Anionic Effect on Electrical Transport Properties of Solid Co2+/3+ Redox Mediators
Abstract
1. Introduction
2. Materials and Methods
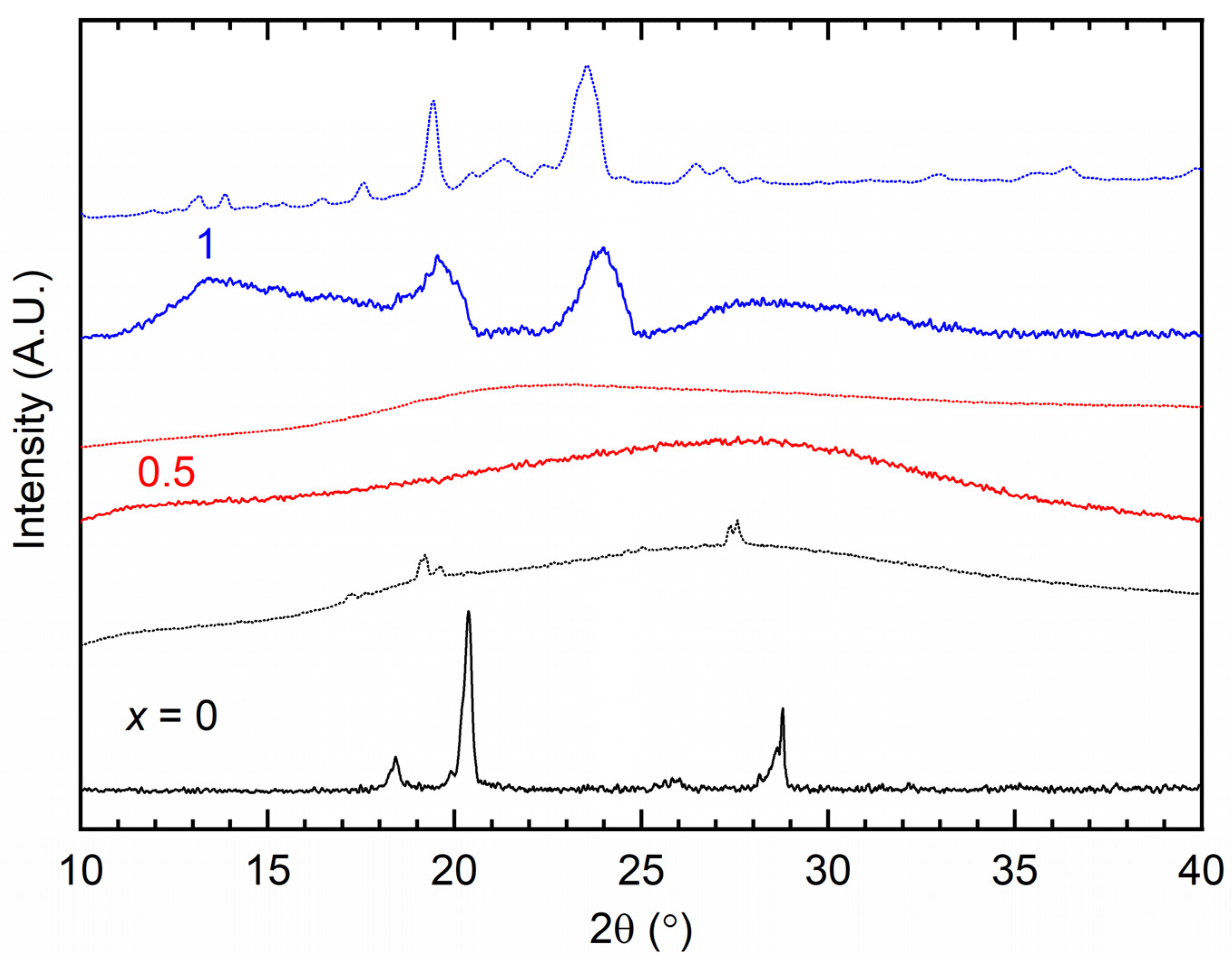
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Luque, A.; Hegedus, S. Handbook of Photovoltaic Science and Engineering, 2nd ed.; John Wiley & Sons, Ltd.: Chichester, UK, 2011. [Google Scholar]
- Green, M.A.; Dunlop, E.D.; Yoshita, M.; Kopidakis, N.; Bothe, K.; Siefer, G.; Hao, X. Solar cell efficiency tables (version 63). Prog. Photovolt. Res. Appl. 2024, 32, 3–13. [Google Scholar] [CrossRef]
- Gupta, R.K.; Shaikh, H.; Imran, A.; Bedja, I.; Ajaj, A.F.; Aldwayyan, A.S. Electrical transport, structural, optical and thermal properties of [(1-x)Succinonitrile: xPEO]-LiTFSI-Co(bpy)3(TFSI)2-Co(bpy)3(TFSI)3 solid redox mediators. Polymers 2022, 14, 1870. [Google Scholar] [CrossRef] [PubMed]
- Hagfeldt, A.; Boschloo, G.; Sun, L.C.; Kloo, L.; Pettersson, H. Dye-sensitized solar cells. Chem. Rev. 2010, 110, 6595–6663. [Google Scholar] [CrossRef] [PubMed]
- Munoz-Garcia, A.B.; Benesperi, I.; Boschloo, G.; Concepcion, J.J.; Delcamp, J.H.; Gibson, E.A.; Meyer, G.J.; Pavone, M.; Pettersson, H.; Hagfeldt, A.; et al. Dye-sensitized solar cells strike back. Chem. Soc. Rev. 2021, 50, 12450–12550. [Google Scholar] [CrossRef]
- Katsaros, G.; Stergiopoulos, T.; Arabatzis, I.M.; Papadokostaki, K.G.; Falaras, P. A solvent-free composite polymer/inorganic oxide electrolyte for high efficiency solid-state dye-sensitized solar cells. J. Photochem. Photobiol. A-Chem. 2002, 149, 191–198. [Google Scholar] [CrossRef]
- Stergiopoulos, T.; Arabatzis, I.M.; Katsaros, G.; Falaras, P. Binary polyethylene oxide/titania solid-state redox electrolyte for highly efficient nanocrystalline tio2 photoelectrochemical cells. Nano Lett. 2002, 2, 1259–1261. [Google Scholar] [CrossRef]
- Nogueira, A.F.; Longo, C.; De Paoli, M.A. Polymers in dye sensitized solar cells: Overview and perspectives. Coord. Chem. Rev. 2004, 248, 1455–1468. [Google Scholar] [CrossRef]
- Li, B.; Wang, L.D.; Kang, B.N.; Wang, P.; Qiu, Y. Review of recent progress in solid-state dye-sensitized solar cells. Sol. Energy Mater. Sol. Cells 2006, 90, 549–573. [Google Scholar] [CrossRef]
- Singh, P.K.; Nagarale, R.K.; Pandey, S.P.; Rhee, H.W.; Bhattacharya, B. Present status of solid state photoelectrochemical solar cells and dye sensitized solar cells using PEO-based polymer electrolytes. Adv. Nat. Sci. Nanosci. Nanotechnol. 2011, 2, 023002. [Google Scholar] [CrossRef]
