Preparation and Characterization of Novel Multifunctional Wound Dressing by Near-Field Direct-Writing Electrospinning and Its Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials Required
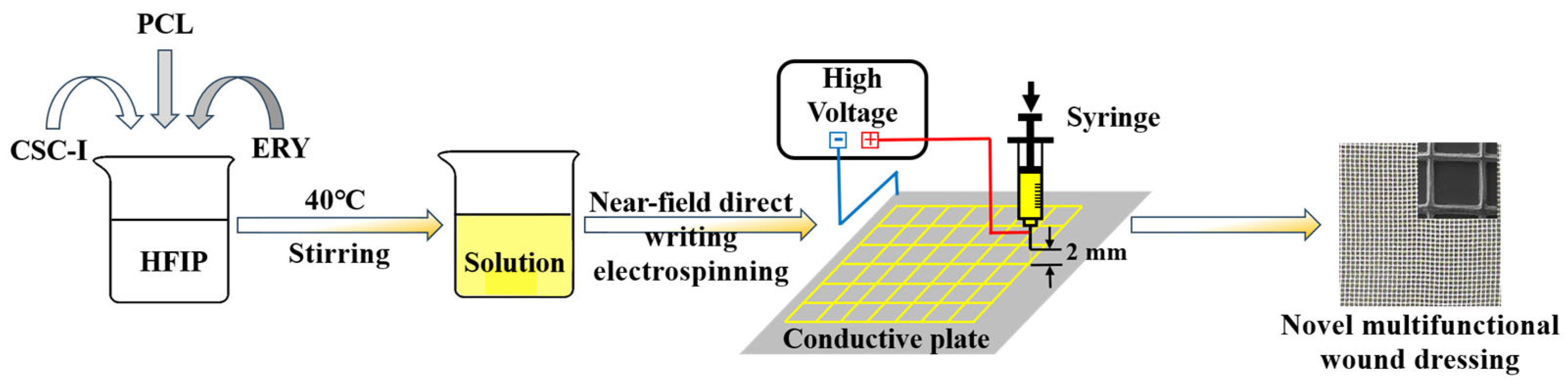
2.2. Experimental Methods
Preparation of Composite Fiber Membrane
2.3. Characterization
2.3.1. Mechanical Property Test
2.3.2. FTIR Analysis
2.3.3. XRD Analysis
2.3.4. Thermal Stability Analysis
2.3.5. SEM Analysis
2.3.6. Hydrophilicity and Water Absorption Performance Test
2.3.7. Determination of Drug Sustained-Release Performance In Vitro
2.3.8. Antibacterial Performance Test
3. Results and Discussion
3.1. SEM Analysis
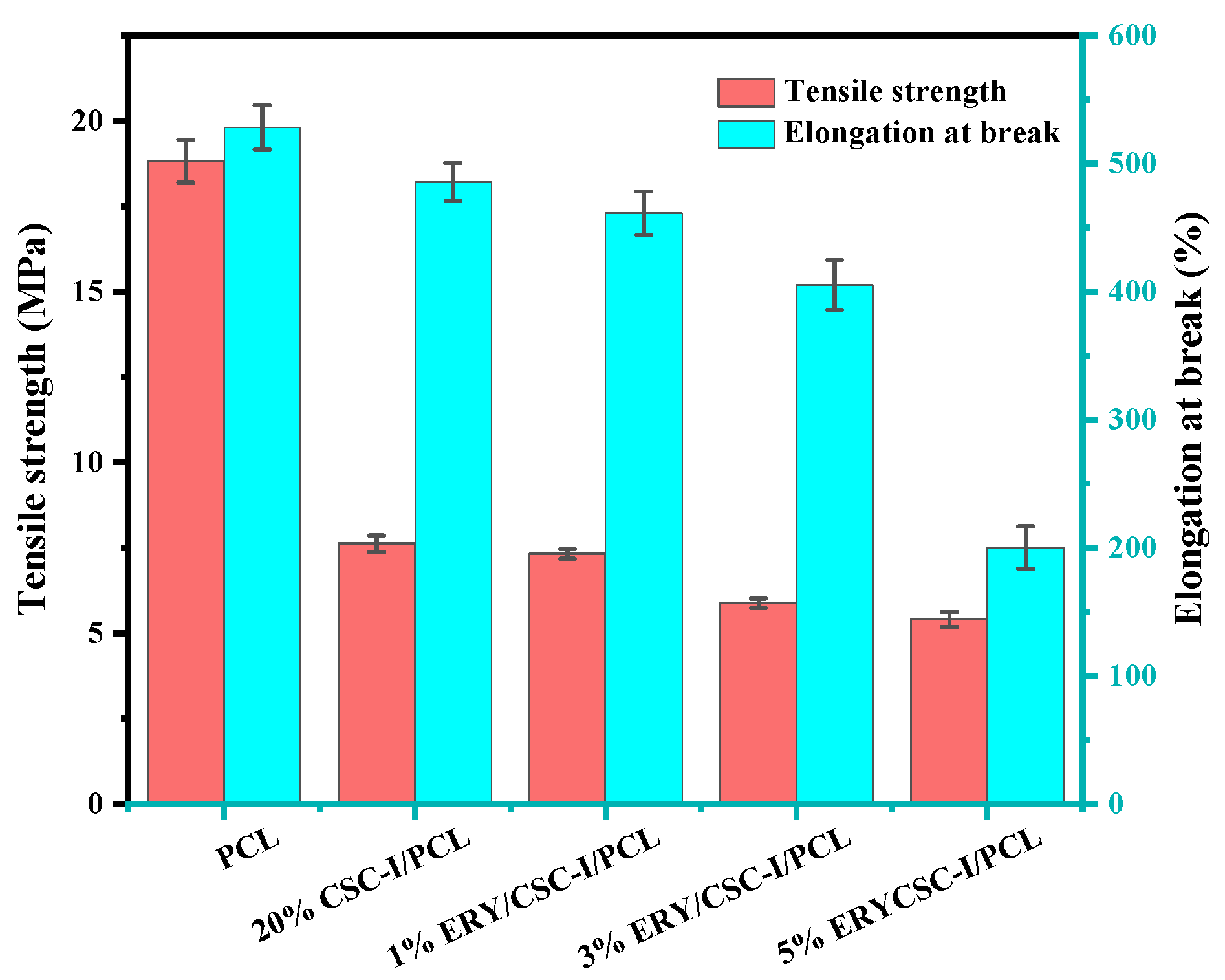
3.2. Mechanical Property Test
3.3. Hydrophilicity Analysis
3.4. Water Absorption Performance Test
3.5. FTIR Analysis
3.6. XRD Analysis
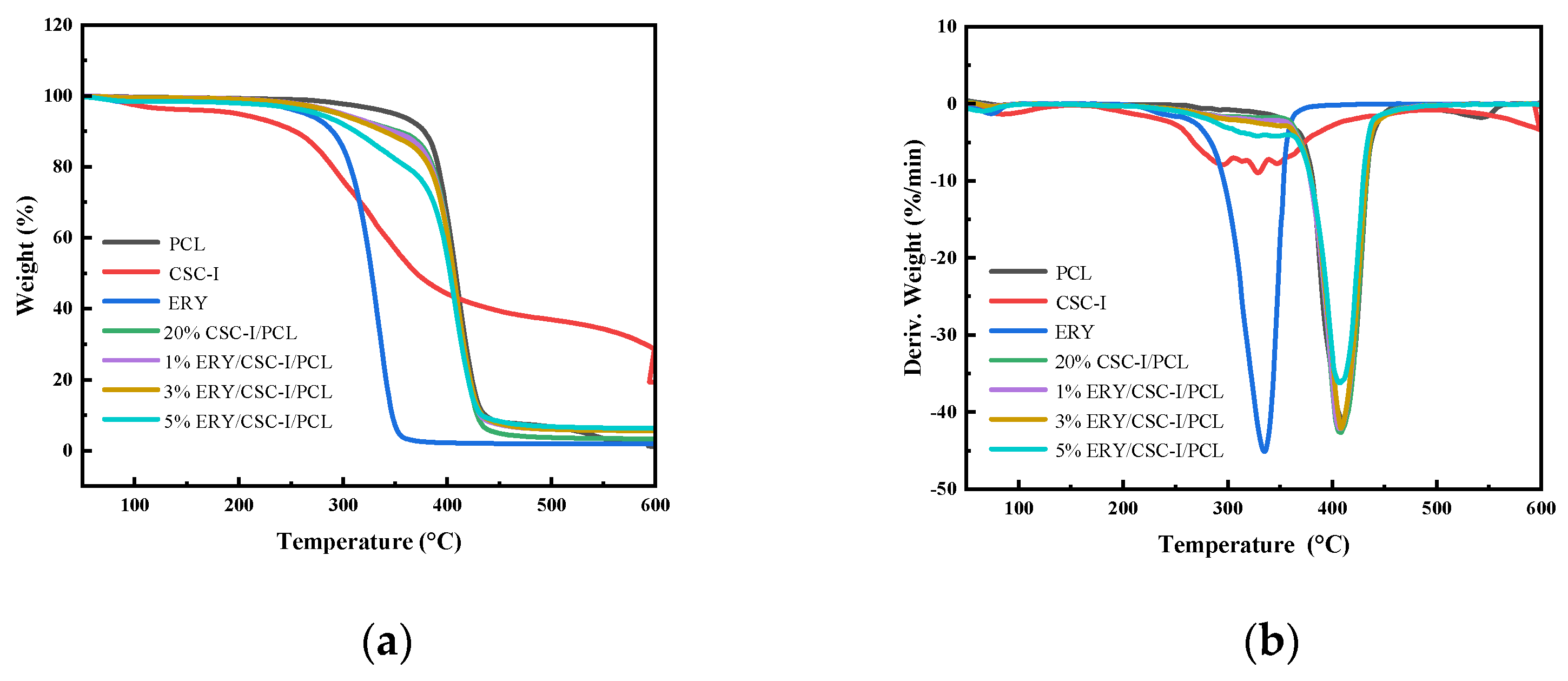
3.7. Thermal Stability Analysis
3.8. Determination of Drug Sustained-Release Performance In Vitro
3.9. Antibacterial Performance Test
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Chambers, E.S.; Vukmanovic-Stejic, M. Skin barrier immunity and ageing. Immunology 2020, 160, 116–125. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Jeong, S.K.; Ahn, S.K. An update of the defensive barrier function of skin. Yonsei Med. J. 2006, 47, 293–306. [Google Scholar] [CrossRef] [PubMed]
- Gruber, R.; Schmuth, M. Barrierefunktion der gesunden Haut. Der Hautarzt 2014, 65, 234–240. [Google Scholar] [CrossRef]
- Derakhshandeh, H.; Kashaf, S.S.; Aghabaglou, F.; Ghanavati, I.O.; Tamayol, A. Smart Bandages: The Future of Wound Care. Trends Biotechnol. 2018, 36, 1259–1274. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Min, T.; Bian, X.; Dong, Y.; Zhang, P.; Wen, Y. Rational Design of Intelligent and Multifunctional Dressing to Promote Acute/Chronic Wound Healing. ACS Appl. Bio Mater. 2022, 5, 4055–4085. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Bi, S.; Yan, D.; Zhou, Z.; Sun, G.; Cheng, X.; Chen, X. Preparation of composite hydroxybutyl chitosan sponge and its role in promoting wound healing. Carbohydr. Polym. 2018, 184, 154–163. [Google Scholar] [CrossRef]
