Extraction of Nanocellulose from the Residue of Sugarcane Bagasse Fiber for Anti-Staphylococcus aureus (S. aureus) Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Extraction of Nanocellulose from Residual Sugarcane Bagasse Fiber
2.3. Yield Percentage
2.4. Characterization
2.4.1. Fourier Transform Infrared Spectroscopy (FTIR)
2.4.2. Field-Emission Scanning Electron Microscopy (FESEM)
2.4.3. Thermal Analysis
2.4.4. X-ray Diffraction (XRD)
2.4.5. X-ray Photoelectron Spectroscopy (XPS)
2.5. Anti-S. aureus Analysis
2.6. Statistical Analysis
3. Results and Discussion
3.1. Yield Percentage of Nanocellulose
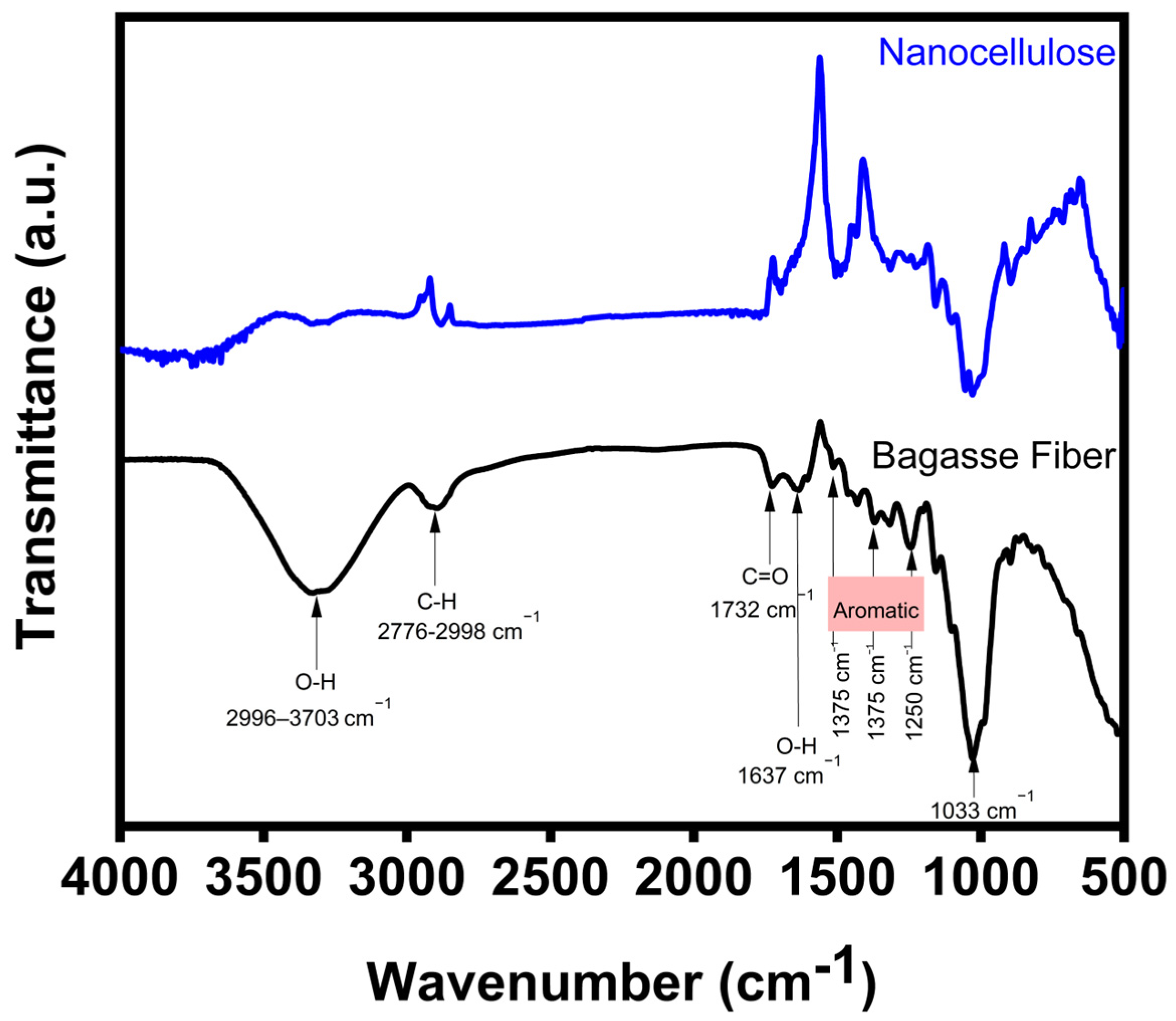
3.2. FTIR
3.3. FESEM
3.4. XRD
3.5. Thermal Analysis
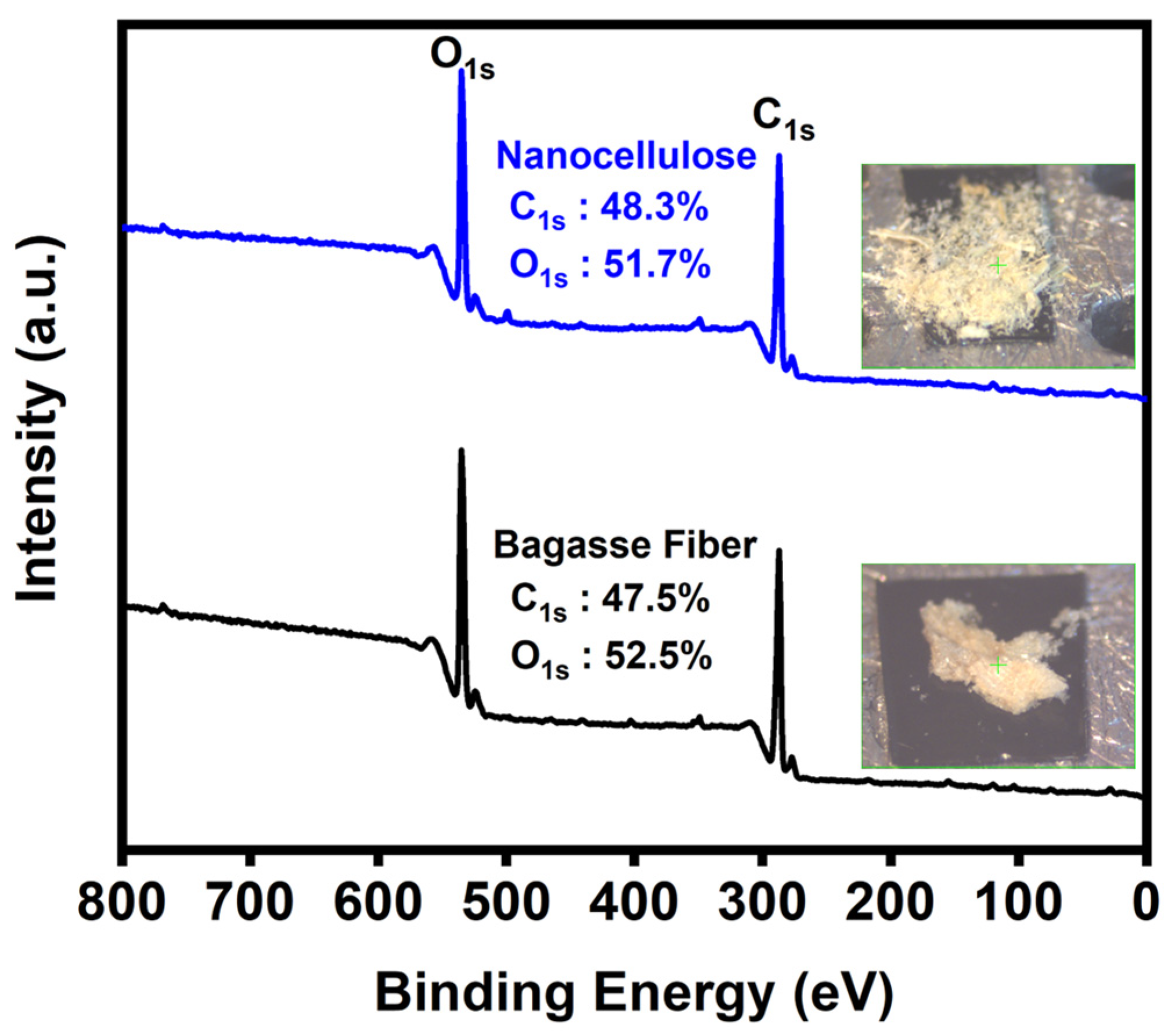
3.6. XPS
3.7. Anti-S. aureus Activity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pipitpukdee, S.; Attavanich, W.; Bejranonda, S. Climate change impacts on sugarcane production in Thailand. Atmosphere 2020, 11, 408. [Google Scholar] [CrossRef]
- Chunhawong, K.; Chaisan, T.; Rungmekarat, S.; Khotavivattana, S. Sugar industry and utilization of its by-products in Thailand: An overview. Sugar Tech 2018, 20, 111–115. [Google Scholar] [CrossRef]
- Sruamsiri, S. Agricultural wastes as dairy feed in Chiang Mai. Anim. Sci. J. 2007, 78, 335–341. [Google Scholar] [CrossRef]
- Kumar, S.; Salam, P.A.; Shrestha, P.; Ackom, E.K. An assessment of Thailand’s biofuel development. Sustainability 2013, 5, 1577–1597. [Google Scholar] [CrossRef]
- Nunta, R.; Techapun, C.; Sommanee, S.; Mahakuntha, C.; Porninta, K.; Punyodom, W.; Phimolsiripol, Y.; Rachtanapun, P.; Wang, W.; Zhuang, X. Valorization of rice straw, sugarcane bagasse and sweet sorghum bagasse for the production of bioethanol and phenylacetylcarbinol. Sci. Rep. 2023, 13, 727. [Google Scholar] [CrossRef]
- Gunawan, G. Energy and Clean Water Potential in a Closed System Sugar Factory. J. Agric. 2023, 2, 139–146. [Google Scholar] [CrossRef]
- Khantayanuwong, S.; Yimlamai, P.; Chitbanyong, K.; Wanitpinyo, K.; Pisutpiched, S.; Sungkaew, S.; Sukyai, P.; Puangsin, B. Fiber morphology, chemical composition, and properties of kraft pulping handsheet made from four Thailand bamboo species. J. Nat. Fibers 2023, 20, 2150924. [Google Scholar] [CrossRef]