- Wu, J.H.; Lan, Z.; Lin, J.M.; Huang, M.L.; Huang, Y.F.; Fan, L.Q.; Luo, G.G. Electrolytes in dye-sensitized solar cells. Chem. Rev. 2015, 115, 2136–2173. [Google Scholar] [CrossRef]
- Su ait, M.S.; Rahman, M.Y.A.; Ahmad, A. Review on polymer electrolyte in dye-sensitized solar cells (DSSCs). Sol. Energy 2015, 115, 452–470. [Google Scholar] [CrossRef]
- Singh, R.; Polu, A.R.; Bhattacharya, B.; Rhee, H.W.; Varlikli, C.; Singh, P.K. Perspectives for solid biopolymer electrolytes in dye sensitized solar cell and battery application. Renew. Sustain. Energy Rev. 2016, 65, 1098–1117. [Google Scholar] [CrossRef]
- Mehmood, U.; Al-Ahmed, A.; Al-Sulaiman, F.A.; Malik, M.I.; Shehzad, F.; Khan, A.U.H. Effect of temperature on the photovoltaic performance and stability of solid-state dye-sensitized solar cells: A review. Renew. Sustain. Energy Rev. 2017, 79, 946–959. [Google Scholar] [CrossRef]
- Venkatesan, S.; Lee, Y.L. Nanofillers in the electrolytes of dye-sensitized solar cells—A short review. Coord. Chem. Rev. 2017, 353, 58–112. [Google Scholar] [CrossRef]
- Iftikhar, H.; Sonai, G.G.; Hashmi, S.G.; Nogueira, A.F.; Lund, P.D. Progress on electrolytes development in dye-sensitized solar cells. Materials 2019, 12, 1998. [Google Scholar] [CrossRef] [PubMed]
- Hasan, M.M.; Islam, M.D.; Rashid, T.U. Biopolymer-based electrolytes for dye-sensitized solar cells: A critical review. Energy Fuels 2020, 34, 15634–15671. [Google Scholar] [CrossRef]
- Wang, N.; Hu, J.J.; Gao, L.G.; Ma, T.L. Current progress in solid-state electrolytes for dye-sensitized solar cells: A mini-review. J. Electron. Mater. 2020, 49, 7085–7097. [Google Scholar] [CrossRef]
- Abu Talip, R.A.; Yahya, W.Z.N.; Bustam, M.A. Ionic liquids roles and perspectives in electrolyte for dye-sensitized solar cells. Sustainability 2020, 12, 7598. [Google Scholar] [CrossRef]
- Dai, Q.; MacFarlane, D.R.; Forsyth, M. High mobility I−/I3− redox couple in a molecular plastic crystal: A potential new generation of electrolyte for solid-state photoelectrochemical cells. Solid State Ion. 2006, 177, 395–401. [Google Scholar] [CrossRef]
- Gupta, R.K.; Bedja, I.; Islam, A.; Shaikh, H. Electrical, structural, and thermal properties of succinonitrile-LiI-I2 redox-mediator. Solid State Ion. 2018, 326, 166–172. [Google Scholar] [CrossRef]
