Depollution of Polymeric Leather Waste by Applying the Most Current Methods of Chromium Extraction
Abstract
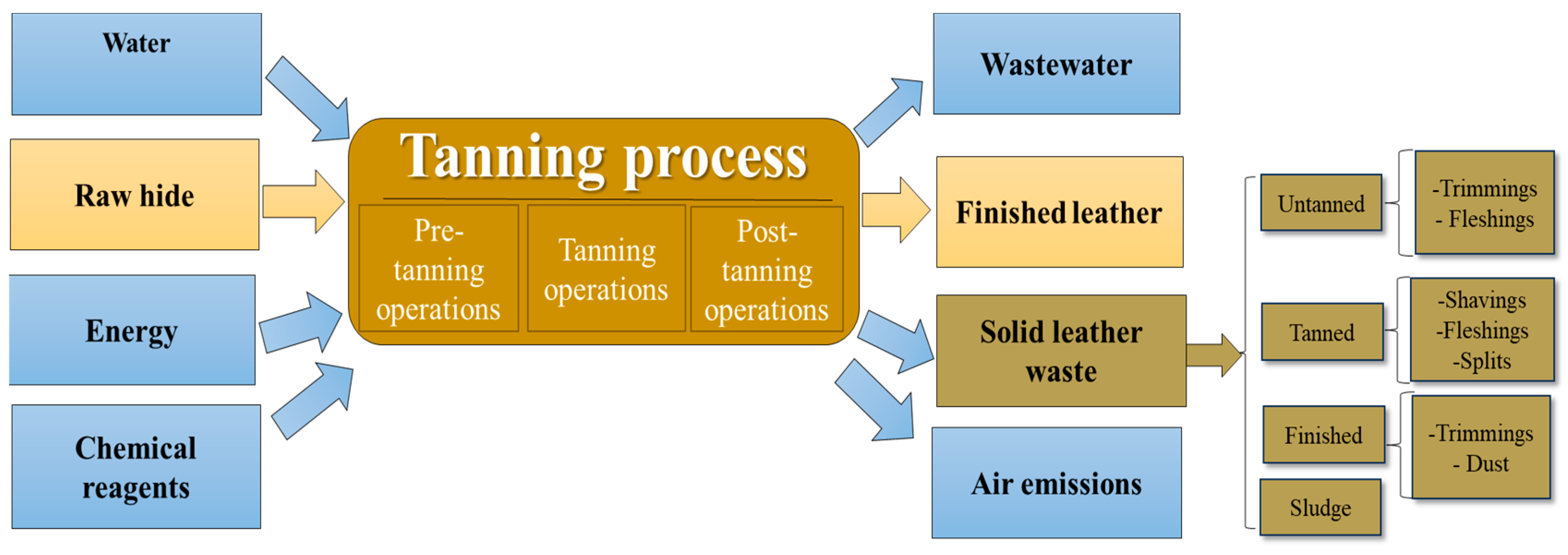
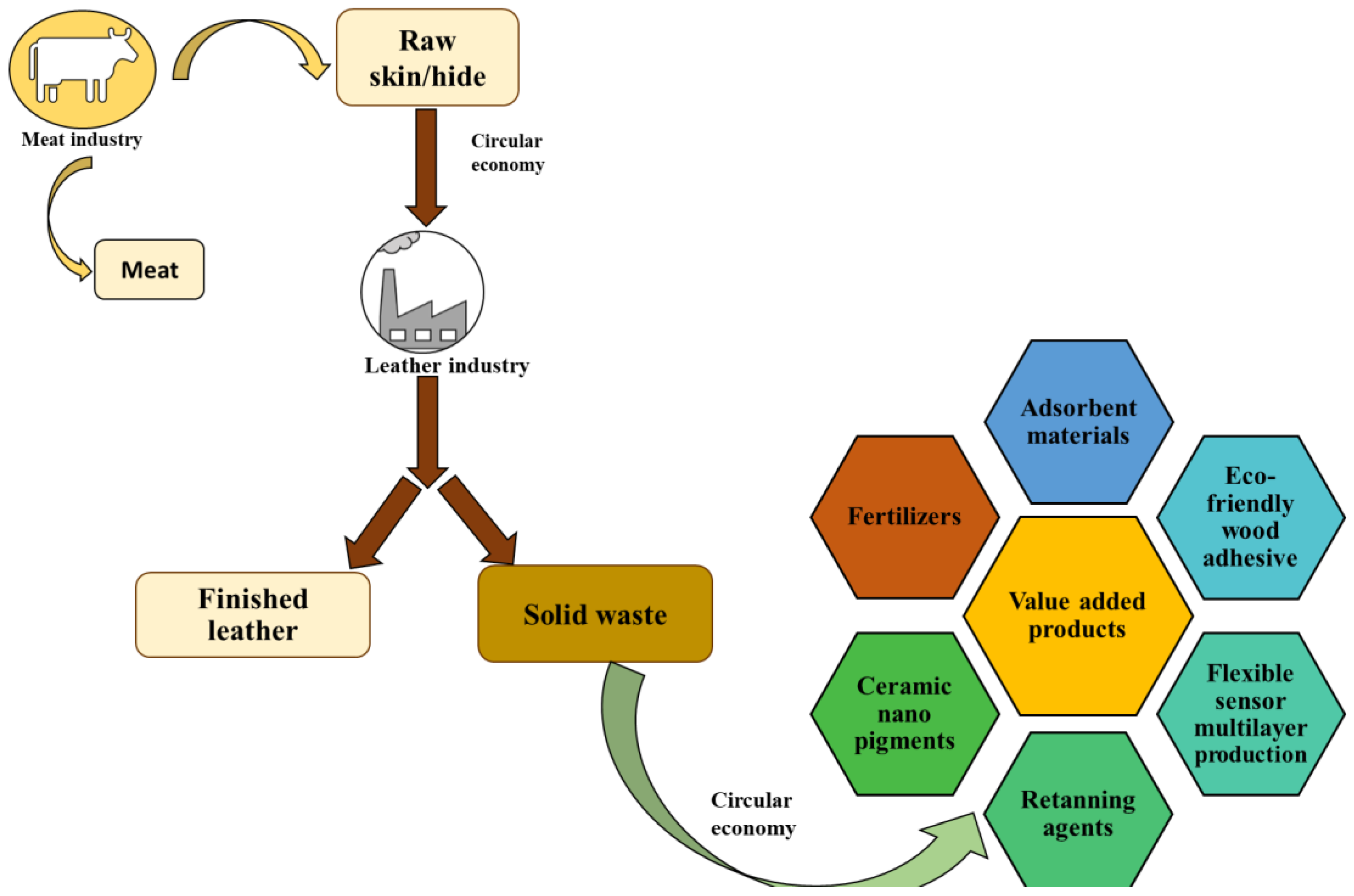
1. Introduction
2. Chromium Extraction Methods
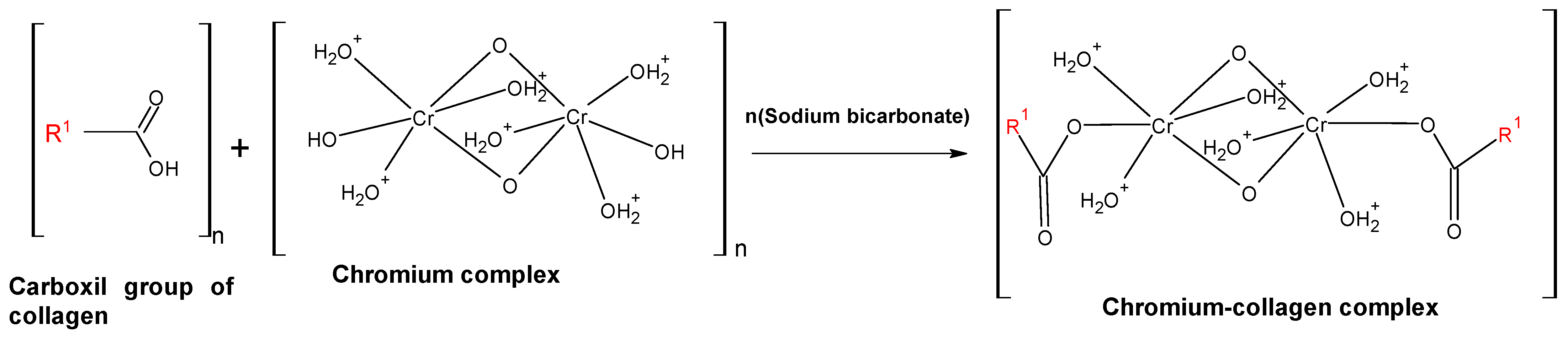
3. Acid Extraction of Chromium
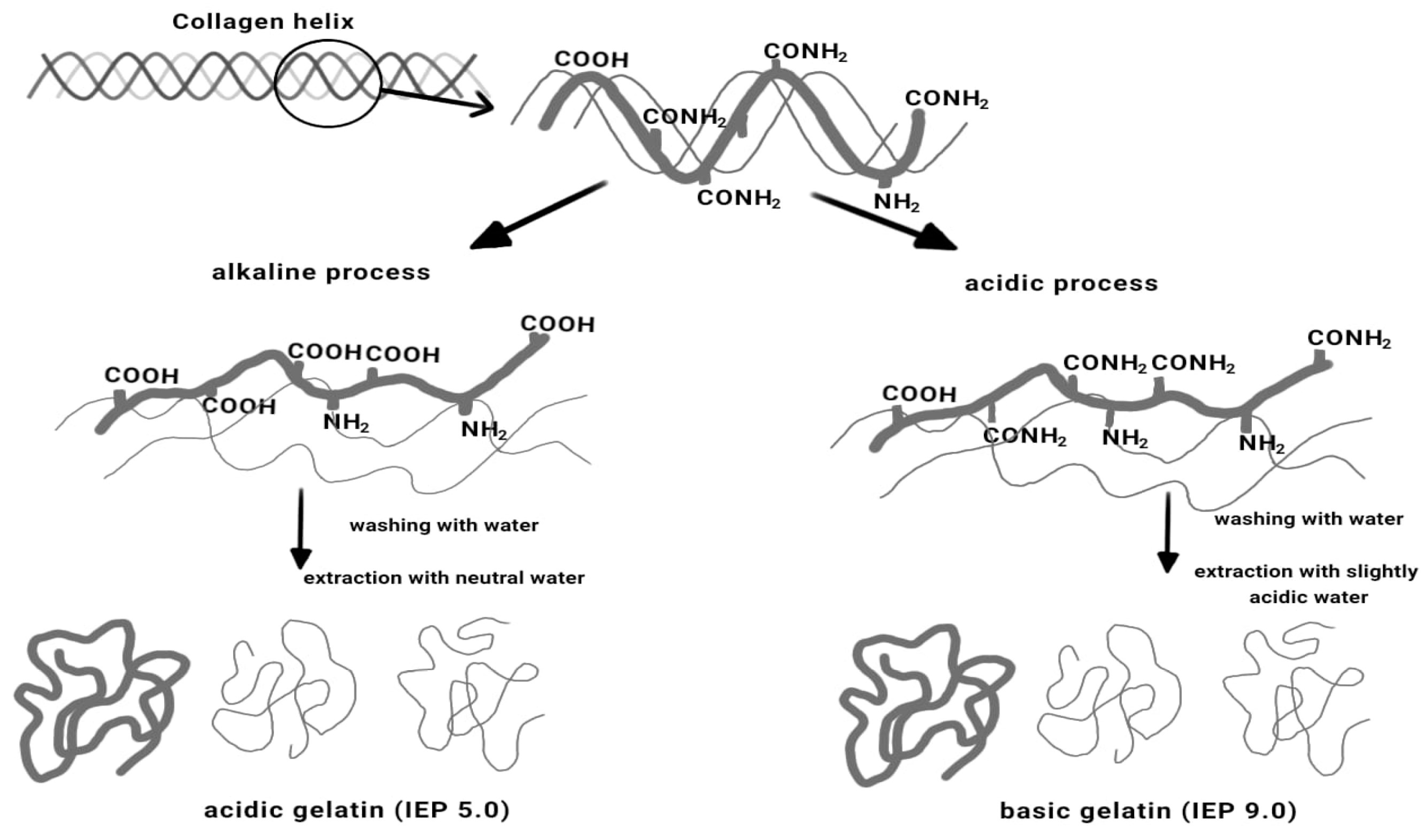
4. Alkaline Extraction of Chromium
5. Enzymatic Extraction of Chromium
6. Ultrasound-Assisted Extraction of Chromium
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Wang, L.; Chen, M.; Li, J.; Jin, Y. A novel substitution-based method for effective leaching of chromium A novel substitution-based method for effective leaching of chromium kinetics and mechanism studies. Waste Manag. 2022, 103, 276–284. [Google Scholar] [CrossRef] [PubMed]
- Satish, M.; Madhan, B.; Sreeram, K.J.; Rao, J.; Nair, B.U. Alternative carrier medium for sustainable leather manufacturing—A review and perspective. J. Clean. Prod. 2016, 112, 49–58. [Google Scholar] [CrossRef]
- Bulijan, J.; Reich, G.; Ludvik, J. Mass Balance in Leather Processing. United Nations Industrial Development Organisation (UNIDO). 9 August 2000. Available online: https://www.unido.org/sites/default/files/2009-05/Mass_balance_in_leather_processing_0.pdf (accessed on 20 December 2023).
