Exploring Mixed Ionic–Electronic-Conducting PVA/PEDOT:PSS Hydrogels as Channel Materials for Organic Electrochemical Transistors
Abstract
1. Introduction
2. Materials and Methods
2.1. Hydrogels Preparation
2.2. Hydrogels Characterization
2.3. Hydrogel-Based OECT Device
2.4. Computational Simulations of Hydrogel-Based OECT Device
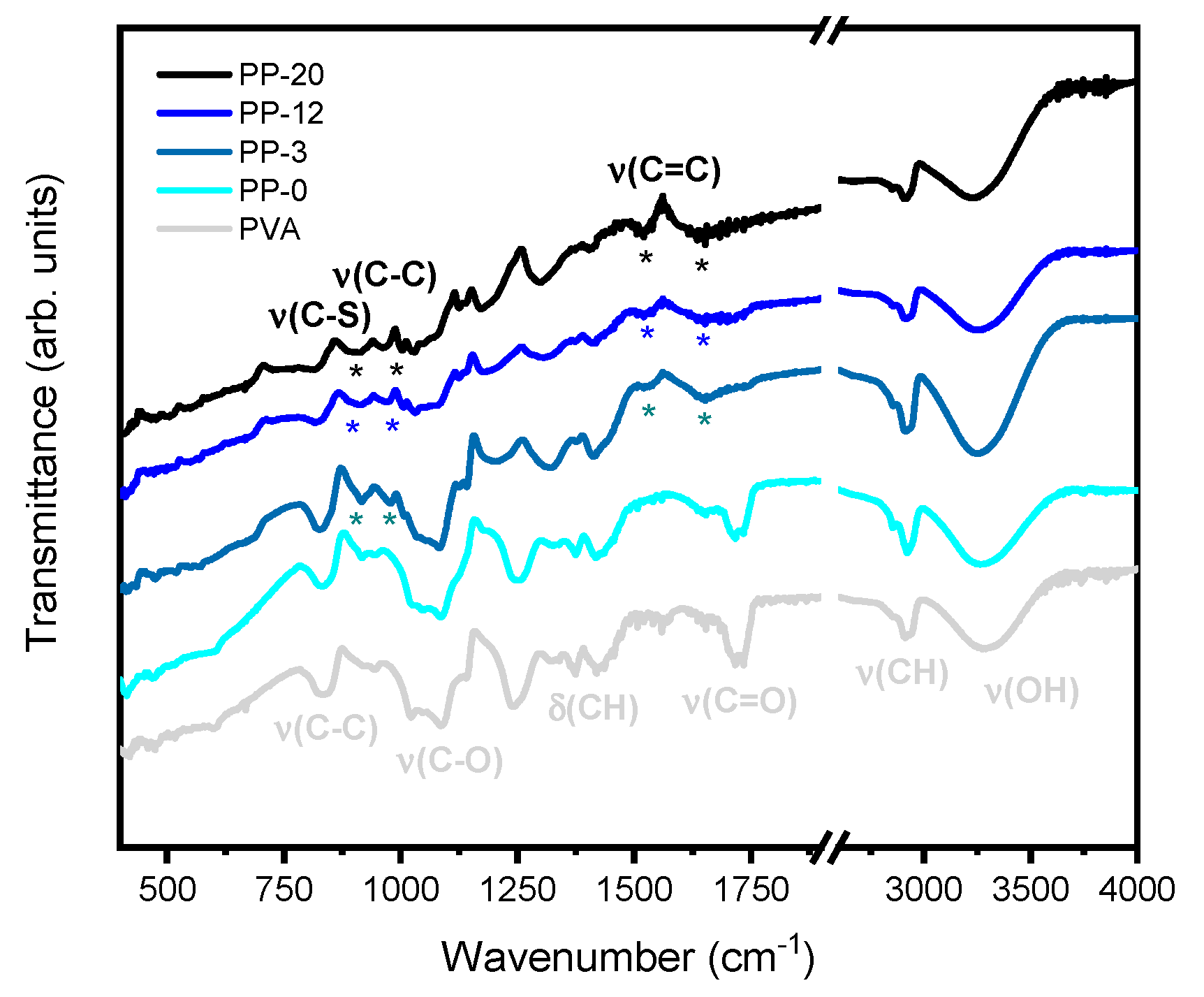
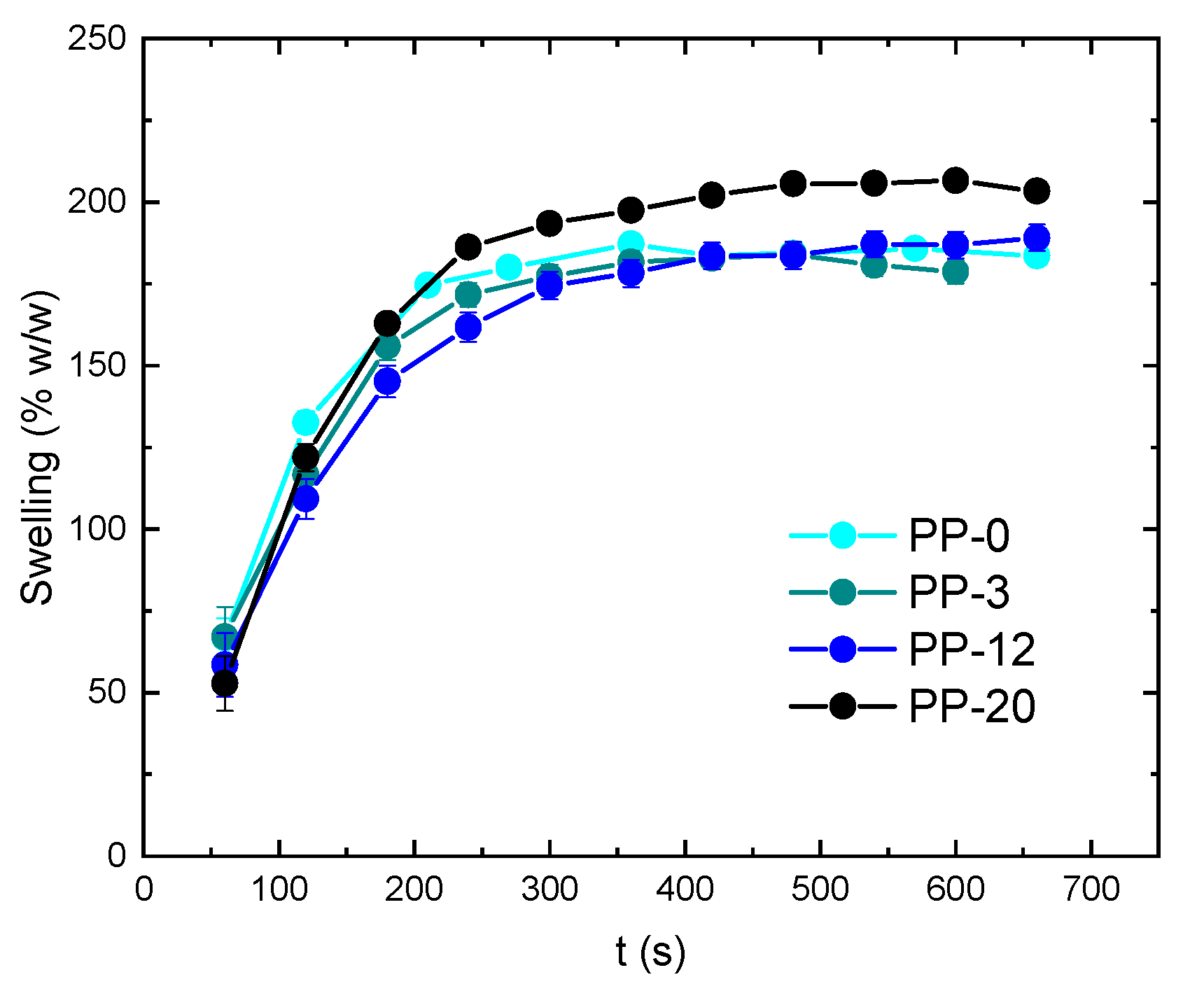
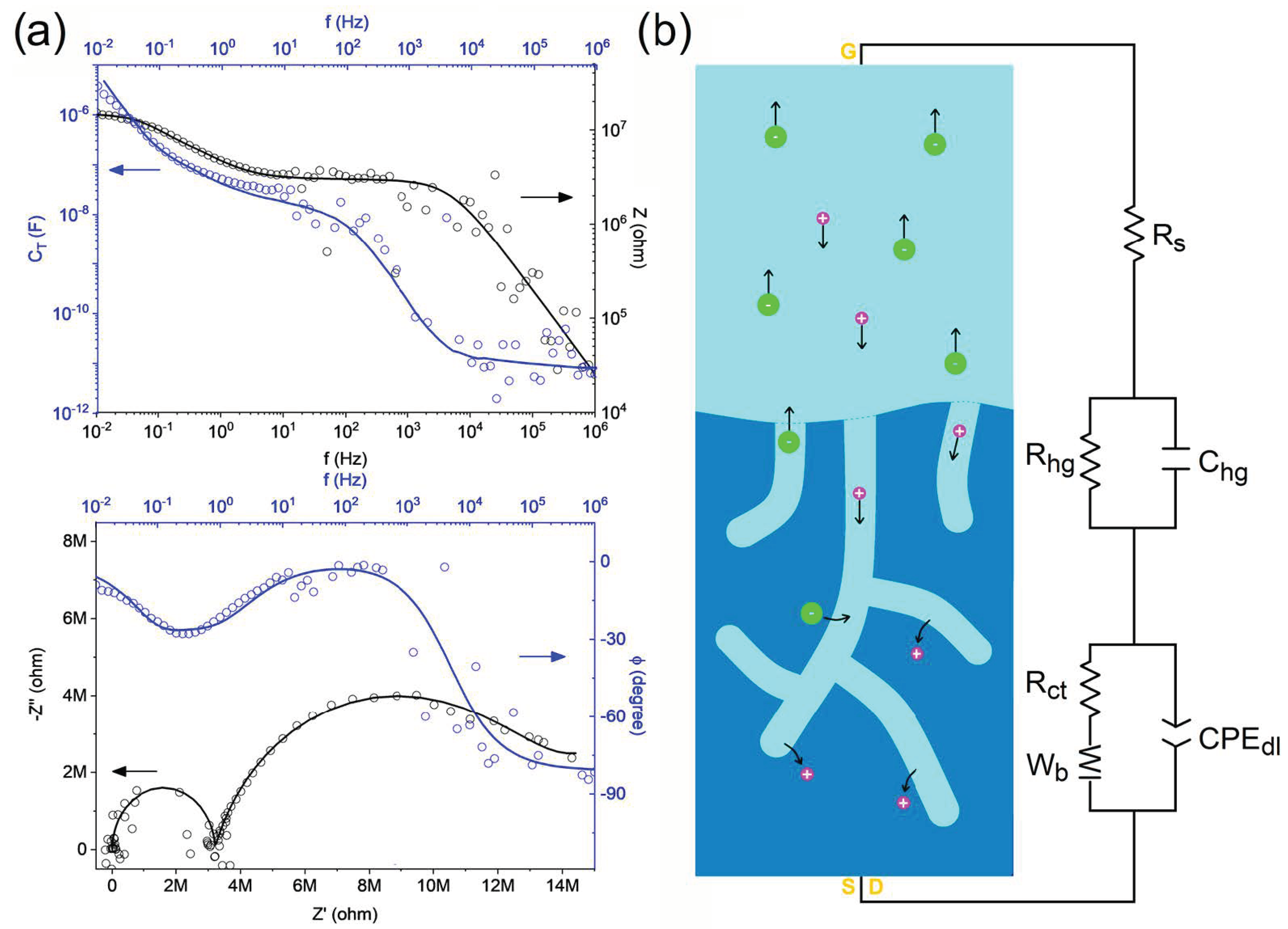
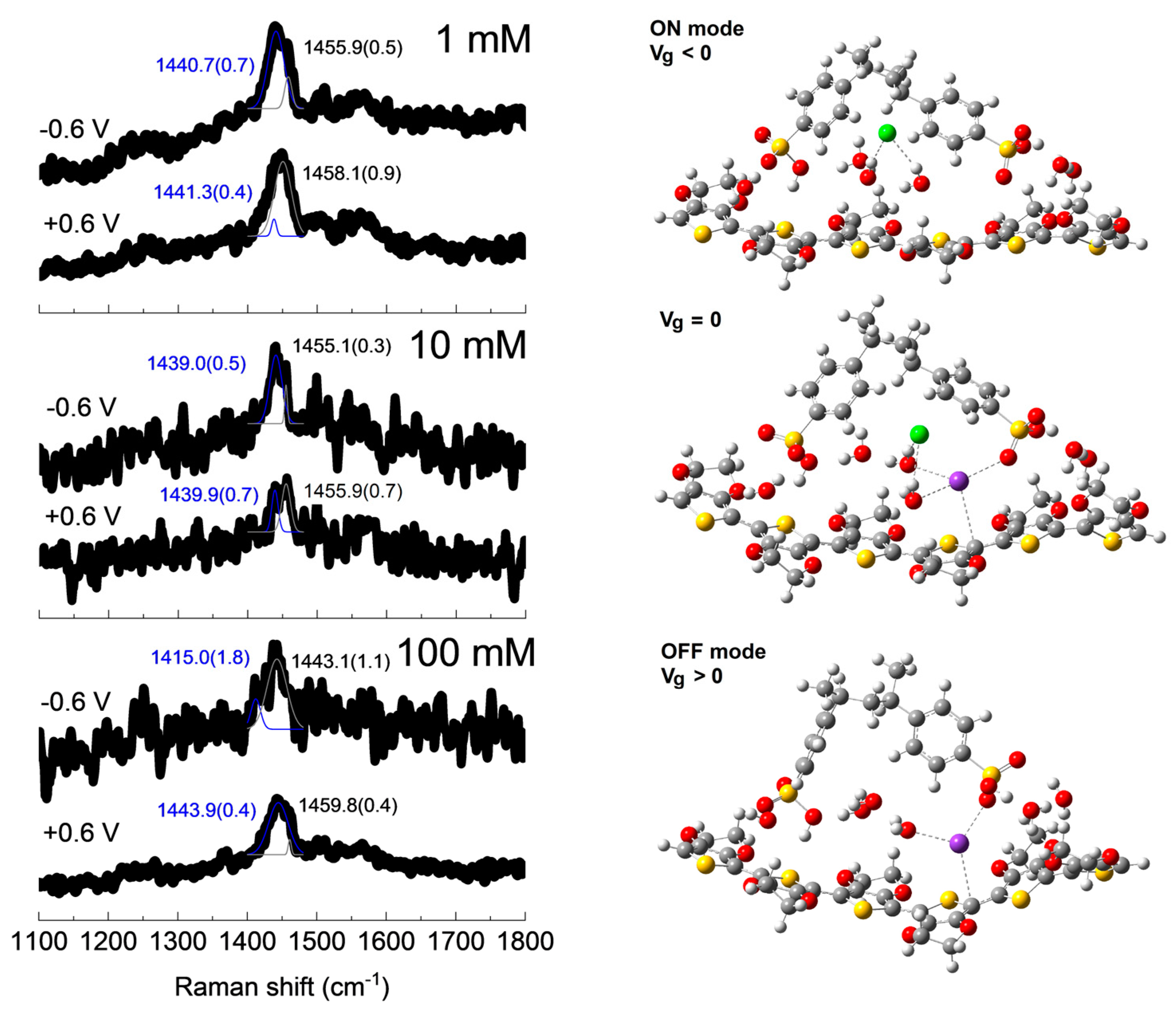
3. Results
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Xia, J.; Khaliliazar, S.; Hamedi, M.M.; Sonkusale, S. Thread-based wearable devices. MRS Bull. 2021, 46, 502–511. [Google Scholar] [CrossRef]
- Kayser, L.V.; Lipomi, D.J. Stretchable Conductive Polymers and Composites Based on PEDOT and PEDOT:PSS. Adv. Mater. 2018, 31, 1806133. [Google Scholar] [CrossRef] [PubMed]
- Facchetti, A. π-Conjugated Polymers for Organic Electronics and Photovoltaic Cell Applications. Chem. Mater. 2011, 23, 733–758. [Google Scholar] [CrossRef]
- Khodagholy, D.; Doublet, T.; Gurfinkel, M.; Quilichini, P.; Ismailova, E.; Leleux, P.; Herve, T.; Sanaur, S.; Bernard, C.; Malliaras, G.G. Highly Conformable Conducting Polymer Electrodes for In Vivo Recordings. Adv. Mater. 2011, 23, H268–H272. [Google Scholar] [CrossRef] [PubMed]
- Liao, C.; Zhang, M.; Yao, M.Y.; Hua, T.; Li, L.; Yan, F. Flexible Organic Electronics in Biology: Materials and Devices. Adv. Mater. 2015, 27, 7493–7527. [Google Scholar] [CrossRef] [PubMed]
- Bendrea, A.-D.; Cianga, L.; Cianga, I. Review Paper: Progress in the Field of Conducting Polymers for Tissue Engineering Applications. J. Biomater. Appl. 2011, 26, 3–84. [Google Scholar] [CrossRef]
- Cho, C.J.; Chen, S.Y.; Kuo, C.C.; Veeramuthu, L.; Au-Duong, A.N.; Chiu, Y.C.; Chang, S.H. Morphology and optoelectronic characteristics of organic field-effect transistors based on blends of polylactic acid and poly(3-hexylthiophene). Polym. J. 2018, 50, 975–987. [Google Scholar] [CrossRef]