- Kang, M.S.; Kim, J.H.; Kim, Y.J.; Won, J.; Park, N.G.; Kang, Y.S. Dye-sensitized solar cells based on composite solid polymer electrolytes. Chem. Commun. 2005, 7, 889–891. [Google Scholar] [CrossRef]
- Kang, M.S.; Kim, J.H.; Won, J.; Kang, Y.S. Dye-sensitized solar cells based on crosslinked poly(ethylene glycol) electrolytes. J. Photochem. Photobiol. A-Chem. 2006, 183, 15–21. [Google Scholar] [CrossRef]
- Zhou, Y.F.; Xiang, W.C.; Chen, S.; Fang, S.B.; Zhou, X.W.; Zhang, J.B.; Lin, Y. Influences of poly(ether urethane) introduction on poly(ethylene oxide) based polymer electrolyte for solvent-free dye-sensitized solar cells. Electrochim. Acta 2009, 54, 6645–6650. [Google Scholar] [CrossRef]
- Kang, M.S.; Kim, J.H.; Won, J.; Kang, Y.S. Oligomer approaches for solid-state dye-sensitized solar cells employing polymer electrolytes. J. Phys. Chem. C 2007, 111, 5222–5228. [Google Scholar] [CrossRef]
- Han, H.W.; Liu, W.; Zhang, J.; Zhao, X.Z. A hybrid poly(ethylene oxide)/poly(vinylidene fluoride)/TiO2 nanoparticle solid-state redox electrolyte for dye-sensitized nanocrystalline solar cells. Adv. Funct. Mater. 2005, 15, 1940–1944. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, J.; Zhou, C.H.; Wu, S.J.; Xu, S.; Liu, W.; Han, H.W.; Chen, B.L.; Zhao, X.Z. Effect of lithium iodide addition on poly(ethylene oxide)-poly(vinylidene fluoride) polymer-blend electrolyte for dye-sensitized nanocrystalline solar cell. J. Phys. Chem. B 2008, 112, 6594–6602. [Google Scholar] [CrossRef]
- Singh, P.K.; Kim, K.W.; Rhee, H.W. Electrical, optical and photoelectrochemical studies on a solid PEO-polymer electrolyte doped with low viscosity ionic liquid. Electrochem. Commun. 2008, 10, 1769–1772. [Google Scholar] [CrossRef]
- Singh, P.K.; Kim, K.W.; Park, N.G.; Rhee, H.W. Mesoporous nanocrystalline TiO2 electrode with ionic liquid-based solid polymer electrolyte for dye-sensitized solar cell application. Synth. Met. 2008, 158, 590–593. [Google Scholar] [CrossRef]
- Singh, P.K.; Kim, K.W.; Rhee, H.W. Ionic liquid (1-methyl 3-propyl imidazolium iodide) with polymer electrolyte for DSSC application. Polym. Eng. Sci. 2009, 49, 862–865. [Google Scholar] [CrossRef]
- Singh, P.K.; Kim, K.W.; Rhee, H.W. Quantum dot doped solid polymer electrolyte for device application. Electrochem. Commun. 2009, 11, 1247–1250. [Google Scholar] [CrossRef]
- Gupta, R.K.; Kim, H.M.; Rhee, H.W. Poly(ethylene oxide): Succinonitrile—A polymeric matrix for fast-ion conducting redox-couple solid electrolytes. J. Phys. D-Appl. Phys. 2011, 44, 205106. [Google Scholar] [CrossRef]