- Tzoumani, I.; Lainioti, G.C.; Aletras, A.; Zainescu, G.; Stefa, S.; Meghea, A.; Kallitsis, J. Modification of Collagen Derivatives with Water-Soluble Polymers for the Development of Cross-Linked Hydrogels for Controlled Release. Materials 2019, 12, 4067. [Google Scholar] [CrossRef]
- Rahmawati, D.; Setyadewi, N.M.; Sugihartono. Extraction and characterization of gelatin from skin trimming pickled waste of tannery. Earth Environ. Sci. 2019, 306, 012022. [Google Scholar] [CrossRef]
- Rigueto, C.V.T.; Nazari, M.T.; Rosseto, M.; Massuda, L.A.; Alessandretti, I.A.; Piccin, J.S.; Gettmer, A. Emerging contaminants adsorption by beads from chromium (III) tanned leather waste recovered gelatin. J. Mol. Liq. 2021, 330, 115638. [Google Scholar] [CrossRef]
- Ayele, M.; Limeneh, D.I.; Tesfaye, T.; Mengie, W.; Abuhay, A.; Haile, A.; Gebino, G. A Review on Utilization Routes of the Leather Industry Biomass. Adv. Mater. Sci. Eng. 2021, 2021, 1503524. [Google Scholar] [CrossRef]
- Rosu, L.; Varganici, C.D.; Crudu, A.M. Ecofriendly wet-white leather vs. conventional tanned wet-blue. A Photochem. Approach. J. Clean. Prod. 2018, 177, 708–720. [Google Scholar] [CrossRef]
- Alibardi, L.; Cossu, R. Pre-treatment of tannery sludge for sustainable landfilling. Waste Manag. 2016, 52, 202–211. [Google Scholar] [CrossRef] [PubMed]
- Famielec, S. Chromium Concentrate Recovery from Solid Tannery Waste in a Thermal Process. Materials 2020, 13, 1533. [Google Scholar] [CrossRef]
- Kanagaraj, J.; Senthilvelan, T.; Panda, R.C.; Kavitha, S. Eco-friendly waste management strategies for greener environment towards sustainable development in leather industry: A comprehensive review. J. Clean. Prod. 2015, 89, 1–17. [Google Scholar] [CrossRef]
- Mella, B.; Glanert, A.C.; Gutterres, M. Removal of chromium from tanning wastewater and its reuse. Process Saf. Environ. Prot. 2015, 95, 195–201. [Google Scholar] [CrossRef]
- Hu, J.; Xiao, Z.; Zhou, R.; Deng, W. Ecological utilization of leather tannery waste with circular economy model. J. Clean. Prod. 2011, 19, 221–228. [Google Scholar] [CrossRef]
- Dixit, S.; Yadav, A.; Dwivedi, P.; Das, M. Toxic hazards of leather industry and technologies to combat threat: A review. J. Clean. Prod. 2015, 87, 39–49. [Google Scholar] [CrossRef]
- Sundar, V.J.; Gnanamani, A.; Muralidharan, C.; Chandrababu, N.K. Recovery and utilization of proteinous wastes of leather making: A review. Rev. Environ. Sci. Biotechnol. 2011, 10, 151–163. [Google Scholar] [CrossRef]
- Kanagaraj, J.; Chandra Babu, N.K.; Mandal, A.B. Recovery and reuse of chromium from chrome tanning waste water aiming towards zero discharge of pollution. J. Clean. Prod. 2008, 16, 1807–1813. [Google Scholar] [CrossRef]
- Nagueira, F.; Castro, I.; Bastos, A.; Souza, G.; Carvalho, J.; Oliveira, L. Recycling of solid waste rich in organic nitrogen from leather industry: Mineral nutrition of rice plants. J. Hazard. Mater. 2011, 186, 1064–1069. [Google Scholar] [CrossRef] [PubMed]
- Şaşmaz, S.; Karaağaç, B.; Uyanık, N. Utilization of chrome-tanned leather wastes in natural rubber and styrene-butadiene rubber blends. J. Mater. Cycles Waste Manag. 2019, 21, 166–175. [Google Scholar] [CrossRef]
- Malek, A.; Hachemi, M.; Didier, V. New approach of depollution of solid chromium leather waste by the use of organic chelates. Economical and environmental impacts. J. Hazard. Mater. 2009, 170, 156–162. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Zhou, L.X.; Wang, S.M.; Fang, D. Removal of Cr from tannery sludge by bioleaching method. J. Environ. Sci. 2006, 18, 885–890. [Google Scholar] [CrossRef]
- Leathersmithe. Available online: www.leathersmithe.com/tanning-methods-and-the.html (accessed on 14 May 2024).
