Green Flame-Retardant Blend Used to Improve the Antiflame Properties of Polypropylene
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Composite Preparation by the Melt Extrusion Process
2.3. Characterization of Composites
2.3.1. Fourier Transform Infrared (FTIR)
2.3.2. Thermogravimetric Analysis (TGA)
2.3.3. Scanning Electron Microscopy (SEM)
2.3.4. Cone Calorimetric
3. Results and Discussion
3.1. Chemical Structure Characterization
3.1.1. Fourier Transform Infrared FTIR (ATR)
3.1.2. X-ray Diffraction (XRD)
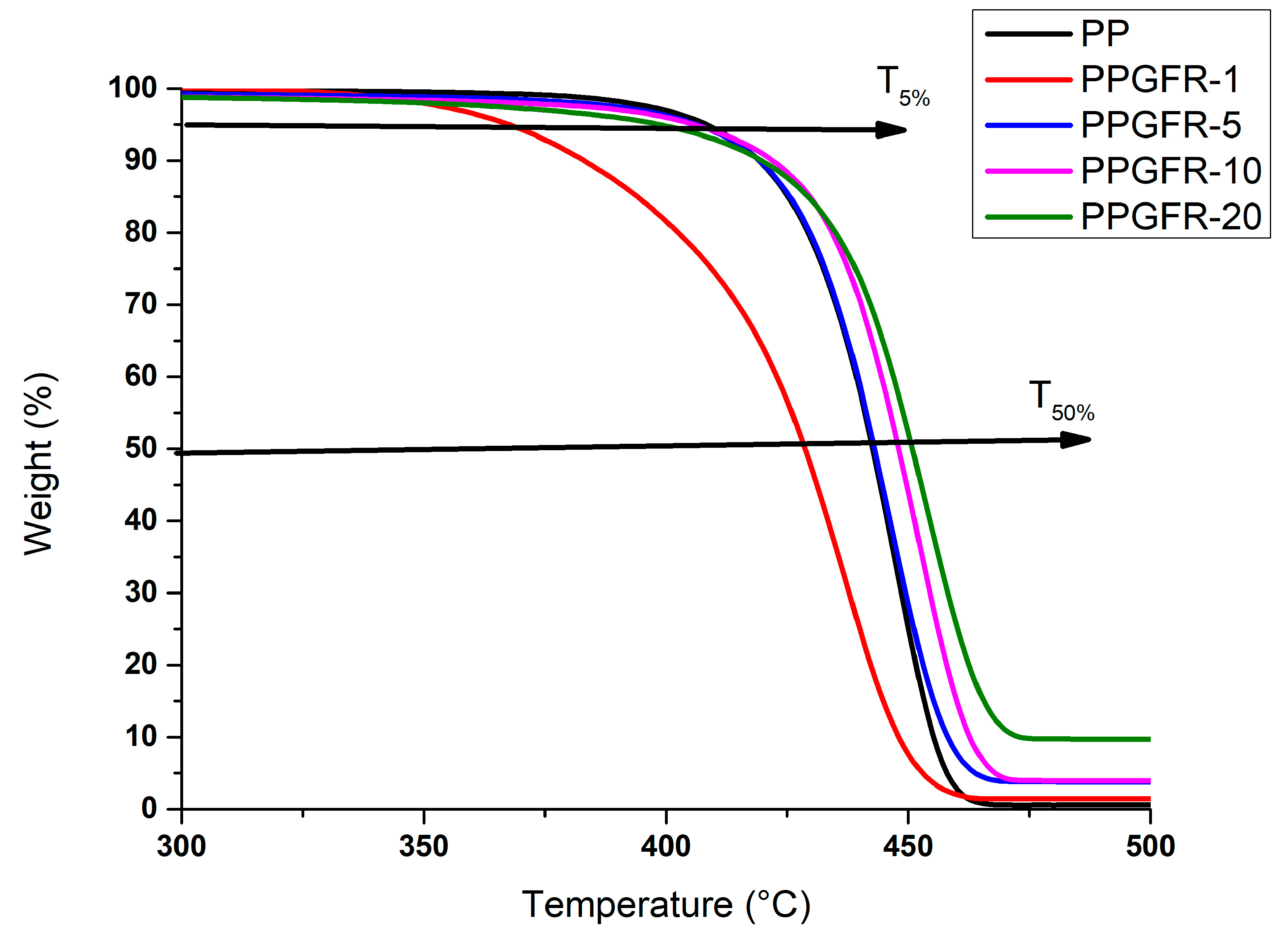
3.2. Thermal Stability Analysis
3.3. Tensile Properties
3.4. Surface Morphology
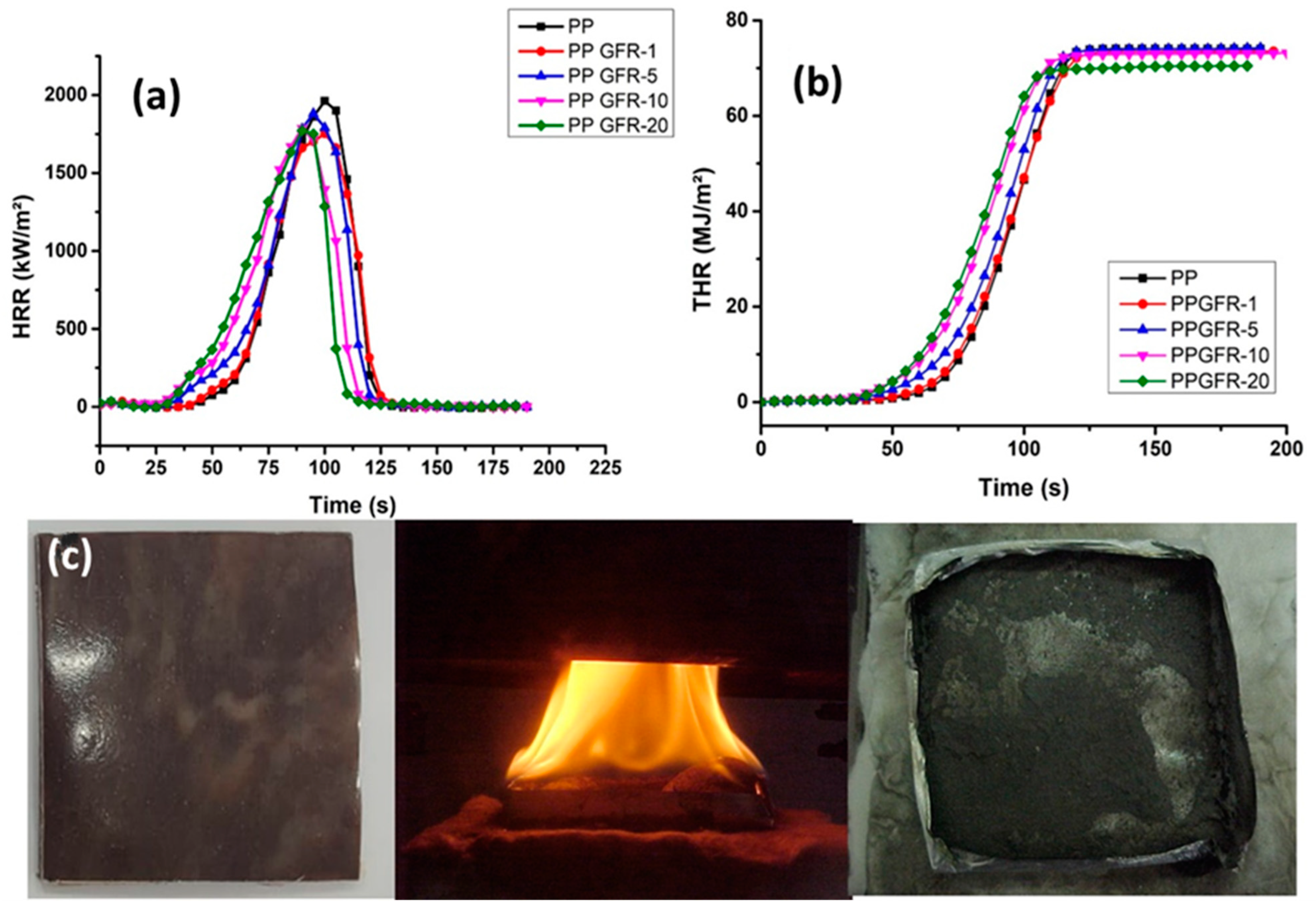
3.5. Flame Retardance Analysis
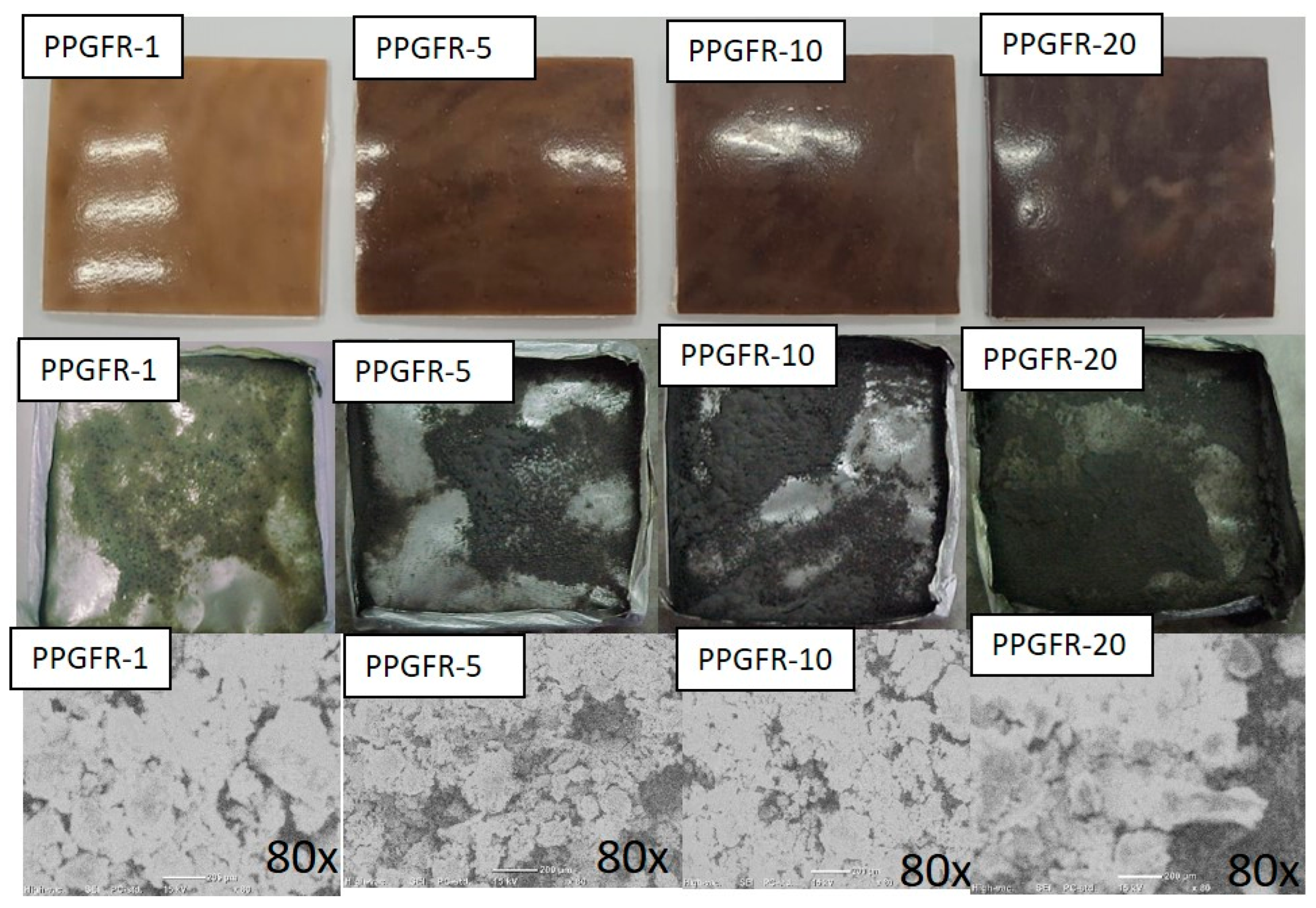
3.6. Analysis of Char Residue
3.6.1. Char Residue Surface Morphology
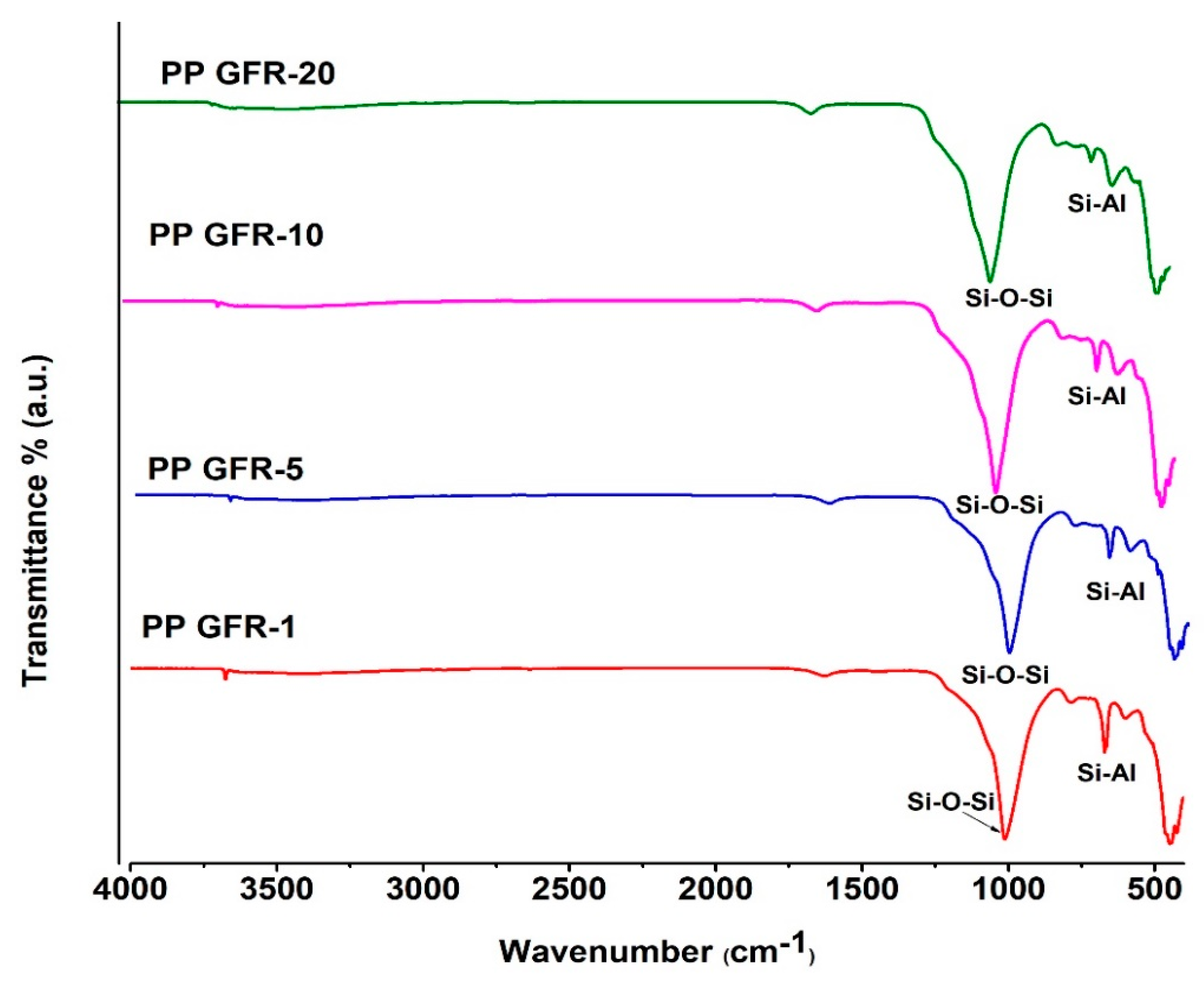
3.6.2. FTIR Spectroscopy
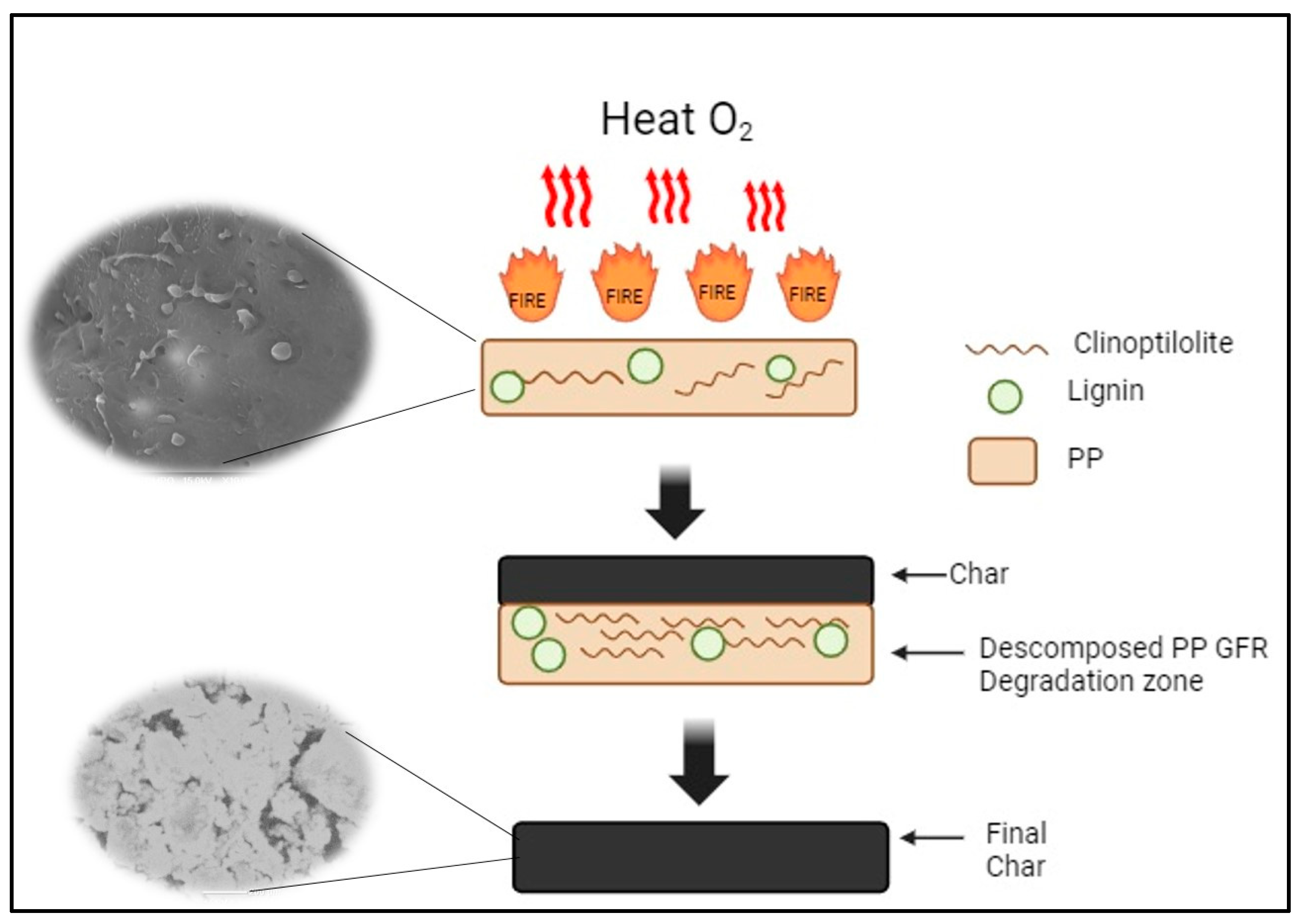
3.6.3. Flame-Retardant Mechanism