- Liang, Y.; Offenhäusser, A.; Ingebrandt, A.; Mayer, D. PEDOT:PSS-Based Bioelectronic Devices for Recording and Modulation of Electrophysiological and Biochemical Cell Signals. Adv. Healthc. Mater. 2021, 10, 2100061. [Google Scholar] [CrossRef]
- Kukhta, N.A.; Marks, A.; Luscombe, C.K. Molecular Design Strategies toward Improvement of Charge Injection and Ionic Conduction in Organic Mixed Ionic−Electronic Conductors for Organic Electrochemical Transistors. Chem. Rev. 2022, 122, 4325–4355. [Google Scholar] [CrossRef] [PubMed]
- Paulsen, B.D.; Tybrandt, K.; Stavrinidou, E.; Rivnay, J. Organic mixed ionic–electronic conductors. Nat. Mater. 2020, 19, 13–26. [Google Scholar] [CrossRef] [PubMed]
- Savva, A.; Hallani, R.; Cendra, C.; Surgailis, J.; Hidalgo, T.C.; Wustoni, S.; Sheelamanthula, R.; Chen, X.; Kirkus, M.; Gio-vannitti, A.; et al. Balancing Ionic and Electronic Conduction for High-Performance Organic Electrochemical Transistors. Adv. Funct. Mater. 2020, 30, 1907657. [Google Scholar] [CrossRef]
- Bischak, C.G.; Flagg, L.Q.; Yan, K.; Rehman, T.; Davies, D.W.; Quezada, R.J.; Onorato, J.W.; Luscombe, C.K.; Diao, Y.; Li, C.Z.; et al. A Reversible Structural Phase Transition by Electrochemically-Driven Ion Injection into a Conjugated Polymer. J. Am. Chem. Soc. 2020, 142, 7434–7442. [Google Scholar] [CrossRef]
- Onorato, J.W.; Wang, Z.; Sun, Y.; Nowak, C.; Flagg, L.Q.; Li, R.; Dong, B.X.; Richter, L.J.; Escobedo, F.A.; Nealey, P.F.; et al. Side chain engineering control of mixed conduction in oligoethylene glycol-substituted polythio-phenes. J. Mater. Chem. A 2021, 9, 21410–21423. [Google Scholar] [CrossRef]
- Lin, P.; Yan, F.; Chan, H.L.W.W. Ion-Sensitive Properties of Organic Electrochemical Transistors. ACS Appl. Mater. Interfaces 2010, 2, 1637–1641. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Lim, T.; Kim, C.-H.; Yeo, C.S.; Seo, K.; Kim, S.-M.; Kim, J.; Park, S.Y.; Ju, S.; Yoon, M.-H. Organic Electrochemical Transistor-Based Channel Dimension-Independent Single-Strand Wearable Sweat Sensors. NPG Asia Mater. 2018, 10, 1086–1095. [Google Scholar] [CrossRef]
