Adhesion and Transparency Enhancement between Flexible Polyimide-PDMS Copolymerized Film and Copper Foil for LED Transparent Screen
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of PDMS-PAA Solution and CPI Copper-Clad Foil
2.3. Characterization
3. Results
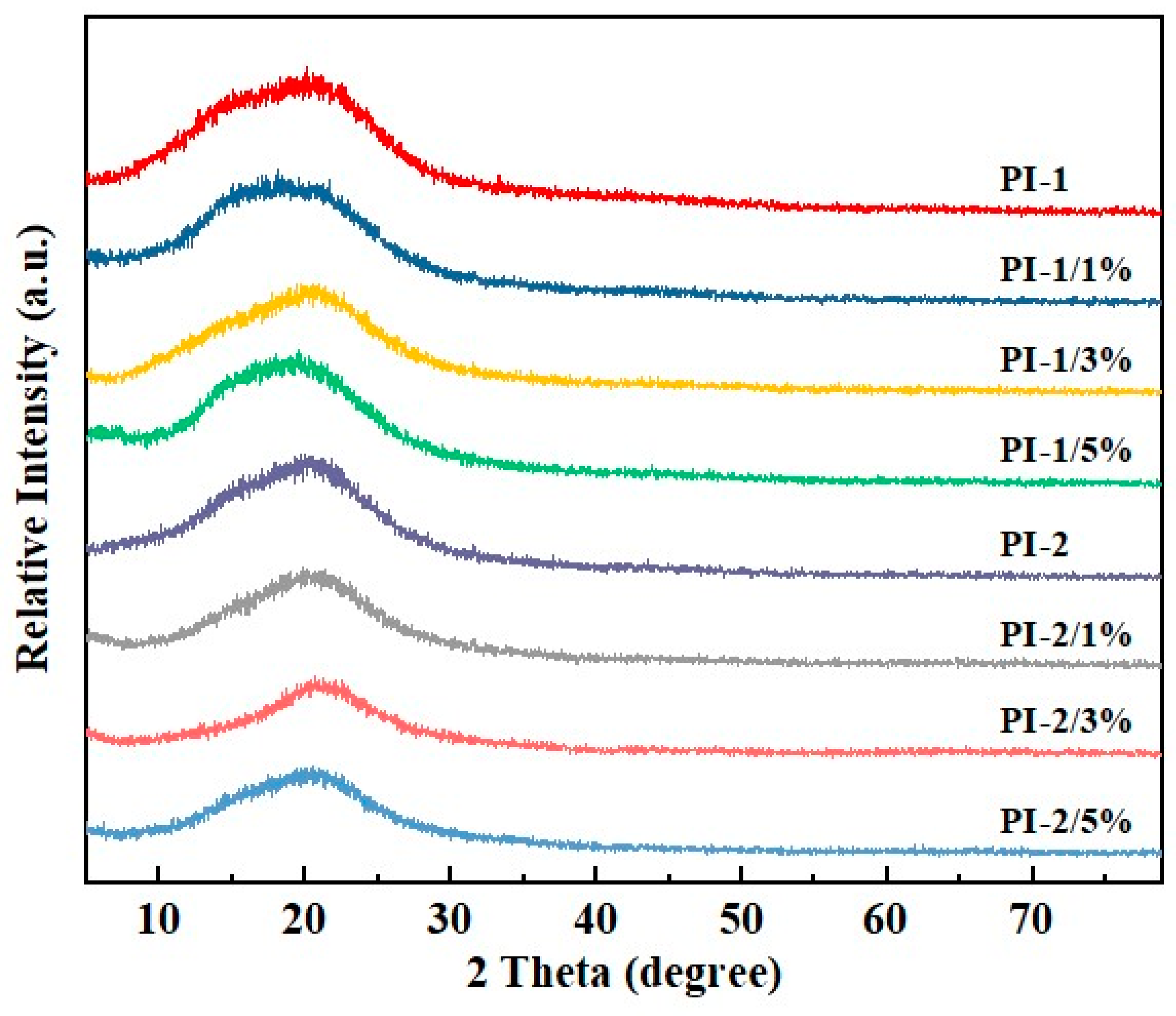
3.1. Chemical Structures
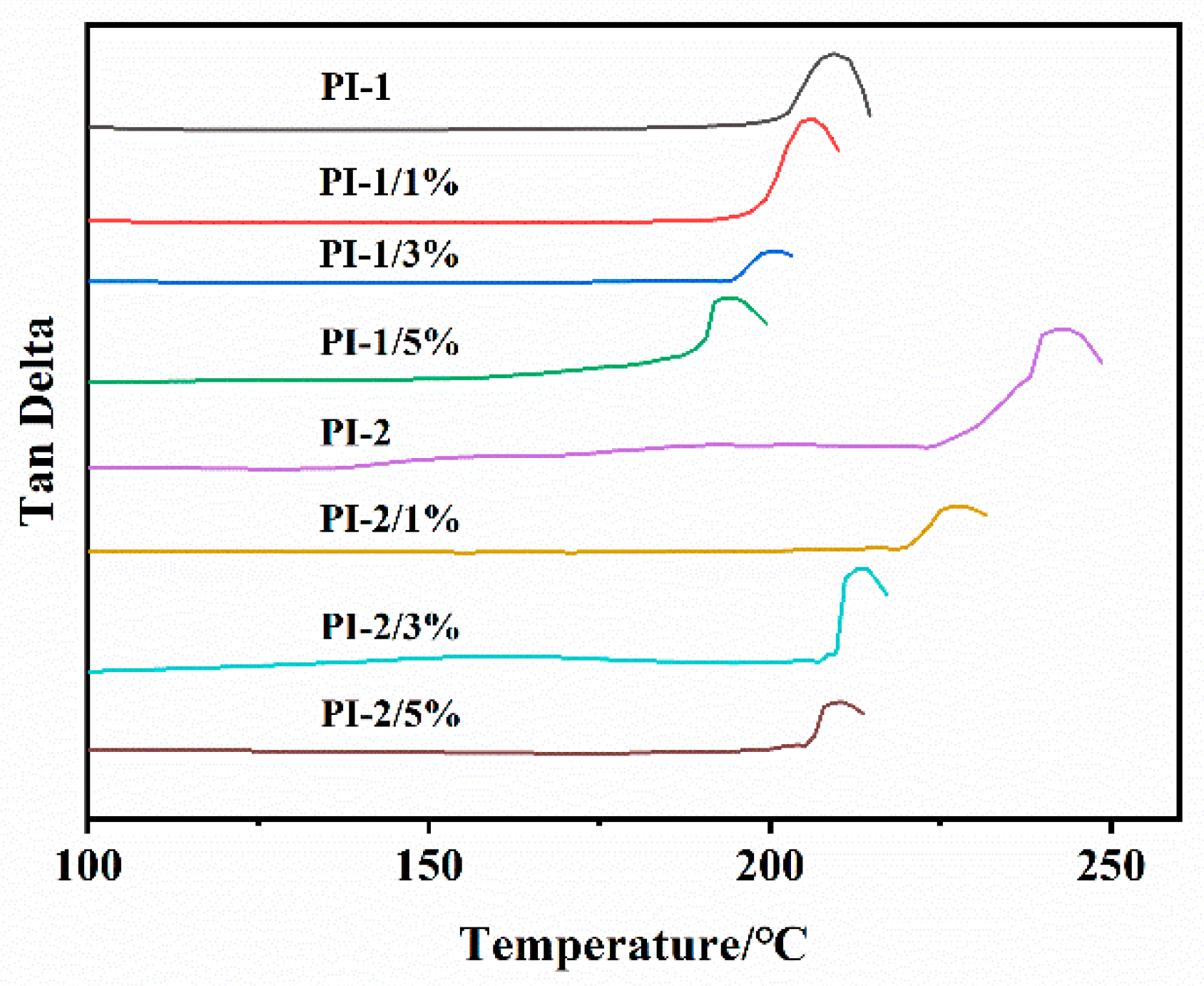
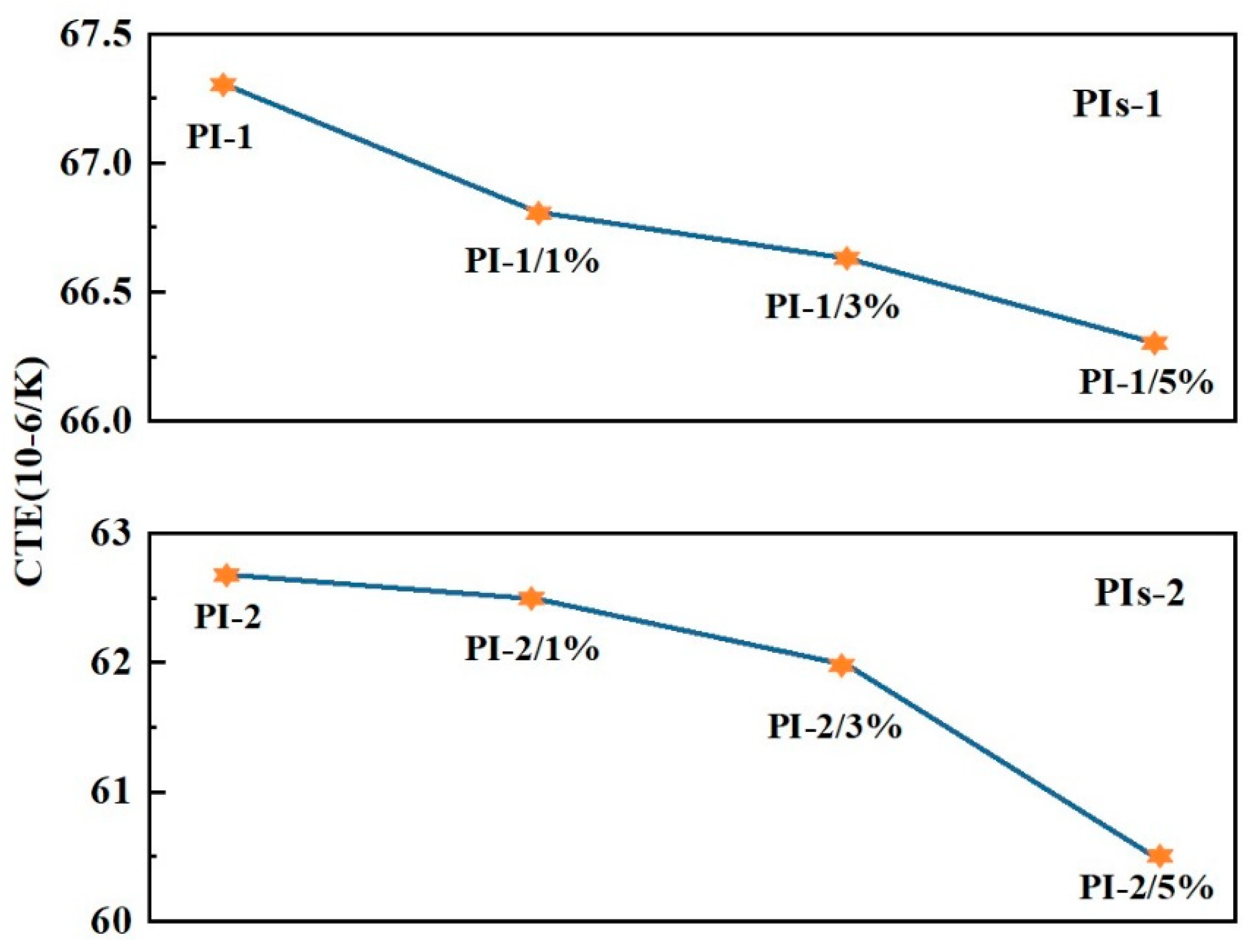
3.2. Thermal Properties
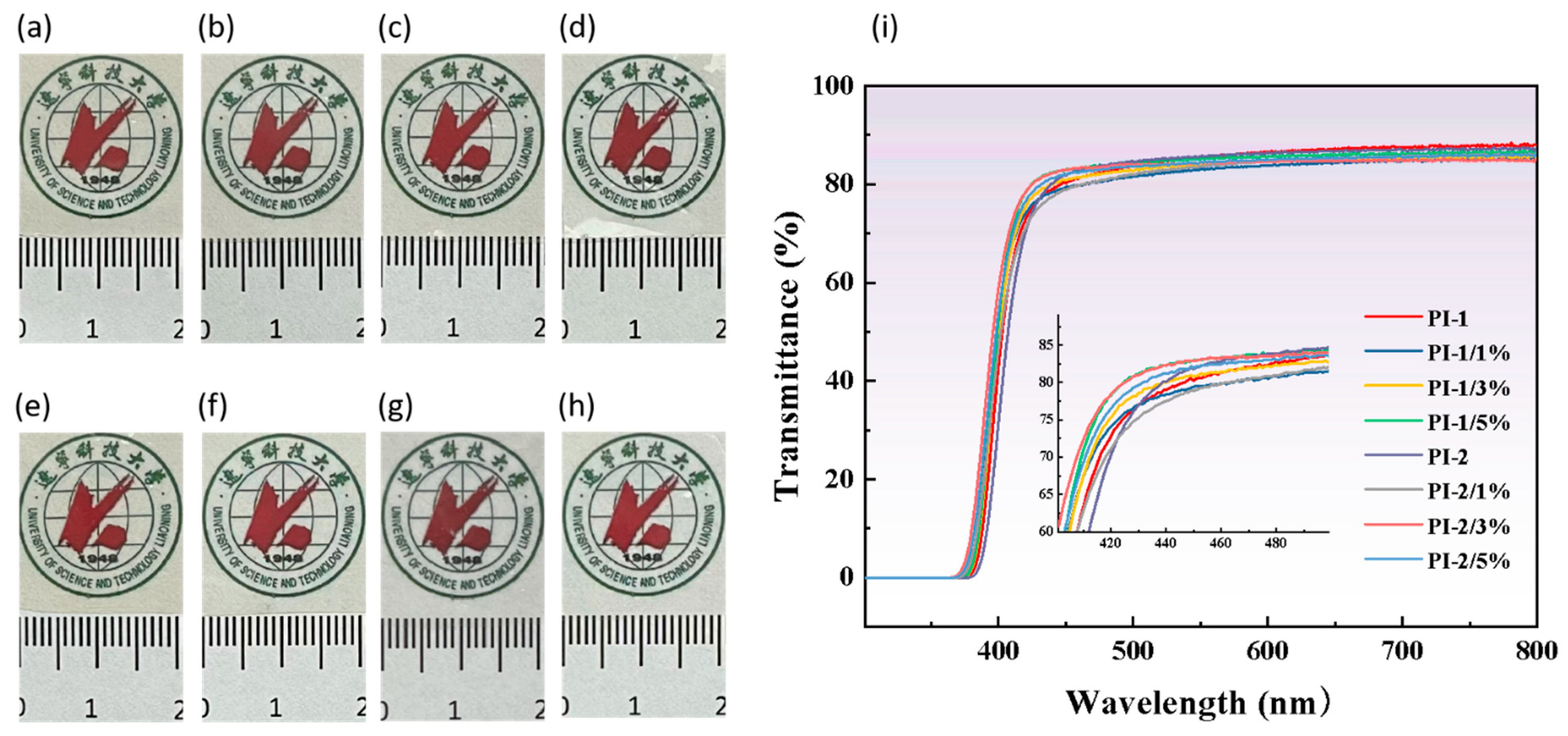
3.3. Optical Properties
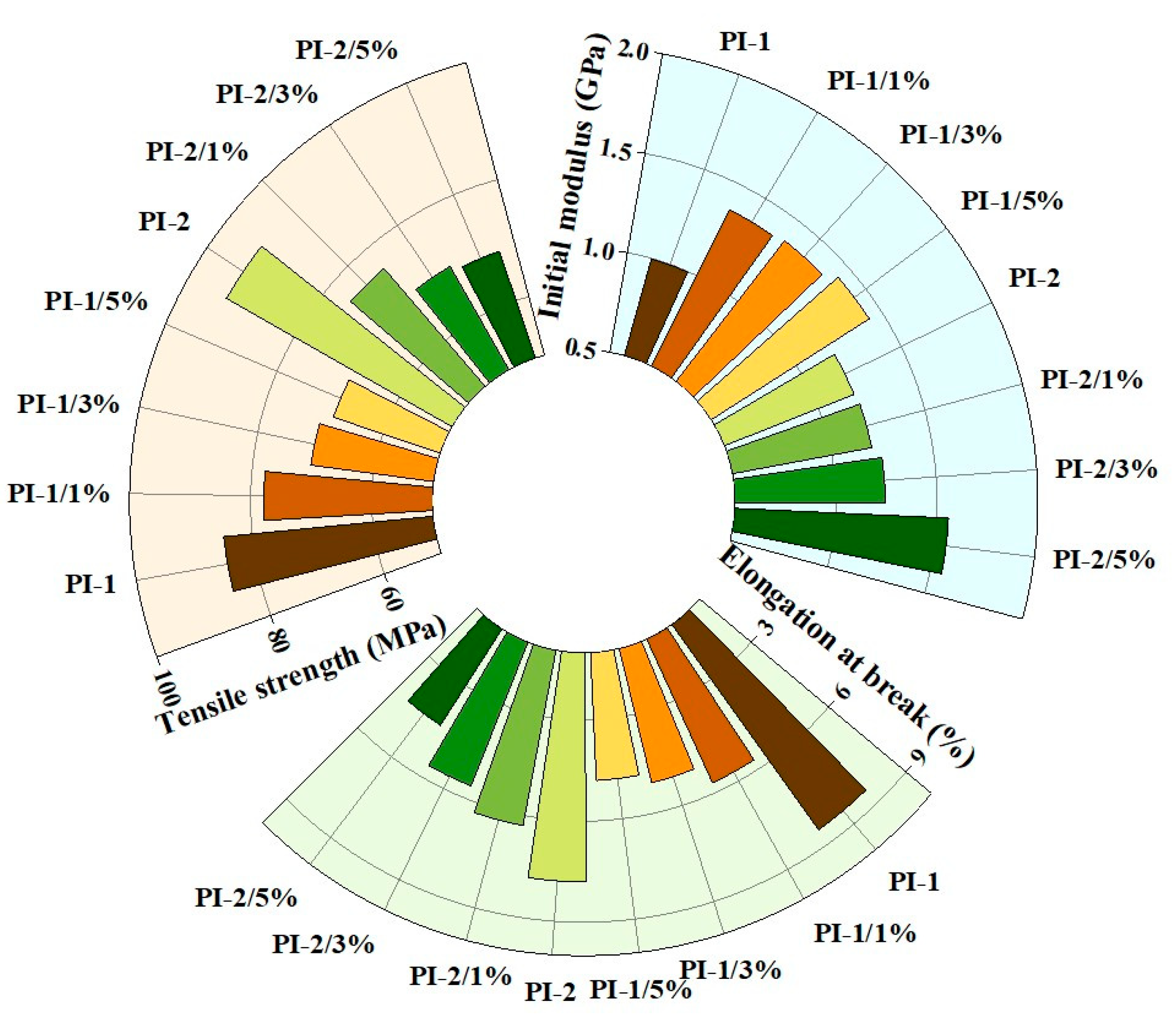
3.4. Mechanical Properties
3.5. Adhesion Testing and Peel Adhesion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- McCoul, D.; Hu, W.; Gao, M.; Mehta, V.; Pei, Q. Recent advances in stretchable and transparent electronic materials. Adv. Electron. Mater. 2016, 2, 1500407. [Google Scholar] [CrossRef]
- Behera, M.; Panda, R.; Dhivya, P.; Joshi, D.; Kumar, R.A. Study of efficient sustainable phosphor in glass (P-i-G) material for white LED applications fabricated by tape casting and screen-printing techniques. Mater. Sci. Eng. B 2023, 298, 116811. [Google Scholar] [CrossRef]
- Yang, L.; Xu, X.; Yuan, Y.; Li, Z.; He, S. Meter-scale transparent conductive circuits based on silver nanowire networks for rigid and flexible transparent light-emitting diode screens. Opt. Mater. Express 2019, 9, 4483–4496. [Google Scholar] [CrossRef]
- Zhang, R.; Wang, B.; Zhu, W.; Li, C.; Wang, H. Preparation and luminescent performances of transparent screen-printed Ce3+: Y3Al5O12 phosphors-in-glass thick films for remote white LEDs. J. Alloys Compd. 2017, 720, 340–344. [Google Scholar] [CrossRef]
- Shalu, C. Opportunities and Challenges in Flexible and Organic LED. In Organic and Inorganic Light Emitting Diodes; CRC Press: Boca Raton, FL, USA, 2023; pp. 107–116. [Google Scholar]
- Tan, J.; Xie, F.; Huang, J.; Liu, X.; Li, H.; Yuan, J.; He, P.; Liu, Y. Design and synthesis of intrinsic black polyimide with full visible-light absorption and low coefficient of thermal expansion for black flexible copper clad laminates. Polym. Test. 2023, 129, 108247. [Google Scholar] [CrossRef]