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hull, T.R.; Kandola, B.K. Fire Retardancy of Polymers: New Strategies and Mechanisms; Royal Society of Chemistry: Cambridge, UK, 2009; pp. 1–14. [Google Scholar]
- Laoutid, F.; Bonnaud, L.; Alexandre, M.; Lopez-Cuesta, J.M.; Dubois, P. New prospects in flame retardant polymer materials: From fundamentals to nanocomposites. Mater. Sci. Eng. R Rep. 2009, 63, 100–125. [Google Scholar] [CrossRef]
- Shamsuri, A.A.; Yusoff, M.Z.M.; Abdan, K.; Jamil, S.N.A.M. Flammability properties of polymers and polymer composites combined with ionic liquids. e-Polymers 2023, 23, 20230060. [Google Scholar] [CrossRef]
- Wang, M.; Yin, G.Z.; Yang, Y.; Fu, W.; Palencia, J.L.D.; Zhao, J.; Wang, N.; Jiang, Y.; Wang, D.Y. Bio-based flame retardants to polymers: A review. Adv. Ind. Eng. Polym. Res. 2023, 6, 132–155. [Google Scholar] [CrossRef]
- Chen, L.; Wang, Y.Z. A review on flame retardant technology in China. Part I: Development of flame retardants. Polym. Adv. Technol. 2010, 21, 1–26. [Google Scholar] [CrossRef]