- Kshirsagar, A.Y.; Vekariya, M.A.; Gupta, V.; Pednekar, A.S.; Mahna, A.; Pantakar, R.; Shaikh, A.; Nagur, B. A Comparative Study of Colostrum Dressing Versus Conventional Dressing in Deep Wounds. J. Clin. Diagn. Res. 2015, 9, PC01. [Google Scholar] [CrossRef] [PubMed]
- Bains, D.; Singh, G.; Kaur, N.; Signh, N. Development of Biological Self-Cleaning Wound-Dressing Gauze for the Treatment of Bacterial Infection. ACS Sustain. Chem. Eng. 2019, 7, 969–978. [Google Scholar] [CrossRef]
- Rezvani Ghomi, E.; Niazi, M.; Ramakrishna, S. The evolution of wound dressings: From traditional to smart dressings. Polym. Adv. Technol. 2023, 34, 520–530. [Google Scholar] [CrossRef]
- Xu, R.; Fang, Y.; Zhang, Z.; Cao, Y.; Yan, Y.; Gan, L.; Xu, J.; Zhou, G. Recent Advances in Biodegradable and Biocompatible Synthetic Polymers Used in Skin Wound Healing. Materials 2023, 16, 5459. [Google Scholar] [CrossRef]
- Curvello, R.; Raghuwanshi, V.S.; Garnier, G. Engineering nanocellulose hydrogels for biomedical applications. Adv. Colloid Interface Sci. 2019, 267, 47–61. [Google Scholar] [CrossRef] [PubMed]
- Moraes, P.R.F.D.; Saska, S.; Barud, H.; Lima, L.R.D.; Martins, V.D.C.A.; Plepis, A.M.D.G.; Ribeiro, S.J.L.; Gaspar, A.M.M. Bacterial Cellulose/Collagen Hydrogel for Wound Healing. Mater. Res. 2016, 19, 106–116. [Google Scholar] [CrossRef]
- Kilmer, C.E.; Battistoni, C.M.; Cox, A.; Breur, G.J.; Panitch, A.; Liu, J.C. Collagen Type I and II Blend Hydrogel with Autologous Mesenchymal Stem Cells as a Scaffold for Articular Cartilage Defect Repair. ACS Biomater. Sci. Eng. 2020, 6, 3464–3476. [Google Scholar] [CrossRef] [PubMed]
- Kosen, Y.; Miyaji, H.; Kato, A.; Sugaya, T.; Kawanami, M. Application of collagen hydrogel/sponge scaffold facilitates periodontal wound healing in class II furcation defects in beagle dogs. J. Periodontal Res. 2012, 47, 626–634. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Xu, Y.; Han, Y.; Cui, J.; Jing, Z.; Li, D.; Liu, J.; Xiao, C.; Li, D. Collagen-Based Hydrogels for Cartilage Regeneration. Orthop. Surg. 2023, 15, 3026–3045. [Google Scholar] [CrossRef]
