A Low-Cost Ecofriendly Oxidation Process to Manufacture High-Performance Polymeric Biosurfactants Derived from Municipal Biowaste
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Treatments
2.2. Products’ Isolation and Characterization
2.3. Analytical Methods
3. Results
3.1. The Reaction of SBP and Water
3.2. The Products’ Solubility and Molecular Weight
3.3. The Products’ Surfactant Properties
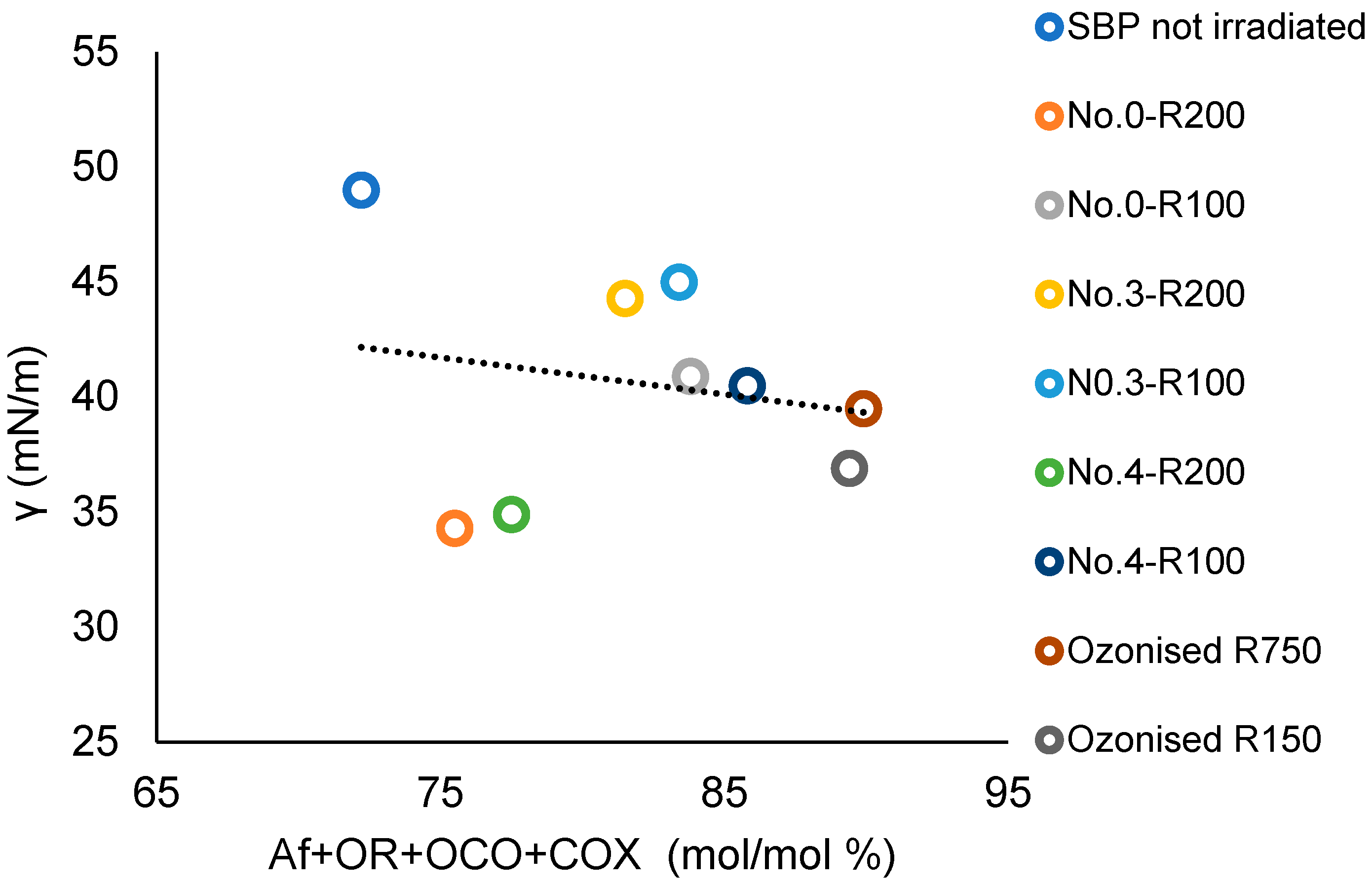
3.4. The Relationship between the Products’ Chemical Composition and Surfactant Properties
3.4.1. Products’ Characterization by 13C Solid-State NMR Spectroscopy
3.4.2. The Products Hydrophilic Lipophilic Balance (HLB)
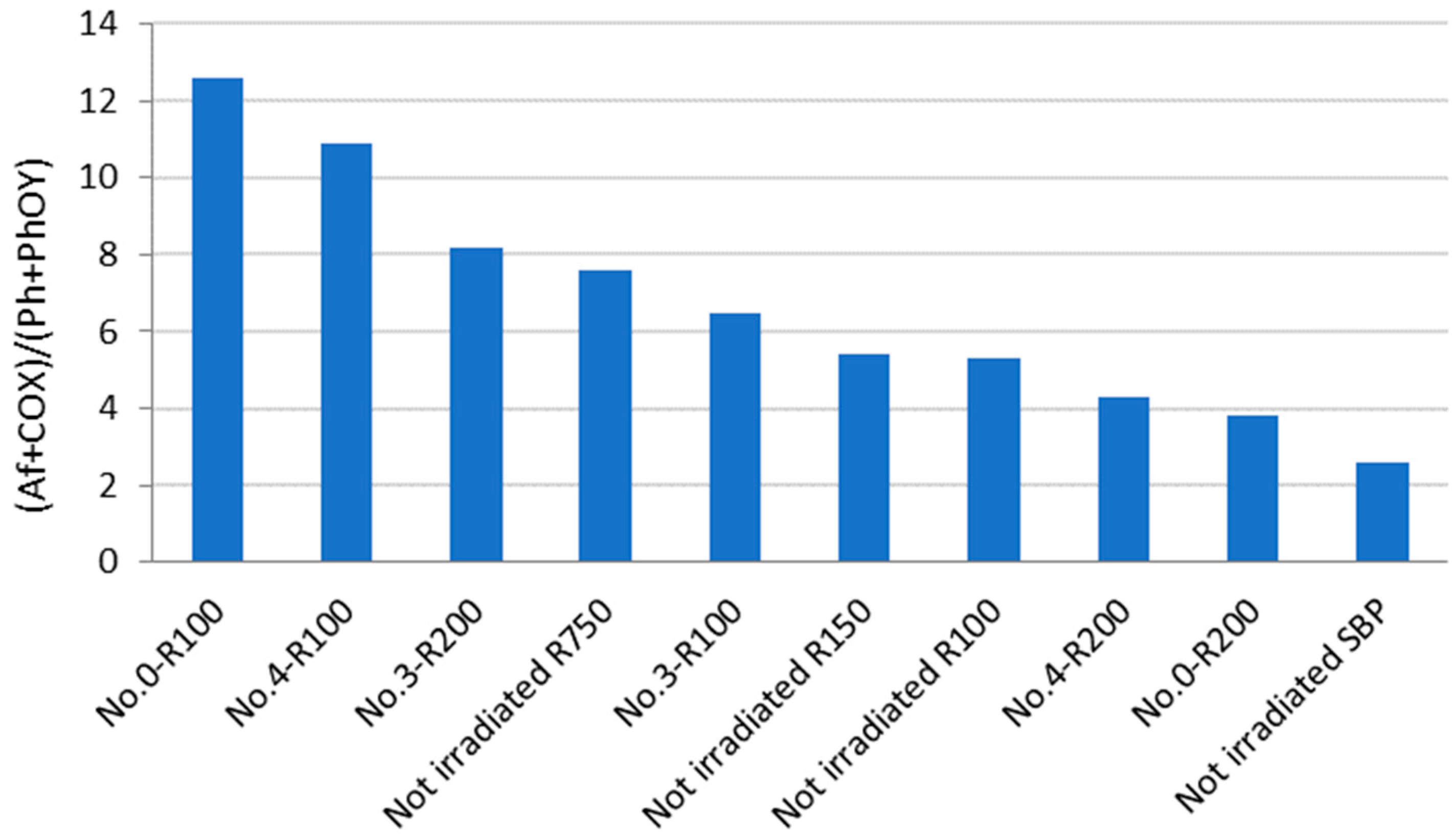
3.4.3. Determination of Carboxylate (-COO−), Amide (-CONR2), Phenol (PhOH), and Phenoxide (PhOR) in the Products Obtained by Irradiation of SBP
4. Discussion
4.1. Key Findings in the Present Work
4.2. Process Scale-Up from Proof-of-Concept to Industrial Level
4.3. Improving Biosurfactants’ Properties
4.4. Challenges and Perspectives for Improving Processes and Products Sustainability: A Comparative Review of Chemical and Bacterial Surfactants
4.4.1. Biowastes as Feedstock for Enhanced Products Sustainability
4.4.2. Improving Biosurfactant Productivity
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Cefic. 2023. Available online: https://cefic.org/a-pillar-of-the-european-economy/facts-and-figures-of-the-european-chemical-industry/profile/ (accessed on 2 December 2023).
- Innospec Inc. Market Report Global Surfactant Market. Acmite Mark. Intell. 2021. Available online: https://www.acmite.com/market-reports/chemicals/global-surfactant-market.html (accessed on 21 May 2024).
- Levinson, M.I. Surfactants production. In Handbook of Detergents, Part F: Production; Zoller, U., Sosis, P., Eds.; CRC Press: Boca Raton, FL, USA, 2009; Chapter 1; pp. 1–38. [Google Scholar]
- Falbe, J. Surfactants in Consumer Products: Theory, Technology and Applicatios; Springer Science & Business Media: New York, NY, USA, 2012. [Google Scholar]
- Rust, D.; Wildes, S. Surfactants: A Market Opportunity Study Update. Available online: https://soynewuses.org/wp-content/uploads/Surfactants-MOS-Jan-2009.pdf (accessed on 16 December 2023).
- GMI. Biosurfactants Market—By Product (Sophorolipids, Rhamnolipids, Alkyl Polyglucosides, Sucrose Esters, Lipopeptides, Phospholipids), By Application (Personal Care, Industrial Cleaners, Food Processing, Textiles, Pharma, Bioremediation), & Forecast, 2023–2032. Report ID: GMI484. Published Date: April 2023. Available online: https://www.gminsights.com/industry-analysis/biosurfactants-market-report?gclid=Cj0KCQiA7OqrBhD9ARIsAK3UXh11BqLKP8Mr8lhe12rBRWWxRe_QWlY7Rbfk69CSvr94eSQ13TpkAQgaAlwXEALw_wcB (accessed on 21 May 2024).
- Connolly, H.E.; Rahman, P.K.S.M.; Banat, I.M.; Lord, R.A. Resource recovery and reduction of oily hazardous wastes via biosurfactant washing and bioremediation. In Trends in Bioremediation and Phytoremediation; Płaza, G., Ed.; Research Signpost: Kerala, India, 2010; pp. 157–172. Available online: https://pure.ulster.ac.uk/ws/portalfiles/portal/11260519/10_Plaza.pdf. (accessed on 2 December 2023).