- Demir, H.; Arkış, E.; Balköse, D.; Ülkü, S. Synergistic effect of natural zeolites on flame retardant additives. Polym. Degrad. Stab. 2005, 89, 478–483. [Google Scholar] [CrossRef]
- Jiao, C.; Chen, X. Flammability and thermal degradation of intumescent flame-retardant polypropylene composites. Polym. Eng. Sci. 2010, 50, 767–772. [Google Scholar] [CrossRef]
- Belardi, G.; Piga, L. Influence of calcium carbonate on the decomposition of asbestos contained in end-of-life products. Thermochim. Acta 2013, 573, 220–228. [Google Scholar] [CrossRef]
- Rad, E.R.; Vahabi, H.; de Anda, A.R.; Saeb, M.R.; Thomas, S. Bio-epoxy resins with inherent flame retardancy. Prog. Org. Coat. 2019, 135, 608–612. [Google Scholar] [CrossRef]
- Seidi, F.; Movahedifar, E.; Naderi, G.; Akbari, V.; Ducos, F.; Shamsi, R.; Vahabi, H.; Saeb, M.R. Flame retardant polypropylenes: A review. Polymers 2020, 12, 1701. [Google Scholar] [CrossRef] [PubMed]
- Vahabi, H.; Jouyandeh, M.; Cochez, M.; Khalili, R.; Vagner, C.; Ferriol, M.; Movahedifar, E.; Ramezanzadeh, B.; Rostami, M.; Ranjbar, Z. Short-lasting fire in partially and completely cured epoxy coatings containing expandable graphite and halloysite nanotube additives. Prog. Org. Coat. 2018, 123, 160–167. [Google Scholar] [CrossRef]
- Zhang, Z.X.; Zhang, J.; Lu, B.-X.; Xin, Z.X.; Kang, C.K.; Kim, J.K. Effect of flame retardants on mechanical properties, flammability and foamability of PP/wood–fiber composites. Compos. Part B Eng. 2012, 43, 150–158. [Google Scholar] [CrossRef]
- Tripathi, D. Practical Guide to Polypropylene; iSmithers Rapra Publishing: Shrewsbury, UK, 2002. [Google Scholar]
- Zhao, W.; Kumar Kundu, C.; Li, Z.; Li, X.; Zhang, Z. Flame retardant treatments for polypropylene: Strategies and recent advances. Compos. Part A Appl. Sci. Manuf. 2021, 145, 106382. [Google Scholar] [CrossRef]
- Available online: https://www.mordorintelligence.com/industry-reports/polypropylene-market (accessed on 15 April 2024).
- Yang, H.; Yu, B.; Xu, X.; Bourbigot, S.; Wang, H.; Song, P. Lignin-derived bio-based flame retardants toward high-performance sustainable polymeric materials. Green Chem. 2020, 22, 2129. [Google Scholar] [CrossRef]