- Han, S.; Qi, Y.; Zhi, X.; Ren, X.; Wang, Z.; He, Z.; Yang, C.; Yu, H.; Liu, J. Synthesis and properties of modified thermoplastic polyimide films with good dielectric properties at high frequency and enhanced thermal stability via incorporation of rigid ester and biphenyl structural units. Polym. Adv. Technol. 2024, 35, e6323. [Google Scholar] [CrossRef]
- Ma, J.; Liu, X.; Wang, R.; Lu, C.; Wen, X.; Tu, G. Research progress and application of polyimide-based nanocomposites. Nanomaterials 2023, 13, 656. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Li, P.; Qin, H.; Liu, N.; Ma, H.; Zhang, Z.; Li, J.; Lu, B.; Pan, X.; Wu, L. Liquid metal/CNTs hydrogel-based transparent strain sensor for wireless health monitoring of aquatic animals. Chem. Eng. J. 2023, 454, 140459. [Google Scholar] [CrossRef]
- Miao, J.; Fan, T. Flexible and stretchable transparent conductive graphene-based electrodes for emerging wearable electronics. Carbon 2023, 202, 495–527. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, H.; Wang, P.; Yu, Y.; Zhou, M.; Xu, B.; Cui, L.; Wang, Q. Stretchable, transparent, self-adhesive, anti-freezing and ionic conductive nanocomposite hydrogels for flexible strain sensors. Eur. Polym. J. 2023, 186, 111824. [Google Scholar] [CrossRef]
- Chen, J.; Ma, Y.; Chen, T.; Du, Y.; Xu, J.; Wang, D.; Yang, J.; Hu, P.; Jing, J.; Yao, B. Transparent high-performance supramolecular plastics operating in all-weather environments. Adv. Funct. Mater. 2023, 33, 2212564. [Google Scholar] [CrossRef]
- Guo, Y.; Wang, Y.; Tao, L.; Wang, T.; Wang, Q.; Zhang, X.; Yang, Z. Engineering Thermal and Light Dual-Triggered Thermosetting Shape Memory Polyimide Nanocomposites with Superior Toughness and Rapid Remote Actuation Properties. Adv. Eng. Mater. 2023, 25, 2201555. [Google Scholar] [CrossRef]