- Chemanalyst. Defoamer Surfactant Price Trends and Forecast. Available online: https://www.chemanalyst.com/Pricing-data/defoamer-surfactant-1240 (accessed on 2 February 2024).
- Made-in-China. Surfactants Price. Available online: https://www.made-in-china.com/products-search/hot-china-products/Surfactants_Price.html (accessed on 3 February 2024).
- Montoneri, E.; Tomasso, L.; Colajanni, N.; Zelano, I.; Alberi, F.; Cossa, G.; Barberis, R. Urban wastes to remediate industrial sites: A case of polycyclic aromatic hydrocarbons contamination and a new process. Int. J. Environ. Sci. Technol. 2014, 11, 251–262. [Google Scholar] [CrossRef]
- Tabasso, S.; Berto, S.; Rosato, R.; Marinos, J.A.T.; Ginepro, M.; Zelano, V.; Daniele, P.G.; Montoneri, E. Chemical modeling of acid-base properties of soluble biopolymers derived from municipal waste treatment materials. Int. J. Mol. Sci. 2015, 16, 3405–3418. [Google Scholar] [CrossRef] [PubMed]
- Montoneri, E.; Fabbri, G.; Quagliotto, P.L.; Baglieri, A.; Padoan, E.; Boero, V.; Negre, M. High molecular weight biosurfactants from mild chemical reactions of fermented municipal biowastes. ChemistrySelect 2020, 5, 2564–2576. [Google Scholar] [CrossRef]
- Montoneri, E.; Rosso, D.; Bucci, G.; Berto, S.; Baglieri, A.; Mendichi, R.; Quagliotto, P.L.; Francavilla, M.; Mainero, D.; Negre, M. Ozonization to upgrade waste-derived soluble lignin-like substances to higher value products. ChemistrySelect 2016, 1, 1613–1629. [Google Scholar] [CrossRef]
- Negre, M.; Montoneri, E.; Antonini, M.; Grillo, G.; Tabasso, S.; Quagliotto, P.; Berto, S.; Mendichi, R.; Cravotto, G.; Baglieri, A. Product yield, quality and energy in the hydrolysis of urban bio-waste compost from laboratory-scale runs. J. Clean. Prod. 2018, 170, 1484–1492. [Google Scholar] [CrossRef]
- Frund, R.; Ludemann, H.D. Thequantitative analysis of solution and CPMAS-C-13 NMR spectra of humic material. Sci. Total Environ. 1989, 81, 157–168. [Google Scholar] [CrossRef]
- Padoan, E.; Montoneri, E.; Baglieri, A.; Francavilla, M.; Negre, M. Mild chemical treatment of unsorted urban food wastes. Molecules 2023, 28, 7670. [Google Scholar] [CrossRef] [PubMed]
- Padoan, E.; Montoneri, E.; Baglieri, A.; Contillo, F.; Francavilla, M.; Negre, M. The Autocatalytic Chemical Reaction of a Soluble Biopolymer Derived from Municipal Biowaste. Molecules 2024, 29, 485. [Google Scholar] [CrossRef]
- Geraili, A.; Romagnoli, J.A. A framework for optimal design of integrated biorefineries under uncertainty. Chem. Eng. Trans. 2015, 43, 1357–1362. [Google Scholar] [CrossRef]
- Sweeney, J.; Murphy, F.; Vance, C. Space, time, and sustainability: The status and future of life cycle assessment frameworks for novel biorefinery systems. Renew. Sustain. Energy Rev. 2022, 159, 112259. [Google Scholar] [CrossRef]
- Guilayn, F.; Rouez, M.; Crest, M.; Patureau, D.; Jimenez, J. Valorization of digestates from urban or centralized biogas plants: A critical review. Rev. Environ. Sci. Biotechnol. 2020, 19, 419–462. [Google Scholar] [CrossRef]
- Consorzio Italiano Compostatori (CIC). Country Report—The State of the Art of Separate Collection, Composting and AD in Italy. 2017. Available online: https://www.compost.it/wp-content/uploads/2019/08/Rapporto-CIC-2017-Eng-v-2.6-web-version.pdf (accessed on 17 October 2023).