- Dwivedi, S.P.; Dixit, A.; Bajaj, R. Development of bio-composite material by utilizing chrome containing leather waste with Al2O3 ceramic particles. Mater. Res. Express 2019, 6, 105105. [Google Scholar] [CrossRef]
- Cardona, N.; Velásquez, S.; Giraldo, D. Characterization of Leather Wastes from Chrome Tanning and its Effect as Filler on the Rheometric Properties of Natural Rubber Compounds. J. Polym. Environ. 2017, 25, 1190–1197. [Google Scholar] [CrossRef]
- Tahiri, S.; DeLaGuardia, M. Treatment and valorization of leather industry solid: A review. J. Am. Leather Chem. Assoc. 2009, 104, 52–67. [Google Scholar]
- The European Commission. EUR-LEX. The European Commission. 18 December 2014. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A32014D0955 (accessed on 6 November 2023).
- Stefan, D.S.; Bosomoiu, M.; Constantinescu, R.R.; Ignat, M. Composite polymers frim leather waste to produce smart fertilizers. Polymers 2021, 13, 4351. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Tan, K.; Wang, X.; Chen, Y. A rapid soil Chromium pollution detection method based on hyperspectral remote sensing data. Int. J. Appl. Earth Obs. Geoinf. 2024, 128, 103759. [Google Scholar] [CrossRef]
- Velusamy, M.; Chakali, B.; Ganesan, S.; Tinwala, F. Investigation on pyrolysis and incineration of chrome-tanned solid waste from tanneries for effective treatment and disposal: An experimental study. Environ. Sci. Pollut. Res. 2019, 27, 29778–29790. [Google Scholar] [CrossRef]
- Achmad, R.T.; Budiwan, B.; Auerkari, E.I. Effects of Chromium on Human Body. Annu. Res. Rev. Biology. 2017, 13, 1–8. [Google Scholar] [CrossRef]
- Amata, A.I. Chromium in Livestock Nutrition: A Review. Glob. Adv. Res. J. Agric. Sci. 2013, 2, 289–306. [Google Scholar]
- Shanker, A. Chromium toxicity in plants. Environ. Int. 2005, 31, 739–753. [Google Scholar] [CrossRef] [PubMed]
- Fendorf, S. Surface reactions of chromium in soils and waters. Geoderma 1995, 67, 55–71. [Google Scholar] [CrossRef]
- Thatoi, H.N.; Dal, B.; Das, N.N.; Pandey, B.D. Chemical and microbial remediation of hexavalent chromium from contaminated soil and mining/metallurgical solid waste: A review. J. Hazard. Mater. 2013, 250–251, 272–291. [Google Scholar]
- Ocak, B. Film-forming ability of collagen hydrolysate extracted from leather solid wastes with chitosan. Environ. Sci. Pollut. Res. 2018, 25, 4643–4655. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, R.; Pati, A.; Subramani, S. A review on management of chrome-tanned leather shavings: A holistic paradigm to combat the environmental issues. Environ. Sci. Pollut. Res. 2014, 21, 11266–11282. [Google Scholar]
- Agency for Toxic Substances and Disease Registry. 18 October 2023. Available online: www.atsdr.cdc.gov/spl/index.html#2022sp (accessed on 18 October 2023).
- Chrysochoou, M.; Johnston, C. Reduction of Chromium(VI) in Saturated Zone Sediments by Calcium Polysulfide and Nanoscale Zerovalent Iron Derived From Green Tea Extract. In GeoCongress 2012: State of the Art and Practice in Geotechnical Engineering; ASCE 2012; Geotechnical Special Publication: Oakland, CA, USA, 2012; p. 3959. [Google Scholar]
- Zayed, A.M.; Terry, N. Chromium in the Environment: Fators Affecting Biological Remediation. Plant Soil 2003, 249, 139–156. [Google Scholar] [CrossRef]
- Verma, S.K.; Sharma, P.C. Current trends in solid tannery waste management. Crit. Rev. Biotechnol. 2022, 43, 805–822. [Google Scholar] [CrossRef]
- de Matos, E.; Scopel, B.; Dettmer, A. Citronella essential oil microencapsulation by complex coacervation with leather waste gelatin and sodium alginate. J. Environ. Chem. Eng. 2018, 6, 1989–1994. [Google Scholar] [CrossRef]