- Ogbonna, V.E.; Popoola, P.I.; Popoola, O.M.; Adeosun, S.O. A review on recent advances on improving polyimide matrix nanocomposites for mechanical, thermal, and tribological applications: Challenges and recommendations for future improvement. J. Thermoplast. Compos. Mater. 2023, 36, 836–865. [Google Scholar] [CrossRef]
- Zhang, X.; Li, P.; Zeng, J.; Li, J.; Wang, B.; Gao, W.; Xu, J.; Chen, K. Dynamic covalent bond enabled strong bio−based polyimide materials with thermally−driven adaptivity, healability and recycling. Chem. Eng. J. 2023, 465, 143017. [Google Scholar] [CrossRef]
- Wu, Z.; Dong, J.; Teng, C.; Li, X.; Zhao, X.; Qin, X.; Ji, C.; Zhang, Q. Polyimide-based composites reinforced by carbon nanotube-grafted carbon fiber for improved thermal conductivity and mechanical property. Compos. Commun. 2023, 39, 101543. [Google Scholar] [CrossRef]
- Sun, J.; Zhuo, S.; Zhang, R. Highly Transparent, Temperature-Resistant, and Flexible Polyimide Aerogels for Solar Energy Collection. ACS Appl. Mater. Interfaces 2023, 15, 37957–37965. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Zhang, Y.; Gu, J. Polyimide nanocomposite foams and aerogels for electromagnetic interference shielding. In Porous Nanocomposites for Electromagnetic Interference Shielding; Elsevier: Amsterdam, The Netherlands, 2024; pp. 261–284. [Google Scholar]
- Sheng, X.; Yun, S.; Wang, S.; Gao, Y.; Zuo, X.; Miao, X.; Shi, X.; Qin, J.; Ma, Z.; Zhang, G. Highly heat-resistant and mechanically strong co−crosslinked polyimide/bismaleimide rigid foams with superior thermal insulation and flame resistance. Mater. Today Phys. 2023, 36, 101154. [Google Scholar] [CrossRef]
- Zhu, C.; Xue, T.; Ma, Z.; Fan, W.; Liu, T. Mechanically strong and thermally insulating polyimide aerogel fibers reinforced by prefabricated long polyimide fibers. ACS Appl. Mater. Interfaces 2023, 15, 12443–12452. [Google Scholar] [CrossRef]
- Huang, C.; Liu, J.; Zhao, L.; Hu, N.; Wei, Q. Advances in atomic oxygen resistant polyimide composite films. Compos. Part A Appl. Sci. Manuf. 2023, 168, 107459. [Google Scholar] [CrossRef]
