3.2.1. Mechanical Properties
Table 2 presents the results of the tensile properties of PPLA/Saqqez gum blends with different contents of two plasticizers, ATBC and PEG, in terms of Young’s modulus, elongation at break and yield strength. The amounts were not determined for Saqqez gum, PLA/Saqqez gum (70/30
w/
w) without plasticizer and 14 wt% ATBC samples due to their high brittleness (n.d.). The available data show that the PPLA/Saqqez gum blends with different contents of ATBC and PEG have varying tensile properties. The inclusion of plasticizers seemed to affect the mechanical properties of the blends.
In terms of Young’s modulus, the blends with PLA plasticized with 16% ATBC had the lowest Young’s modulus (1100 ± 0.01 MPa). However, the Young’s modulus was lower in the samples containing 16% and 18% ATBC than in those containing PEG. This suggests that the presence of ATBC improves the stiffness of the blend. Indeed, the addition of plasticizers to a polymer reduces its resistance to deformation, resulting in a lower modulus of elasticity. This is due to an increase in the free volume between the polymer chains, which promotes greater chain mobility and flexibility. Thus, plasticized polymers require less force to deform than polymers without plasticizers [
19]. PLA is known for its high fragility and brittle nature, which is characterized by a high Young’s modulus [
40]. Therefore, the addition of plasticizers to PLA aims to reduce its fragility [
24]. It should be noted that the Young’s modulus of the samples containing 16% and 18% ATBC is lower than that of neat PLA, which has previously been reported to be 3500 MPa [
41]. According to Pivsa-Art et al. (2013), the increase in Young’s modulus is due to phase separation that occurs in immiscible compounds [
42]. Therefore, it is likely that the ATBC treatments lacked phase separation and demonstrated adequate miscibility.
In terms of elongation at break, the PPLA/Saqqez gum blend with 16% ATBC content has the highest value (130.6 ± 0.1%), followed by the PPLA/Saqqez gum blend with 18% ATBC content (120.4 ± 0.1). Among all samples, the PLA/Saqqez gum with 14% PEG has the lowest elongation at break (80.4 ± 0.1).
By comparison, the elongation of neat PLA, reported to be 8% [
43], indicates that all plasticized blends could effectively improve the stretchability of the blend. It is widely recognized that plasticizers lead to increased flexibility and elongation at break of polymers [
44,
45]. Lim and Hoag (2013) stated that the elongation percentage is a valuable parameter for evaluating the effectiveness of plasticizers based on their type and quantity [
19]. Previous studies have shown that the addition of plasticizers, such as PEG and ATBC, can increase the elongation at the break of PLA [
23], which is consistent with the results of this study. Additionally, Zhao et al. (2020) found that ATBC had a practical function at a weight increase of 20% [
40]. According to Courgneau et al. (2011), the increase in elongation at the break is related to the decrease in Young’s modulus, strength at yield and storage modulus [
24].
The results indicate that the PPLA/Saqqez gum blend with 16% ATBC content demonstrates the highest yield strength value (15.4 ± 0.9 MPa), followed by PPLA/Saqqez gum with 14% PEG content (15.2 ± 0.2 MPa). On the other hand, the PLA/Saqqez gum with 16% ATBC exhibits the lowest yield strength value (12.2 ± 0.9). Since then, this value has been reported to be 58 MPa for neat PLA [
46], suggesting that the presence of plasticizers, particularly ATBC, reduces the yield strength of the blend compared to neat PLA. Yield strength is defined as the stress required to produce a specific amount of plastic deformation, as stated by Subramaniam et al., (2019) [
47]. Kodal et al. (2019) suggest that the inclusion of plasticizers in PLA enhances its ductility and processability. The addition of 10% plasticizer by weight to PLA reduces its Young’s modulus and yield strength while improving its elongation at the break, regardless of the type of plasticizer used. Generally, the yield strength and Young’s modulus of PLA decrease with increasing plasticizer content, unless low molecular weight plasticizer migration to the polymer surface occurs during its service life [
43]. Courgneau et al. (2011) reported that PLA and ATBC are miscible up to 17 wt%, but phase separation occurs at concentrations above 5 wt% when blended with PEG [
24].
Overall,
Table 2 has demonstrated that, in this study, ATBC had a much greater effect on the tensile properties of the blends than PEG, due to the possible phase separation of PEG.