- Tsagaraki, E.; Karachaliou, E.; Delioglanis, I.; Kouzi, E. D2.1 Bio-Based Products and Applications Potential. 2017. Available online: https://www.bioways.eu/download.php?f=150&l=en&key=441a4e6a27f83a8e828b802c37adc6e1 (accessed on 10 October 2023).
- Chen, G.Q. New challenges and opportunities for industrial biotechnology. Microb. Cell Fact. 2012, 11, 111. [Google Scholar] [CrossRef]
- Speight, J.G. (Ed.) Other Feedstocks—Coal, Oil Shale, and Biomass. In Handbook of Petrochemical Processes; CRC Press: Boca Rtaon, FL, USA, 2019; ISBN 9781498729703/9780429155611. [Google Scholar] [CrossRef]
- Saeed, S.H. Handbook of Petrochemical Processes; Speight, J.G., Ed.; CRC Press: Boca Rtaon, FL, USA, 2019. [Google Scholar]
- OECD. Biobased Chemicals and Bioplastics: Finding the Right Policy Balance. In OECD Science, Technology and Industry Policy Papers; OECD Publishing: Paris, France, 2014; Volume 17. [Google Scholar]
- Iles, A.; Martin, A.N. Expanding bioplastics production: Sustainable business innovation in the chemical industry. J. Clean. Prod. 2013, 45, 38–49. [Google Scholar] [CrossRef]
- Panoutsou, C.; Mianiatis, K. Sustainable Biomass Availability in the EU, to 2050. Concawe Report RED II Annex IX A/B. 2021. Available online: https://www.concawe.eu/publication/sustainable-biomass-availability-in-the-eu-to-2050/ (accessed on 17 October 2023).
- Vaneeckhaute, C.; Lebuf, V.; Michels, E.; Belia, E.; Vanrolleghem, P.A.; Tack, F.M.G.; Meers, E. Nutrient recovery from digestate: Systematic technology review and product classification. Waste Biomass Valorization 2017, 8, 21–40. [Google Scholar] [CrossRef]
- Barret, R. Importance and Evaluation of Lipophilicity. Ther. Chem. 2018, 53–78. [Google Scholar] [CrossRef]
- Sowada, R.; Germany, L.; McGowan, J.C. Calculation of HLB values. Tenside Surf. Det. 1992, 29, 109–113. [Google Scholar] [CrossRef]
- Pasquali, R.C.; Taurozzi, M.P.; Bregni, C. Some considerations about the hydrophilic–lipophilic balance system. Int. J. Pharm. 2008, 356, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Montoneri, E.; Fabbri, G.; Grillo, G.; Tabasso, S.; Cravotto, G.; Quagliotto, P.L.; Baglieri, A.; Boero, V.; Negre, M. Mild Hydrogenation of Urban Biowaste Hydrolysates to Biopolymers with Improved Properties. ChemistrySelect 2019, 4, 4168–4177. [Google Scholar] [CrossRef]
- Davies, J.T. A quantitative kinetic theory of emulsion. In Proceedings of the 2nd International Congress Surface Activity, London, UK; 1957; pp. 426–438. Available online: http://www.firp.ula.ve/archivos/historicos/57_Chap_Davies.pdf (accessed on 3 February 2024).
- Guo, X.; Rong, Z.; Ying, X. Calculation of hydrophile–lipophile balance for polyethoxylated surfactants by group contribution methods. J. Colloid. Interface Sci. 2006, 298, 441–450. [Google Scholar] [CrossRef]
- Malato, S.; Blanco, J.; Alarcòn, D.J.; Maldonado, M.I.; Fernàndez-Ibànez, P.; Gernjak, W. Photocatalytic decontamination and disinfection of water with solar collectors. Catal. Today 2007, 122, 137–149. [Google Scholar] [CrossRef]
- Avetta, P.; Bella, F.; Bianco Prevot, A.; Laurenti, E.; Montoneri, E.; Arques, A.; Carlos, L. Waste cleaning waste: Photodegradation of monochlorophenols in the presence of waste derived photosensitizer. ACS Sustain. Chem. Eng. 2013, 1, 1545–1550. [Google Scholar] [CrossRef]
- Galushchinskiy, A.; González-Gómez, R.; McCarthy, K.; Farràs, P.; Savateev, A. Progress in development of photocatalytic processes for synthesis of fuels and organic compounds under outdoor solar light. Energy Fuels 2022, 36, 4625–4639. [Google Scholar] [CrossRef] [PubMed]
- Mission Innovation. Innovation Challenge 5: Converting Sunlight into Solar Fuels and Chemicals Roadmap 2020–2050. Available online: http://mission-innovation.net/wp-content/uploads/2021/03/Converting-Sunlight-into-Solar-Fuels-and-Chemicals-MI-Challenge-5-roadmap-Feb-2021-final.pdf (accessed on 3 February 2024).