- Sharf, A.; Gasmeleed, A.; Musa, A.E. Extraction of Chromium Six from Chrome Shavings. J. For. Prod. Ind. 2013, 2, 21–26. [Google Scholar]
- Yang, J.; Shan, Z.; Zhang, Y.; Chen, L. Stabilization and cyclic utilization of chrome leather shavings. Environ. Sci. Pollut. Res. 2018, 26, 4680–4689. [Google Scholar] [CrossRef] [PubMed]
- da Costa Cunha, G.; Peixoto, J.A.; de Souza, D.R.; Cruz Romao, L.P.; Macedo, A.S. Recycling of chromium wastes from tanning industry to produce ceramic nanopigments. Green Chem. 2016, 18, 5342–5356. [Google Scholar] [CrossRef]
- He, B.; Du, Y.; Xu, H.; Ma, J. Synthesis of Ceramic Pigments with Chromium Content from Leather Waste. Trans. Indian Ceram. Soc. 2021, 2021, 103–109. [Google Scholar] [CrossRef]
- Majee, S.; Halder, G.; Mandal, T. Formulating Nitrogen-Phosphorous-Potassium enriched organic manure from solid waste: A novel approach of waste valorization. Process Saf. Environ. Prot. 2019, 132, 160–168. [Google Scholar] [CrossRef]
- Wang, X.; Yue, O.; Liu, X.; Hou, M.; Zheng, M. A novel bio-inspired multi-functional collagen aggregate based flexible sensor with multi-layer and internal 3D network structure. Chem. Eng. J. 2020, 392, 123672. [Google Scholar] [CrossRef]
- Yang, M.; Li, Y.; Dang, X. An eco-friendly wood adhesive based on waterborne polyurethane grafted with gelatin derived from chromium shavings waste. Environ. Res. 2022, 206, 112266. [Google Scholar] [CrossRef] [PubMed]
- Younas, H.; Nazir, A.; Bareen, F. Application of microbe-impregnated tannery solid waste biochar in soil enhances growth performance of sunflower. Environ. Sci. Pollut. Res. 2022, 29, 57669–57687. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, O.; Kantarli, I.C.; Yuksel, M.; Saglam, M. Conversion of leather wastes to useful products. Resour. Conserv. Recycl. 2007, 49, 436–448. [Google Scholar] [CrossRef]
- Zhou, C.; Zhou, H.; Yuan, Y.; Peng, J.; Yao, G.; Zhou, P.; Lai, B. Coupling adsorption and in-situ Fenton-like oxidation by waste leather-derived materials in continuous flow mode towards sustainable removal of trace antibiotics. Chem. Eng. J. 2021, 420, 130370. [Google Scholar] [CrossRef]
- Senthil, R.; Kavukcu, S.B.; Cakir, S.; Turkmen, H. Utilization of various solid leather wastes for the production of blended bricks. Clean Technol. Environ. Policy 2022, 24, 1889–1901. [Google Scholar] [CrossRef]
- Zainescu, G.; Deselnicu, V.; Constantinescu, R.; Georgescu, D. Biocomposites from tanned leather fibres with applications in constructions. Leather Footwear J. 2018, 18, 203–206. [Google Scholar] [CrossRef]
- Jeyasingh, J.; Philip, L. Bioremediation of chromium contaminated soil: Optimization of operating parameters under laboratory conditions. J. Hazard. Mater 2005, 118, 113–120. [Google Scholar] [CrossRef]
- Sethunathan, N.; Meghraj, M.; Smith, L.; Kamalideen, S.P.B.; Avudainayagam, S.; Naidu, R. Microbial role in the failure of natural attenuation of chromium (VI) in long-term tannery waste contaminated soil. Agric. Ecosyst. Environ. 2005, 105, 657–661. [Google Scholar] [CrossRef]
- Cavington, A. Tanning Chemistry: The Science of Leather; Royal Society of Chemistry: Cambridge, UK, 2009. [Google Scholar]
- Pati, A.; Chaudhary, R. Soybean plant growth study conducted using purified protein hydrolysate-based fertilizer made from chrome-tanned leather ysate-based fertilizer made from chrome-tanned leather. Environ. Sci. Pollut. Res. 2015, 22, 20316–20321. [Google Scholar] [CrossRef]
- Taylor, M. Effect of processing variables on ash content of galable and hydrolyzed portein products isolated from treatment of chromium leather waste. J. Am. Leather Chem. Assoc. 1993, 88, 358–367. [Google Scholar]