- Dong, R.; Guo, B. Smart wound dressings for wound healing. Nano Today 2021, 41, 101290. [Google Scholar] [CrossRef]
- Sill, T.J.; Recum, H.A. Electrospinning: Applications in drug delivery and tissue engineering. Biomaterials 2008, 29, 1989–2006. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.; Chang, C.; Li, S.; Lin, L. Near-Field Electrospinning. Nano Lett. 2006, 6, 839–842. [Google Scholar] [CrossRef]
- He, X.; Zheng, J.; Yu, G.; You, M.; Yu, M.; Ning, X.; Long, Y. Near-Field Electrospinning: Progress and Applications. J. Phys. Chem. C 2017, 121, 8663–8678. [Google Scholar] [CrossRef]
- Bisht, G.S.; Canton, G.; Mirsepassi, A.; Kulinsky, L.; Oh, S.; Dunn-Rankin, D.; Madou, M.J. Controlled Continuous Patterning of Polymeric Nanofibers on Three-Dimensional Substrates Using Low-Voltage Near-Field Electrospinning. Nano Lett. 2011, 11, 1831–1837. [Google Scholar] [CrossRef]
- Pan, H.; Jiang, H.; Chen, W. Interaction of dermal fibroblasts with electrospun composite polymer scaffolds prepared from dextran and poly lactide-co-glycolide. Biomaterials 2006, 27, 3209–3220. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.H.; Khil, M.S.; Kim, H.Y.; Lee, H.U.; Jahng, K.Y. An improved hydrophilicity via electrospinning for enhanced cell attachment and proliferation. J. Biomed. Mater. Res. Part B Appl. Biomater. 2006, 78, 283–290. [Google Scholar] [CrossRef] [PubMed]
- Lee, I.S.; Kwon, O.H.; Meng, W.; Kang, I.; Ito, Y. Nanofabrication of Microbial Polyester by Electrospinning Promotes Cell Attachment. Macromol. Res. 2004, 12, 374–378. [Google Scholar] [CrossRef]
- Patel, S.; Kurpinski, K.; Quigley, R.; Gao, H.; Hsiao, B.S.; Poo, M.; Li, S. Bioactive Nanofibers: Synergistic Effects of Nanotopography and Chemical Signaling on Cell Guidance. Nano Lett. 2007, 7, 2122–2128. [Google Scholar] [CrossRef]