- Zafeer, M.K.; Prabhu, R.; Rao, S.; Mahesha, G.; Bhat, K.S. Mechanical Characteristics of Sugarcane Bagasse Fibre Reinforced Polymer Composites: A Review. Cogent Eng. 2023, 10, 2200903. [Google Scholar] [CrossRef]
- Fatma, S.; Hameed, A.; Noman, M.; Ahmed, T.; Shahid, M.; Tariq, M.; Sohail, I.; Tabassum, R. Lignocellulosic biomass: A sustainable bioenergy source for the future. Protein Pept. Lett. 2018, 25, 148–163. [Google Scholar] [CrossRef]
- Bajpai, P. Pretreatment of Lignocellulosic Biomass. In Pretreatment of Lignocellulosic Biomass for Biofuel Production; Springer: Singapore, 2016; pp. 7–12. ISBN 978-981-10-0687-6. [Google Scholar]
- Kargarzadeh, H.; Mariano, M.; Gopakumar, D.; Ahmad, I.; Thomas, S.; Dufresne, A.; Huang, J.; Lin, N. Advances in cellulose nanomaterials. Cellulose 2018, 25, 2151–2189. [Google Scholar] [CrossRef]
- Ilyas, R.; Sapuan, S.; Atiqah, A.; Ibrahim, R.; Abral, H.; Ishak, M.; Zainudin, E.; Nurazzi, N.; Atikah, M.; Ansari, M. Sugar palm (Arenga pinnata [Wurmb.] Merr) starch films containing sugar palm nanofibrillated cellulose as reinforcement: Water barrier properties. Polym. Compos. 2020, 41, 459–467. [Google Scholar] [CrossRef]
- Asyraf, M.; Ishak, M.; Sapuan, S.; Yidris, N.; Ilyas, R. Woods and composites cantilever beam: A comprehensive review of experimental and numerical creep methodologies. J. Mater. Res. Technol. 2020, 9, 6759–6776. [Google Scholar] [CrossRef]
- Omran, A.A.B.; Mohammed, A.A.B.A.; Sapuan, S.M.; Ilyas, R.A.; Asyraf, M.R.M.; Rahimian Koloor, S.S.; Petrů, M. Micro- and Nanocellulose in Polymer Composite Materials: A Review. Polymers 2021, 13, 231. [Google Scholar] [CrossRef] [PubMed]
- Daim, W.; Uyama, H.; Lim, S.A. Nanocellulose synthesized from sugarcane bagasse (S. officinarum) via alkaline-mechanical process and its characterization. ASEAN J. Sci. Technol. Dev. 2024, 40, 8. [Google Scholar] [CrossRef]