- Wang, D.; Ma, J.; Li, P.; Fan, L.; Wu, Y.; Zhang, Z.; Xu, C.; Jiang, L. Flexible hard coatings with self-evolution behavior in a low earth orbit environment. ACS Appl. Mater. Interfaces 2021, 13, 46003–46014. [Google Scholar] [CrossRef]
- Minton, T.K.; Wright, M.E.; Tomczak, S.J.; Marquez, S.A.; Shen, L.; Brunsvold, A.L.; Cooper, R.; Zhang, J.; Vij, V.; Guenthner, A.J. Atomic oxygen effects on POSS polyimides in low earth orbit. ACS Appl. Mater. Interfaces 2012, 4, 492–502. [Google Scholar] [CrossRef]
- Sun, Y.; Tao, L.; Wu, M.; Dastan, D.; Rehman, J.; Li, L.-X.; An, B. Multi-atomic Loaded C2N1 Catalysts for CO2 Reduction to CO or Formic Acid. Nanoscale 2024, 16, 9791–9801. [Google Scholar] [CrossRef] [PubMed]
- Gu, M.; Tao, L.; Dastan, D.; Dang, J.; Fang, T.; An, B. Metal-modified C3N1 monolayer sensors for battery instability monitoring. J. Mater. Chem. A 2024. [Google Scholar] [CrossRef]
- Huang, Y.; Tian, X.; Lv, S.; Fu, R.K.; Chu, P.K. Mechanical and optical characteristics of multilayer inorganic films on polyimide for anti-atomic-oxygen erosion. Appl. Surf. Sci. 2012, 258, 5810–5814. [Google Scholar] [CrossRef]
- Kantor, Z.; Wu, T.; Zeng, Z.; Gaan, S.; Lehner, S.; Jovic, M.; Bonnin, A.; Pan, Z.; Mazrouei−Sebdani, Z.; Opris, D.M. Heterogeneous silica−polyimide aerogel−in−aerogel nanocomposites. Chem. Eng. J. 2022, 443, 136401. [Google Scholar] [CrossRef]
- Huang, H.; Wu, C.; Wu, S.; Pan, R.; Yin, L.; Jin, X.; Pan, Y.; Wang, H.; Yan, X.; Hong, C. Super-flexible, thermostable and superhydrophobic polyimide/silicone interpenetrating aerogels for conformal thermal insulating and strain sensing applications. Chem. Eng. J. 2022, 441, 136032. [Google Scholar] [CrossRef]
- Feng, L.; Iroh, J.O. Polyimide-b-polysiloxane copolymers: Synthesis and properties. J. Inorg. Organomet. Polym. Mater. 2013, 23, 477–488. [Google Scholar] [CrossRef]
- Othman, M.B.H.; Ramli, M.R.; Tyng, L.Y.; Ahmad, Z.; Akil, H.M. Dielectric constant and refractive index of poly (siloxane-imide) block copolymer. Mater. Des. 2011, 32, 3173–3182. [Google Scholar] [CrossRef]