- Tang, H.; Yan, F.; Lin, P.; Xu, J.; Chan, H.L.W. Highly Sensitive Glucose Biosensors Based on Organic Electrochemical Tran-sistors Using Platinum Gate Electrodes Modified with Enzyme and Nanomaterials. Adv. Funct. Mater. 2011, 21, 2264–2272. [Google Scholar] [CrossRef]
- Kergoat, L.; Piro, B.; Simon, D.T.; Pham, M.-C.; Noël, V.; Berggren, M. Detection of Glutamate and Acetylcholine with Organic Electrochemical Transistors Based on Conducting Polymer/Platinum Nanoparticle Composites. Adv. Mater. 2014, 26, 5658–5664. [Google Scholar] [CrossRef] [PubMed]
- Bihar, E.; Deng, Y.; Miyake, T.; Saadaoui, M.; Malliaras, G.G.; Rolandi, M. A Disposable Paper Breathalyzer with an Alcohol Sensing Organic Electrochemical Transistor. Sci. Rep. 2016, 6, 27582. [Google Scholar] [CrossRef] [PubMed]
- Tao, W.; Lin, P.; Hu, J.; Ke, S.; Song, J.; Zeng, X. A Sensitive DNA Sensor Based on an Organic Electrochemical Transistor Using a Peptide Nucleic Acid-Modified Nanoporous Gold Gate Electrode. RSC Adv. 2017, 7, 52118–52124. [Google Scholar] [CrossRef]
- Lin, P.; Luo, X.; Hsing, I.M.; Yan, F. Organic Electrochemical Transistors Integrated in Flexible Microfluidic Systems and Used for Label-Free DNA Sensing. Adv. Mater. 2011, 23, 4035–4040. [Google Scholar] [CrossRef] [PubMed]
- Zeglio, E.; Inganäs, O. Active Materials for Organic Electrochemical Transistors. Adv. Mater. 2018, 30, 1800941. [Google Scholar] [CrossRef] [PubMed]
- Rivnay, J.; Inal, S.; Collins, B.A.; Sessolo, M.; Stavrinidou, E.; Strakosas, X.; Tassone, C.; Delongchamp, D.M.; Malliaras, G.G. Structural Control of Mixed Ionic and Electronic Transport in Conducting Polymers. Nat. Commun. 2016, 7, 11287. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Surendran, A.; Ko, J.; Filonik, O.; Herzig, E.M.; Müller-Buschbaum, P.; Leong, W.L. Ionic-Liquid Doping Enables High Transconductance, Fast Response Time, and High Ion Sensitivity in Organic Electrochemical Transistors. Adv. Mater. 2019, 31, 1805544. [Google Scholar] [CrossRef] [PubMed]