3.2.2. Thermal Properties
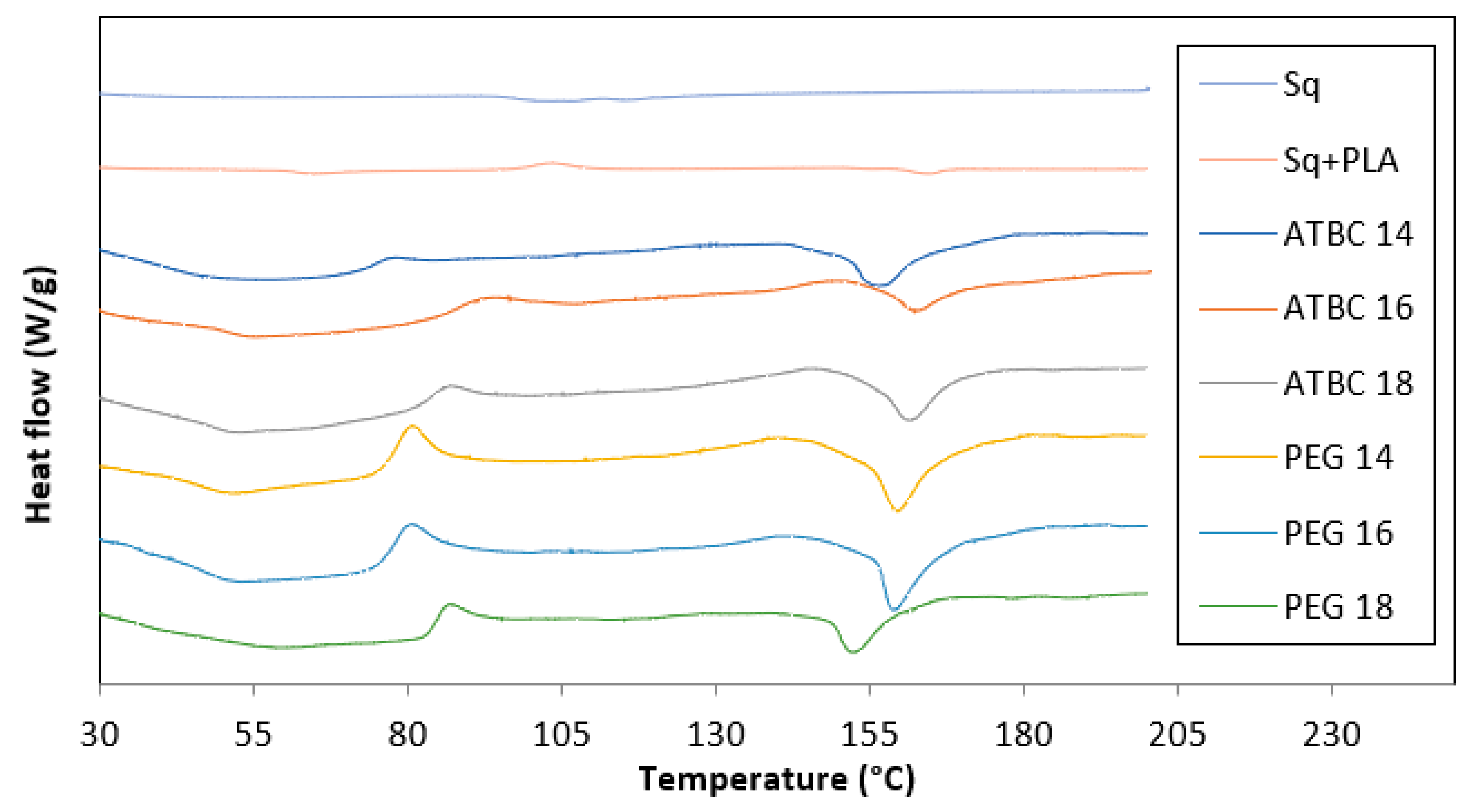
The thermal analysis of the prepared treatments was carried out using DSC. The thermal characteristics of the phase changes, including T
g, T
c and T
m, were recorded and presented in
Table 3 and
Table 4. The thermogram curves indicated similar thermal behaviors for all samples of PPLA/Saqqez gum blends, as shown in
Figure 2. Based on the results, the T
g of neat PLA was obtained at 55 °C, which was confirmed by previous studies [
48,
49]. The T
g of Saqqez gum and PLA/Saqqez gum blend without any plasticizer was found to be 58.3 °C and 50 °C, respectively.
Table 2 shows that the lowest T
g among plasticized blends was 46.2 °C for the blend containing 16% ATBC. The T
g of the blend containing 18% PEG was 50.4 °C, similar to the PLA/Saqqez gum blend without plasticizer.
Plasticizers operate by reducing the interactions between the polymer chains, making them more mobile at lower temperatures [
19]. Their main thermal effect is to lower the T
g by cutting the polymer chain during the process [
24]. Plasticizers generally act as modifiers in PLA blends, improving the plasticizing effect and reducing the T
g, thereby increasing PLA’s ductility [
48,
50,
51,
52]. The T
g values of pure polymers, polymer blends, copolymers and polymer-based composites reflect their miscibility as a function of composition and determine their properties [
53]. Zeng et al. (2015) suggest that the miscibility of a polymer blend can be determined by its morphology (homogeneous or heterogeneous) and the T
g of the blend. A homogeneous blend with a single T
g between the T
g values of both components is considered a completely miscible blend, while a blend with a fine phase morphology and improved properties is referred to as a compatible or partially miscible blend [
52]. Therefore, due to the homogeneity of all treatments and a single, lower T
g for both components of the blend, they may be considered a partially miscible blend within a range of compatibility.
Fox’s equation describes the glass transition temperature (T
g) of a miscible compound of a copolymer, two polymers or a plasticized polymer. This equation represents a more accurate free or random volume for the two components in the blends. The predicted T
g value in this equation is less than the experimental value given by a simple linear law of compounds [
54].
When comparing the T
g values obtained from the DSC analysis with the values predicted via Fox’s equation, deviations from the equation were observed. As shown in
Table 3, samples without plasticizer and samples containing 14% ATBC showed more deviations, while samples containing 16% ATBC and 18% ATBC, as well as all samples containing PEG, showed less deviations.