- Dufresne, A. Nanocellulose: Potential reinforcement in composites. In Natural Polymers Volume 2: Nanocomposites; John, M.J., Thomas, S., Eds.; Royal Society of Chemistry: Cambridge, UK, 2012; pp. 1–32. ISBN 978-1-84973-531-5. [Google Scholar]
- Chinga-Carrasco, G. Potential and limitations of nanocelluloses as components in biocomposite inks for three-dimensional bioprinting and for biomedical devices. Biomacromolecules 2018, 19, 701–711. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Han, X.; Zhang, C.; Liu, K.; Duan, G. Source of nanocellulose and its application in nanocomposite packaging material: A review. Nanomaterials 2022, 12, 3158. [Google Scholar] [CrossRef] [PubMed]
- Spagnuolo, L.; D’Orsi, R.; Operamolla, A. Nanocellulose for Paper and Textile Coating: The Importance of Surface Chemistry. ChemPlusChem 2022, 87, e202200204. [Google Scholar] [CrossRef] [PubMed]
- Norrrahim, M.N.F.; Nurazzi, N.M.; Jenol, M.A.; Farid, M.A.A.; Janudin, N.; Ujang, F.A.; Yasim-Anuar, T.A.T.; Najmuddin, S.U.F.S.; Ilyas, R.A. Emerging development of nanocellulose as an antimicrobial material: An overview. Mater. Adv. 2021, 2, 3538–3551. [Google Scholar] [CrossRef]
- Phanthong, P.; Reubroycharoen, P.; Hao, X.; Xu, G.; Abudula, A.; Guan, G. Nanocellulose: Extraction and application. Carbon Resour. Convers. 2018, 1, 32–43. [Google Scholar] [CrossRef]
- Souza, E.; Gottschalk, L.; Freitas-Silva, O. Overview of nanocellulose in food packaging. Recent Pat. Food Nutr. Agric. 2020, 11, 154–167. [Google Scholar] [CrossRef]
- Naettip, S.; Wongsuwanphon, S.; Khamsakhon, S.; Insri, C.; Kanyamee, O.; Siri, C.; Suphanchaimat, R. Staphylococcal Food Poisoning Outbreak from a Community Gathering, Wang Nuea District, Lampang Province, Northern Thailand, July 2022. Outbreak Surveill. Investig. Response (OSIR) J. 2023, 16, 93–104. [Google Scholar] [CrossRef]
- Pal, M.; Ketchakmadze, D.; Durglishvili, N.; Ketchakmadze, K. Staphylococcus aureus: A major pathogen of food poisoning: A rare research report. Nutr. Food Process. 2022, 5, 1–3. [Google Scholar]
- Kim, I.; Viswanathan, K.; Kasi, G.; Thanakkasaranee, S.; Sadeghi, K.; Seo, J. ZnO nanostructures in active antibacterial food packaging: Preparation methods, antimicrobial mechanisms, safety issues, future prospects, and challenges. Food Rev. Int. 2022, 38, 537–565. [Google Scholar] [CrossRef]