- Kee, S.; Kim, N.; Kim, B.S.; Park, S.; Jang, Y.H.; Lee, S.H.; Kim, J.J.; Kim, J.J.; Kwon, S.; Lee, K. Controlling Molecular Ordering in Aqueous Conducting Polymers Using Ionic Liquids. Adv. Mater. 2016, 28, 8625–8631. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Surendran, A.; Moser, M.; Chen, S.; Muhammad, B.T.; Maria, I.P.; McCulloch, I.; Leong, W.L. Universal Spray-Deposition Process for Scalable, High-Performance, and Stable Organic Electrochemical Transistors. ACS Appl. Mater. Interfaces 2020, 12, 20757–20764. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhu, C.; Pfattner, R.; Yan, H.; Jin, L.; Chen, S.; Molina-Lopez, F.; Lissel, F.; Liu, J.; Rabiah, N.I.; et al. A Highly Stretchable, Transparent, and Conductive Polymer. Sci. Adv. 2017, 3, e1602076. [Google Scholar] [CrossRef] [PubMed]
- Keene, S.T.; van der Pol, T.P.A.; Zakhidov, D.; Weijtens, C.H.L.; Janssen, R.A.J.; Salleo, A.; van de Burgt, Y. Enhance-ment-Mode PEDOT: PSS Organic Electrochemical Transistors Using Molecular De-Doping. Adv. Mater. 2020, 32, 2000270. [Google Scholar] [CrossRef] [PubMed]
- Rivnay, J.; Leleux, P.; Sessolo, M.; Khodagholy, D.; Hervé, T.; Fiocchi, M.; Malliaras, G.G. Organic electrochemical transistors with maximum transconductance at zero gate bias. Adv. Mater. 2013, 25, 7010–7014. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Kumar, P.; Nouas, A.S.; Fontaine, L.; Tang, H.; Cicoira, F. Solvent-induced changes in PEDOT: PSS films for organic electrochemical transistors. APL Mater. 2015, 3, 014911. [Google Scholar] [CrossRef]
- Osazuwa, P.O.; Lo, C.-Y.; Feng, X.; Nolin, A.; Dhong, C.; Kayser, L.V. Surface Functionalization with (3-Glycidyloxypropyl)trimethoxysilane (GOPS) as an Alternative to Blending for Enhancing the Aqueous Stability and Electronic Performance of PEDOT:PSS Thin Films. ACS Appl. Mater. Interfaces 2023, 15, 54711–54720. [Google Scholar] [CrossRef]
- del Agua, I.; Mantione, D.; Ismailov, U.; Sanchez-Sanchez, A.; Aramburu, N.; Malliaras, G.G.; Mecerreyes, D.; Ismailova, E. DVS-Crosslinked PEDOT: PSS Free-Standing and Textile Electrodes toward Wearable Health Monitoring. Adv. Mater. Technol. 2018, 3, 1700322. [Google Scholar] [CrossRef]