According to Lee & Litt (2000) [
55], deviations from Fox’s equation in copolymer systems can be explained by steric effects and sequence distributions. However, the effect of polarity on the composition’s T
g has not been fully elucidated. Campbell et al. (2000) also believed the systems that do not follow Fox’s equation are less uniform or weak mixtures [
54]. In the miscible blends without strong interactions between two polymers, the T
g curve usually follows Fox’s equation, whereas in blend systems with strong interactions, such as interchain electron donor–acceptor complex formation or hydrogen bonding, the T
g curves show positive deviations from Fox’s equation. Courgneau et al. (2011) investigated the impact of two plasticizers, ATBC and PEG-300, on PLA [
24]. The study found that adding up to 17% ATBC by weight reduces the glass transition temperature without causing phase separation, confirming the miscibility of PLA and ATBC. Previous studies have shown that PEG serves as a plasticizer and a compatibilizing agent [
56,
57], as well as a polymeric surfactant that reduces surface tension and increases interfacial adhesion between dispersed and matrix polymer phases in polymer blends [
58]. However, PEG is not as effective as ATBC in reducing T
g. Courgneau et al. (2011) attributed this function to the phase separation of PEG above 5% [
24]. However, some authors have reported phase separation in PEG above 20% [
59] and even at 30% by weight [
60,
61].
According to Qian et al. (2010), the distinct T
g of an amorphous mixture is the average T
g of its components, indicating homogeneity at the molecular level [
62]. However, this approach is limited by the need for a phase separation region larger than approximately 30 nm in DSC measurements of T
g.
As previously mentioned, plasticizers increase the free space in the polymer network [
48]. At certain temperature points, this increased freedom of mobility leads to the spontaneous arrangement of a crystalline structure at the crystallization point. Therefore, the crystallization process is expected to occur earlier and at lower temperatures as the plasticizer content increases. Another effect of adding plasticizers to the polymer is the reduction in the crystallization peak [