- Min, T.; Zhu, Z.; Sun, X.; Yuan, Z.; Zha, J.; Wen, Y. Highly efficient antifogging and antibacterial food packaging film fabricated by novel quaternary ammonium chitosan composite. Food Chem. 2020, 308, 125682. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.; Qian, Y.; Wei, J.; Zhou, C. Polymeric antimicrobial food packaging and its applications. Polymers 2019, 11, 560. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Cha, R.; Mou, K.; Zhao, X.; Long, K.; Luo, H.; Zhou, F.; Jiang, X. Nanocellulose-Based Antibacterial Materials. Adv. Healthc. Mater. 2018, 7, 1800334. [Google Scholar] [CrossRef] [PubMed]
- Gond, R.K.; Gupta, M.K.; Jawaid, M. Extraction of nanocellulose from sugarcane bagasse and its characterization for potential applications. Polym. Compos. 2021, 42, 5400–5412. [Google Scholar] [CrossRef]
- Sanuja, S.; Agalya, A.; Umapathy, M. Studies on magnesium oxide reinforced chitosan bionanocomposite incorporated with clove oil for active food packaging application. Int. J. Polym. Mater. 2014, 63, 733–740. [Google Scholar] [CrossRef]
- Rodríguez, F.J.; Coloma, A.; Galotto, M.J.; Guarda, A.; Bruna, J.E. Effect of organoclay content and molecular weight on cellulose acetate nanocomposites properties. Polym. Degrad. Stab. 2012, 97, 1996–2001. [Google Scholar] [CrossRef]
- Rambo, M.K.; Ferreira, M. Determination of cellulose crystallinity of banana residues using near infrared spectroscopy and multivariate analysis. J. Braz. Chem. Soc. 2015, 26, 1491–1499. [Google Scholar] [CrossRef]
- Tougaard, S.; Jansson, C. Comparison of validity and consistency of methods for quantitative XPS peak analysis. Surf. Interface Anal. 1993, 20, 1013–1046. [Google Scholar] [CrossRef]
- Kong, P.; Thangunpai, K.; Zulfikar, A.; Masuo, S.; Abe, J.P.; Enomae, T. Preparation of Green Anti-Staphylococcus aureus Inclusion Complexes Containing Hinoki Essential Oil. Foods 2023, 12, 3104. [Google Scholar] [CrossRef] [PubMed]