- Lei, X.; Chen, Y.; Qiao, M.; Tian, L.; Zhang, Q. Hyperbranched polysiloxane (HBPSi)-based polyimide films with ultralow dielectric permittivity, desirable mechanical and thermal properties. J. Mater. Chem. C 2016, 4, 2134–2146. [Google Scholar] [CrossRef]
- Yilgör, E.; Yilgör, I. Silicone containing copolymers: Synthesis, properties and applications. Prog. Polym. Sci. 2014, 39, 1165–1195. [Google Scholar] [CrossRef]
- Tian, K.; Chen, C.; Xiong, L.; Chen, X.; Fu, Q.; Deng, H. Fast-Crosslinking Enabled Self-Roughed Polydimethylsiloxane Transparent Superhydrophobic Coating and Its Application in Anti-Liquid-Interference Electrothermal Device. Small 2023, 2308051. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.-I.; Jeon, H.-J. Conductive Nanofiber Web Film with Polydimethylsiloxane Sidewalls Selectively Coated through a Plasma Process for High Performance Flexible Transparent Electrodes. Langmuir 2023, 39, 17480–17487. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Feng, F.; Ji, Y.; Peng, J.; He, L.; Cui, J. Stretchable polydimethylsiloxane/aligned electrospun cellulose acetate nanofibers composites with high transparency and fracture resistance. Polym. Compos. 2024, 45, 3120–3130. [Google Scholar] [CrossRef]
- Hao, T.; Zhang, L.; Ji, H.; Zhou, Q.; Feng, T.; Song, S.; Wang, B.; Liu, D.; Ren, Z.; Liu, W. A Stretchable, Transparent, and Mechanically Robust Silver Nanowire–Polydimethylsiloxane Electrode for Electrochromic Devices. Polymers 2023, 15, 2640. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Wu, Q.; Chen, K.; Shi, D.; Zhang, Y.; Li, H.; Zhao, Z. PDMS-b-PI-b-PDMS triblock copolymer self−supported membranes with microphase separated structures for efficient ethanol dehydration. Sep. Purif. Technol. 2024, 330, 125262. [Google Scholar] [CrossRef]