11]. The results show that all samples have exothermic crystallization points. Increasing the ATBC content from 16 to 18% decreased the crystallization temperature by approximately 7 degrees. Previous studies have confirmed that certain plasticizers, such as PEG and citrate derivatives, can reduce the crystallization temperature [
57,
63]. According to Abdelwahab et al. (2012), a homogeneous phase is indicated during the heating process when the mixture reaches its crystallization temperature [
33]. Additionally, the difference in flexibility of the chains and their ability to form a crystalline structure is related to the change in crystallization peaks.
All samples showed an endothermic peak between 100 and 180 °C in their thermograms. The melting temperature of the treatments changed slightly with increasing amounts of plasticizers. However, compared to the melting temperature of PLA/Saqqez gum without any plasticizer, which was recorded at 155 °C, the treatments with 14% and 16% ATBC showed a decreasing trend.
Wu and Liao (2005) stated that adding plasticizer to the blend is expected to decrease the melting point temperature [
64]. However, Abdelwahab et al. (2012) reported no changes in the melting temperature of PLA/PHB (poly hydroxybutyrate) plasticized with Lapol [
33]. Previous studies have shown that the endothermic melting temperature is influenced by the molecular motion of the polymer chains and the degradation of the molecular structure. This can be related to the evaporation of moisture from the sample or the degradation of the softener, which can affect the properties of the materials and their application [
50,
65].
Generally, adding plasticizers (ATBC and PEG) to the blend can effectively alter the crystallization temperature. However, it does not significantly change the glass transition temperature and melting temperature.
3.2.3. Morphological Properties
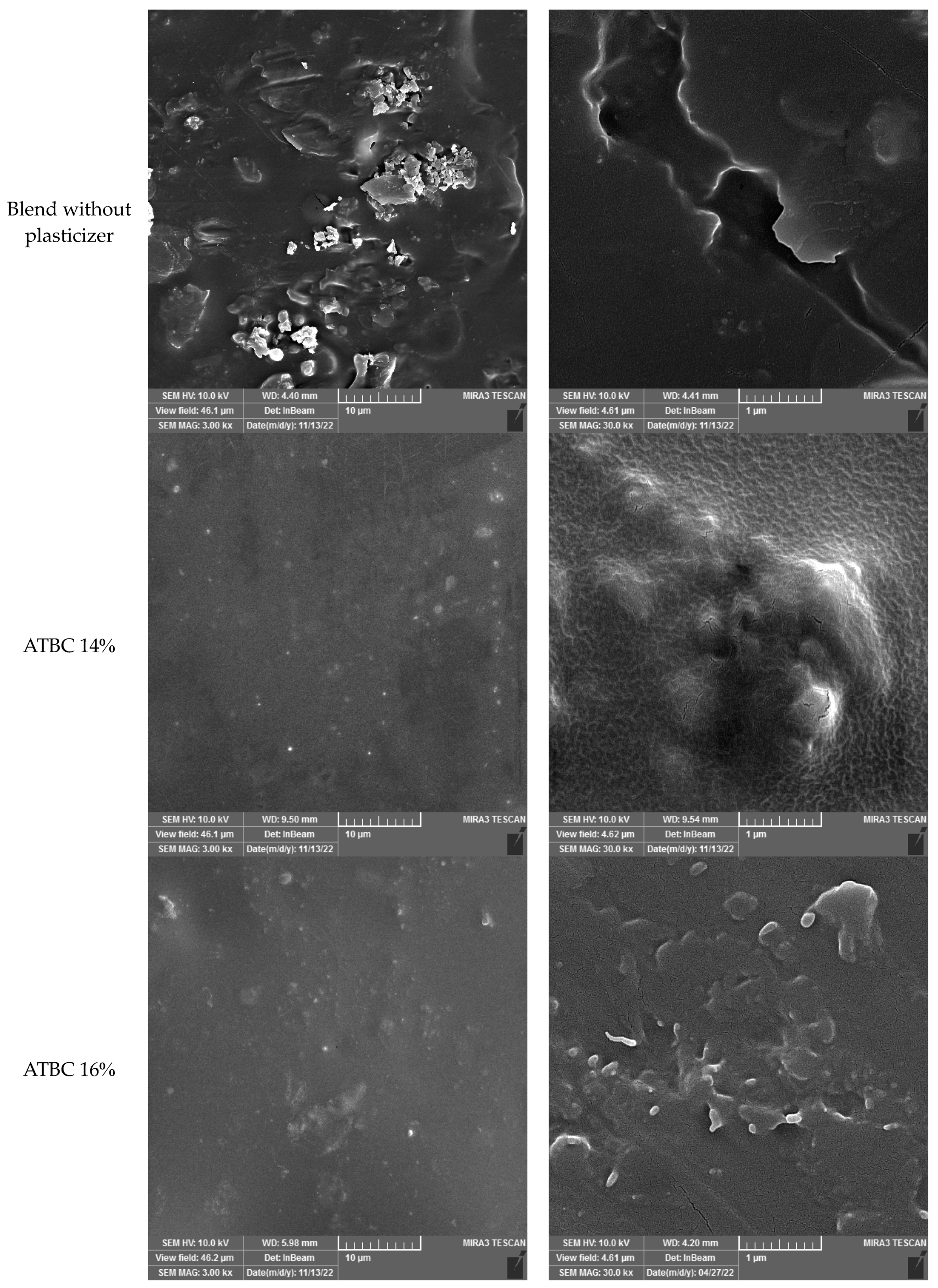
FESEM was used to analyze the fracture surface morphologies of the samples. This allowed for investigation into the dispersion of Saqqez gum particles, differences between samples with and without plasticizers and the function of plasticizers in the blend. Additionally, the miscibility between the matrices and materials was clarified.