- Gupta, R.K.; Rhee, H.W. Highly conductive redox-couple solid polymer electrolyte system: Blend-KI-I2 for dye-sensitized solar cells. Adv. OptoElectron. 2011, 2011, 102932. [Google Scholar] [CrossRef]
- Gupta, R.K.; Rhee, H.W. Effect of succinonitrile on electrical, structural, optical, and thermal properties of poly(ethylene oxide)-succinonitrile/LiI-I2 redox-couple solid polymer electrolyte. Electrochim. Acta 2012, 76, 159–164. [Google Scholar] [CrossRef]
- Gupta, R.K.; Rhee, H.W. Plasticizing effect of K+ ions and succinonitrile on electrical conductivity of poly(ethylene oxide)-succinonitrile/KI-I2 redox-couple solid polymer electrolyte. J. Phys. Chem. B 2013, 117, 7465–7471. [Google Scholar] [CrossRef]
- Gupta, R.K.; Shaikh, H.; Imran, A.; Bedja, I.; Aldwayyan, A.S. Structural, thermal, and electrical properties of poly(ethylene oxide)-tetramethyl succinonitrile blend for redox mediators. Polymers 2022, 14, 3728. [Google Scholar] [CrossRef]
- Gupta, R.K.; Shaikh, H.; Imran, A.; Bedja, I.; Aldwayyan, A.S. Tetramethyl succinonitrile as a solid plasticizer in a poly(ethylene oxide)8 -LiI-I2 solid polymer electrolyte. Macromol. Rapid Commun. 2022, 43, e2100764. [Google Scholar] [CrossRef]
- Gupta, R.K.; Rhee, H.W.; Bedja, I.; AlHazaa, A.N.; Khan, A. Effect of laponite(R) nanoclay dispersion on electrical, structural, and photovoltaic properties of dispersed poly(ethylene oxide)-succinonitrile -LiI-I2 solid polymer electrolyte. J. Power Sources 2021, 490, 229509. [Google Scholar] [CrossRef]
- Bhattacharya, B.; Lee, J.Y.; Geng, J.; Jung, H.T.; Park, J.K. Effect of cation size on solid polymer electrolyte based dye-sensitized solar cells. Langmuir 2009, 25, 3276–3281. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.K.; Bedja, I. Cationic effect on dye-sensitized solar cell properties using electrochemical impedance and transient absorption spectroscopy techniques. J. Phys. D-Appl. Phys. 2017, 50, 245501. [Google Scholar] [CrossRef]
- Lide, D.R. CRC Handbook of Chemistry and Physics, 89th ed.; CRC Press/Taylor and Francis: Boca Raton, FL, USA, 2009; pp. 3–6. [Google Scholar]
- Arya, A.; Sharma, A.L. A glimpse on all-solid-state Li-ion battery (ASSLIB) performance based on novel solid polymer electrolytes: A topical review. J. Mater. Sci. 2020, 55, 6242–6304. [Google Scholar] [CrossRef]