- Scopel, B.; Baldasso, C.; Mettmer, A.; Santana, R. Hydrolysis of Chromium Tanned Leather Waste: Turning Waste into Valuable Materials—A Review. J. Am. Leather Chem. Assoc. 2018, 113, 122–129. [Google Scholar]
- Masilamani, D.; Madhan, B.; Shanmugam, G.; Sarvanan, P. Extraction of collagen from raw trimming wastes of tannery: A waste to wealth approach. J. Clean. Prod. 2016, 113, 338–344. [Google Scholar] [CrossRef]
- Bhagwat, P.K.; Dandge, P.B. Collagen and collagenolytic proteases: A review. Biocatal. Agric. Biotechnol. 2018, 15, 43–55. [Google Scholar] [CrossRef]
- Said, N.S.; Sarbon, N.M. Physical and Mechanical Characteristics of Gelatin-Based Films as a Potential Food Packaging Material: A Review. Membranes 2022, 12, 442. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, H.; Sanlier, S.H. Preparation of magnetic gelatin nanoparticles and investigating the possible use as chemotherapeutic agent. Artif. Cells Nanomed. Biotechnol. 2013, 41, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Li, J.; Jin, Y.; Chen, M. Study on the removal of chromium(III) from leather waste by a two-step method. J. Ind. Eng. Chem. 2019, 79, 172–180. [Google Scholar] [CrossRef]
- Ferreira, M.; Almeida, M.; Pinho, S.; Santos, I. Finished leather waste chromium acid extraction and anaerobic biodegradation of the products. Waste Manag. 2010, 30, 1091–1100. [Google Scholar] [CrossRef] [PubMed]
- Bizzi, C.; Zanatta, R.; Santos, D.; Giacobe, K.; Dallago, R.; Mello, P.; Flores, E. Ultrassound assisted extraction of chromium from residual tannd leaher: An inovative strategy for the reuse of waste in tanning industry. Ultrason. Sonochem. 2020, 64, 104682. [Google Scholar] [CrossRef]
- Tian, Z.; Wang, Y.; Wang, H.; Zhang, K. Regeneration of native collagen from hazardous waste: Chrome tanned leather shavings by acid method. Environ. Sci. Pollut. Res. 2020, 27, 31300–31310. [Google Scholar] [CrossRef]
- Brown, D.A. Investigation of carboxylic acids for the extraction of chromium (III) from the leather waste and the posiible re-use of the extracted chromium tanning industry. Environ. Technol. Lett. 1986, 7, 289–298. [Google Scholar] [CrossRef]
- Wang, L.; Chen, M.; Li, J.; Jin, Y.; Zhang, Y.; Wang, Y. A novel substitution—Based method for effective leaching of chromium (III) from chromium- tanned leather waste: The termodynamics, kinetics and mechanism studies. Waste Manag. 2020, 103, 276–284. [Google Scholar] [CrossRef] [PubMed]
- Popiolski, A.; Dallago, R.; Steffens, J.; Mignoni, M.; Venquiaruto, L.; Santos, D.; Duarte, F. Ultrasound-Assisted Extraction of Cr from Residual Tannery Leather: Feasibility of Ethylenediaminetetraacetic Acid as the Extraction Solution. ACS Omega 2018, 3, 16074–16080. [Google Scholar] [CrossRef] [PubMed]
- Pedrotti, M.; Santos, D.; Cauduro, V.; Bizii, C.; Flores, E. Ultrasound-assisted extraction of chromium from tanned leather shavings: A promising continous flow technology for the treatment of solid waste. Ultrason. Sonochem. 2022, 89, 106124. [Google Scholar] [CrossRef]
- Wionczyk, B.; Apostoluk, W.; Charewicz, W.; Adamski, Z. Recovery of chromium (III) from waste of uncolored chromium leaters. Part I. Kinetic studies on alkaline hydrolytic decomposition of the waste. Sep. Purif. Technol. 2011, 81, 223–236. [Google Scholar] [CrossRef]
- Hinojosa, J.A.B.; Marrufo, L. Optimization of Alkaline Hydrolysis of Chrome Shavings to Recover Collagen Hydrolysate and Chromium Hydroxide. Leather Footwear J. 2020, 20, 15–28. [Google Scholar] [CrossRef]
- Pahlawan, I.F.; Sutyasmi, S.; Griyanitasari, G. Hydrolysis of leather shavings waste for protein binder. In Proceedings of the International Conference on Green Agro-industry and Bioeconomy, Malang, Indonesia, 18–20 September 2019. [Google Scholar]