According to Mao et al. (2019), the properties of polymer blends, including toughness, rigidity and thermal resistance, are not solely determined by the properties of each component, but also by the morphology, specifically the shape and distribution of the components [
66]. To better understand the structure of the blend and evaluate the micrographs, it is important to state the role of each component and the effect of plasticization.
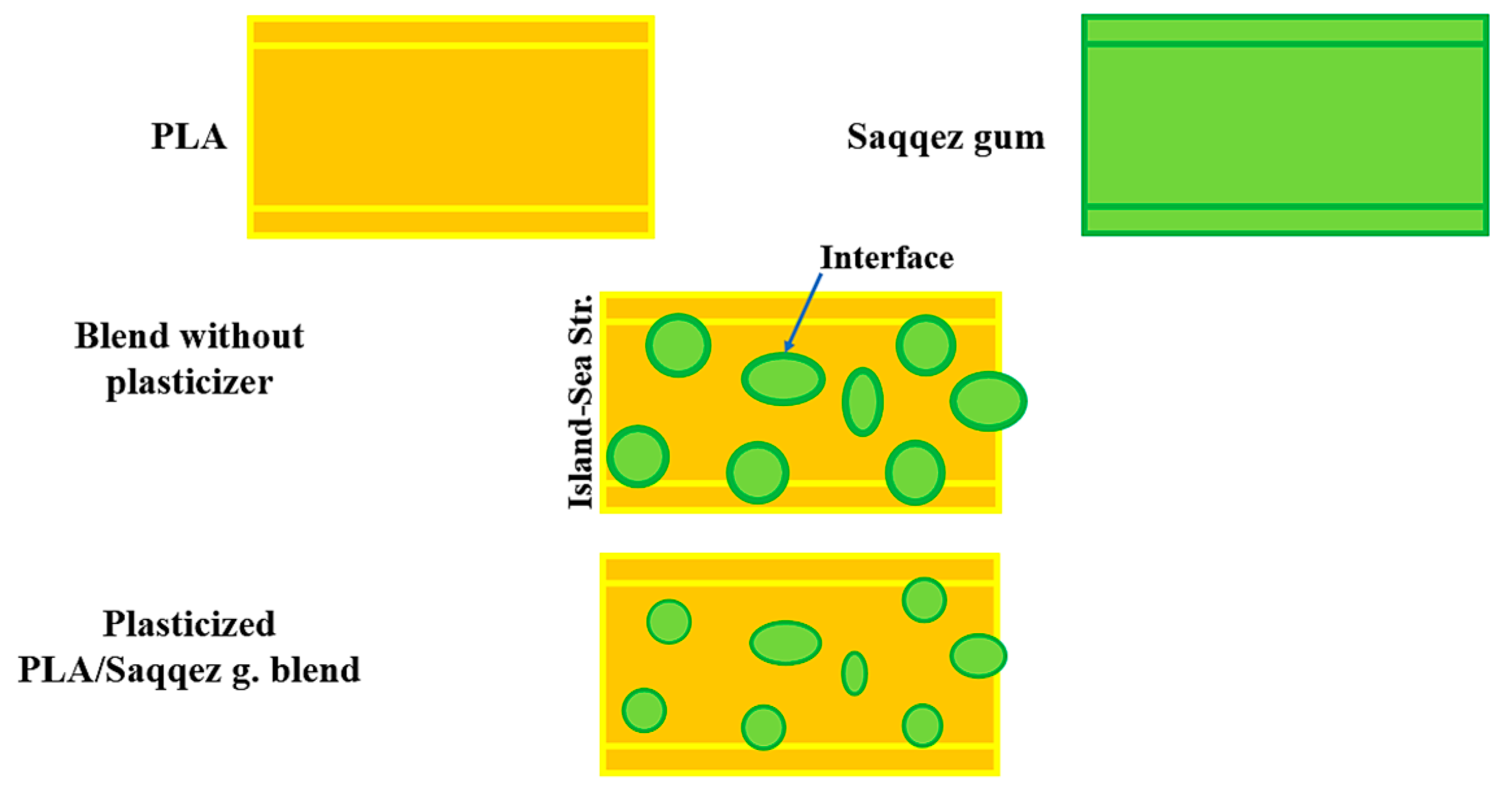
Figure 3 provides visual illustrations of this. Saqqez gum particles are used to toughen the brittle polymeric matrix of PLA, act as stress concentrators in the blend and induce a large number of crazes during the deformation process. Polymer blends can sometimes be structured as sea-island or core-shell structures, where two phases are present as discrete spherical domains embedded in a surrounding matrix. In this study, Saqqez gum was dispersed as the “island” or minor phase in PPLA and PPLA as the “sea” phase in blends. This structure was confirmed via SEM micrographs presented in
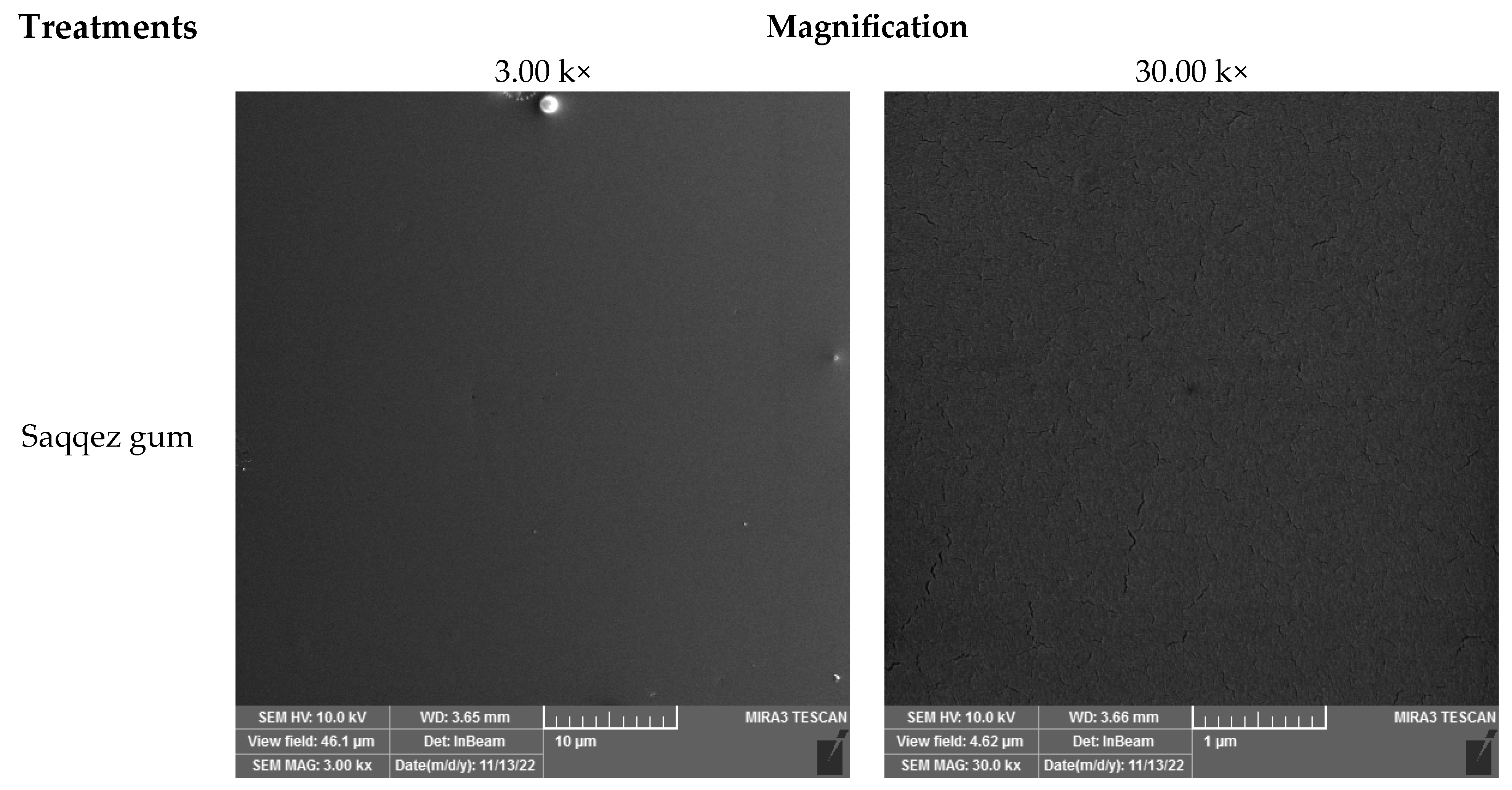
Figure 4. Previous studies have shown that the fracture surfaces of neat PLAs are smooth [
67], and form the major continuous phase [
67,
68,
69]. SEM images also indicate that the Saqqez gum surface is smooth and cavity-free. Wei et al. (2021) found that immiscible polymer blends exhibit various morphologies, including sea-island structures, depending on the ratio of polymer compounds, interfacial adhesion and processing conditions [
70]. The addition of a plasticizer to the PLA matrix is suggested to improve interfacial adhesion and dispersion. Chemical interactions between the components resulted in a diffused interface and good adhesion, leading to a homogeneous matrix without separation at the interface. This indicates plasticization in the PLA matrix [
71]. In addition, plasticizers can significantly reduce the size of island agglomerates and increase the dispersion between the blend components [
72], as shown in
Figure 3. It is widely acknowledged that smaller particle sizes facilitate improved interfacial interactions, resulting in better mechanical properties of blends [
69], as confirmed by the results of tensile tests conducted on plasticized samples.