- Largeot, C.; Portet, C.; Chmiola, J.; Taberna, P.-L.; Gogotsi, Y.; Simon, P. Relation between the ion size and pore size for an electric double-layer capacitor. J. Am. Chem. Soc. 2008, 130, 2730–2731. [Google Scholar] [CrossRef] [PubMed]
- Mathew, S.; Yella, A.; Gao, P.; Humphry-Baker, R.; Curchod, B.F.E.; Ashari-Astani, N.; Tavernelli, I.; Rothlisberger, U.; Nazeeruddin, M.K.; Gratzel, M. Dye-sensitized solar cells with 13% efficiency achieved through the molecular engineering of porphyrin sensitizers. Nat. Chem. 2014, 6, 242–247. [Google Scholar] [CrossRef] [PubMed]
- Aifantis, K.E.; Kumar, R.V.; Hu, P. Rechargeable Ion Batteries: Materials, Design, and Applications of Li-Ion Cells and Beyond; WILEY-VCH GmbH: Weinheim, Germany, 2023. [Google Scholar]
- Gupta, R.K.; Jung, H.Y.; Whang, C.M. Transport properties of a new Li+ ion-conducting ormolyte: (SiO2-PEG)-LiCF3SO3. J. Mater. Chem. 2002, 12, 3779–3782. [Google Scholar] [CrossRef]
- Alhefeiti, M.; Chandra, F.; Gupta, R.K.; Saleh, N. Dyeing non-recyclable polyethylene plastic with photoacid phycocyanobilin from spirulina algae: Ultrafast photoluminescence studies. Polymers 2022, 14, 4811. [Google Scholar] [CrossRef]
- Monk, P. Fundamentals of Electroanalytical Chemistry; John Wiley & Sons: Chichester, UK, 2001. [Google Scholar]
- Careem, M.A.; Noor, I.S.M.; Arof, A.K. Impedance spectroscopy in polymer electrolyte characterization. In Polymer Electrolytes: Characterization Techniques and Energy Applications; Winie, T., Arof, A.K., Thomas, S., Eds.; Wiley: Weinheim, Germany, 2020; pp. 23–64. [Google Scholar]
- Haq, N.; Shakeel, F.; Alanazi, F.K.; Shaikh, H.; Bedja, I.; Gupta, R.K. Utilization of poly(ethylene terephthalate) waste for preparing disodium terephthalate and its application in a solid polymer electrolyte. J. Appl. Polym. Sci. 2019, 136, 47612. [Google Scholar] [CrossRef]
- Fan, L.Z.; Hu, Y.S.; Bhattacharyya, A.J.; Maier, J. Succinonitrile as a versatile additive for polymer electrolytes. Adv. Funct. Mater. 2007, 17, 2800–2807. [Google Scholar] [CrossRef]
- Patel, M.; Chandrappa, K.G.; Bhattacharyya, A.J. Increasing ionic conductivity and mechanical strength of a plastic electrolyte by inclusion of a polymer. Electrochim. Acta 2008, 54, 209–215. [Google Scholar] [CrossRef]
- Agrawal, R.C.; Gupta, R.K. Superionic solids: Composite electrolyte phase—An overview. J. Mater. Sci. 1999, 34, 1131–1162. [Google Scholar] [CrossRef]