- Subramanian, S.; Karuppannan, S.K.; Ramalingam, R.; Dowlath, M.J.H.; Mohamed Khalith, S.B.; Anjum Musthafa, S.; Chitra, V.; Munuswamy-Ramanujam, G.; Arunachalam, K.D. Effect of gamma sterilization on Gymnema sylvestre leaf extract fused Polycaprolactone nanofiber for effective wound dressing applications. Mater. Lett. 2021, 300, 130145. [Google Scholar] [CrossRef]
- Jing, X.; Mi, H.; Wang, X.; Peng, X.; Turng, L. Shish-Kebab-Structured Poly(ε-Caprolactone) Nanofibers Hierarchically Decorated with Chitosan–Poly(ε-Caprolactone) Copolymers for Bone Tissue Engineering. ACS Appl. Mater. Interfaces 2015, 7, 6955–6965. [Google Scholar] [CrossRef] [PubMed]
- Chia, H.N.; Wu, B.M. Recent advances in 3D printing of biomaterials. J. Biol. Eng. 2015, 9, 4. [Google Scholar] [CrossRef]
- Woodruff, M.A.; Hutmacher, D.W. The return of a forgotten polymer—Polycaprolactone in the 21st century. Prog. Polym. Sci. 2010, 35, 1217–1256. [Google Scholar] [CrossRef]
- Hulmes, D.J.S. Building Collagen Molecules, Fibrils, and Suprafibrillar Structures. J. Struct. Biol. 2002, 137, 2–10. [Google Scholar] [CrossRef]
- Wu, S.; Applewhite, A.J.; Niezgoda, J.; Snyder, R.; Shah, J.; Cullen, B.; Schultz, G.; Harrison, J.; Hill, R.; Howell, M.; et al. Oxidized Regenerated Cellulose/Collagen Dressings: Review of Evidence and Recommendations. Adv. Ski. Wound Care 2017, 30, S1–S18. [Google Scholar] [CrossRef]
- Singh, O.; Gupta, S.S.; Soni, M.; Moses, S.; Shukla, S.; Mathur, R.K. Collagen dressing versus conventional dressings in burn and chronic wounds: A retrospective study. J. Cutan. Aesthet. Surg. 2011, 4, 12–16. [Google Scholar] [CrossRef]
- Ding, C.; Tian, M.; Fengm, R.; Dang, Y.; Zhang, M. Novel Self-Healing Hydrogel with Injectable, pH-Responsive, Strain Sensitive, Promoting Wound-Healing, and Hemostatic Properties Based on Collagen and Chitosan. Acs Biomater. Sci. Eng. 2020, 6, 3855–3867. [Google Scholar] [CrossRef]
- Wang, T.; Yang, L.; Wang, G.; Han, L.; Chen, K.; Liu, P.; Xu, S.; Li, D.; Xie, Z.; Mo, X.; et al. Biocompatibility, hemostatic properties, and wound healing evaluation of tilapia skin collagen sponges. J. Bioact. Compat. Polym. 2021, 36, 44–58. [Google Scholar] [CrossRef]