- De Chirico, A.; Armanini, M.; Chini, P.; Cioccolo, G.; Provasoli, F.; Audisio, G. Flame retardants for polypropylene based on lignin. Polym. Degrad. Stab. 2003, 79, 139. [Google Scholar] [CrossRef]
- Melro, E.; Filipe, A.; Sousa, D.; Medronho, B.; Romano, A. Revisiting lignin: A tour through its structural features, characterization methods, and applications. New J. Chem. 2021, 45, 6986–7013. [Google Scholar] [CrossRef]
- Li, J.; Li, B.; Zhang, X.; Su, R. The study of flame retardants on thermal degradation and charring process of manchurian ash lignin in the condensed phase. Polym. Degrad. Stab. 2001, 73, 493. [Google Scholar] [CrossRef]
- Gallina, G.; Bravin, E.; Badalucco, C.; Audisio, G.; Armanini, M.; De Chirico, A.; Provasoli, F. Application of cone calorimeter for the assessment of class of flame retardants for polypropylene. Fire Mater. 1998, 22, 15. [Google Scholar] [CrossRef]
- Lu, S.Y.; Hamerton, I. Recent developments in the chemistry of halogen-free flame retardant polymers. Prog. Polym. Sci. 2002, 27, 1661–1712. [Google Scholar] [CrossRef]
- Kurt, R.; Mengeloglu, F.; Meric, H. The effects of boron compounds synergists with ammonium polyphosphate on mechanical properties and burning rates of wood-HDPE polymer composites. Eur. J. Wood Prod. 2012, 70, 177. [Google Scholar] [CrossRef]
- Feng, C.; Zhang, Y.; Liu, S.; Chik, Z.; Xu, J. Synergistic effects of 4A zeolite on the flame retardant properties and thermal stability of a novel halogen-free PP/IFR composite. Polym. Adv. Technol. 2013, 24, 478. [Google Scholar] [CrossRef]
- Iyer, K.A.; Torkelson, J.M. Sustainable Green Hybrids of Polyolefins and Lignin Yield Major Improvements in Mechanical Properties When Prepared via Solid-State Shear. ACS Sustain. Chem. Eng. 2015, 3, 959–968. [Google Scholar] [CrossRef]
- ASTM E 1354; Standard Test Method for Heat and Visible Smoke Release Rates for Materials and Products Using an Oxygen Consumption Calorimeter. ASTM: West Conshohocken, PA, USA, 2010.
