Surface Reconstruction of Silicone-Based Amphiphilic Polymers for Mitigating Marine Biofouling
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
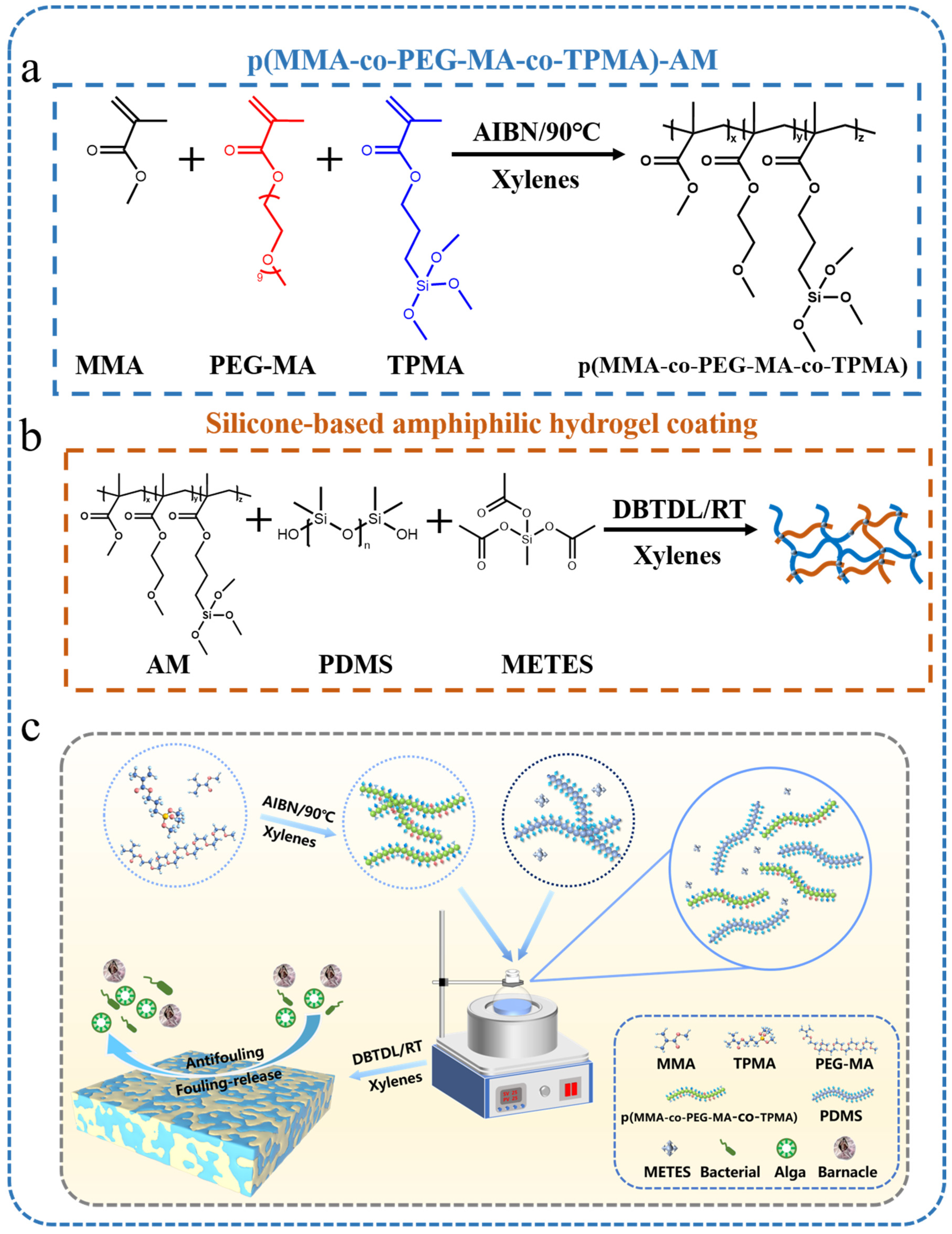
2.2. Synthesis of Poly(MMA-co-PEG-MA-co-TPMA)
2.3. Coating Preparation
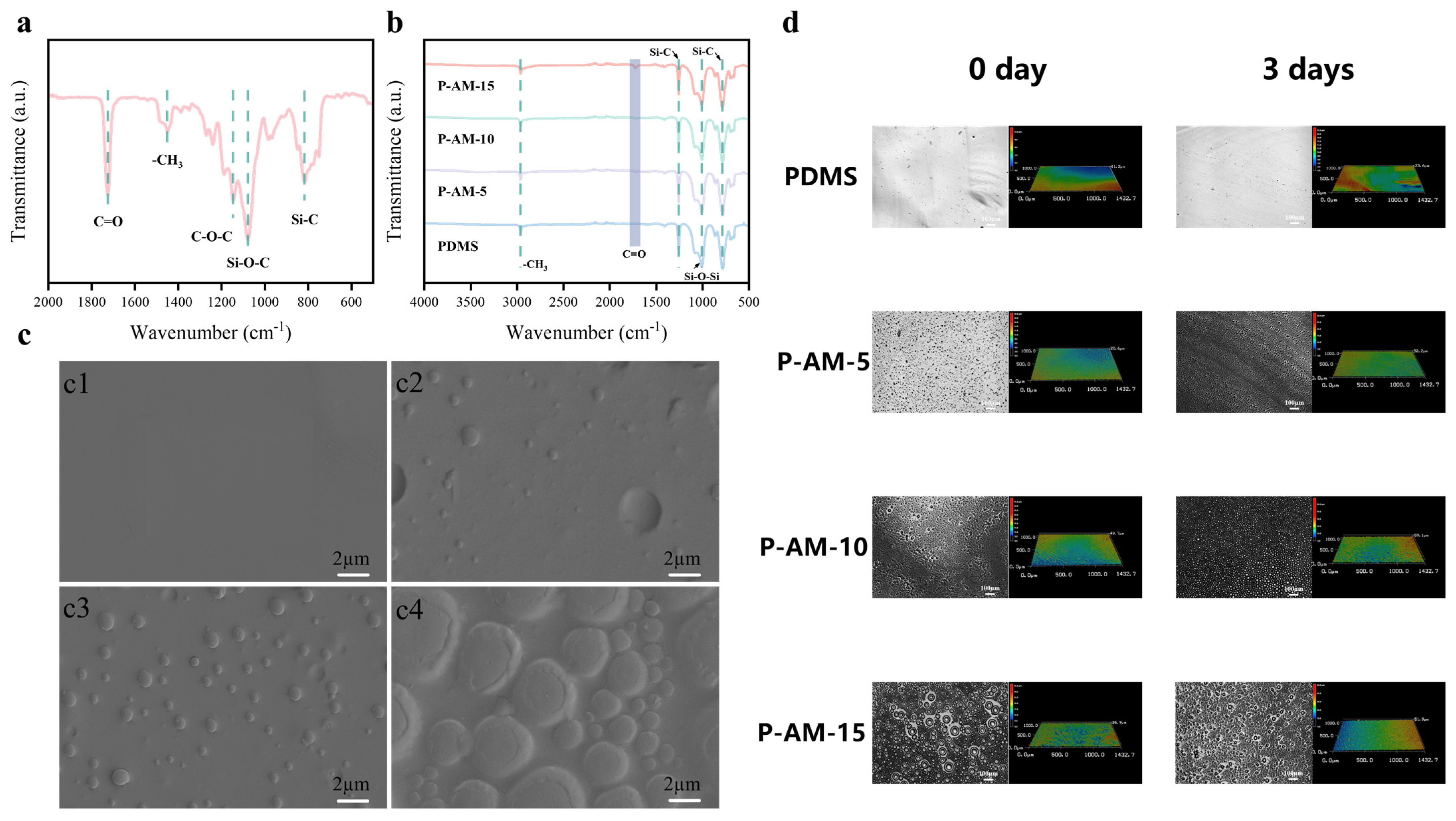
2.4. Characterization
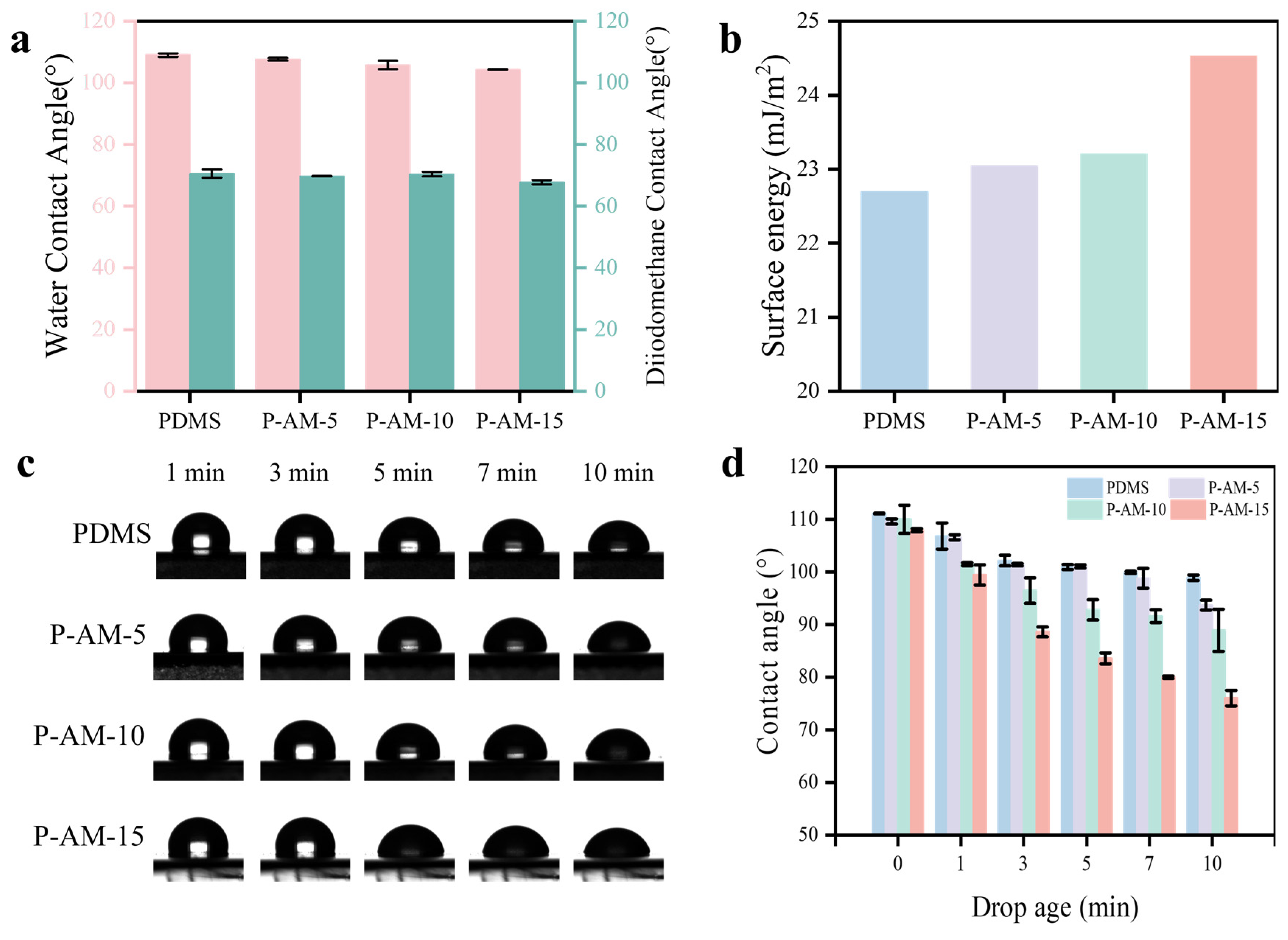
2.5. Contact Angle Measurement
2.6. FITC-BSA Adsorption Tests
2.7. Bacterial Adhesion Test
2.8. Algal Resistance Tests
2.9. Marine Field Test
3. Results and Discussion
3.1. Chemical Characterization of P-AM Coatings
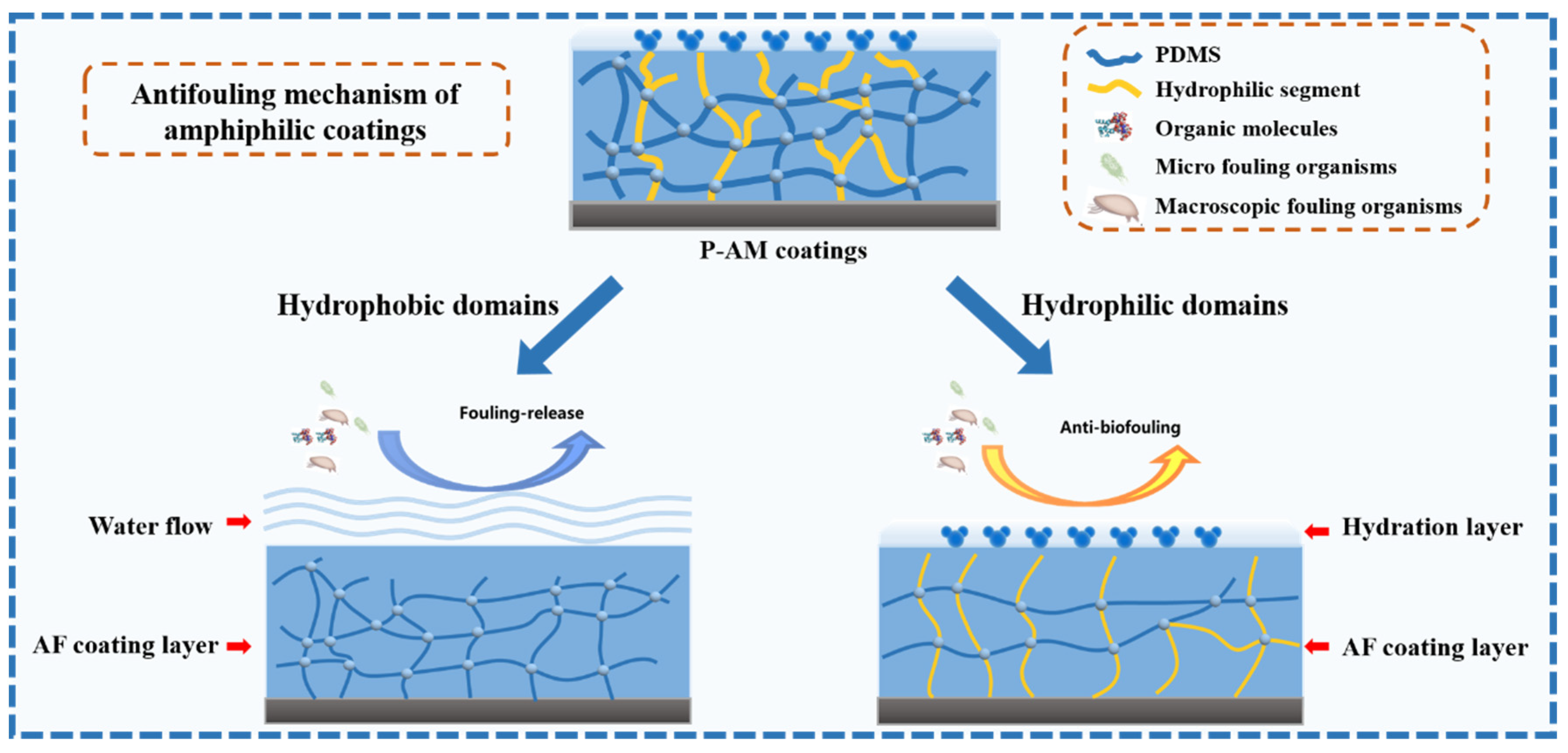
3.2. Antifouling Properties of the P-AM Coatings
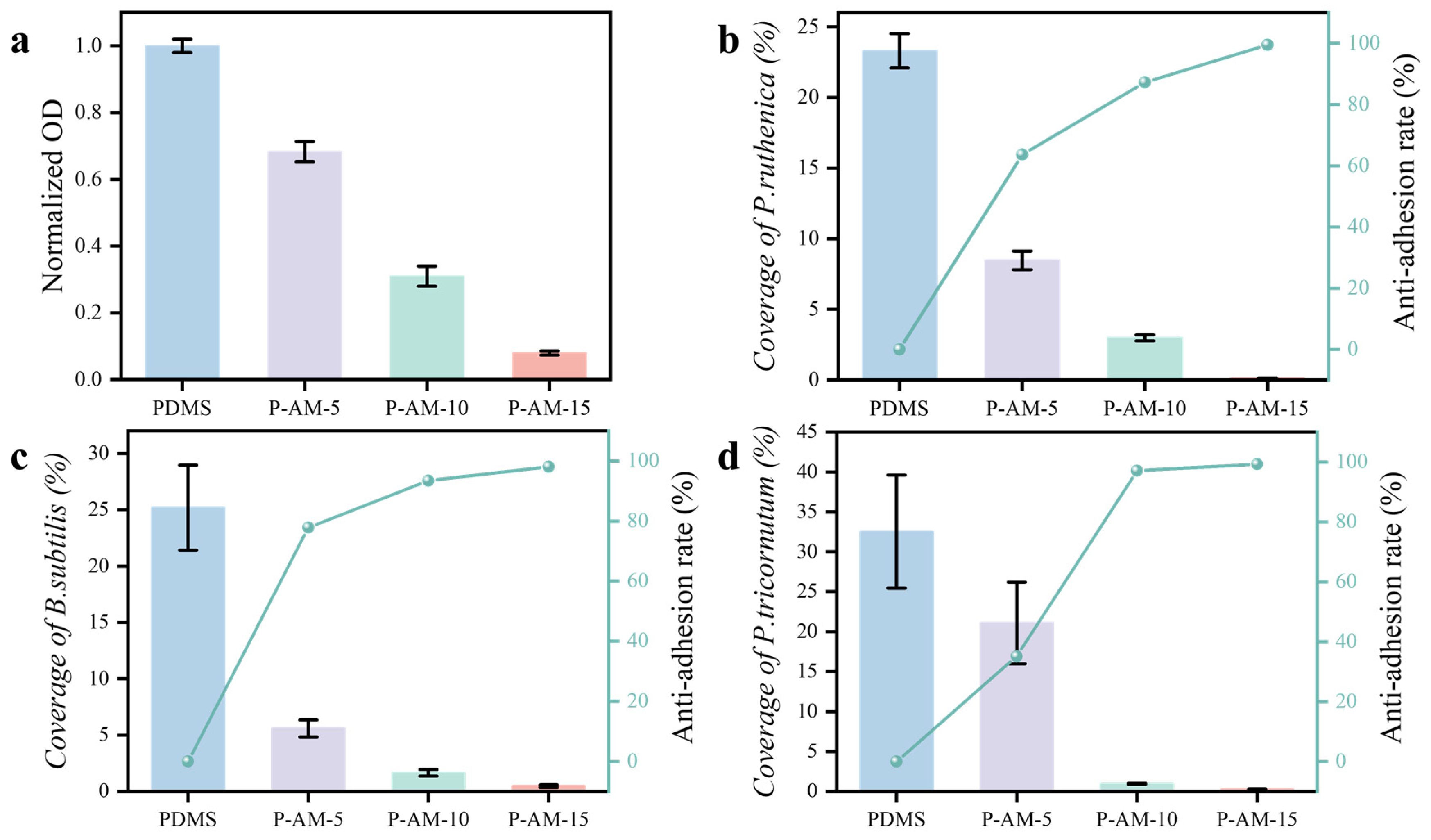
3.2.1. FITC-BSA Adsorption Tests
3.2.2. Bacterial Adhesion Test
3.2.3. Alga Resistance Tests
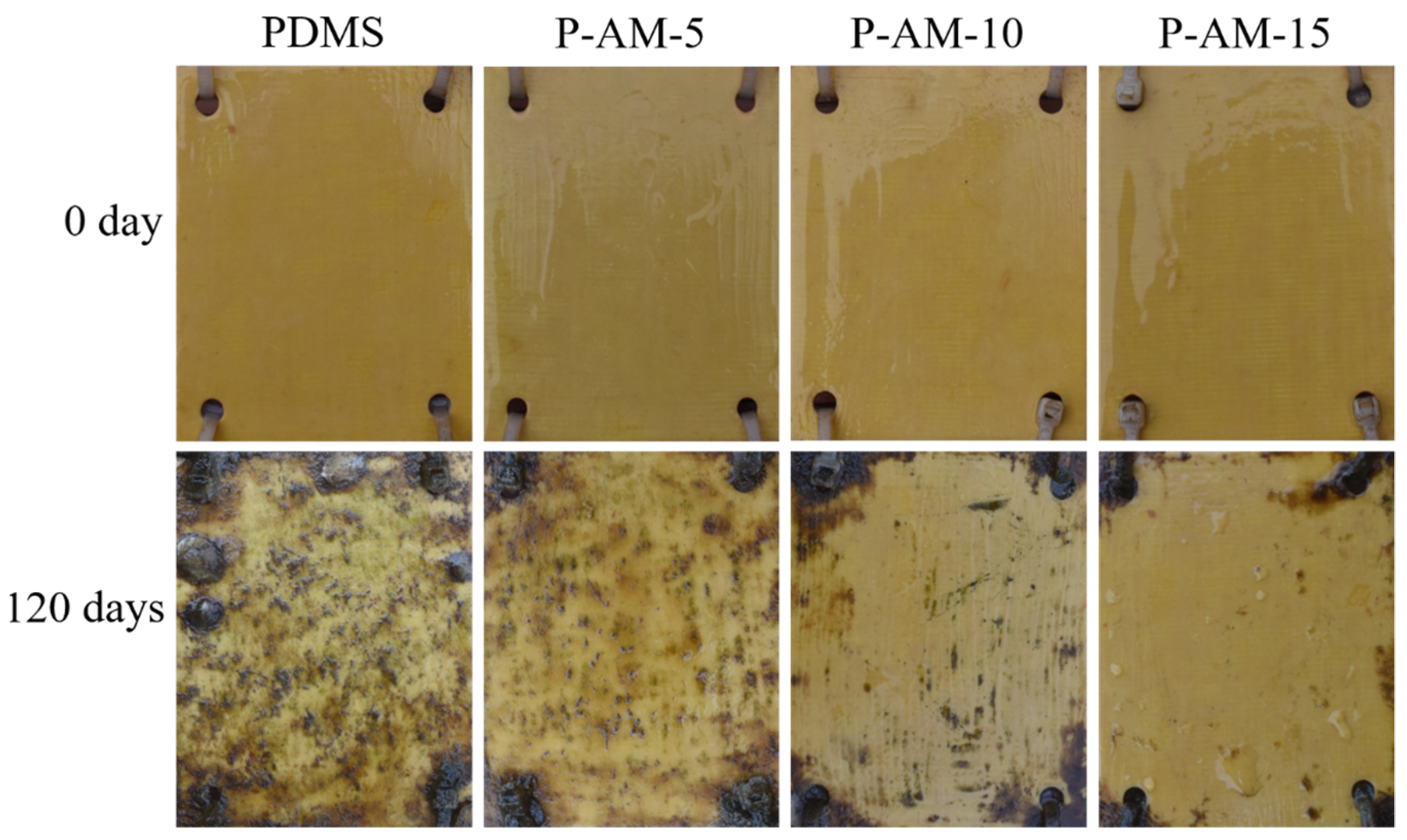
3.2.4. Marine Field Test
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Yebra, D.M.; Kiil, S.; Dam-Johansen, K. Antifouling technology—Past, present and future steps towards efficient and environmentally friendly antifouling coatings. Prog. Org. Coat. 2004, 50, 75–104. [Google Scholar] [CrossRef]