- Mantione, D.; del Agua, I.; Schaafsma, W.; ElMahmoudy, M.; Uguz, I.; Sanchez-Sanchez, A.; Sardon, H.; Castro, B.; Malliaras, G.G.; Mecerreyes, D. Low-temperature cross-linking of PEDOT: PSS films using divinylsulfone. ACS Appl. Mater. Interfaces 2017, 9, 18254–18262. [Google Scholar] [CrossRef] [PubMed]
- Noh, J.-S. Highly Conductive and Stretchable Poly(Dimethylsiloxane):Poly(3,4-Ethylenedioxythiophene):Poly(Styrene Sul-fonic Acid) Blends for Organic Interconnects. RSC Adv. 2014, 4, 1857–1863. [Google Scholar] [CrossRef]
- Su, X.; Wu, X.; Chen, S.; Nedumaran, A.M.; Stephen, M.; Hou, K.; Czarny, B.; Leong, W.L. A Highly Conducting Polymer for Self-Healable, Printable, and Stretchable Organic Electrochemical Transistor Arrays and Near Hysteresis-Free Soft Tactile Sensors. Adv. Mater. 2022, 34, 2200682. [Google Scholar] [CrossRef] [PubMed]
- Gao, N.; He, Y.; Tao, X.; Xu, X.-Q.; Wu, X.; Wang, Y. Crystal-confined freestanding ionic liquids for reconfigurable and repairable electronics. Nat. Commun. 2019, 10, 547. [Google Scholar] [CrossRef] [PubMed]
- Ge, G.; Mandal, K.; Haghniaz, R.; Li, M.; Xiao, X.; Carlson, L.; Jucaud, V.; Dokmeci, M.R.; Ho, G.W.; Khademhosseini, A. Deep Eutectic Solvents-Based Ionogels with Ultrafast Gelation and High Adhesion in Harsh Environments. Adv. Funct. Mater. 2023, 33, 2207388. [Google Scholar] [CrossRef] [PubMed]
- Hui, Y.; Yao, Y.; Qian, Q.; Luo, J.; Chen, H.; Qiao, Z.; Yu, Y.; Tao, L. Three-dimensional printing of soft hydrogel electronics. Nat. Electron. 2022, 5, 893–903. [Google Scholar] [CrossRef]
- Ko, J.; Wu, X.; Surendran, A.; Muhammad, B.T.; Leong, W.L. Self-Healable Organic Electrochemical Transistor with High Transconductance, Fast Response, and Long-Term Stability. ACS Appl. Mater. Interfaces 2020, 12, 33979–33988. [Google Scholar] [CrossRef]
- Wang, W.; Li, Z.; Li, M.; Fang, L.; Chen, F.; Han, S.; Lan, L.; Chen, J.; Chen, Q.; Wang, H.; et al. High-Transconductance, Highly Elastic, Durable and Recyclable All-Polymer Electrochemical Transistors with 3D Micro-Engineered Interfaces. Nano-Micro Lett. 2022, 14, 184. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.-H.; Azimi, M.; Fan, J.; Nagarajan, H.; Wang, M.; Cicoira, F. All-printed and stretchable organic electrochemical transistors using a hydrogel electrolyte. Nanoscale 2023, 15, 3263–3272. [Google Scholar] [CrossRef] [PubMed]