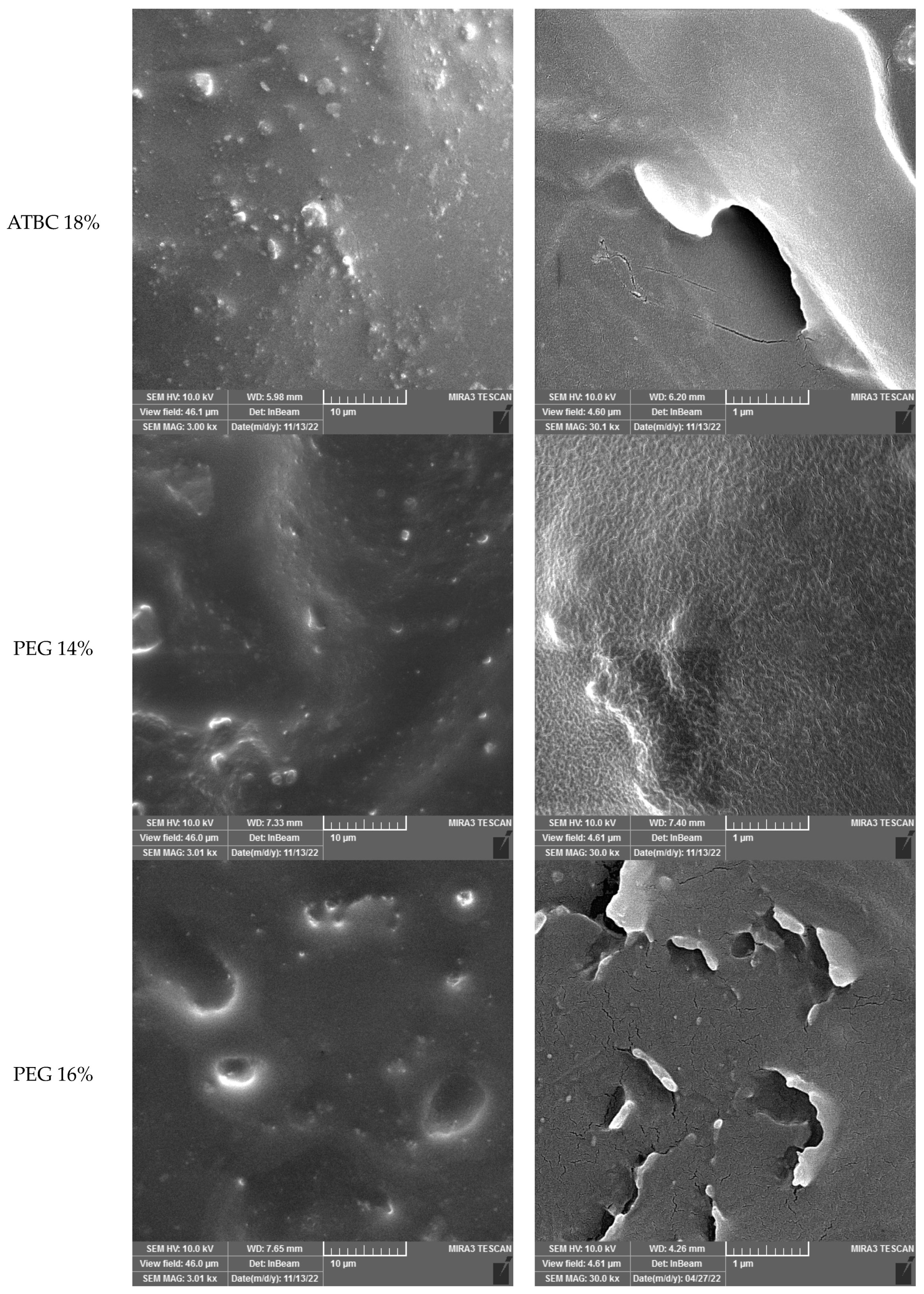
Micrographs of the sample without plasticizer reveal significant heterogeneity in the blend, with fractures and cavities displaying sharp edges that indicate the presence of sea-island structures. The Saqqez gum domains, which have a broad size and distribution and an irregular shape, are dispersed throughout the PLA matrix. By adding the plasticizers, the homogeneity of the samples containing both plasticizers was significantly increased compared to the sample without plasticizer.