- Jung, K.; Kim, S.; Joo, K.; Lim, S.; Shin, J.; Roh, J.; Kim, E.; Park, C.W.; Kim, W. Oral Intake of Enzymatically Decomposed AP Collagen Peptides Improves Skin Moisture and Ceramide and Natural Moisturizing Factor Contents in the Stratum Corneum. Nutrients 2021, 13, 4372. [Google Scholar] [CrossRef]
- Champney, W.S.; Pelt, J. The Ketolide Antibiotic ABT-773 Is a Specific Inhibitor of Translation and 50S Ribosomal Subunit Formation in Streptococcus pneumoniae Cells. Curr. Microbiol. 2002, 45, 155–160. [Google Scholar] [CrossRef] [PubMed]
- Champney, W.S. Bacterial ribosomal subunit synthesis: A novel antibiotic target. Curr. Drug Targets. Infect. Disord. 2001, 1, 19. [Google Scholar] [CrossRef] [PubMed]
- Usary, J.; Champney, W.S. Erythromycin inhibition of 50S ribosomal subunit formation in Escherichia coli cells. Mol. Microbiol. 2001, 40, 951–962. [Google Scholar] [CrossRef]
- Chen, Z.; Wei, B.; Mo, X.; Lim, C.T.; Ramakrishna, S.; Cui, F. Mechanical properties of electrospun collagen–chitosan complex single fibers and membrane. Mater. Sci. Eng. C 2009, 29, 2428–2435. [Google Scholar] [CrossRef]
- Prasad, T.; Shabeena, E.A.; Vinod, D.; Kumary, T.V.; Anil Kumar, P.R. Characterization and in vitro evaluation of electrospun chitosan/polycaprolactone blend fibrous mat for skin tissue engineering. J. Mater. Sci. Mater. Med. 2015, 26, 28. [Google Scholar] [CrossRef]
- Pandey, V.K.; Upadhyay, S.N.; Niranjan, K.; Mishra, P.K. Antimicrobial biodegradable chitosan-based composite Nano-layers for food packaging. Int. J. Biol. Macromol. 2020, 157, 212–219. [Google Scholar] [CrossRef]
- Wang, M.; Ge, R.; Zhang, F.; Yu, D.; Liu, Z.; Li, X.; Shen, H.; Williams, G.R. Electrospun fibers with blank surface and inner drug gradient for improving sustained release. Biomater. Adv. 2023, 150, 213404. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Zhang, J.; Hanm, J.; Yao, B. A numerical solution to the effects of surface roughness on water–coal contact angle. Sci. Rep. 2021, 11, 459. [Google Scholar] [CrossRef]