- Doshi, S.M.; Barry, A.; Schneider, A.; Parambil, N.; Mulzer, C.; Yahyazadehfar, M.; Samadi-Dooki, A.; Foltz, B.; Warrington, K.; Wessel, R. Adhesion Characterization and Enhancement between Polyimide-Silica Composite and Nodulated Copper for Applications in Next−Generation Microelectronics. ACS Appl. Mater. Interfaces 2024, 16, 2692–2703. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, M.; Horii, S. Heat−Resistant Polymers with Intense, Visible Photoluminescence Functionality and Fluorescence Probing Application. Macromol 2023, 3, 245–274. [Google Scholar] [CrossRef]
- Barzic, A.I.; Albu, R.M.; Stoica, I.; Varganici, C.D.; Hulubei, C. Polyimides containing cycloaliphatic units and chalcogen atoms as alternative shielding coatings for solar cells. Polym. Bull. 2023, 80, 4503–4522. [Google Scholar] [CrossRef]
- Low, J.-H.; Chee, P.-S.; Lim, E.-H. Cavity-backed double H-slot antenna with IPMC flaps for designing frequency-switchable On/In-metal semi-active tag. IEEE Trans. Antennas Propag. 2022, 71, 288–298. [Google Scholar] [CrossRef]
- Li, H.; Wang, X.; Gong, Y.; Zhao, H.; Liu, Z.; Tao, L.; Peng, Y.; Ma, K.; Hu, Z.; Dastan, D. Polyimide/crown ether composite film with low dielectric constant and low dielectric loss for high signal transmission. RSC Adv. 2023, 13, 7585–7596. [Google Scholar] [CrossRef]
- Li, H.; Wang, X.; Ding, Z.; Gao, W.; Liu, Y.; Ma, K.; Hu, Z.; Wang, Y. Crown Ether Copolymerized Polyimide Film: Enhanced Mechanical, Thermal Properties and Low Dielectric Constant under High Frequency. Polymers 2024, 16, 1188. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Wang, X.; Wu, T.; Gong, Y.; Zhao, H.; Liu, Z.; Dastan, D.; Ma, K.; Hu, Z. Mechanical enhancement and dielectric properties of SiO2 contained polyimides under high frequency. J. Mater. Sci. Mater. Electron. 2023, 34, 2310. [Google Scholar] [CrossRef]
- Li, H.; Wang, X.; Gong, Y.; Zhao, H.; Liu, Z.; Tao, L.; Dastan, D.; Ma, K.; Hu, Z.; Sun, M. APDS modified several bisphenol A polyimides with low dielectric constant under high frequency. J. Polym. Res. 2023, 30, 407. [Google Scholar] [CrossRef]
- Nawaz, H.; Akhter, Z.; Iqbal, N. Study of physicochemical properties of meta and ortho trifluoromethyl substituted isomeric aromatic polyimides. Polym. Bull. 2017, 74, 3889–3906. [Google Scholar] [CrossRef]
