foam. Appl. Sci. 2022, 12, 12936. [Google Scholar] [CrossRef]
- Deng, Y.; Dewil, R.; Appels, L.; Ansart, R.; Baeyens, J.; Kang, Q. Reviewing the thermo-chemical recycling of waste polyurethane foam. J. Environ. Manag. 2021, 278, 111527. [Google Scholar] [CrossRef]
- Hulme, A.J.; Goodhead, T.C. Cost effective reprocessing of polyurethane by hot compression moulding. J. Mater. Process. Technol. 2003, 139, 322–326. [Google Scholar] [CrossRef]
- He, P.; Lu, H.; Ruan, H.; Wang, C.; Zhang, Q.; Huang, Z.; Liu, J. Mechanochemistry: An Efficient Way to Recycle Thermoset Polyurethanes. Polymers 2022, 14, 3277. [Google Scholar] [CrossRef]
- Beran, R.; Zarybnicka, L.; Machova, D. Recycling of rigid polyurethane foam: Micro-milled powder used as active filler in polyurethane adhesives. J. Appl. Polym. Sci. 2020, 137, 49095. [Google Scholar] [CrossRef]
- Duan, R.; Wei, L.; Coates, P.; Kelly, A.; Wang, C.; Chen, B.; Zhou, Z.; Lu, C. Reinforcing mechanically reclaimed polyurethane foam wastes-based elastomer with modified recycled polyester fibers. J. Polym. Sci. 2023, in press. [Google Scholar] [CrossRef]
- Guo, L.; Wang, W.; Guo, X.; Hao, K.; Liu, H.; Xu, Y.; Liu, G.; Guo, S.; Bai, L.; Ren, D.; et al. Recycling of flexible polyurethane foams by regrinding scraps into powder to replace polyol for re-foaming. Materials 2022, 15, 6047. [Google Scholar] [CrossRef]
- Datta, E.; Głowińska, J.; Włoch, M. Mechanical Recycling via Regrinding, Rebonding. Adhesive Pressing, and Molding. In Recycling of Polyurethane Foams, 5th ed.; Sabu, T., Krishnan, K., Martin, G.T., Ajay, V.R., Abitha, V.K., Eds.; William Andrew Publishing: New York, NY, USA, 2018; pp. 57–65. [Google Scholar] [CrossRef]
- Hicks, D.A.; Kirk, A.C.; Stapleton, R.J. Performance of MDI pour-in-place automotive seating incorporating recycled content. J. Cell. Plast. 1996, 32, 191–211. [Google Scholar] [CrossRef]
- Wang, S.; Panyukov, S.; Rubinstein, M.; Craig, S.L. Quantitative adjustment to the molecular energy parameter in the Lake–Thomas theory of polymer fracture energy. Macromolecules 2019, 52, 2772–2777. [Google Scholar] [CrossRef]
- He, P.; Ruan, H.; Wang, C.; Lu, H. Mechanical properties and thermal conductivity of thermal insulation board containing recycled thermosetting polyurethane and thermoplastic. Polymers 2021, 13, 4411. [Google Scholar] [CrossRef]
- Vale, M.; Mateus, M.M.; dos Santos, R.G.; De Castro, C.N.; De Schrijver, A.; Bordado, J.C.; Marques, A.C. Replacement of petroleum-derived diols by sustainable biopolyols in one component polyurethane foams. J. Clean. Prod. 2019, 212, 1036–1043. [Google Scholar] [CrossRef]


| Sample | Component Mass (g) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Recycled Powder | 5616 | LHS-100 | T-80 | A33 | T9 | 5870 | MC | Deionized Water | |
| Original F-PUF | 0 | 150 | 150 | 133.05 | 0.66 | 0.54 | 3.6 | 12 | 9.9 |
| F-PUF containing recycled powder | 15 | 135 | 150 | 132.08 | 0.66 | 0.54 | 3.6 | 12 | 9.9 |
| Sample | Cross-Linking Chains Proportion A1 (%) | Dangling Chains Proportion B1 (%) |
|---|---|---|
| Original F-PUF | 72.73 ± 0.14 | 27.27 ± 0.14 |
| F-PUF powder with 1 extrusion | 70.30 ± 0.29 | 29.70 ± 0.29 |
| F-PUF powder with 3 extrusions | 68.90 ± 0.28 | 31.10 ± 0.28 |
| F-PUF powder with 5 extrusions | 68.43 ± 0.43 | 31.25 ± 0.43 |
| Sample | Density (Kg/m3) | Resilience (%) | ILD (lbf) | Compression Set (%) | Air Permeability (L/m2·s) | Tensile Strength (Kpa) | Tear Strength (N/cm) |
|---|---|---|---|---|---|---|---|
| Original F-PUF | 23.90 ± 0.21 | 43.6 ± 0.4 | 26.4 ± 0.3 | 2.0 ± 0.2 | 660.8 ± 0.6 | 73.1 ± 0.4 | 2.2 ± 0.2 |
| F-PUF containing recycled powder | 22.71 ± 0.18 | 43.4 ± 0.3 | 21.3 ± 0.2 | 2.0 ± 0.3 | 815.7 ± 0.7 | 73.0 ± 0.5 | 2.3 ± 0.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, L.; Wang, F.; Chai, H.; Liu, G.; Jian, X.; Zhao, J.; Liu, K.; Liu, H.; Liu, T.; Zhang, X.; et al. Mechanochemical Recycling of Flexible Polyurethane Foam Scraps for Quantitative Replacement of Polyol Using Wedge-Block-Reinforced Extruder. Polymers 2024, 16, 1633. https://doi.org/10.3390/polym16121633
Guo L, Wang F, Chai H, Liu G, Jian X, Zhao J, Liu K, Liu H, Liu T, Zhang X, et al. Mechanochemical Recycling of Flexible Polyurethane Foam Scraps for Quantitative Replacement of Polyol Using Wedge-Block-Reinforced Extruder. Polymers. 2024; 16(12):1633. https://doi.org/10.3390/polym16121633
Chicago/Turabian StyleGuo, Lei, Fu Wang, Hailin Chai, Gongxu Liu, Xingao Jian, Jinyang Zhao, Kexin Liu, Haichao Liu, Tiewei Liu, Xiangping Zhang, and et al. 2024. "Mechanochemical Recycling of Flexible Polyurethane Foam Scraps for Quantitative Replacement of Polyol Using Wedge-Block-Reinforced Extruder" Polymers 16, no. 12: 1633. https://doi.org/10.3390/polym16121633
APA StyleGuo, L., Wang, F., Chai, H., Liu, G., Jian, X., Zhao, J., Liu, K., Liu, H., Liu, T., Zhang, X., Wang, Y., & Liu, F. (2024). Mechanochemical Recycling of Flexible Polyurethane Foam Scraps for Quantitative Replacement of Polyol Using Wedge-Block-Reinforced Extruder. Polymers, 16(12), 1633. https://doi.org/10.3390/polym16121633