- Samanta, S.; Srivastava, R. Catalytic conversion of CO2 to chemicals and fuels: The collective thermocatalytic/photocatalytic/electrocatalytic approach with graphitic carbon nitride. Mater. Adv. 2020, 1, 1506–1545. [Google Scholar] [CrossRef]
- Xiang, Z.; Han, W.; Deng, J.; Zhu, W.; Zhang, Y.; Wang, H. Photocatalytic Conversion of Lignin into Chemicals and Fuels. ChemSusChem 2020, 13, 4199–4213. [Google Scholar] [CrossRef]
- Chen, H.; Wan, K.; Zheng, F.; Zhang, Z.; Zhang, H.; Zhang, Y.; Long, D. Recent Advances in Photocatalytic Transformation of Carbohydrates Into Valuable Platform Chemicals. Front. Chem. Eng. 2021, 3, 615309. [Google Scholar] [CrossRef]
- Yang, C.; Li, R.; Zhang, K.A.I.; Lin, W.; Landfester, K.; Wang, X. Heterogeneous photoredox flow chemistry for the scalable organosynthesis of fine chemicals. Nat. Commun. 2020, 11, 1239. [Google Scholar] [CrossRef]
- Reed, N.L.; Yoon, T.P. Oxidase reactions in photoredox catalysis. Chem. Soc. Rev. 2021, 50, 2954–2967. [Google Scholar] [CrossRef]
- Oelgemoller, M. Solar Photochemical Synthesis: From the Beginnings of Organic Photochemistry to the Solar Manufacturing of Commodity Chemicals. Chem. Rev. 2016, 116, 9664–9682. [Google Scholar] [CrossRef]
- Bellardita, M.; Loddo, V.; Palmisano, L. Formation of High Added Value Chemicals by Photocatalytic Treatment of Biomass. Mini-Rev. Org. Chem. 2020, 17, 884–901. [Google Scholar]
- Ramachandran, S.; Fontanille, P.; Pandey, A.; Larroche, C. Gluconic acid: Properties, applications and microbial production. Food Technol. Biotechnol. 2006, 44, 185–195. [Google Scholar]
- Zhang, H.D.; Li, N.; Pan, X.J.; Wu, S.B.; Xie, J. Oxidative conversion of glucose to gluconic acid by iron(III) chloride in water under mild conditions. Green Chem. 2016, 18, 2308–2312. [Google Scholar] [CrossRef]
- Ghorpade, V.; Hanna, M. Industrial Applications for Levulinic Acid. In Cereals; Campbell, G.M., Webb, C., McKee, S.L., Eds.; Springer: Boston, MA, USA, 1997; Available online: https://link.springer.com/chapter/10.1007/978-1-4757-2675-6_7 (accessed on 23 December 2023). [CrossRef]
- Manas, M.G.; Campos, J.; Sharninghausen, L.S.; Lin, E.; Grabtree, R.H. Selective catalytic oxidation of sugar alcohols to lactic acid. Green Chem. 2015, 17, 594–600. [Google Scholar] [CrossRef]
- Albert, J.; Wasserscheid, P. Expanding the scope of biogenic substrates for the selective production of formic acid from wateri nsoluble and wet waste biomass. Green Chem. 2015, 17, 5164–5171. [Google Scholar] [CrossRef]
- Chou, C.F.; Chou, T.C. Paired electrooxidation IV. Decarboxylation of sodium gluconate to D-arabinose. J. Appl. Electrochem. 2003, 33, 741–745. [Google Scholar] [CrossRef]
- Nagtode, V.S.; Cardoza, C.; Yasin, H.K.A.; Mali, S.N.; Tambe, S.M.; Roy, P.; Singh, K.; Goel, A.; Amin, P.D.; Thorat, B.R.; et al. Green Surfactants (Biosurfactants): A Petroleum-Free Substitute for Sustainability_Comparison, Applications, Market, and Future Prospects. ACS Omega 2023, 8, 11674–11699. [Google Scholar] [CrossRef] [PubMed]
- Dierickx, S.; Castelein, M.; Remmery, J.; De Clercq, V.; Lodens, S.; Baccile, N.; De Maeseneire, S.L.; Roelants, S.L.K.W.; Soetaert, W.K. From bumblebee to bioeconomy: Recent developments and perspectives for sophorolipid biosynthesis. Biotechnol. Adv. 2021, 54, 107788. Available online: https://hal.sorbonne-universite.fr/hal-03273220/document (accessed on 23 December 2023). [CrossRef]
- Randhawa, K.K.S.; Rahman, P.K.S.M. Rhamnolipid biosurfactants—Past, present, and future scenario of global market. Front. Microbiol. Sec. Microbiotechnol. 2014, 5, 454. [Google Scholar]
- Rahman, P.K.S.M.; Randhawa, K.K.S. Editorial: Microbiotechnology Based Surfactants and Their Applications. Front. Microbiol. Sec. Microbiotechnol. 2015, 6, 1344. [Google Scholar] [CrossRef]
- Arkhipov, V.P.; Arkhipov, R.; Filippov, A. Rhamnolipid Biosurfactant: Use for the Removal of Phenol from Aqueous Solutions by Micellar Solubilization. ACS Omega 2023, 8, 30646–30654. [Google Scholar] [CrossRef]
- Pala, M.; Everaert, J.; Ollivier, A.; Raeymaekers, R.; Quataert, K.; Roelants, S.L.K.W.; Soetaert, W.; Stevens, C.V. Ozonolysis of Symmetrical Bola Sophorosides Yields Key Precursors for the Development of Functional Sophorolipid Derivatives. ACS Sustain. Chem. Eng. 2022, 10, 12234–12244. [Google Scholar] [CrossRef]
- Mulligan, C.N. Sustainable Production of Biosurfactants Using Waste Substrates. In Advancements in Biosurfactants Research; Aslam, R., Mobin, M., Aslam, J., Zehra, S., Eds.; Springer: Cham, Switzerland, 2023. [Google Scholar] [CrossRef]
- Miao, Y.; To, M.H.; Siddiqui, M.A.; Wang, H.; Lodens, S.; Chopra, S.S.; Kaur, G.; Roelants, S.L.K.W.; Lin, C.S.K. Sustainable biosurfactant production from secondary feedstock—Recent advances, process optimization and perspectives. Front. Chem. Sec. Green Sustain. Chem. 2024, 12, 1327113. [Google Scholar] [CrossRef] [PubMed]
- Maddaloni, M.; Vassalini, I.; Alessandri, I. Green Routes for the Development of Chitin/Chitosan Sustainable Hydrogels. Sustain. Chem. 2020, 1, 325–344. [Google Scholar] [CrossRef]
- Gustavsson, J.; Cederberg, C.; Sonesson, U.; van Otterdijk, R.; Meybeck, A. Global Food Losses and Food Waste—Extent, Causes and Prevention; Food and Agriculture Organization of the United Nations: Rome, Italy, 2011. [Google Scholar]
- Ellen Macarthur Foundation. Cities in the Circular Economy: An Initial Exploration. Available online: https://www.ellenmacarthurfounda-tion.org/assets/downloads/publications/Cities-in-the-CE_An-Initial-Exploration.pdf (accessed on 12 December 2021).
- Sheldon-Coulson, G.A. Production of Levulinic Acid in Urban Biorefineries. Master’s Thesis, Massachusetts Institute of Technology, Engineering Systems Division, Technology and Policy Program, Cambridge, MA, USA, 2011. Available online: https://www.semanticscholar.org/paper/Production-of-levulinicacid-in-urban-biorefineries-Sheldon-Coulson/116f70becc058aef0af6beb323dfa5c56c422ea3 (accessed on 12 December 2021).
- Wong, J.W.C.; Kaur, G.; Mehariya, S.; Karthikeyan, O.P.; Chen, G. Food waste treatment by anaerobic co-digestion with saline sludge and its implications for energy recovery in Hong Kong. Bioresour. Technol. 2018, 268, 824–828. [Google Scholar] [CrossRef] [PubMed]
- Waste & Resources Action Programme. Anaerobic Digestate—Financial Impact Assessment. Available online: http://www.organicsrecycling.org.uk/uploads/category1060/Financial_impact_assessment_for_anaerobic_digestate.pdf (accessed on 3 February 2020).