- Jin, W.; Johnston, P.V.; Elder, D.L.; Manner, K.T.; Garrett, K.E.; Kaminsky, W.; Xu, R.; Robinson, B.H.; Dalton, L.R. Structure–function relationship exploration for enhanced thermal stability and electro-optic activity in monolithic organic NLO chromophores. J. Mater. Chem. C 2016, 4, 3119–3124. [Google Scholar] [CrossRef]
- Lan, Z.; Chen, X.; Zhang, X.; Zhu, C.; Yu, Y.; Wei, J. Transparent, high glass-transition temperature, shape memory hybrid polyimides based on polyhedral oligomeric silsesquioxane. Polymers 2019, 11, 1058. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.-J.; Zheng, M.-S.; Chen, G.; Dang, Z.-M.; Zha, J.-W. High-temperature polyimide dielectric materials for energy storage: Theory, design, preparation and properties. Energy Environ. Sci. 2022, 15, 56–81. [Google Scholar] [CrossRef]
- Huang, S.; Zhang, Y.; Lai, X.; Lv, X.; Li, J.; Qiu, S.; Zhang, G.; Sun, R. A comprehensive study on the effect of molecular chain flexibility on the low-temperature curing ability of polyimides. J. Mater. Chem. C 2024, 12, 177–186. [Google Scholar] [CrossRef]
- Zhou, X.; Min, Y.; Zhao, C.; Chen, C.; Ke, M.-K.; Xu, S.-L.; Chen, J.-J.; Wu, Y.; Yu, H.-Q. Constructing sulfur and oxygen super-coordinated main-group electrocatalysts for selective and cumulative H2O2 production. Nat. Commun. 2024, 15, 193. [Google Scholar] [CrossRef]
- Yang, H.; Du, J. Crystallinity, Rheology, and Mechanical Properties of Low-/High-Molecular-Weight PLA Blended Systems. Molecules 2023, 29, 169. [Google Scholar] [CrossRef]
- Jin, C.; Wang, C.; Song, S.; Zhang, Y.; Wan, J.; He, L.; Qiao, Z.; E, P. Grafting Amino Groups onto Polyimide Films in Flexible Copper-Clad Laminates Using Helicon Plasma. Materials 2023, 16, 6214. [Google Scholar] [CrossRef] [PubMed]
- Cho, K.; Lee, D.; Ahn, T.O.; Seo, K.H.; Jeong, H.M. Adhesion behavior of PDMS-containing polyimide to glass. J. Adhes. Sci. Technol. 1998, 12, 253–269. [Google Scholar] [CrossRef]