- Seta, F.T.; An, X.; Liu, L.; Zhang, H.; Yang, J.; Zhang, W.; Nie, S.; Yao, S.; Cao, H.; Xu, Q. Preparation and characterization of high yield cellulose nanocrystals (CNC) derived from ball mill pretreatment and maleic acid hydrolysis. Carbohydr. Polym. 2020, 234, 115942. [Google Scholar] [CrossRef] [PubMed]
- Imman, S.; Khongchamnan, P.; Wanmolee, W.; Laosiripojana, N.; Kreetachat, T.; Sakulthaew, C.; Chokejaroenrat, C.; Suriyachai, N. Fractionation and characterization of lignin from sugarcane bagasse using a sulfuric acid catalyzed solvothermal process. RSC Adv. 2021, 11, 26773–26784. [Google Scholar] [CrossRef]
- Oh, S.Y.; Yoo, D.I.; Shin, Y.; Seo, G. FTIR analysis of cellulose treated with sodium hydroxide and carbon dioxide. Carbohydr. Res. 2005, 340, 417–428. [Google Scholar] [CrossRef] [PubMed]
- Melesse, G.T.; Hone, F.G.; Mekonnen, M.A. Extraction of Cellulose from Sugarcane Bagasse Optimization and Characterization. Adv. Mater. Sci. Eng. 2022, 2022, 1712207. [Google Scholar] [CrossRef]
- Trilokesh, C.; Uppuluri, K.B. Isolation and characterization of cellulose nanocrystals from jackfruit peel. Sci. Rep. 2019, 9, 16709. [Google Scholar] [CrossRef] [PubMed]
- Kargarzadeh, H.; Ahmad, I.; Abdullah, I.; Dufresne, A.; Zainudin, S.Y.; Sheltami, R.M. Effects of hydrolysis conditions on the morphology, crystallinity, and thermal stability of cellulose nanocrystals extracted from kenaf bast fibers. Cellulose 2012, 19, 855–866. [Google Scholar] [CrossRef]
- Jonoobi, M.; Khazaeian, A.; Tahir, P.M.; Azry, S.S.; Oksman, K. Characteristics of cellulose nanofibers isolated from rubberwood and empty fruit bunches of oil palm using chemo-mechanical process. Cellulose 2011, 18, 1085–1095. [Google Scholar] [CrossRef]
- Nacos, M.K.; Katapodis, P.; Pappas, C.; Daferera, D.; Tarantilis, P.; Christakopoulos, P.; Polissiou, M. Kenaf xylan–a source of biologically active acidic oligosaccharides. Carbohydr. Polym. 2006, 66, 126–134. [Google Scholar] [CrossRef]
- Abraham, E.; Deepa, B.; Pothen, L.; Cintil, J.; Thomas, S.; John, M.J.; Anandjiwala, R.; Narine, S. Environmental friendly method for the extraction of coir fibre and isolation of nanofibre. Carbohydr. Polym. 2013, 92, 1477–1483. [Google Scholar] [CrossRef] [PubMed]