- Dafforn, K.A.; Lewis, J.A.; Johnston, E.L. Antifouling strategies: History and regulation, ecological impacts and mitigation. Mar. Pollut. Bull. 2011, 62, 453–465. [Google Scholar] [CrossRef] [PubMed]
- Schultz, M.P. Effects of coating roughness and biofouling on ship resistance and powering. Biofouling 2007, 23, 331–341. [Google Scholar] [CrossRef] [PubMed]
- Schultz, M.P.; Bendick, J.A.; Holm, E.R.; Hertel, W.M. Economic impact of biofouling on a naval surface ship. Biofouling 2011, 27, 87–98. [Google Scholar] [CrossRef] [PubMed]
- Dahllof, I.; Agrenius, S.; Blanck, H. The Effect of TBT on the Structure of a Marine Sediment Community—A Boxcosm Study. Mar. Pollut. Bull. 2001, 42, 689–695. [Google Scholar] [CrossRef] [PubMed]
- Ahn, I.Y.; Chung, K.H.; Choi, H.J. Influence of glacial runoff on baseline metal accumulation in the Antarctic limpet Nacella concinna from Ring George Island. Mar. Pollut. Bull. 2004, 49, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Omae, I. General Aspects of Tin-Free Antifouling Paints. Chem. Rev. 2003, 103, 3431–3448. [Google Scholar] [CrossRef]