- Kaur, G.; Wong, J.W.C.; Kumar, R.; Patria, R.D.; Bhardwaj, A.; Uisan, K.; Johnravindar, D. Value Addition of Anaerobic Digestate from Biowaste: Thinking Beyond Agriculture. Curr. Sustain. Renew. Energy Rep. 2020, 7, 48–55. [Google Scholar] [CrossRef]
- Cerda, A.; Mejias, L.; Rodríguez, P.; Rodríguez, A.; Artola, A.; Font, X.; Gea, T.; Sánchez, A. Valorisation of digestate from biowaste through solid-state fermentation to obtain value added bioproducts: A first approach. Bioresour. Technol. 2019, 271, 409–416. [Google Scholar] [CrossRef] [PubMed]
- Cesaro, A. The valorization of the anaerobic digestate from the organic fractions of municipal solid waste: Challenges and perspectives. J. Environ. Manag. 2021, 280, 111742. [Google Scholar] [CrossRef] [PubMed]
- Patria, R.D.; Wong, J.W.C.; Johnravindar, D.; Uisan, K.; Kumar, R.; Kaur, G. FoodWaste Digestate-Based Biorefinery Approach for Rhamnolipids Production: A Techno-Economic Analysis. Sustain. Chem. 2021, 2, 237–253. [Google Scholar] [CrossRef]
- Montoneri, E.; Koutinas, M.; Padoan, E.; Negro, V.; Licignano, C.; Leone, S.; Photiou, P.; Kallis, M.; Vyrides, I.; Liendo, F.; et al. Integrated chemical and biochemical technology to produce biogas with reduced ammonia content from municipal biowaste. Validating lab-scale research in real operational environment. Environ. Sci. Adv. 2022, 1, 746–768. [Google Scholar] [CrossRef]
- Mohanty, S.S.; Koul, Y.; Varjani1, S.; Pandey, A.; Ngo, H.H.; Chang, J.; Wong, J.W.C.; Bui, X. A critical review on various feedstocks as sustainable substrates for biosurfactants production: A way towards cleaner production. Microb Cell Fact 2021, 20, 120. [Google Scholar] [CrossRef]
- Begum, W.; Saha, B.; Mandal, U. A comprehensive review on production of biosurfactants by bio-degradation of waste carbohydrate feedstocks: An approach towards sustainable development. RSC Adv. 2023, 13, 25599. [Google Scholar] [CrossRef]
- Isikgor, F.K.; Becer, C.R. Lignocellulosic biomass: A sustainable platform for the production of bio-based chemicals and polymers. Polym. Chem. 2015, 6, 4497–4559. [Google Scholar] [CrossRef]