- Tirella, A.; Mattei, G.; Ahluwalia, A. Strain rate viscoelastic analysis of soft and highly hydrated biomaterials. J. Biomed. Mater. Res. A 2014, 102, 3352–3360. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Wieland, M.; Omiecienski, B.; Kim, Y.; Park, J.; Kim, G.; Ludwigs, S.; Yoon, M.-H. Delicate modulation of mixed conducting properties of PEDOT:PSS via crosslinking with polyvinyl alcohol. Flex. Print. Electron. 2022, 7, 044005. [Google Scholar] [CrossRef]
- Mansur, H.S.; Sadahira, C.M.; Souza, A.N.; Mansur, A.A.P. FTIR spectroscopy characterization of poly (vinyl alcohol) hydrogel with different hydrolysis degree and chemically crosslinked with glutaraldehyde. Mater. Sci. Eng. C 2008, 28, 539–548. [Google Scholar] [CrossRef]
- Dong, J.L.; Yu, L.S.H.; Xie, J.W. A Simple and Versatile Method for the Formation of Acetals/Ketals Using Trace Conventional Acids. ACS Omega 2018, 3, 4974–4985. [Google Scholar] [CrossRef] [PubMed]
- Kohn, W.; Sham, L.J. Self-Consistent Equations Including Exchange and Correlation Effects. Phys. Rev. 1965, 140, A1133–A1138. [Google Scholar] [CrossRef]
- Hohenberg, P.; Kohn, W. Inhomogeneous Electron Gas. Phys. Rev. B 1964, 136, 864–871. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16; Gaussian. Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Becke, A.D. Density-Functional Thermochemistry. Iii. The Role of Exact Exchange. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar] [CrossRef]
- Lee, C.; Yang, W.; Parr, R.G. Development of the Colle-Salvetti Correlation-Energy Formula into a Functional of the Electron Density. Phys. Rev. B 1988, 37, 785–789. [Google Scholar] [CrossRef] [PubMed]
- Stephens, P.J.; Devlin, F.J.; Chabalowski, C.F.; Frisch, M.J. Ab Initio Calculation of Vibrational Absorption and Circular Dichroism Spectra Using Density Functional Force Fields. J. Phys. Chem. 1994, 98, 11623–11627. [Google Scholar] [CrossRef]