A good homogeneity is observed in the samples containing ATBC (14 and 16%). This finding is consistent with the results reported by Yu et al. (2008) in their study of PLA/ATBC/CB (carbon black), where ATBC was found to reduce the size of CB agglomerates [
29]. However, it appears that the proportion of sea-island structures increased by 18%, and they also grew in size, resulting in more distinct interfaces between the continuous phase and dispersed Saqqez particles. Previous studies have confirmed that the addition of ATBC leads to an increase in the diameter of the dispersed phase [
73,
74]. According to Aliotta et al. (2021), the addition of plasticizer reduces viscosity, which in turn increases the diameter of the dispersed domain [
73]. In blends with 15 wt% or more of ATBC content, the dispersed particles become closer to each other, forming larger domains. In samples containing PEG, similar to ATBC, Saqqez domains (dispersed phase) were homogeneously dispersed in a smaller size PLA matrix in 14% and 16% of samples compared to the sample without plasticizer. In a sample containing 18% PEG, heterogeneity occurred. Previous studies have suggested that this may be related to phase separation in mixtures containing PEG above 17% [
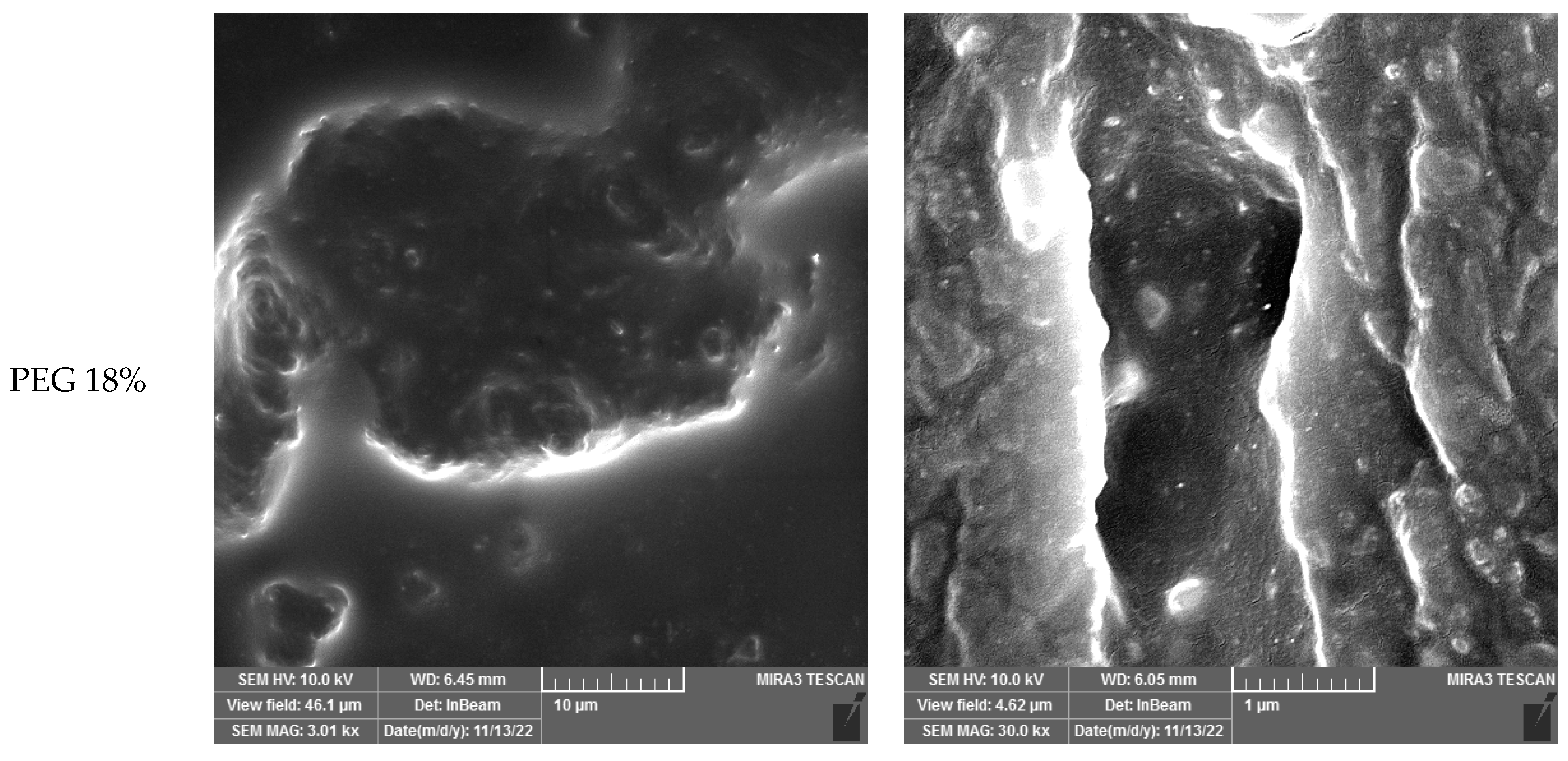
24]. The micrographs revealed that the Saqqez gum droplets coalesced during melt-blending, even at 18 wt% of ATBC and PEG. This suggests a range of miscibility.