- Morsy, R.; Hosny, M.; Reicha, F.; Elnimr, T. Developing a potential antibacterial long-term degradable electrospun gelatin-based composites mats for wound dressing applications. React. Funct. Polym. 2017, 114, 8–12. [Google Scholar] [CrossRef]
- Ghosal, K.; Thomas, S.; Kalarikkal, N.; Gnanamani, A. Collagen coated electrospun polycaprolactone (PCL) with titanium di-oxide (TiO2) from an environmentally benign solvent: Preliminary physico-chemical studies for skin substitute. J. Polym. Res. 2014, 21, 410. [Google Scholar] [CrossRef]
- Avramov Ivić, M.L.; Petrović, S.D.; Mijin, D.Ž.; Vanmoos, F.; Orlović, D.Ž.; Marjanović, D.Ž.; Radović, V.V. The electrochemical behavior of erythromycin A on a gold electrode. Electrochim. Acta 2008, 54, 649–654. [Google Scholar] [CrossRef]
- Cai, J.; Xiong, Z.; Zhou, M.; Tan, J.; Zeng, F.; Ma, H.; Lin, S.; Xiong, H. Thermal properties and crystallization behavior of thermoplastic starch/poly(ε-caprolactone) composites. Carbohydr. Polym. 2014, 102, 746–754. [Google Scholar] [CrossRef]







Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, D.; Lin, D.; Li, Y.; Xu, S.; Cao, Q.; Zhou, W. Preparation and Characterization of Novel Multifunctional Wound Dressing by Near-Field Direct-Writing Electrospinning and Its Application. Polymers 2024, 16, 1573. https://doi.org/10.3390/polym16111573
Li D, Lin D, Li Y, Xu S, Cao Q, Zhou W. Preparation and Characterization of Novel Multifunctional Wound Dressing by Near-Field Direct-Writing Electrospinning and Its Application. Polymers. 2024; 16(11):1573. https://doi.org/10.3390/polym16111573
Chicago/Turabian StyleLi, Dingfan, Dongsong Lin, Yun Li, Sikun Xu, Qingyun Cao, and Wuyi Zhou. 2024. "Preparation and Characterization of Novel Multifunctional Wound Dressing by Near-Field Direct-Writing Electrospinning and Its Application" Polymers 16, no. 11: 1573. https://doi.org/10.3390/polym16111573
APA StyleLi, D., Lin, D., Li, Y., Xu, S., Cao, Q., & Zhou, W. (2024). Preparation and Characterization of Novel Multifunctional Wound Dressing by Near-Field Direct-Writing Electrospinning and Its Application. Polymers, 16(11), 1573. https://doi.org/10.3390/polym16111573