- Gupta, R.K.; Jung, H.Y.; Wi, C.J.; Whang, C.M. Solid State Ionics: Trends in the New Millennium; Chowdari, B.V.R., Prabaharan, S.R.S., Yahaya, M., Talib, I.A., Eds.; World Scientific: Singapore, 2002; pp. 369–376. [Google Scholar]
- Wen, S.J.; Richardson, T.J.; Ghantous, D.I.; Striebel, K.A.; Ross, P.N.; Cairns, E.J. Ftir characterization of PEO + LiN(CF3SO2)2 electrolytes. J. Electroanal. Chem. 1996, 408, 113–118. [Google Scholar] [CrossRef]
- Rey, I.; Lassègues, J.C.; Grondin, J.; Servant, L. Infrared and raman study of the peo-litfsi polymer electrolyte. Electrochim. Acta 1998, 43, 1505–1510. [Google Scholar] [CrossRef]
- Bernson, A.; Lindgren, J. Ion aggregation and morphology for poly(ethylene oxide)-based polymer electrolytes containing rare-earth-metal salts. Solid State Ion. 1993, 60, 31–36. [Google Scholar] [CrossRef]
- Huang, W.; Frech, R.; Wheeler, R.A. Molecular structures and normal vibrations of trifluoromethane sulfonate (CF3SO3−) and its lithium ion pairs and aggregates. J. Phys. Chem. 1994, 98, 100–110. [Google Scholar] [CrossRef]
- Rhodes, C.P.; Frech, R. A symmetry-based analysis of raman and infrared spectra of the compounds (poly(ethylene oxide))3 LiCF3SO3 and (poly(ethylene oxide))NaCF3SO3. Solid State Ion. 2000, 136–137, 1131–1137. [Google Scholar] [CrossRef]
- Castellucci, E.; Angeloni, L.; Neto, N.; Sbrana, G. Ir and raman spectra of a 2,2′-bipyridine single crystal: Internal modes. Chem. Phys. 1979, 43, 365–373. [Google Scholar] [CrossRef]
- Gupta, R.K.; Rhee, H.W. Detailed investigation into the electrical conductivity and structural properties of poly(ethylene oxide)-succinonitrile -Li(CF3SO2)2N solid polymer electrolytes. Bull. Korean Chem. Soc. 2017, 38, 356–363. [Google Scholar] [CrossRef]
- Colthup, N.B.; Daly, L.H.; Wiberley, S.E. Introduction to Infrared and Raman Spectroscopy, 3rd ed.; Academic Press: San Diego, CA, USA, 1990. [Google Scholar]
- Alarco, P.J.; Abu-Lebdeh, Y.; Abouimrane, A.; Armand, M. The plastic-crystalline phase of succinonitrile as a universal matrix for solid-state ionic conductors. Nat. Mater. 2004, 3, 476–481. [Google Scholar] [CrossRef] [PubMed]
- Martin-Vosshage, D.; Chowdari, B.V.R. X-ray photoelectron spectroscopy studies on poly(ethylene oxide) with sodium triflate. J. Electrochem. Soc. 1993, 140, 3531. [Google Scholar] [CrossRef]
- Andersson, A.M.; Herstedt, M.; Bishop, A.G.; Edström, K. The influence of lithium salt on the interfacial reactions controlling the thermal stability of graphite anodes. Electrochim. Acta 2002, 47, 1885–1898. [Google Scholar] [CrossRef]