- Kim, J.; Chisholm, B.J.; Bahr, J. Adhesion study of silicone coatings: The interaction of thickness, modulus and shear rate on adhesion force. Biofouling 2007, 23, 113–120. [Google Scholar] [CrossRef]
- Kaffashi, A.; Jannesari, A.; Ranjbar, Z. Silicone fouling-release coatings: Effects of the molecular weight of poly(dimethylsiloxane) and tetraethyl orthosilicate on the magnitude of pseudobarnacle adhesion strength. Biofouling 2012, 28, 729–741. [Google Scholar] [CrossRef]
- Callow, J.; Callow, M. Trends in the development of environmentally friendly fouling-resistant marine coatings. Nat. Commun. 2011, 2, 244. [Google Scholar] [CrossRef]
- Sokolova, A.; Cilz, N.; Daniels, J.; Stafslien, S.J.; Brewer, L.H.; Wendt, D.E.; Detty, M.R. A comparison of the antifouling/foul-release characteristics of non-biocidal xerogel and commercial coatings toward micro- and macrofouling organisms. Biofouling 2012, 28, 511–523. [Google Scholar] [CrossRef] [PubMed]
- Molino, P.J.; Childs, S.; Hubbard, M.R.E.; Carey, J.M.; Burgman, M.A.; Wetherbee, R. Development of the primary bacterial microfouling layer on antifouling and fouling release coatings in temperate and tropical environments in Eastern Australia. Biofouling 2009, 25, 149–162. [Google Scholar] [CrossRef] [PubMed]
- Feng, S.; Wang, Q.; Gao, Y.; Huang, Y.; Qing, F.L. Synthesis and characterization of a novel amphiphilic copolymer capable as anti-biofouling coating material. J. Appl. Polym. Sci. 2010, 114, 2071–2078. [Google Scholar] [CrossRef]
- Park, D.; Weinman, C.J.; Finlay, J.A.; Fletcher, B.R.; Paik, M.Y.; Sundaram, H.S.; Dimitriou, M.D.; Sohn, K.E.; Callow, M.E.; Callow, J.A. Amphiphilic surface active triblock copolymers with mixed hydrophobic and hydrophilic side chains for tuned marine fouling-release properties. Langmuir ACS J. Surf. Colloids 2010, 26, 9772–9781. [Google Scholar] [CrossRef] [PubMed]
- Leonardi, A.K.; Ober, C.K. Polymer-Based Marine Antifouling and Fouling Release Surfaces: Strategies for Synthesis and Modification. Annu. Rev. Chem. Biomol. Eng. 2019, 10, 241–264. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Finlay, J.A.; Betts, D.E.; Merkel, T.J.; Luft, J.C.; Callow, M.E.; Callow, J.A.; DeSimone, J.M. Amphiphilic Co-networks with Moisture-Induced Surface Segregation for High-Performance Nonfouling Coatings. Langmuir 2011, 27, 10365–10369. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Betts, D.E.; Finlay, J.A.; Brewer, L.; Callow, M.E.; Callow, J.A.; Wendt, D.E.; DeSimone, J.M. Photocurable Amphiphilic Perfluoropolyether/Poly(ethylene glycol) Networks for Fouling-Release Coatings. Macromolecules 2011, 44, 878–885. [Google Scholar] [CrossRef]
- Pollack, K.A.; Imbesi, P.M.; Raymond, J.E.; Wooley, K.L. Hyperbranched Fluoropolymer-Polydimethylsiloxane-Poly(ethylene glycol) Cross-Linked Terpolymer Networks Designed for Marine and Biomedical Applications: Heterogeneous Nontoxic Antibiofouling Surfaces. ACS Appl. Mater. Interfaces 2014, 6, 19265–19274. [Google Scholar] [CrossRef] [PubMed]
- Govorun, E.N.; Chertovich, A.V. Microphase separation in random multiblock copolymers. J. Chem. Phys. 2017, 146, 034903. [Google Scholar] [CrossRef]
- Zhao, W.; Yang, J.; Guo, H.; Xu, T.; Li, Q.; Wen, C.; Sui, X.; Lin, C.; Zhang, J.; Zhang, L. Slime-resistant marine anti-biofouling coating with PVP-based copolymer in PDMS matrix. Chem. Eng. Sci. 2019, 207, 790–798. [Google Scholar] [CrossRef]