- Vosko, S.H.; Wilk, L.; Nusair, M. Accurate Spin-Dependent Electron Liquid Correlation Energies for Local Spin Density Calculations: A Critical Analysis. Can. J. Phys. 1980, 58, 1200–1211. [Google Scholar] [CrossRef]
- Abral, H.; Atmajayaa, A.; Mahardikaa, M.; Hafizulhaqa, F.; Kadriadi; Handayani, D.; Sapuanc, S.M.; Ilyas, R.A. Effect of ultra-sonication duration of polyvinyl alcohol (PVA) gel on characterizations of PVA film. J. Mater. Res. Technol. 2020, 9, 2477–2486. [Google Scholar] [CrossRef]
- Otsuka, E.; Kudo, S.; Sugiyama, M.; Suzuki, A. Effects of Microcrystallites on Swelling Behavior in Chemically Crosslinked Poly(vinyl alcohol) Gels. J. Polym. Sci. Part B Polym. Phys. 2010, 49, 96–102. [Google Scholar] [CrossRef]
- Akhter, S.; Allan, K.; Buchanan, D.; Cook, J.A.; Campion, A.; White, J.M. XPS and IR study of X/ray induced degradation of PVA polymer film. Appl. Surf. Sci. 1988, 35, 241–258. [Google Scholar] [CrossRef]
- Peng, Y.; Yan, B.; Li, Y.; Lan, J.; Shi, L.; Ran, R. Antifreeze and moisturizing high conductivity PEDOT/PVA hydrogels for wearable motion sensor. J. Mater. Sci. 2020, 55, 1280–1291. [Google Scholar] [CrossRef]
- Romero, M.; Mombrú, D.; Pignanelli, F.; Faccio, R.; Mombrú, A.W. Mixed Ionic-Electronic Transport for PEDOT:PSS-Based Zero-Gated Organic Electrochemical Transistors Using Impedance Spectroscopy and Micro-Raman Imaging. ACS Appl. Electron. Mater. 2023, 5, 4863–4874. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, Y.; Lin, S.; Wang, Q. Preparation and properties of glutaraldehyde crosslinked poly(vinyl alcohol) membrane with gradient structure. J. Polym. Res. 2020, 27, 228. [Google Scholar] [CrossRef]
- Cooney, T.F.; Wang, L.; Sharma, S.K.; Gauldie, R.W.; Montana, A.J. Raman spectral study of solid and dissolved poly(vinyl alcohol) and ethylene-vinyl alcohol copolymer. J. Polym. Sci. Part B Polym. Phys. 1994, 32, 1163–1174. [Google Scholar] [CrossRef]
- Mombrú, D.; Romero, M.; Faccio, R.; Mombrú, A.W. Raman Microscopy Insights on the Out-of-Plane Electrical Transport of Carbon Nanotube-Doped PEDOT:PSS Electrodes for Solar Cell Applications. J. Phys. Chem. B 2018, 122, 2694–2701. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Liu, Z.; Du, X.; Ringer, S.P. Electrodeposited PEDOT films on ITO with a flower-like hierarchical structure. Synth. Met. 2010, 160, 1636–1641. [Google Scholar] [CrossRef]