- Morales-Ugarte, J.E.; Santini, C.C.; Bouchet, R.; Benayad, A. New interpretation of x-ray photoelectron spectroscopy of imidazolium ionic liquid electrolytes based on ionic transport analyses. J. Phys. Chem. B 2020, 124, 7625–7635. [Google Scholar] [CrossRef]
- Zhang, Q.; Liu, K.; Ding, F.; Li, W.; Liu, X.; Zhang, J. Enhancing the high voltage interface compatibility of LiNi0.5Co0.2Mn0.3O2 in the succinonitrile-based electrolyte. Electrochim. Acta 2019, 298, 818–826. [Google Scholar] [CrossRef]
- Andersson, E.K.W.; Sångeland, C.; Berggren, E.; Johansson, F.O.L.; Kühn, D.; Lindblad, A.; Mindemark, J.; Hahlin, M. Early-stage decomposition of solid polymer electrolytes in li-metal batteries. J. Mater. Chem. A 2021, 9, 22462–22471. [Google Scholar] [CrossRef]
- Gupta, R.K.; Shaikh, H.; Imran, A.; Bedja, I.; Ajaj, A.F.; Aldwayyan, A.S. Towards All-Solid-State Dye-Sensitized Solar Cells Using Co(II/III) Redox Couple-Based Solid Polymer Electrolytes; 13-ENE886-02; King Saud University: Riyadh, Saudi Arabia, 2023; p. 1. [Google Scholar]
- Yella, A.; Mathew, S.; Aghazada, S.; Comte, P.; Gratzel, M.; Nazeeruddin, M.K. Dye-sensitized solar cells using cobalt electrolytes: The influence of porosity and pore size to achieve high-efficiency. J. Mater. Chem. C 2017, 5, 2833–2843. [Google Scholar] [CrossRef]
- Juodkazis, K.; Juodkazytė, J.; Jelmakas, E.; Kalinauskas, P.; Valsiūnas, I.; Miečinskas, P.; Juodkazis, S. Photoelectrolysis of water: Solar hydrogen–achievements and perspectives. Opt. Express 2010, 18, A147–A160. [Google Scholar] [CrossRef] [PubMed]
- Hwang, D.; Lee, H.; Jang, S.Y.; Jo, S.M.; Kim, D.; Seo, Y.; Kim, D.Y. Electrospray preparation of hierarchically-structured mesoporous TiO2 spheres for use in highly efficient dye-sensitized solar cells. ACS Appl. Mater. Interfaces 2011, 3, 2719–2725. [Google Scholar] [CrossRef]
- Hwang, D.; Kim, D.Y.; Jang, S.Y.; Kim, D. Superior photoelectrodes for solid-state dye-sensitized solar cells using amphiphilic TiO2. J. Mater. Chem. A 2013, 1, 1228–1238. [Google Scholar] [CrossRef]
- Alduraibi, M.; Hezam, M.; Al-Ruhaimi, B.; El-Toni, A.M.; Algarni, A.; Abdel-Rahman, M.; Qing, W.; Aldwayyan, A. Rapid room-temperature synthesis of mesoporous TiO2 sub-microspheres and their enhanced light harvesting in dye-sensitized solar cells. Nanomaterials 2020, 10, 413. [Google Scholar] [CrossRef]