- Guo, H.; Yang, J.; Xu, W.; Lin, T.; Zhang, C.; Zhang, J.; Zhang, L. Direct formation of amphiphilic crosslinked networks based on PVP as a marine anti-biofouling coating. Chem. Eng. J. 2019, 374, 1353–1363. [Google Scholar] [CrossRef]
- Zeng, H.; Xie, Q.; Ma, C.; Zhang, G. Silicone Elastomer with Surface-Enriched, Nonleaching Amphiphilic Side Chains for Inhibiting Marine Biofouling. ACS Appl. Polym. Mater. 2019, 1, 1689–1696. [Google Scholar] [CrossRef]
- Gudipati, C.S.; Finlay, J.A.; Callow, J.A.; Callow, M.E.; Wooley, K.L. The Antifouling and Fouling-Release Perfomance of Hyperbranched Fluoropolymer (HBFP)−Poly(ethylene glycol) (PEG) Composite Coatings Evaluated by Adsorption of Biomacromolecules and the Green Fouling Alga Ulva. Langmuir 2005, 21, 3044–3053. [Google Scholar] [CrossRef]
- Yao, X.; Liu, J.; Yang, C.; Yang, X.; Wei, J.; Xia, Y.; Gong, X.; Suo, Z. Hydrogel Paint. Adv. Mater. 2019, 31, 1903062. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Liu, Y.; Wang, C.; Wang, S.; Müller-Steinhagen, H. Effect of surface free energy on the adhesion of biofouling and crystalline fouling. Chem. Eng. Sci. 2005, 60, 4858–4865. [Google Scholar] [CrossRef]
- Owens, D.K.; Wendt, R.C. Estimation of the surface free energy of polymers. J. Appl. Polym. Sci. 1969, 13, 1741–1747. [Google Scholar] [CrossRef]
- Lejars, M.; Margaillan, A.; Bressy, C. Fouling Release Coatings: A Nontoxic Alternative to Biocidal Antifouling Coatings. Chem. Rev. 2012, 112, 4347–4390. [Google Scholar] [CrossRef] [PubMed]
- Lu, G.; Tian, S.; Li, J.; Xu, Y.; Liu, S.; Pu, J. Fabrication of bio-based amphiphilic hydrogel coating with excellent antifouling and mechanical properties. Chem. Eng. J. 2021, 409, 128134. [Google Scholar] [CrossRef]
- Xie, M.; Wang, Y.; Zhao, W. Design novel three-dimensional network nanostructure for lubricant infused on titanium alloys towards long-term anti-fouling. Colloids Surf. B Biointerfaces 2021, 197, 111375. [Google Scholar] [CrossRef]
- ASTM D6990-05; Standard Practice for Evaluating Biofouling Resistance and Physical Performance of Marine Coating Systems. ASTM: West Conshohocken, PA, USA, 2011.