- de Oliveira, U.R.; Espindola, L.S.; da Silva, I.R.; da Silva, I.N.; Rocha, H.M. A systematic literature review on green supply chain management: Research implications and future perspectives. J. Clean. Prod. 2018, 187, 537–561. [Google Scholar] [CrossRef]
- Scarso, A.; Strukul, G. Sustainability Trends in Homogeneous Catalytic Oxidations. In Handbook of Advanced Methods and Processes in Oxidation Catalysis. From Laboratory to Industry; Imperial College Press: London, UK, 2014; Chapter 5; pp. 679–766. [Google Scholar]
- Anastas, P.; Eghbali, N. Green Chemistry: Principles and Practice. Chem. Soc. Rev. 2010, 39, 301–312. [Google Scholar] [CrossRef] [PubMed]
- Biagini, D.; Montoneri, E.; Rosato, R.; Lazzaroni, C.; Dinuccio, E. Reducing ammonia and GHG emissions from rabbit rearing through a feed additive produced from green urban residues. Sustain. Prod. Consum. 2021, 27, 1–9. [Google Scholar] [CrossRef]
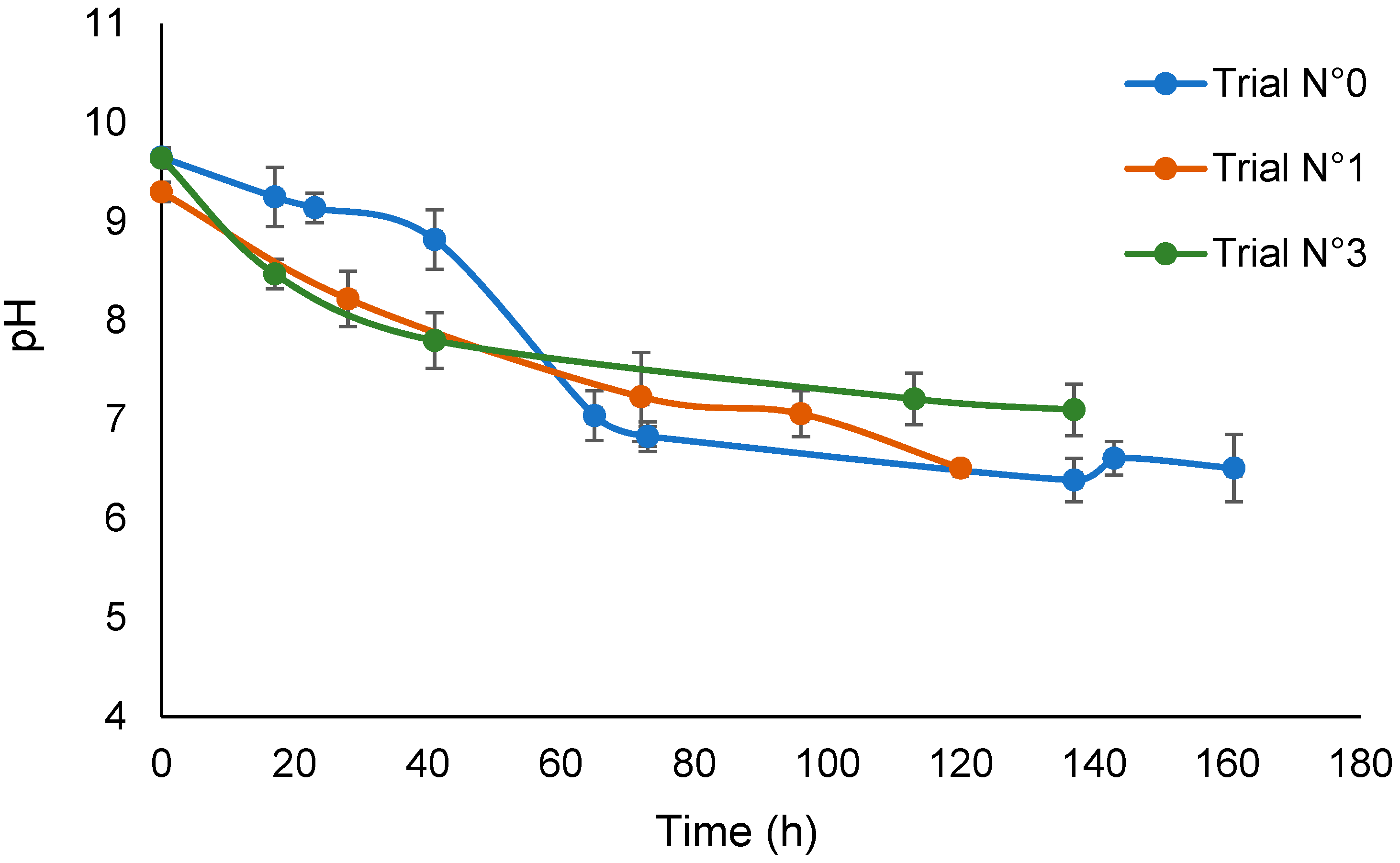






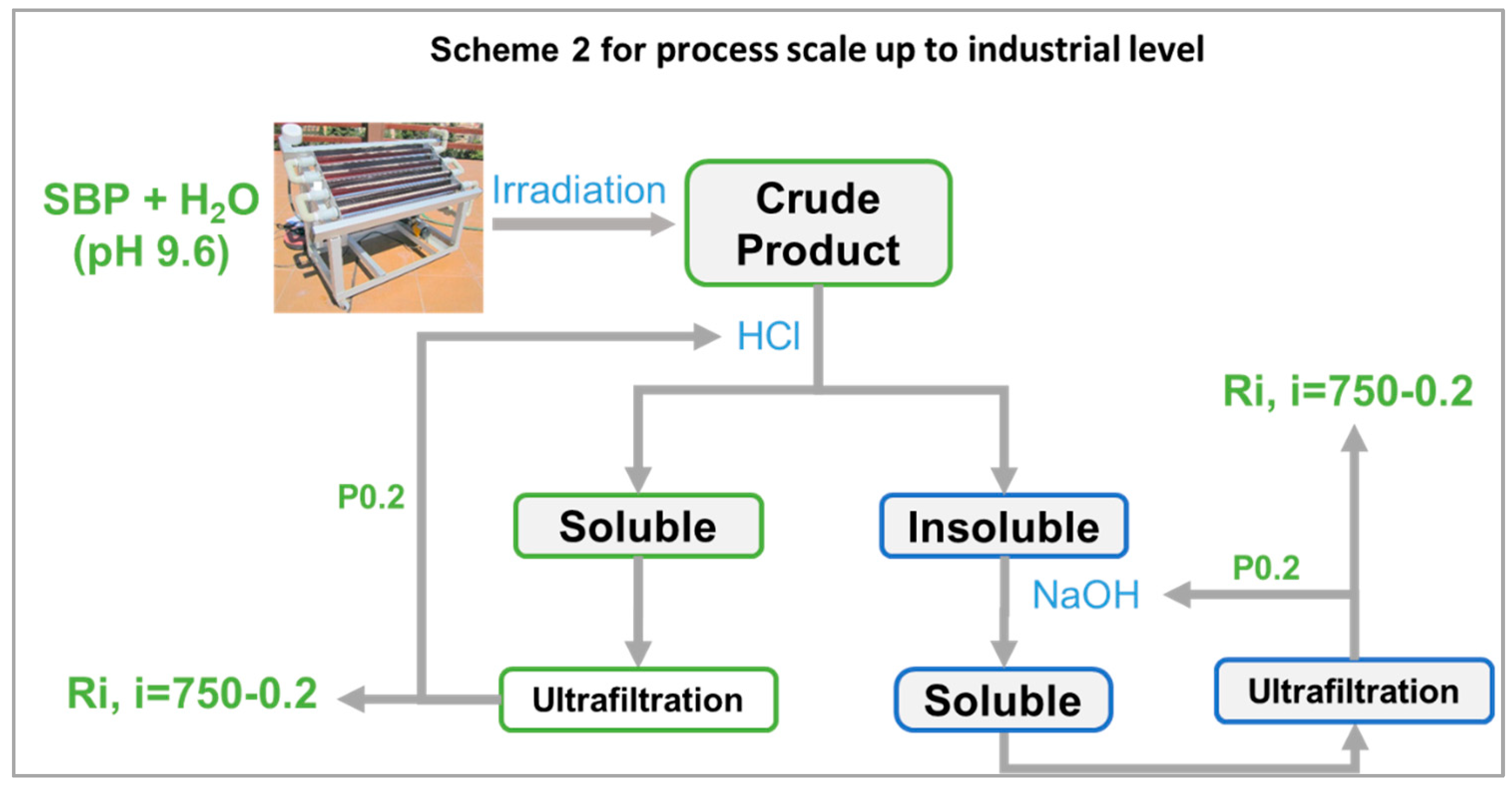
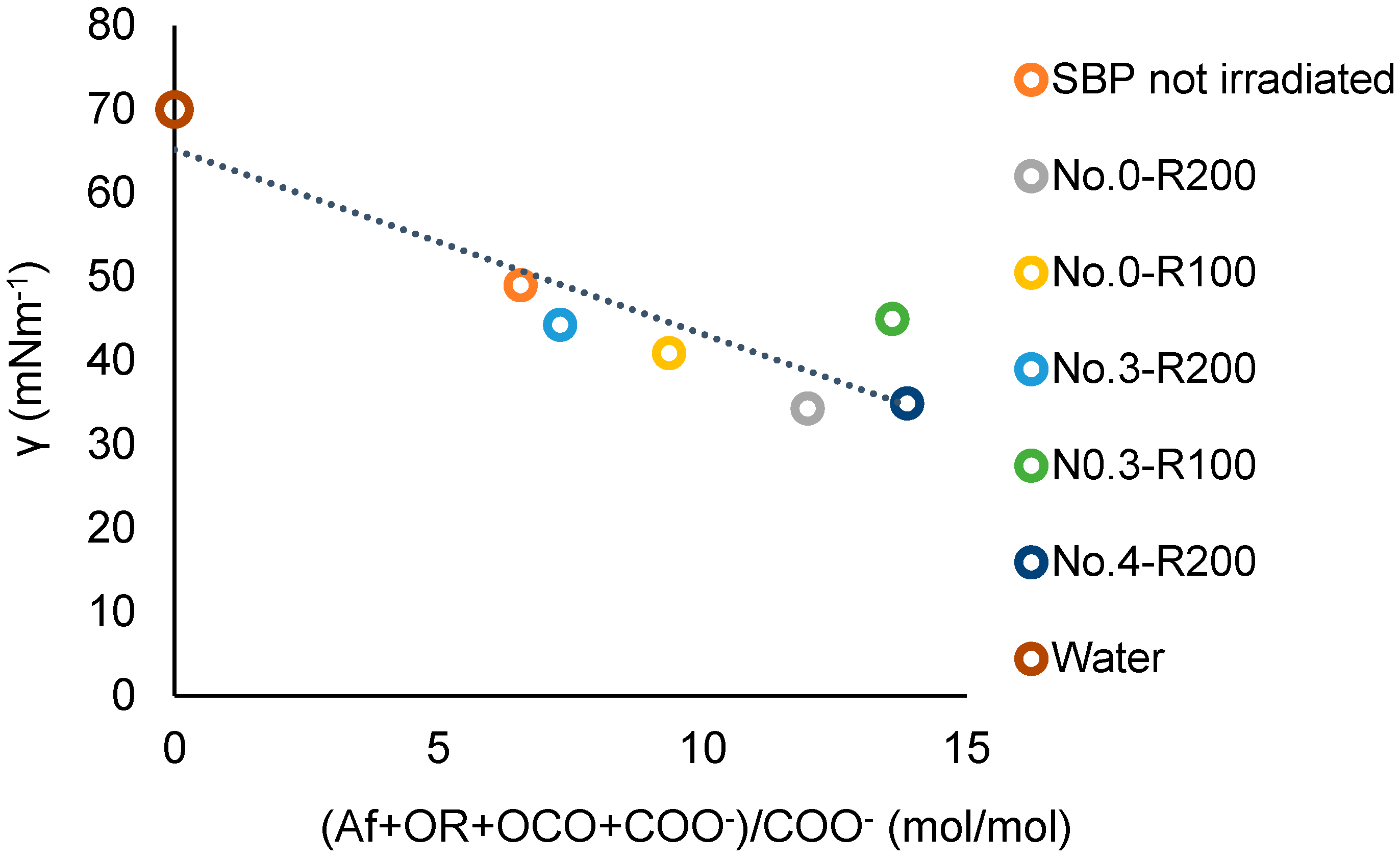
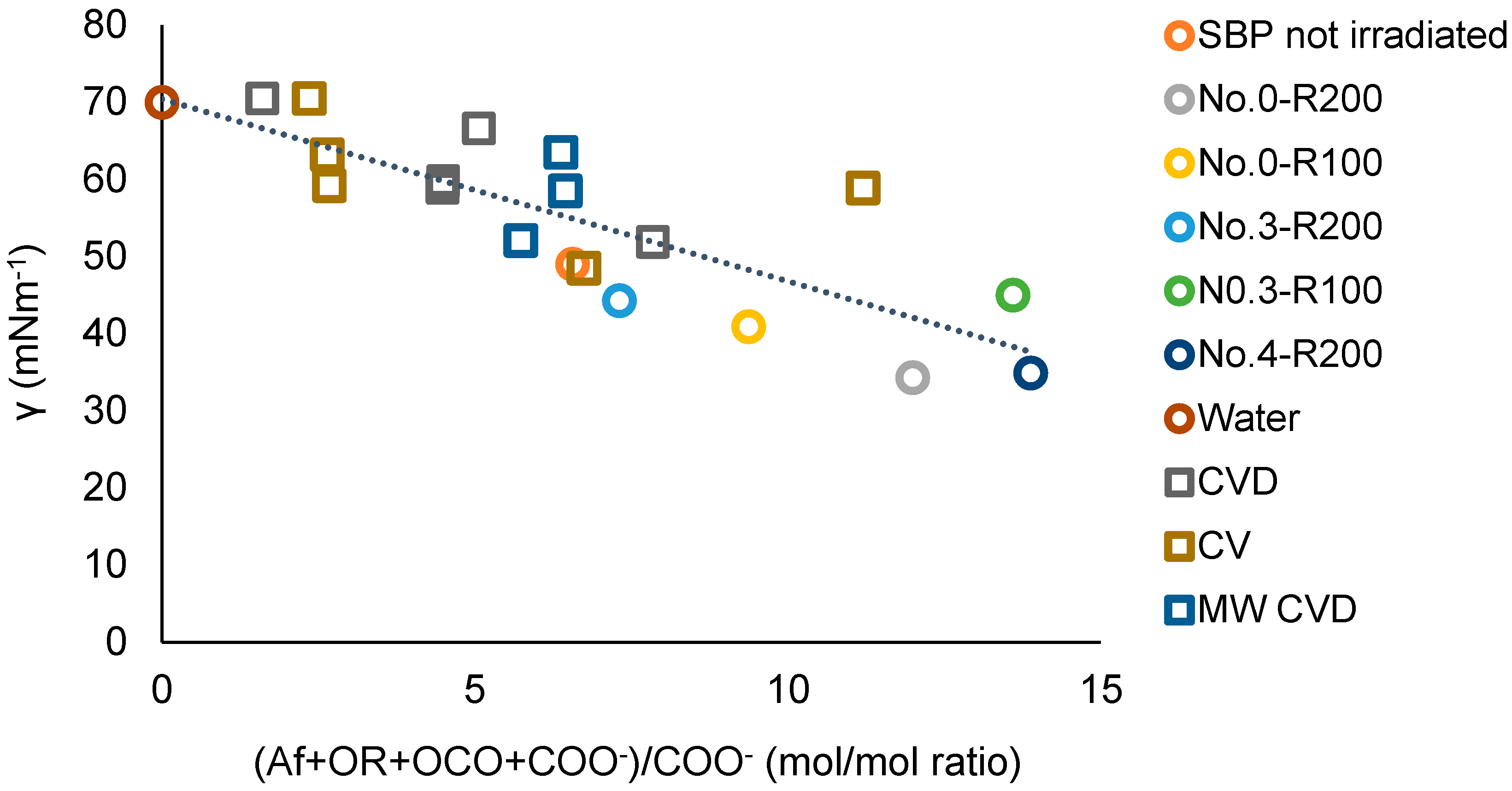
| Treatment No. | H2O2 | KOH | Irradiation Days | pH | |
|---|---|---|---|---|---|
| Start | End | ||||
| None a | no | no | 0 | ||
| 0 | no | no | 7 | 9.6 | 6.5 |
| 1 | yes | no | 5 | 9.8 | 6.5 |
| 2 | yes | yes | 7 | 9.8 | 9.8 |
| yes | no | +12 | 9.8 | 7.1 | |
| yes | no | +9 | 7.1 | 7.0 | |
| Total irradiation days for No.2 | 28 | ||||
| 3 | yes | no | 6 | 9.6 | 7.1 |
| 4 | yes | yes | 6 | 9.7 | 9.7 |
| Post-Treatment No. a | Mass w/w % and C mol/mol % Yields, Relative to Pristine SBP, for Insoluble and Soluble Matter in Water at pH < 1 | |||
|---|---|---|---|---|
| Insoluble Matter | Soluble Matter | |||
| Mass | C | Mass | C | |
| None | 65 ± 7 | 71 ± 7 | 30 ± 6 | 9.4 ± 3 |
| 0 | 48 ± 1 | 63 ± 4 | 53 ± 8 | 20 ± 2 |
| 1 | 28 ± 4 | 38 ± 2 | n.d. b | n.d. b |
| 2 | 35 ± 5 | 47 ± 5 | n.d. b | n.d. b |
| 3 | 35 ± 2 | 46 ± 3 | 61 ± 4 | 42 ± 1 |
| 4 | 37 ± 3 | 48 ± 6 | 61 ± 9 | 30 ± 3 |