- Johar, N.; Ahmad, I.; Dufresne, A. Extraction, preparation and characterization of cellulose fibres and nanocrystals from rice husk. Ind. Crops Prod. 2012, 37, 93–99. [Google Scholar] [CrossRef]
- Nishiyama, Y. Structure and properties of the cellulose microfibril. J. Wood Sci. 2009, 55, 241–249. [Google Scholar] [CrossRef]
- Li, M.; Wang, L.-J.; Li, D.; Cheng, Y.-L.; Adhikari, B. Preparation and characterization of cellulose nanofibers from de-pectinated sugar beet pulp. Carbohydr. Polym. 2014, 102, 136–143. [Google Scholar] [CrossRef]
- Yang, H.; Yan, R.; Chen, H.; Lee, D.H.; Zheng, C. Characteristics of hemicellulose, cellulose and lignin pyrolysis. Fuel 2007, 86, 1781–1788. [Google Scholar] [CrossRef]
- Teixeira, S.R.; Arenales, A.; De Souza, A.E.; Magalhães, R.d.S.; Peña, A.F.V.; Aquino, D.; Freire, R. Sugarcane bagasse: Applications for energy production and ceramic materials. J. Solid Waste Technol. Manag. 2015, 41, 229–238. [Google Scholar] [CrossRef]
- Sahu, P.; Gupta, M. Effect of ecofriendly coating and treatment on mechanical, thermal and morphological properties of sisal fibre. Indian J. Fibre Text. Res. 2019, 44, 199–204. [Google Scholar]
- Chen, X.; Yu, J.; Zhang, Z.; Lu, C. Study on structure and thermal stability properties of cellulose fibers from rice straw. Carbohydr. Polym. 2011, 85, 245–250. [Google Scholar] [CrossRef]
- Kuzmenko, V.; Wang, N.; Haque, M.; Naboka, O.; Flygare, M.; Svensson, K.; Gatenholm, P.; Liu, J.; Enoksson, P. Cellulose-derived carbon nanofibers/graphene composite electrodes for powerful compact supercapacitors. RSC Adv. 2017, 7, 45968–45977. [Google Scholar] [CrossRef]
- Wang, Q.; Xie, D.; Chen, J.; Liu, G.; Yu, M. Superhydrophobic paper fabricated via nanostructured titanium dioxide-functionalized wood cellulose fibers. J. Mater. Sci. 2020, 55, 7084–7094. [Google Scholar] [CrossRef]
- Tian, H.; Li, W.; Chen, C.; Yu, H.; Yuan, H. Antibacterial Activity and Mechanism of Oxidized Bacterial Nanocellulose with Different Carboxyl Content. Macromol. Biosci. 2023, 23, 2200459. [Google Scholar] [CrossRef] [PubMed]