- Gao, P.; Pu, W.; Wei, P.; Kong, M. Molecular dynamics simulations on adhesion energy of PDMS-silica interface caused by molecular structures and temperature. Appl. Surf. Sci. 2022, 577, 151930. [Google Scholar] [CrossRef]
- Hilbich, D.D. A New, Low-Cost, PDMS Metallization Process for Highly Conductive Flexible and Stretchable Electronics. Ph.D. Thesis, Simon Fraser University, Burnaby, BC, Canada, 2017. [Google Scholar]





| System Name | n(6FAPB) a (mmol) | n(BAPS) a (mmol) | n(BPADA) a (mmol) | Mass Fraction of PDMS (%) b | Remark |
|---|---|---|---|---|---|
| PI-1 | 15 | / | 15 | / | film |
| PI-1/1% | 15 | / | 15 | 1 | film |
| PI-1/3% | 15 | / | 15 | 3 | film |
| PI-1/5% | 15 | / | 15 | 5 | film |
| PI-2 | / | 15 | 15 | / | film |
| PI-2/1% | / | 15 | 15 | 1 | film |
| PI-2/3% | / | 15 | 15 | 3 | film |
| PI-2/5% | / | 15 | 15 | 5 | film |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Zhao, Y.; Li, H.; Gao, W.; Liu, Y.; Sun, A.; Ma, K.; Hu, Z.; Wang, Y. Adhesion and Transparency Enhancement between Flexible Polyimide-PDMS Copolymerized Film and Copper Foil for LED Transparent Screen. Polymers 2024, 16, 1591. https://doi.org/10.3390/polym16111591
Wang X, Zhao Y, Li H, Gao W, Liu Y, Sun A, Ma K, Hu Z, Wang Y. Adhesion and Transparency Enhancement between Flexible Polyimide-PDMS Copolymerized Film and Copper Foil for LED Transparent Screen. Polymers. 2024; 16(11):1591. https://doi.org/10.3390/polym16111591
Chicago/Turabian StyleWang, Xinming, Yuting Zhao, Heming Li, Weiguo Gao, Yan Liu, Anning Sun, Ke Ma, Zhizhi Hu, and Yongqi Wang. 2024. "Adhesion and Transparency Enhancement between Flexible Polyimide-PDMS Copolymerized Film and Copper Foil for LED Transparent Screen" Polymers 16, no. 11: 1591. https://doi.org/10.3390/polym16111591
APA StyleWang, X., Zhao, Y., Li, H., Gao, W., Liu, Y., Sun, A., Ma, K., Hu, Z., & Wang, Y. (2024). Adhesion and Transparency Enhancement between Flexible Polyimide-PDMS Copolymerized Film and Copper Foil for LED Transparent Screen. Polymers, 16(11), 1591. https://doi.org/10.3390/polym16111591