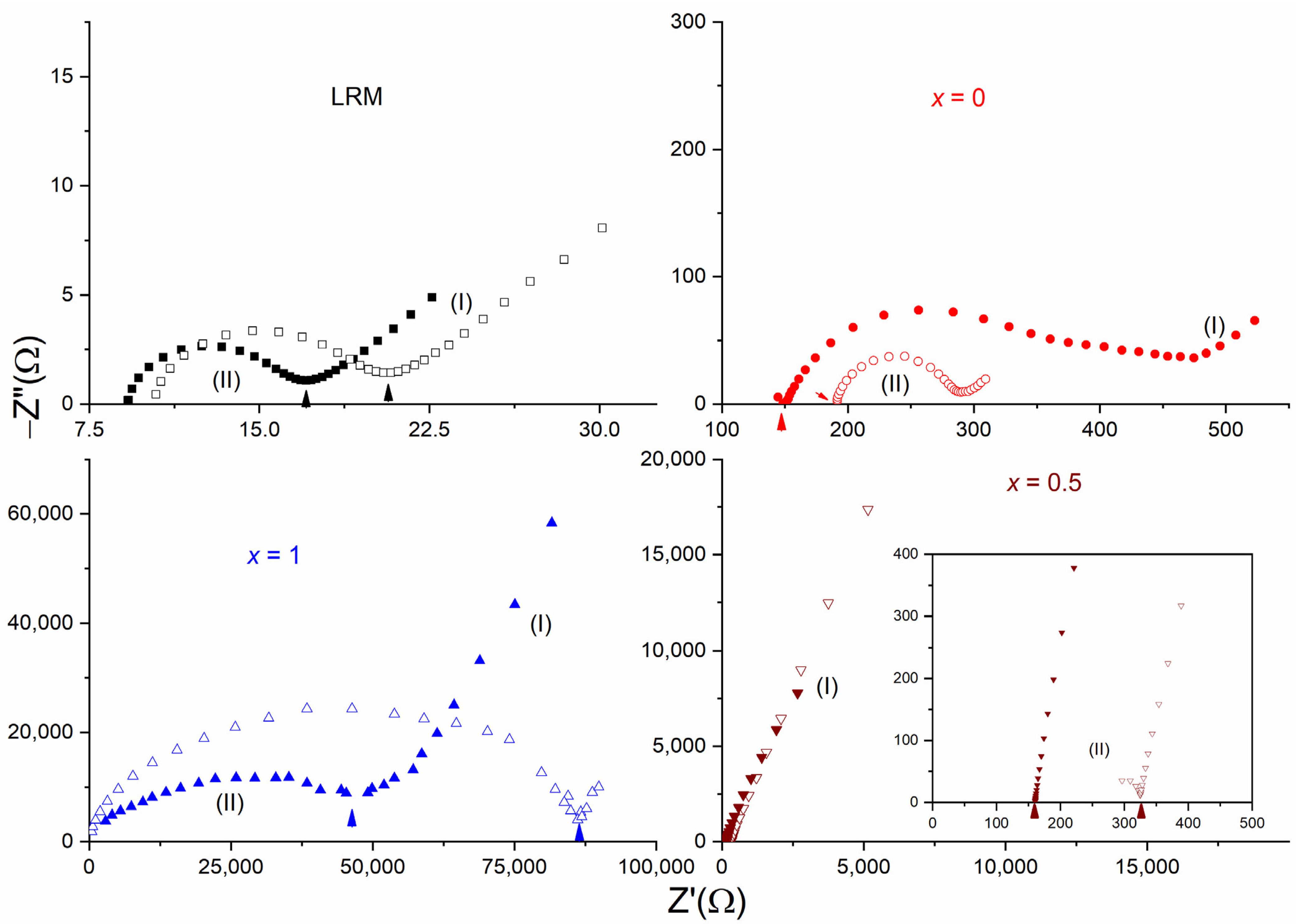

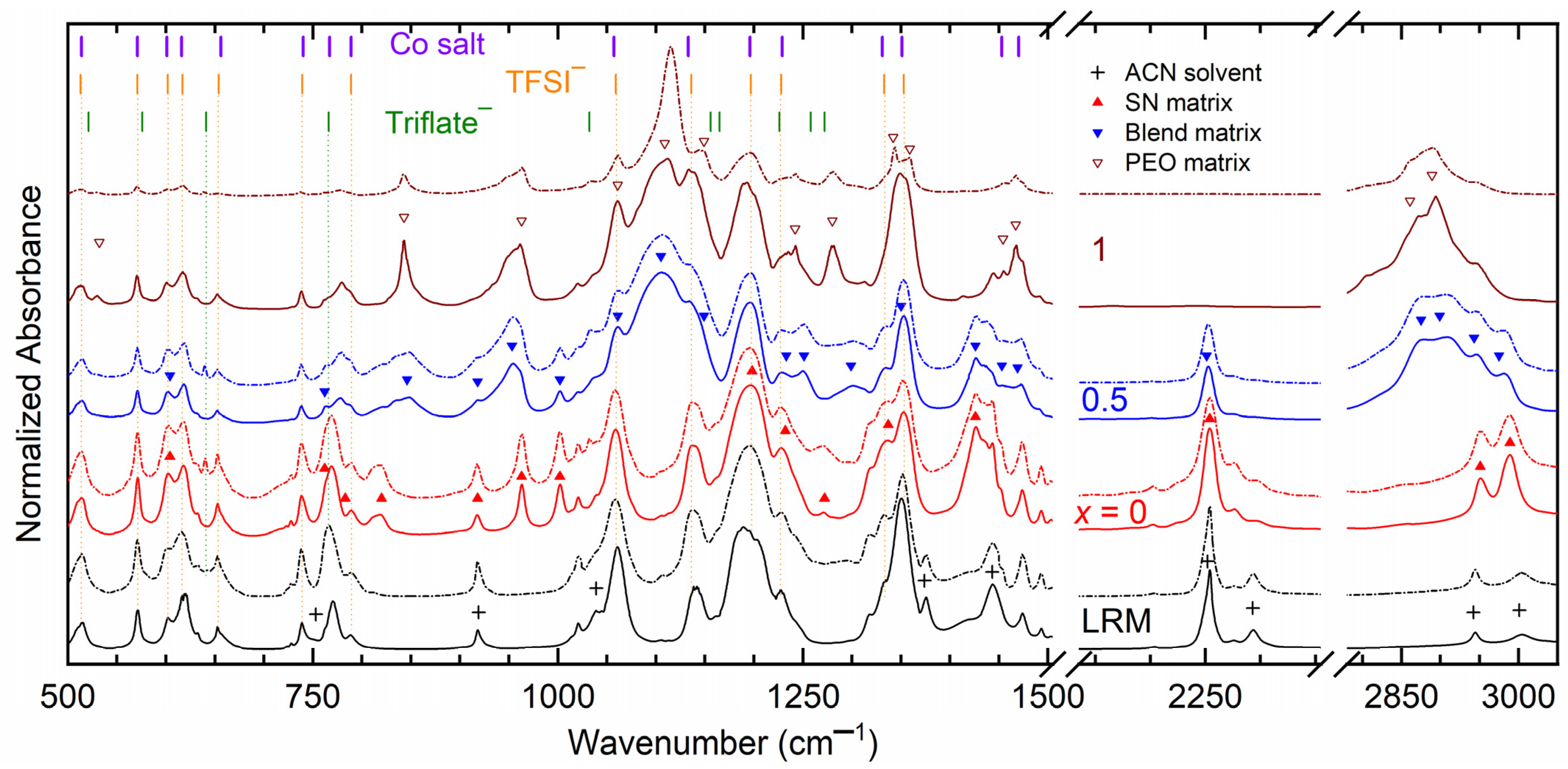
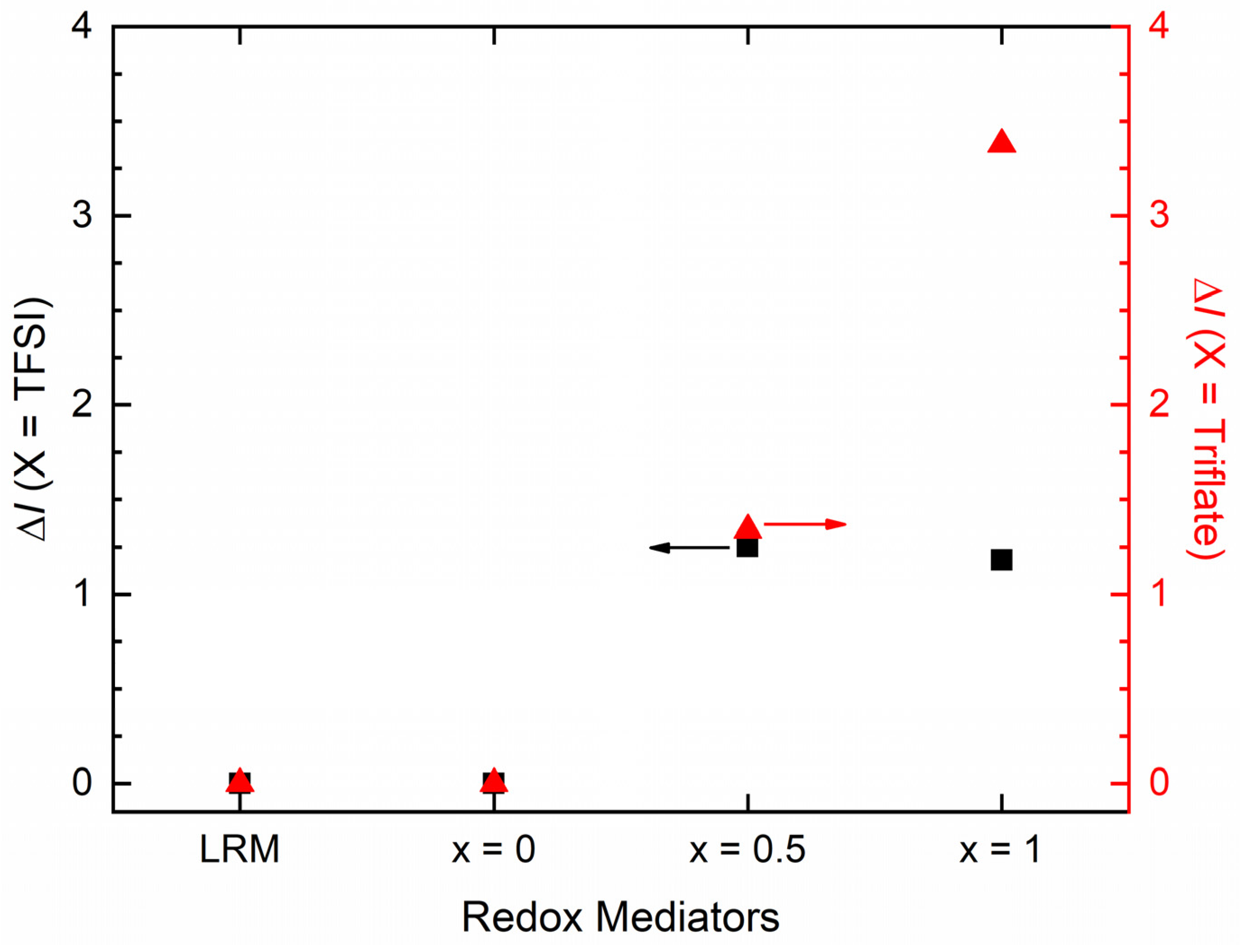
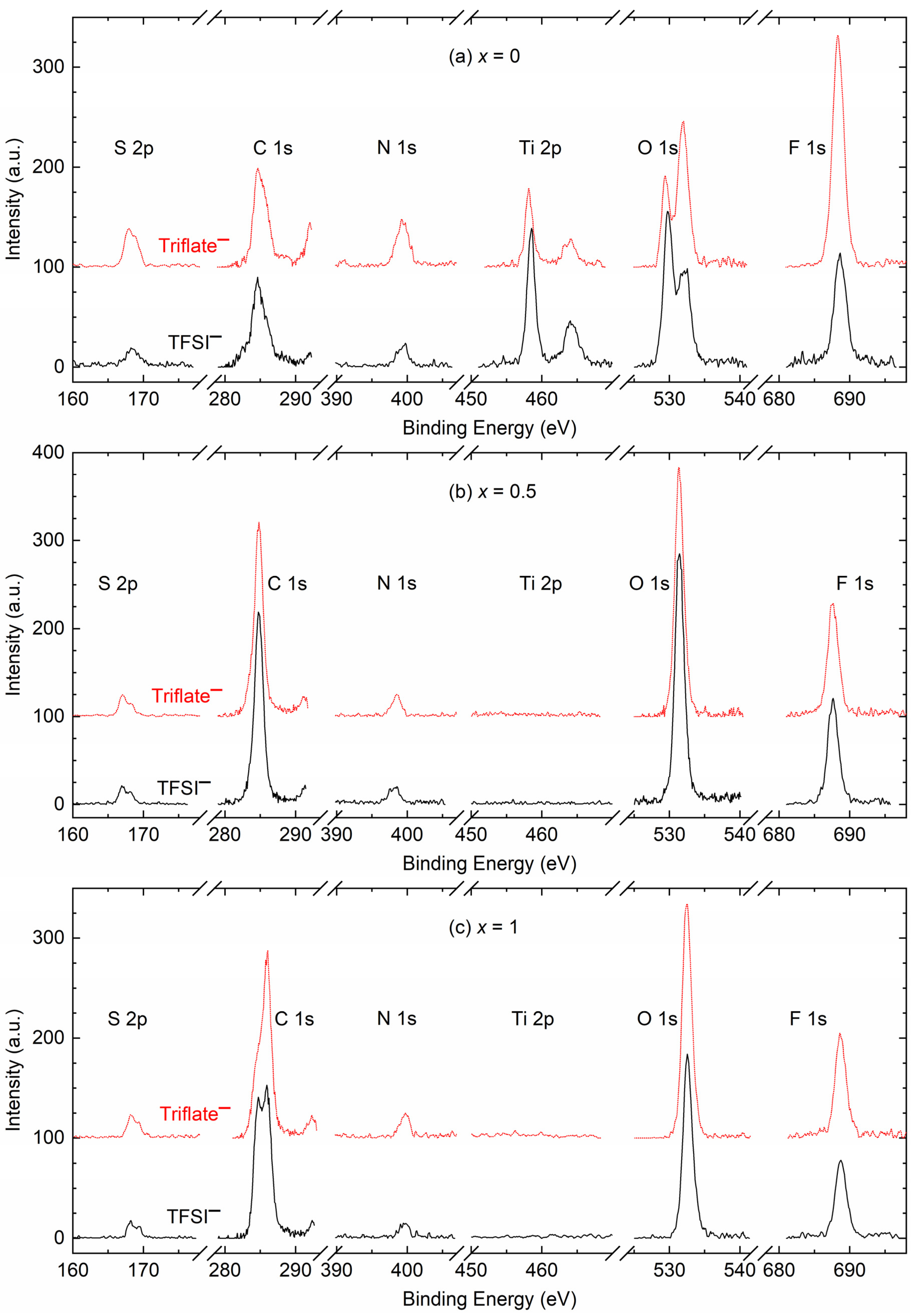
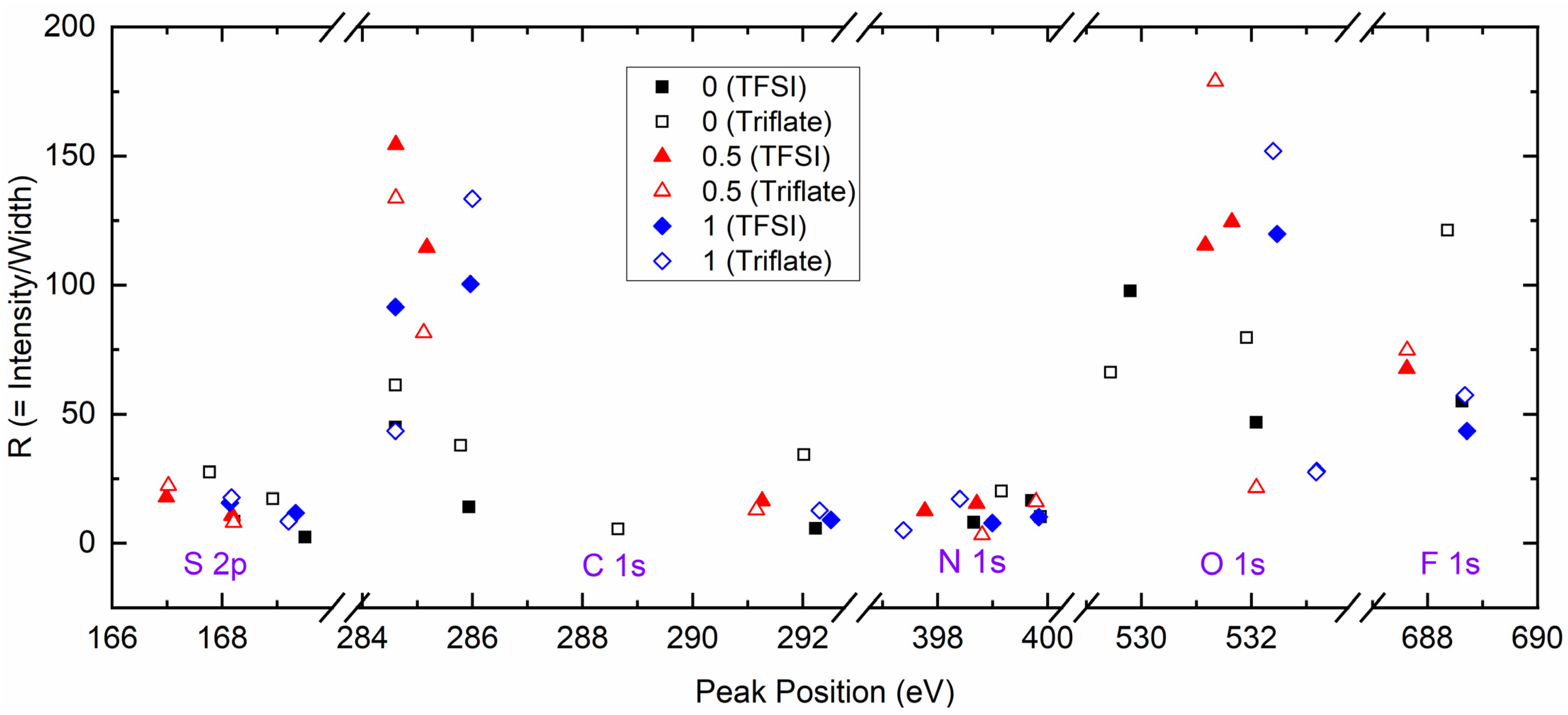
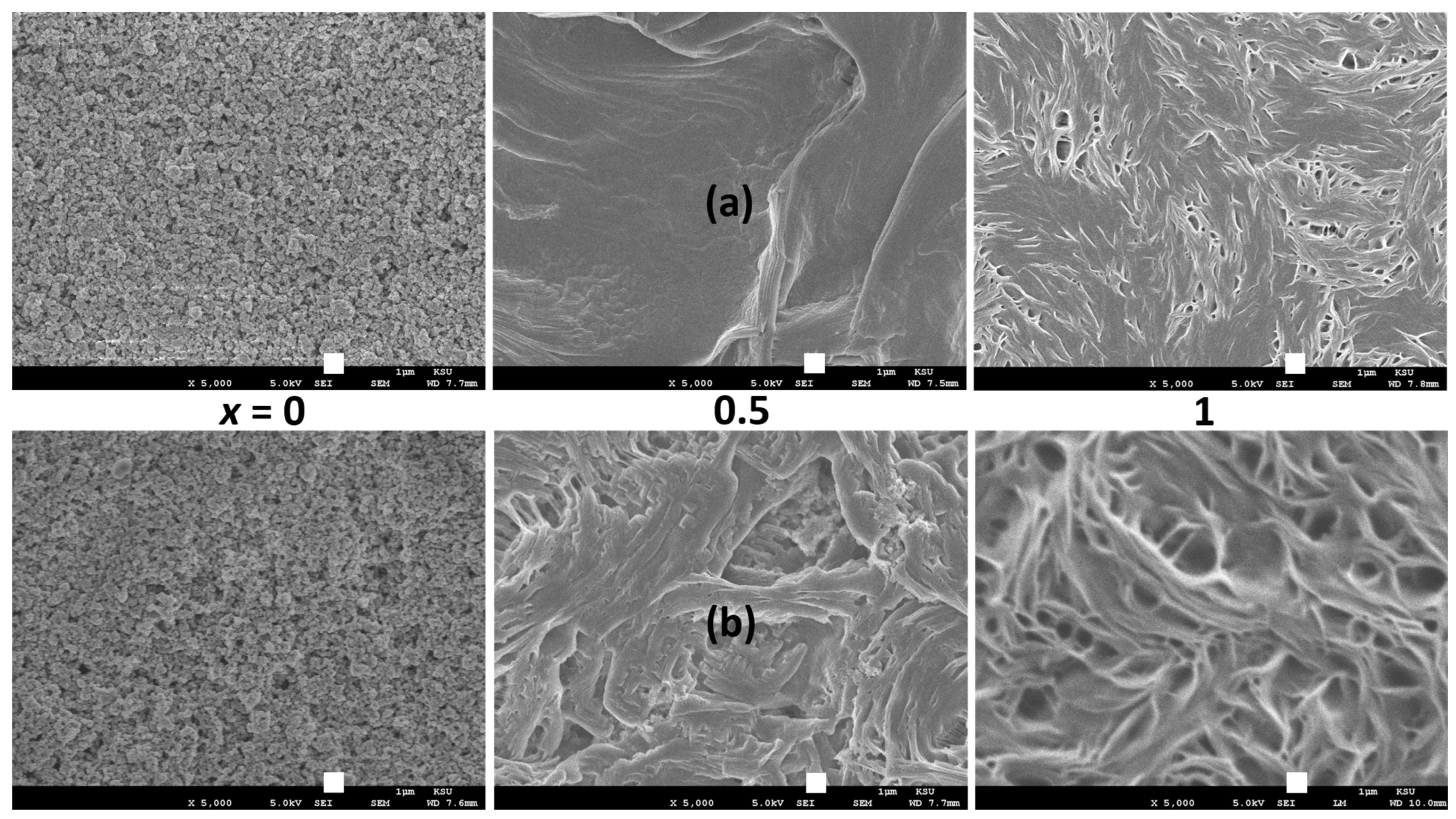
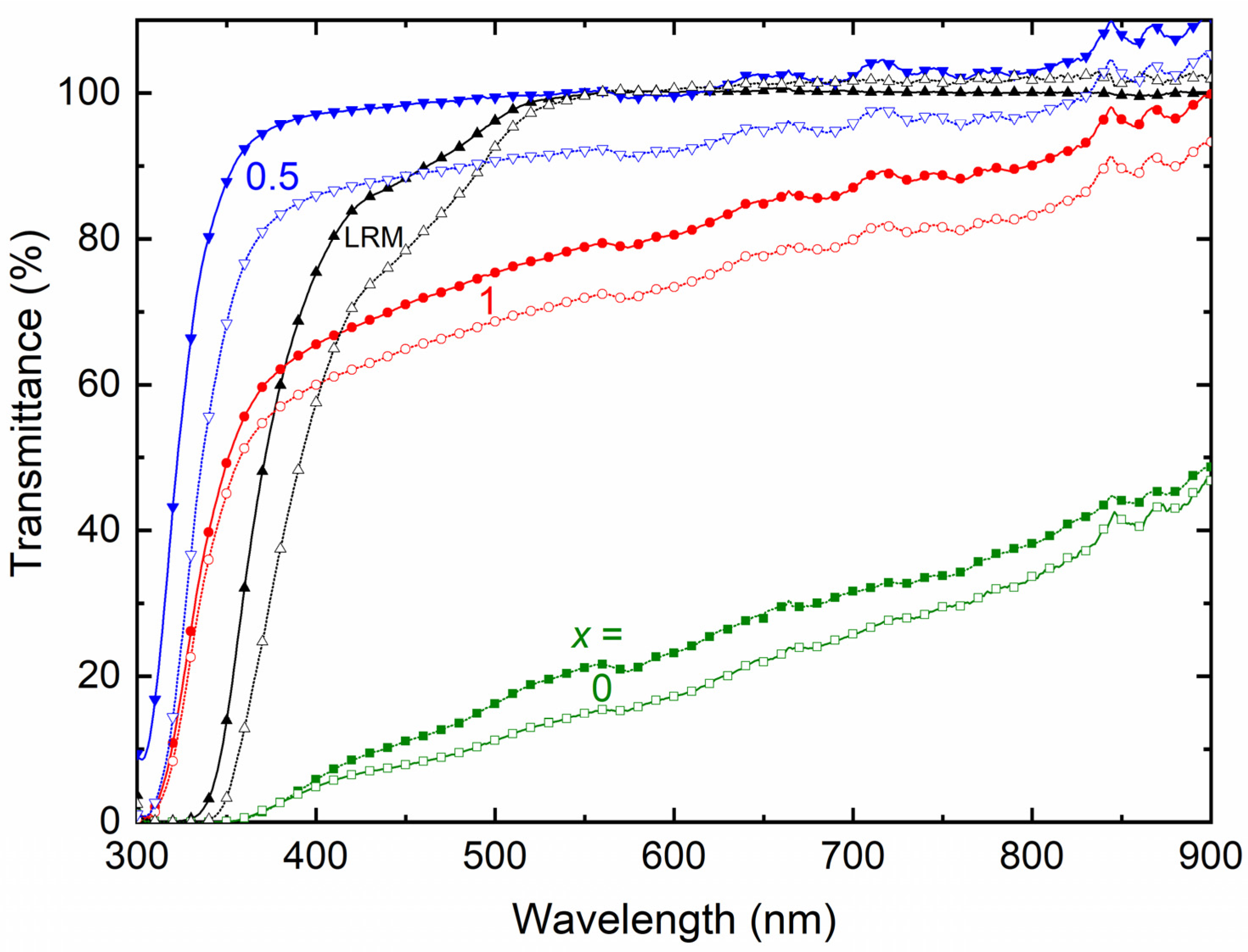
| x (Wt. Fraction) | σ25°C (S cm−1) | Log σ − T−1 Nature | Activation Energy (eV) in Regions I and II | ||
|---|---|---|---|---|---|
| TFSI− | Triflate− | TFSI− | Triflate− | ||
| 0 | 2.1 × 10−3 | 1.5 × 10−3 | Arrhenius | 0.56 (I), 0.16 (II) | 0.77 (I), 0.13 (II) |
| 0.5 | 7.2 × 10−4 | 3.1 × 10−4 | VTF | 0.05 | 0.06 |
| 1 | 9.7 × 10−7 | 6.3 × 10−7 | Arrhenius | 1.07 (I), 0.36 (II) | 1.22 (I), 0.44 (II) |
| LRM | 1.7 × 10−2 | 1.6 × 10−2 | Arrhenius | 0.15 | 0.16 |
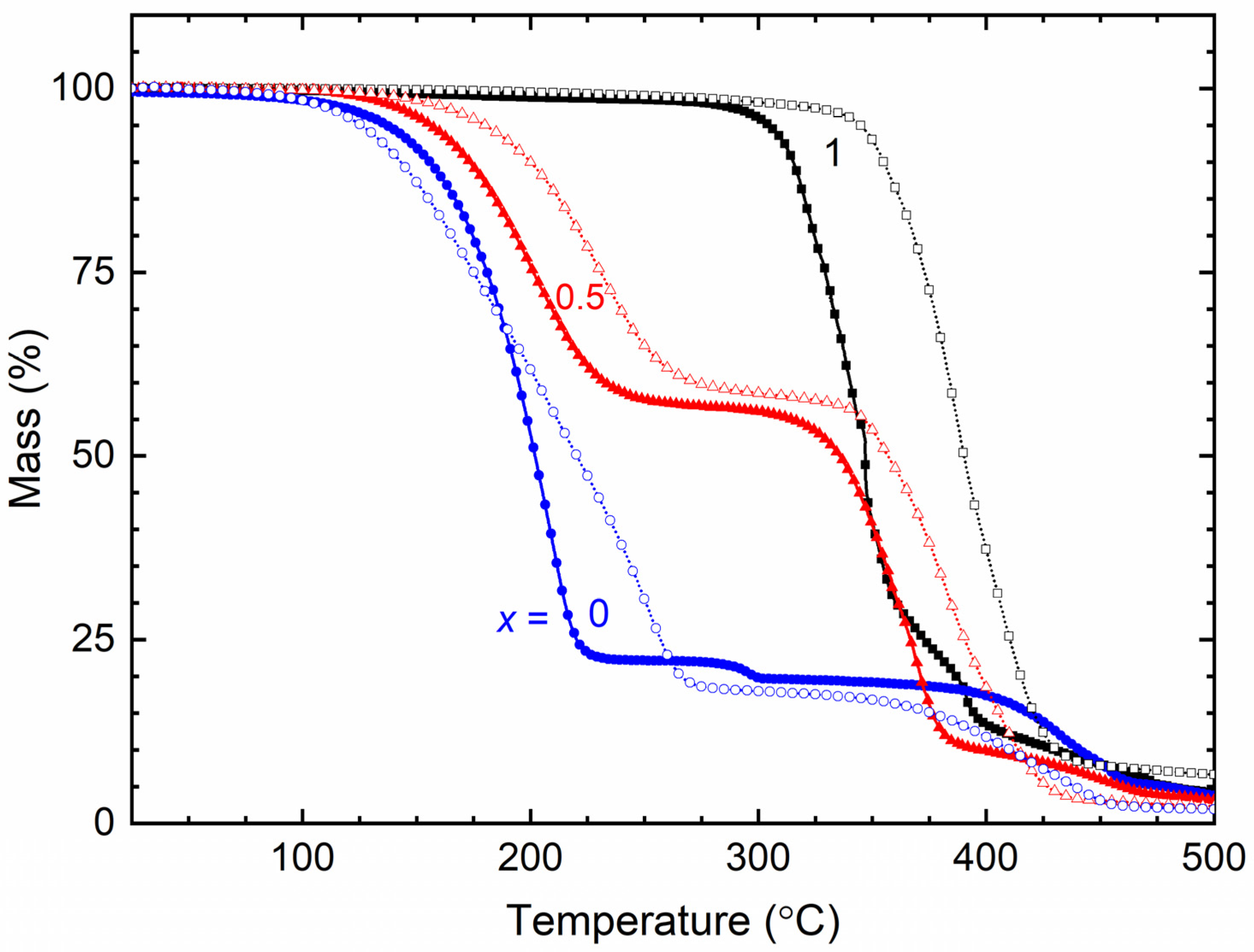
| x (Wt. Fraction) | 2θ (°) | T (%) in Regions I, II, and III | ||
|---|---|---|---|---|
| TFSI− | Triflate− | TFSI− | Triflate− | |
| 0 | 20.4, 28.8 | 19.2, 27.6 | 0 (I), 21.6 (II), 48.7 (III) | 0 (I), 15.1 (II), 46.8 (III) |
| 0.5 | 27.6 | 23.1 | 88.1 (I), 99.8 (II), 110.5 (III) | 68.8 (I), 91.8 (II), 105.4 (III) |
| 1 | 19.5, 24 | 19.4, 23.6 | 49.6 (I), 78.9 (II), 99.9 (III) | 45.4 (I), 71.9 (II), 93.3 (III) |
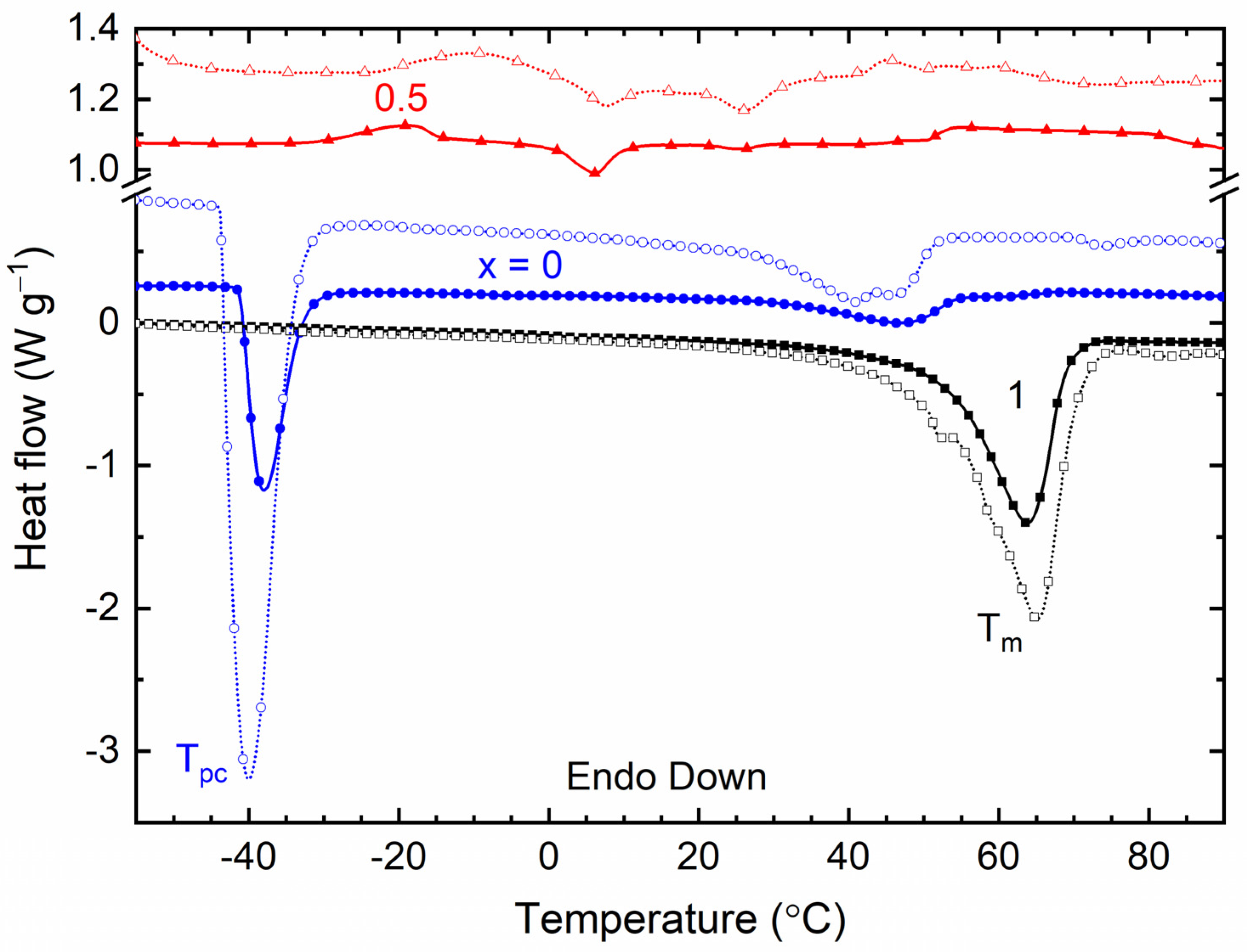
| x (Wt. Fraction) | Tm (°C) | Tm-Area (a.u.) | Tpc (°C) | |||
|---|---|---|---|---|---|---|
| TFSI− | Triflate− | TFSI− | Triflate− | TFSI− | Triflate− | |
| 0 | 47 | 41 and 45.8 | 15.6 | 43.2 | −37.8 | −40 |
| 0.5 | 4 | 7.8 and 26 | 2.4 | 17.5 | - | - |
| 1 | 63.8 | 65.2 | 92.7 | 160.4 | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gupta, R.K.; Imran, A.; Khan, A. Anionic Effect on Electrical Transport Properties of Solid Co2+/3+ Redox Mediators. Polymers 2024, 16, 1436. https://doi.org/10.3390/polym16101436
Gupta RK, Imran A, Khan A. Anionic Effect on Electrical Transport Properties of Solid Co2+/3+ Redox Mediators. Polymers. 2024; 16(10):1436. https://doi.org/10.3390/polym16101436
Chicago/Turabian StyleGupta, Ravindra Kumar, Ahamad Imran, and Aslam Khan. 2024. "Anionic Effect on Electrical Transport Properties of Solid Co2+/3+ Redox Mediators" Polymers 16, no. 10: 1436. https://doi.org/10.3390/polym16101436
APA StyleGupta, R. K., Imran, A., & Khan, A. (2024). Anionic Effect on Electrical Transport Properties of Solid Co2+/3+ Redox Mediators. Polymers, 16(10), 1436. https://doi.org/10.3390/polym16101436