| Treatment No. a | Product | R750 | R150 | R100 | R5 | P0.2 | Total b | |
|---|---|---|---|---|---|---|---|---|
| None (not-irradiated SBP) | crude | 58 ± 3 | 20 ± 2 | 5 ± 2 | 2 ± 1 | 15 ± 4 | 100 | |
| R500 | R200 | R100 | R50 | R10 | P10 | |||
| 0 | insoluble | 8.7 ± 1.6 | 21.7 ± 4 | 7.4 ± 3.1 | 7.9 ± 4.2 | 45.7 | ||
| soluble | 1.9 ± 0.6 | 5.0 ± 2.7 | 22.8 ± 5 | 23.7 ± 5 | 53.4 | |||
| 3 | insoluble | 2.7 ± 0.4 | 29.8 ± 7 | 4.3 ± 2.3 | 0.2 ± 0.1 | 0.9 ± 0.2 | 3.7 ± 1.8 | 41.6 |
| soluble | 1.9 ± 0.8 | 13.4 ± 3 | 3 ± 0.4 | 33.4 ± 7 | 14 ± 2 | 65.7 | ||
| 4 | insoluble | 1.5 ± 0.6 | 31.6 ± 6 | 6.1 ± 2 | 0.3 ± 0.1 | 4.3 ± 0.2 | 43.8 | |
| soluble | 1.5 ± 0.4 | 1.1 ± 0.5 | 17.8 ± 6 | 1.9 ± 0.2 | 36.8 ± 8 | 59.1 |
| Treatment No./Products | Ri | γ (2 g/L) | CMC (g/L) | γcmc |
|---|---|---|---|---|
| 0 | R200 | 34.3 ± 0.0 | 0.4 | 39 |
| R100 | 40.9 ± 1.3 | 0.5 | 46 | |
| 3 | R200 | 44.3 ± 0.3 | 0.5 | 46 |
| R100 | 45.0 ± 1.8 | |||
| 4 | R200 | 34.5 ± 0.2 | 0.4 | 35 |
| R100 | 40.5 ± 0.9 | 0.5 | 48 | |
| Pristine SBP [12] | R750 | 56.8 ± 0.8 | ||
| R150 | 57.3 ± 0.0 | |||
| R100 | 54.2 ± 0.7 | |||
| Pristine SBP ozonized 64 h [12] | R750 | 39.5 ± 1.0 | ||
| R150 | 36.8 ± 0.6 | 0.47 | 38 | |
| R100 | 43.6 ± 2.3 |
| Structural Unit | Group-Contribution Number [31] |
|---|---|
| -CONR2 | 1.9–2.7 |
| -CH(NH3)+-COO− | 4.3 |
| -COO− | 12.7–21 |
| -COO-ester | 1.1–2.3 |
| -COOH | 2.09 |
| -NR2, -NR2+ | 7.0–9.4 |
| Sample | PhOH | -COOH I | -COOH II | Total -COOH |
|---|---|---|---|---|
| SBP | 0.72 ± 0.10 | 2.11 ± 0.11 | 0.97 ± 0.17 | 3.07 ± 0.18 |
| No.0-R200 | 0.48 ± 0.09 | 1.76 ± 0.14 | 0.40 ± 0.03 | 2.15 ± 0.15 |
| No.0-R100 | 0.73 ± 0.34 | 1.86 ± 0.25 | 0.50 ± 0.01 | 2.35 ± 0.26 |
| No.3-R200 | 0.25 ± 0.00 | 1.61 ± 0.08 | 0.30 ± 0.07 | 1.91 ± 0.00 |
| No.3-R100 | 0.49 ± 0.10 | 1.86 ± 0.02 | 0.48 ± 0.05 | 2.34 ± 0.07 |
| No.4-R200 | 0.40 ± 0.09 | 1.61 ± 0.08 | 0.32 ± 0.06 | 1.92 ± 0.01 |
| SBP | No.0-R200 | No.0-R100 | No.3-R200 | No.3-R100 | No.4-R200 | |
|---|---|---|---|---|---|---|