- Guo, H.; Chen, P.; Tian, S.; Ma, Y.; Zhang, L. Amphiphilic Marine Antifouling Coatings Based on a Hydrophilic Polyvinylpyrrolidone and Hydrophobic Fluorine–Silicon-Containing Block Copolymer. Langmuir 2020, 36, 14573–14581. [Google Scholar] [CrossRef]
- Zhang, Q.; Hong, J.; Hoogenboom, R. A triple thermoresponsive schizophrenic diblock copolymer. Polym. Chem 2013, 4, 4322. [Google Scholar] [CrossRef]
- Matsumoto, M.; Takenaka, M.; Sawamoto, M.; Terashima, T. Self-assembly of amphiphilic block pendant polymers as microphase separation materials and folded flower micelles. Polym. Chem 2019, 10, 4954. [Google Scholar] [CrossRef]
- Senkum, H.; Kelly, P.V.; Gramlich, W.M. Water-Stable Thin-Film Nanostructures from Amphiphilic Cationic Bottlebrush Block Copolymers by Grafting-through Ring-Opening Metathesis Polymerization. Macromolecules 2021, 54, 7987–7997. [Google Scholar] [CrossRef]
- Su, X.; Yang, M.; Hao, D.; Guo, X.; Jiang, L. Marine antifouling coatings with surface topographies triggered by phase segregation. J. Colloid Interface Sci. 2021, 598, 104–112. [Google Scholar] [CrossRef] [PubMed]
- Brady, R.F. A fracture mechanical analysis of fouling release from nontoxic antifouling coatings. Prog. Org. Coat. 2001, 43, 188–192. [Google Scholar] [CrossRef]
- Zhang, H.; Chiao, M. Anti-fouling Coatings of Poly(dimethylsiloxane) Devices for Biological and Biomedical Applications. J. Med. Biol. Eng. 2015, 35, 143–155. [Google Scholar] [CrossRef] [PubMed]
- Salta, M.; Wharton, J.A.; Blache, Y.; Stokes, K.R. Marine biofilms on artificial surfaces: Structure and dynamics. Environ. Microbiol. 2013, 15, 2879–2893. [Google Scholar] [CrossRef] [PubMed]
- Xie, Q.; Zeng, H.; Peng, Q.; Bressy, C.; Zhang, G. Self-Stratifying Silicone Coating with Nonleaching Antifoulant for Marine Anti-Biofouling. Adv. Mater. Interfaces 2019, 6, 1900535. [Google Scholar] [CrossRef]
- Wang, Y.; Pitet, L.M.; Finlay, J.A.; Brewer, L.H.; Cone, G.; Betts, D.E.; Callow, M.E.; Callow, J.A.; Wendt, D.E.; Hillmyer, M.A. Investigation of the role of hydrophilic chain length in amphiphilic perfluoropolyether/poly(ethylene glycol) networks: Towards high-performance antifouling coatings. Biofouling 2011, 27, 1139–1150. [Google Scholar] [CrossRef]
- Krishnan, S.; Ayothi, R.; Hexemer, A.; Finlay, J.A.; Sohn, K.E.; Perry, R.; Ober, C.K.; Kramer, E.J.; Callow, M.E.; Callow, J.A. Anti-biofouling properties of comblike block copolymers with amphiphilic side chains. Langmuir ACS J. Surf. Colloids 2006, 22, 5075–5086. [Google Scholar] [CrossRef]
- Stein, J.; Truby, K.; Wood, C.D.; Stein, J.; Gardner, M.; Swain, G.; Kavanagh, C.; Kovach, B.; Schultz, M.; Wiebe, D. Silicone Foul Release Coatings: Effect of the Interaction of Oil and Coating Functionalities on the Magnitude of Macrofouling Attachment Strengths. Biofouling 2003, 19, 71–82. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wei, C.; Zhang, Y.; Tang, Z.; Zhang, C.; Wu, J.; Wu, B. Surface Reconstruction of Silicone-Based Amphiphilic Polymers for Mitigating Marine Biofouling. Polymers 2024, 16, 1570. https://doi.org/10.3390/polym16111570
Wei C, Zhang Y, Tang Z, Zhang C, Wu J, Wu B. Surface Reconstruction of Silicone-Based Amphiphilic Polymers for Mitigating Marine Biofouling. Polymers. 2024; 16(11):1570. https://doi.org/10.3390/polym16111570
Chicago/Turabian StyleWei, Chuanying, Yan Zhang, Zhen Tang, Changan Zhang, Jianhua Wu, and Bo Wu. 2024. "Surface Reconstruction of Silicone-Based Amphiphilic Polymers for Mitigating Marine Biofouling" Polymers 16, no. 11: 1570. https://doi.org/10.3390/polym16111570
APA StyleWei, C., Zhang, Y., Tang, Z., Zhang, C., Wu, J., & Wu, B. (2024). Surface Reconstruction of Silicone-Based Amphiphilic Polymers for Mitigating Marine Biofouling. Polymers, 16(11), 1570. https://doi.org/10.3390/polym16111570