- Ferreira, M.J.; Almeida, M.F.; Pinho, S.C.; Gomes, J.R.; Rodrigues, J.L. Alkaline hydrolysis of chromium tanned leather scrap fibers and anaerobic biodegration of the products. Waste Biomass Valoriz. 2014, 5, 551–562. [Google Scholar] [CrossRef]
- Poulopoulou, V.; Katakis, D.; Vrachnou, E. A method of removal chromium from tanned leather wastes. J. Air Waste Manag. Assoc. 1998, 48, 846–852. [Google Scholar] [CrossRef] [PubMed]
- Ding, W.; Xuepin, L.; Zhang, W.; Shi, B. Dechroming of chromium containing leather waste with low hydrolysis degree of colagen. J. Soc. Leather Technol. Chem. 2015, 99, 129–133. [Google Scholar]
- Asava, A.; Sang, P.; Onyuka, A. Recovery of Collagen Hydrolysate from Chrome Leather Shaving Tannery Waste through Two-Step Hydrolysis using Magnesium Oxide and Bating Enzyme. Soc. Leather Technol. Chem. J. 2019, 103, 80–85. [Google Scholar]
- Dettmer, A.; Oliveira dos Santos, R.M. Protein extraction from chromium tanned leather waste by Bacillus subtilis enzymes. J. AQEIC 2014, 65, 93–100. [Google Scholar]
- Qiang, X.H.; Feng, H. Collagen Extracted from Chrome Shavings Using Alkali and Enzyme. In Proceedings of the 2011 International Conference on Remote Sensing, Environment and Transportation Engineering, Nanjing, China, 24–26 June 2011. [Google Scholar]
- Tahiri, S.; Mohamed, B.; Albizane, A.; Massaoudi, A. Extraction of proteins from chrome shavings with sodium hydroxide and reuse of chromium in the tanning process. J. Am. Leather Chem. Assoc. 2004, 99, 16–25. [Google Scholar]
- Pecha, J.; Barinova, M.; Kolomaznik, K.; Nguyen, T.N. Technological-economic optimization of enzymatic hydrolysis used for the processing of chrome-tanned leather waste. Process Saf. Environ. Prot. 2021, 152, 220–229. [Google Scholar] [CrossRef]
- Rahaman, A.; Raihan, M.; Islam, M.D. Recovery of chromium from chrome shaving dust. Eur. Acad. Res. 2017, 4, 9441–9448. [Google Scholar]
- Pollet, B.; Ashokkumar, M. Introduction to Ultrasound, Sonochemistry and Sonoelectrochemistry; Springer: Berlin/Heidelberg, Germany, 2019. [Google Scholar]
- Mason, T.J.; Cobley, A.J.; Graves, J.E.; Morgan, D. New evidence for the inverse dependence of mechanical and chemicaleffects on the frequency of ultrasound. Ultrason. Sonochem. 2011, 18, 226–230. [Google Scholar] [CrossRef]
- Tadeo, J.L.; Sanchez-Brunete, C.; Albero, B.; Garcia-Valcarcel, A. Application of ultrasound-assisted extraction to the determi-nation of contaminants in food and soil samples. J. Chromatogr. A 2010, 1217, 2415–2440. [Google Scholar] [CrossRef]

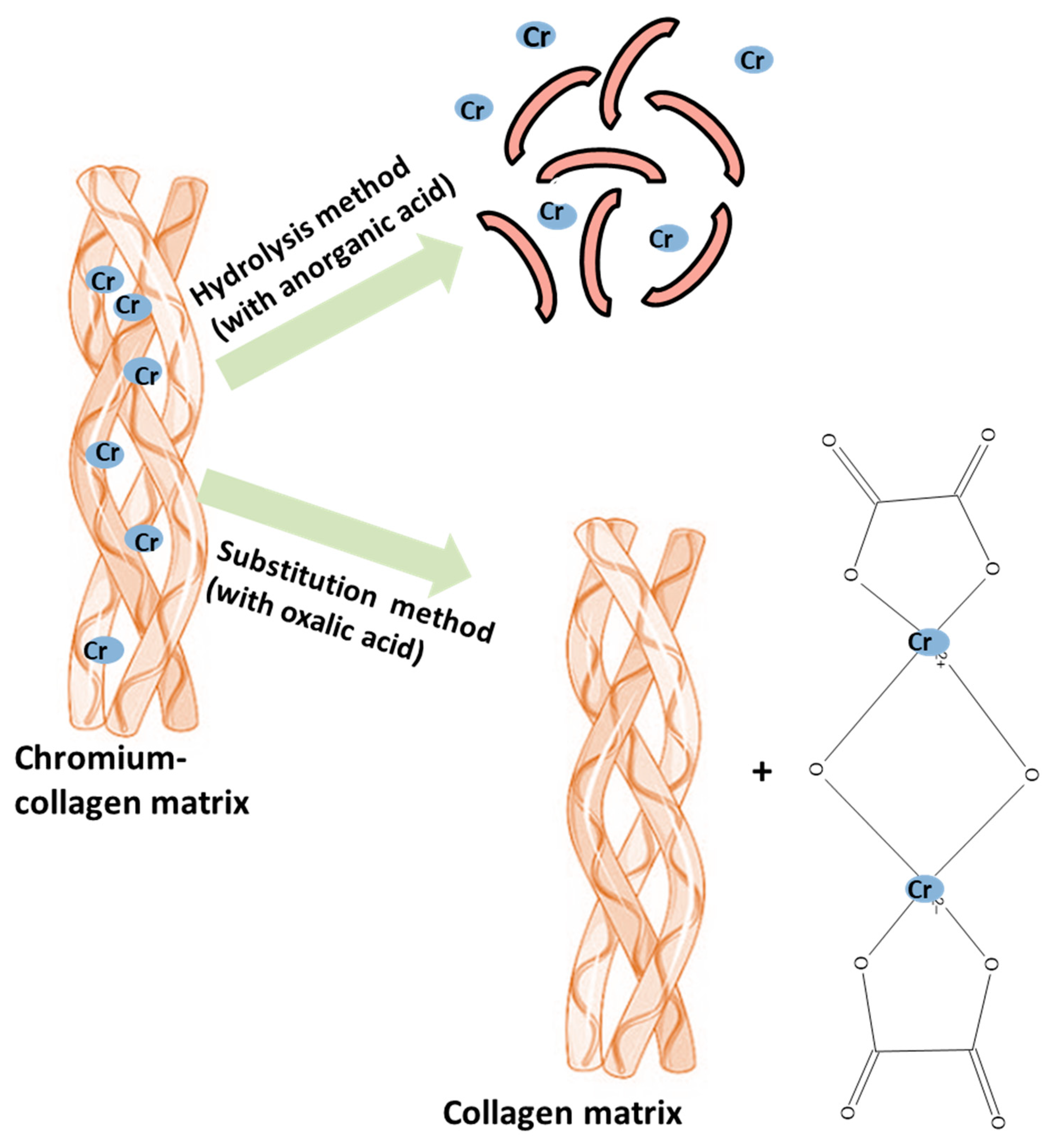
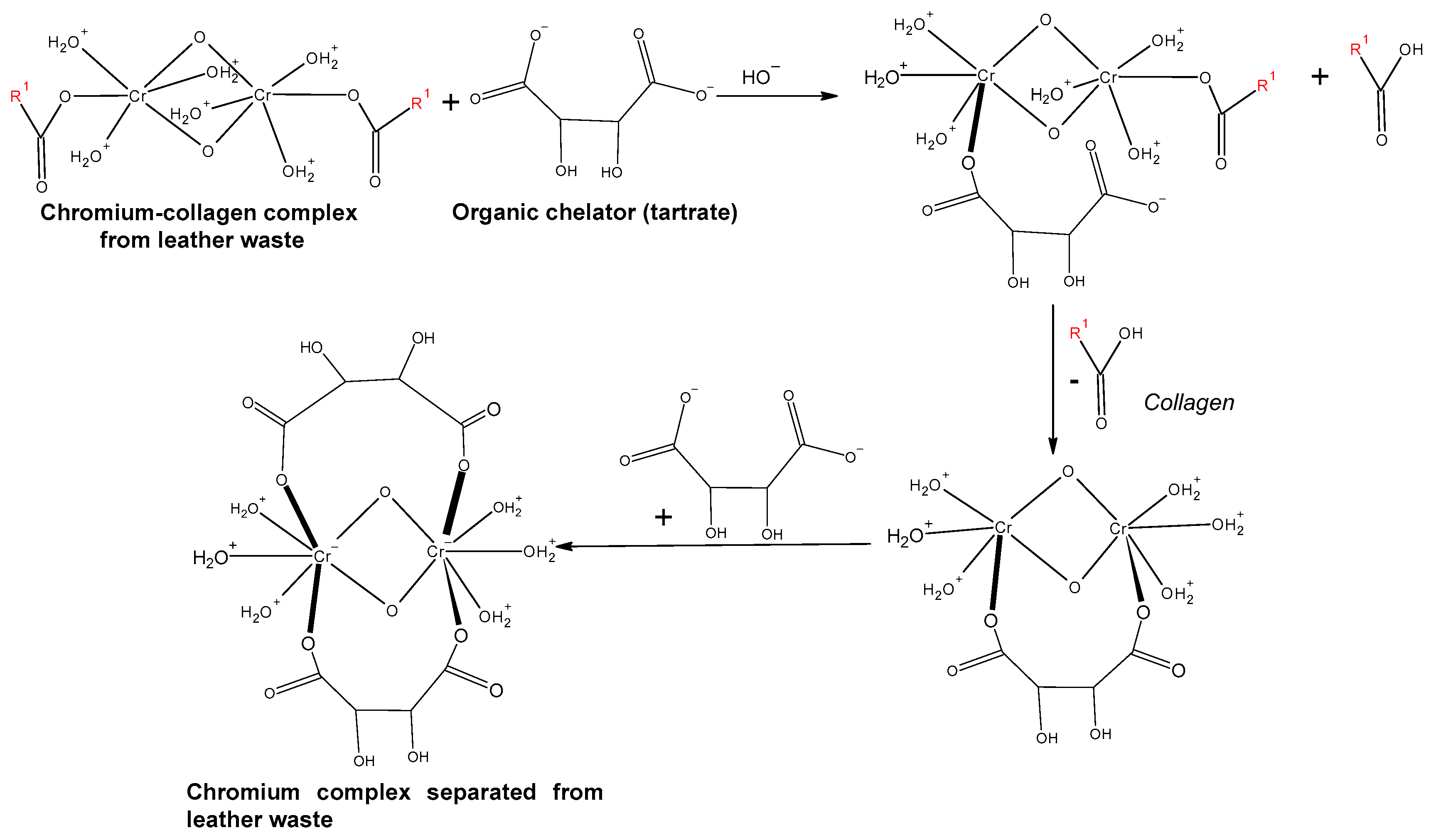

| Chromium Removal Method | Chromium Removal Method | Chromium Extraction Yield | The Degree of Collagen Hydrolysis | Reference |
|---|---|---|---|---|
| Acid extraction | - Concentration of extraction solution = 8% H2SO4 - H2SO4:sample ratio = 11:1 - Time = 2.5 h - Temperature = 343 K | >95% | - | [63] |
| Acid extraction | - Concentration of extraction solution = 25 mL/L H2SO4 | 30–60% ± 5% | 3–6 ± 1% | [64] |
| - Time = 3 or 6 days | ||||
| Acid extraction | - Sample amount = 150 mg | 92% | - | [65] |
| - Concentration of extraction solution = 3 mol/L HNO3 | ||||
| - Temperature = 30 °C | ||||
| - Time = 30 min - Amplitude = 90% US bath = 37 kHz | ||||
| Acid extraction | - H2C2O4/H2SO4/sample ratio = 2:1:1 - Time = 12 h - Processing times = 1 h - Stirring speed = 250 r/min - Temperature = 40 °C | 95.6% | 90.6% | [66] |
| Acid extraction | - Extraction agent: oxalic acid - Time = 36 h - Room temperature - pH = 5.5 - Cr–oxalic acid ratio = 1:3 | 71% | - | [67] |