- Sakmeche, N.; Aeiyach, S.; Aaron, J.-J.; Jouini, M.; Lacroix, J.C.; Lacaze, P.-C. Improvement of the electrosynthesis and physicochemical properties of poly(3,4-ethylenedioxythiophene) using a sodium dodecyl sulfate micellar aqueous medium. Langmuir 1999, 15, 2566–2574. [Google Scholar] [CrossRef]
- Ohayon, D.; Druet, V.; Inal, S. A guide for the characterization of organic electrochemical transistors and channel materials. Chem. Soc. Rev. 2023, 52, 1001–1023. [Google Scholar] [CrossRef] [PubMed]
- Orazem, M.E.; Tribollet, B. Electrochemical Impedance Spectroscopy; John Wiley & Sons: Hoboken, NJ, USA, 2008. [Google Scholar]
- Chang, B.-Y. Conversion of a Constant Phase Element to an Equivalent Capacitor. J. Electrochem. Sci. Technol. 2020, 11, 318–321. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gregorio, T.; Mombrú, D.; Romero, M.; Faccio, R.; Mombrú, Á.W. Exploring Mixed Ionic–Electronic-Conducting PVA/PEDOT:PSS Hydrogels as Channel Materials for Organic Electrochemical Transistors. Polymers 2024, 16, 1478. https://doi.org/10.3390/polym16111478
Gregorio T, Mombrú D, Romero M, Faccio R, Mombrú ÁW. Exploring Mixed Ionic–Electronic-Conducting PVA/PEDOT:PSS Hydrogels as Channel Materials for Organic Electrochemical Transistors. Polymers. 2024; 16(11):1478. https://doi.org/10.3390/polym16111478
Chicago/Turabian StyleGregorio, Tatiana, Dominique Mombrú, Mariano Romero, Ricardo Faccio, and Álvaro W. Mombrú. 2024. "Exploring Mixed Ionic–Electronic-Conducting PVA/PEDOT:PSS Hydrogels as Channel Materials for Organic Electrochemical Transistors" Polymers 16, no. 11: 1478. https://doi.org/10.3390/polym16111478
APA StyleGregorio, T., Mombrú, D., Romero, M., Faccio, R., & Mombrú, Á. W. (2024). Exploring Mixed Ionic–Electronic-Conducting PVA/PEDOT:PSS Hydrogels as Channel Materials for Organic Electrochemical Transistors. Polymers, 16(11), 1478. https://doi.org/10.3390/polym16111478