| Af | 37.6 ± 1.1 | 49.2 ± 1.2 | 61.6 ± 2.7 | 53.4 ± 2.9 | 39.8 ± 0.9 | 48.4 ± 1.1 |
| NR + OMe | 7.9 ± 0.2 | 8.1 ± 0.5 | 10.1 ± 0.4 | 10 ± 0.5 | 5.5 ± 0.8 | 8.2 ± 0.5 |
| OR | 15.6 ± 0.4 | 10.4 ± 0.8 | 7.5 ± 0.3 | 10.1 ± 0.6 | 7.3 ± 1.1 | 12.4 ± 0.4 |
| OCO | 4.6 ± 0.1 | 2.3 ± 0.3 | 0.4 ± 0.0 | 2.1 ± 0.1 | 3.7 ± 0.5 | 2.8 ± 0.2 |
| Ph | 14.4 ± 0.2 | 12.5 ± 0.7 | 4.6 ± 0.3 | 7.6 ± 0.4 | 5.4 ± 0.8 | 10.2 ± 0.3 |
| PhOH | 2.3 ± 0.1 | 1.3 ± 0.1 | 1.2 ± 0.2 | 0.7 ± 0.1 | 2.2 ± 0.2 | 1.0 ± 0.1 |
| PhOR | 3.1 ± 0.1 | 2.6 ± 0.1 | 0.2 ± 0.1 | 0.2 ± 0.1 | 3.5 ± 0.2 | 3.2 ± 0.0 |
| COO− | 10.4 ± 0.3 | 5.6 ± 0.2 | 8.3 ± 0.1 | 5.2 ± 0.1 | 10.5 ± 1.0 | 4.9 ± 0.1 |
| CONR2 | 4.0 ± 0.3 | 8.0 ± 0.2 | 6.1 ± 0.6 | 10.7 ± 0.9 | 22.1 ± 1.8 | 9.0 ± 1.4 |
| C b | 2.98 | 3.73 | 2.89 | 3.66 | 2.23 | 3.89 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Padoan, E.; Contillo, F.; Marafante, M.; Montoneri, E.; Francavilla, M.; Berto, S.; Baglieri, A. A Low-Cost Ecofriendly Oxidation Process to Manufacture High-Performance Polymeric Biosurfactants Derived from Municipal Biowaste. Polymers 2024, 16, 1479. https://doi.org/10.3390/polym16111479
Padoan E, Contillo F, Marafante M, Montoneri E, Francavilla M, Berto S, Baglieri A. A Low-Cost Ecofriendly Oxidation Process to Manufacture High-Performance Polymeric Biosurfactants Derived from Municipal Biowaste. Polymers. 2024; 16(11):1479. https://doi.org/10.3390/polym16111479
Chicago/Turabian StylePadoan, Elio, Francesco Contillo, Matteo Marafante, Enzo Montoneri, Matteo Francavilla, Silvia Berto, and Andrea Baglieri. 2024. "A Low-Cost Ecofriendly Oxidation Process to Manufacture High-Performance Polymeric Biosurfactants Derived from Municipal Biowaste" Polymers 16, no. 11: 1479. https://doi.org/10.3390/polym16111479
APA StylePadoan, E., Contillo, F., Marafante, M., Montoneri, E., Francavilla, M., Berto, S., & Baglieri, A. (2024). A Low-Cost Ecofriendly Oxidation Process to Manufacture High-Performance Polymeric Biosurfactants Derived from Municipal Biowaste. Polymers, 16(11), 1479. https://doi.org/10.3390/polym16111479