| Acid extraction | - Concentration of potassium tartrate = 0.5 M - NaOH solution concentration = 0.25 M - Room temperature - Time = 72 h | 95% | - | [19] |
| Acid extraction | - Concentration of sodium oxalate = 2% - Sodium oxalate/sample ratio = 200 mL/g - Thickness of sample = 0.5 mm - Temperature = 333 K - Time = 5 h - Stirring speed = 150 rpm | 98% | >95% | [68] |
| Acid extraction | - Sample amount = 3 g - Cr3+/EDTA ratio = 1:3 - Temperature = 80 °C - Time = 30 de minutes - Ultrasonic bath = 25 kHz Amplitude = 100% - 5 washing cycles with water (V = 50 mL), at a temperature of 50 °C, for 3 min each | 98% | - | [69] |
| Acid extraction | - EDTA/Cr 3+ ratio = 3:1 - US power = 150 W - Frequency = 20 KHz - Residence time = 60 min - Temperature = 70 °C | 71.7% | - | [70] |
| Alkaline extraction | - Concentration of extraction solution = 0.2 M NaOH - NaOH/sample ratio = 80 cm3/g - Time = 1 h - Temperature = 60 °C | 90% | ~100% | [71] |
| Alkaline extraction | - Concentration of extraction solution = 0.47 M NaOH - Time = 90 min - Temperature = 70 °C | 750.8 g | 87.165% | [72] |
| Alkaline extraction | - Concentration of extraction solution = 3% NaOH - Time = 180 min - Temperature = 90 °C - NaOH/sample ratio = 5:1 | ~100% | - | [73] |
| Alkaline extraction | - Concentration of extraction solution = 4 M NaOH - NaOH/sample ratio = 0.15 - Time = 90 min - Temperature = 423 K | 85% | 98% | [74] |
| Alkaline extraction | - H2SO4 concentration = 0.1 N H2SO4 - Dose of Gamma radiation 60Co = 60 Krad - Concentration of extraction solution = 1 N NaOH | ~100% | 25–40% | [75] |
| Alkaline extraction | - Concentration of extraction solution 1 = 2 g/L NaOH, stirring for 30 min at 30 °C, urea concentration = 40 g/L - Concentration of extraction solution 2 = 50 g/L H2SO4, stirring for 1 h at 30 °C - Concentration of extraction solution 3 = 40 g/L CaOH, stirring for 2 ore at 30 °C - Concentration of extraction solution 4 = 50 g/L H2SO4, stirring for 1 h at 30 °C | 97% | 10% | [76] |
| Enzymatic extraction | - Extraction solution concentration = 6% MgO - Bating enzyme concentration = 0.75% - Time = 30 h - Temperature = 33–37 °C - pH = 8.3–8.5 | 99.99% | - | [77] |
| Enzymatic extraction | - Extraction solution MgO - Stirring speed = 60 rpm - Temperature = 70 °C - Time = 6 h - Bacillus subtilis enzyme A proteolytic activity = 130.5 U/mL - pH = 9 - Time = 15 h - Temperature = 45 °C - Stirring speed = 60 rpm | ~100% | - | [78] |
| Enzymatic extraction | - Extraction solution concentration = 3% MgO - Extraction solution concentration = 3% CaO - Temperature = 80 °C - Time = 4 h - 1398 neutral protease concentration = 0.125% - Temperature = 46 °C | ~100% | >60% | [79] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Codreanu, A.-M.N.; Stefan, D.S.; Kim, L.; Stefan, M. Depollution of Polymeric Leather Waste by Applying the Most Current Methods of Chromium Extraction. Polymers 2024, 16, 1546. https://doi.org/10.3390/polym16111546
Codreanu A-MN, Stefan DS, Kim L, Stefan M. Depollution of Polymeric Leather Waste by Applying the Most Current Methods of Chromium Extraction. Polymers. 2024; 16(11):1546. https://doi.org/10.3390/polym16111546
Chicago/Turabian StyleCodreanu (Manea), Ana-Maria Nicoleta, Daniela Simina Stefan, Lidia Kim, and Mircea Stefan. 2024. "Depollution of Polymeric Leather Waste by Applying the Most Current Methods of Chromium Extraction" Polymers 16, no. 11: 1546. https://doi.org/10.3390/polym16111546
APA StyleCodreanu, A.-M. N., Stefan, D. S., Kim, L., & Stefan, M. (2024). Depollution of Polymeric Leather Waste by Applying the Most Current Methods of Chromium Extraction. Polymers, 16(11), 1546. https://doi.org/10.3390/polym16111546