3.2.4. Water-Absorption Behavior
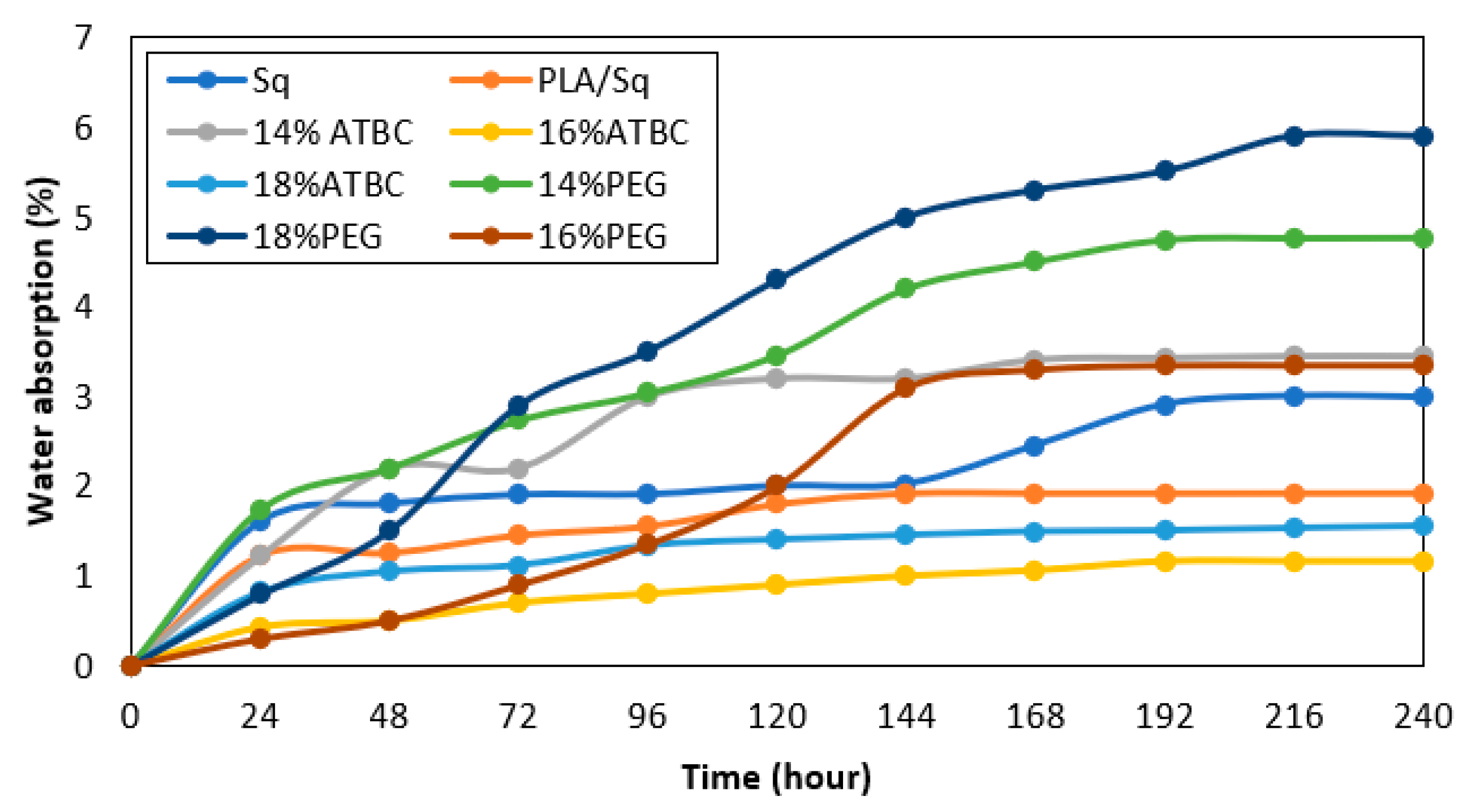
Table 5 presents data on the coefficients of water absorption, sorption, diffusion and permeability coefficients of the samples after 240 h (10 days). The highest and lowest values of water absorption in the saturation stage (%) were associated with PEG 18% and ATBC 16%, respectively. It can be inferred that ATBC 16% exhibits the greatest resistance to environmental moisture. This absorption behavior is also evident in all samples, as illustrated in
Figure 5. However, with the addition of plasticizer, the water-absorption gradually increased, except for 16%, which could be due to the greater cohesion of its structure compared to the other treatments. The water absorption of 18% may indicate an incoherent structure, which is supported by the results of other tests in this investigation. However, it could also be a sign of a higher water-absorption capacity in this sample. The measurement of water absorption in materials determines the quantity of water they absorb, while water sorption refers to their capacity to attract and retain water molecules. Both parameters are related to the interaction between water and the material. Water sorption affects the water-absorption capacity of a material. The sorption coefficient (S) represents the ability of a substance to sorb or adsorb water. Therefore, higher values indicate a greater sorption capacity. The study results show that PEG 18% has the highest value, while ATBC 16% has the lowest value, similar to water absorption.
Similarly, the highest and lowest values for the diffusion coefficient (D) and the permeability coefficient (P) belong to PEG 18% and ATBC 16%, respectively. These coefficients represent the rate at which water molecules penetrate through the samples and the ease of water flow through the samples, respectively.
The study confirms that a sample with a higher water-absorption capacity is likely to have higher sorption, diffusion and permeability coefficients.
A thorough review of the water-absorption properties of natural fiber-reinforced PLA composites was conducted by Rahman & Mustapa (2021) [
75]. In addition, Kamaludin et al. (2021) investigated the water-absorption kinetics of PLA/chitosan composites and found that the addition of chitosan increased water uptake, suggesting a greater interaction between water and filler, which in turn led to a decrease in tensile properties [
76]). In another investigation on the water-absorption behaviour of cellulose-reinforced PLA biocomposites, Penjumras et al. (2015) reported that water absorption increased with increasing cellulose content and exposure time [
77].