- El-Sheekh, M.M.; Yousuf, W.E.; Kenawy, E.-R.; Mohamed, T.M. Biosynthesis of cellulose from Ulva lactuca, manufacture of nanocellulose and its application as antimicrobial polymer. Sci. Rep. 2023, 13, 10188. [Google Scholar] [CrossRef] [PubMed]
- Dou, X.-Q.; Zhang, D.; Feng, C.; Jiang, L. Bioinspired Hierarchical Surface Structures with Tunable Wettability for Regulating Bacteria Adhesion. ACS Nano 2015, 9, 10664–10672. [Google Scholar] [CrossRef] [PubMed]




| No. | Length (nm) | Diameter (nm) | Aspect Ratio (L/D) |
|---|---|---|---|
| 1 | 711 | 43 | 16.53 |
| 2 | 230 | 27 | 8.52 |
| 3 | 281 | 172 | 1.63 |
| 4 | 129 | 12 | 10.75 |
| 5 | 500 | 160 | 3.13 |
| 6 | 352 | 203 | 1.73 |
| 7 | 906 | 742 | 1.22 |
| 8 | 887 | 47 | 18.87 |
| 9 | 285 | 12 | 23.75 |
| 10 | 148 | 4 | 37.00 |
| Average | 442.9 | 142.2 | 3.11 |
| With Nanocellulose | Without Nanocellulose (Control) | |
|---|---|---|
| OD600 value | 2.10 ± 0.06 | 3.44 ± 0.14 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Charoensopa, K.; Thangunpai, K.; Kong, P.; Enomae, T.; Ploysri, W. Extraction of Nanocellulose from the Residue of Sugarcane Bagasse Fiber for Anti-Staphylococcus aureus (S. aureus) Application. Polymers 2024, 16, 1612. https://doi.org/10.3390/polym16111612
Charoensopa K, Thangunpai K, Kong P, Enomae T, Ploysri W. Extraction of Nanocellulose from the Residue of Sugarcane Bagasse Fiber for Anti-Staphylococcus aureus (S. aureus) Application. Polymers. 2024; 16(11):1612. https://doi.org/10.3390/polym16111612
Chicago/Turabian StyleCharoensopa, Krairop, Kotchaporn Thangunpai, Peifu Kong, Toshiharu Enomae, and Wat Ploysri. 2024. "Extraction of Nanocellulose from the Residue of Sugarcane Bagasse Fiber for Anti-Staphylococcus aureus (S. aureus) Application" Polymers 16, no. 11: 1612. https://doi.org/10.3390/polym16111612
APA StyleCharoensopa, K., Thangunpai, K., Kong, P., Enomae, T., & Ploysri, W. (2024). Extraction of Nanocellulose from the Residue of Sugarcane Bagasse Fiber for Anti-Staphylococcus aureus (S. aureus) Application. Polymers, 16(11), 1612. https://doi.org/10.3390/polym16111612