- ASTM D3801-20a; Standard Test Method for Measuring the Comparative Burning Characteristics of Solid Plastics in a Vertical Position. ASTM: West Conshohocken, PA, USA, 2020.
- Andrade-Guel, M.; Cabello-Alvarado, C.; Avila-Orta, C.A.; Pérez-Alvarez, M.; Cadenas-Pliego, G.; Reyes-Rodríguez, P.Y.; Rios-González, L. Green Flame-Retardant Composites Based on PP/TiO2/Lignin Obtained by Melt-Mixing Extrusion. Polymers 2022, 14, 1300. [Google Scholar] [CrossRef] [PubMed]
- Gopanna, A.; Mandapati, R.N.; Thomas, S.P.; Rajan, K.; Chavali, M. Fourier transform infrared spectroscopy (FTIR), Raman spectroscopy, and wide-angle X-ray scattering (WAXS) of polypropylene (PP)/cyclic olefin copolymer (COC) blend for qualitative and quantitative analysis. Polym. Bull. 2019, 76, 4259–4274. [Google Scholar] [CrossRef]
- Părpăriţă, E.; Nistor, M.T.; Popescu, M.C.; Vasile, C. TG/FT–IR/MS study on thermal decomposition of polypropylene/biomass composites. Polym. Degrad. Stab. 2014, 109, 13–20. [Google Scholar] [CrossRef]
- Herth, E.; Zeggari, R.; Rauch, J.Y.; Remy-Martin, F.; Boireau, W. Investigation of amorphous SiOx layer on gold surface for Surface Plasmon Resonance measurements. Microelectron. Eng. 2016, 163, 43–48. [Google Scholar] [CrossRef]
- Dias OA, T.; Sain, M.; Cesarino, I.; Leão, A.L. Development of high bio-content polypropylene composites with different industrial lignins. Polym. Adv. Technol. 2019, 30, 70–78. [Google Scholar] [CrossRef]
- Abdelwahab, M.A.; Misra, M.; Mohanty, A.K. Injection molded biocomposites from polypropylene and lignin: Effect of compatibilizers on interfacial adhesion and performance. Ind. Crops Prod. 2019, 132, 497–510. [Google Scholar] [CrossRef]
- Cabello-Alvarado, C.; Andrade-Guel, M.; Ávila-Orta, C.A.; Gamero-Melo, P.; Reyes-Rodríguez, P.Y.; Quiñones-Jurado, Z.V.; Cadenas-Pliego, G.; Bartolo-Pérez, P.; Soriano-Corral, F. Composites based on nylon 6/clinoptilolite by ultrasound-assisted extrusion for enhanced flame retardant and mechanical properties. Polym. Bull. 2022, 79, 1803–1819. [Google Scholar] [CrossRef]
- Wu, W.; Liu, T.; Deng, X.; Sun, Q.; Cao, X.; Feng, Y.; Wang, B.; Roy, V.A.L.; Li, R.K.Y. Ecofriendly UV-protective films based on poly (propylene carbonate) biocomposites filled with TiO2 decorated lignin. Int. J. Biol. Macromol. 2019, 126, 1030–1036. [Google Scholar] [CrossRef]
- Nezamzadeh-Ejhieh, A.; Khodabakhshi-Chermahini, F. Incorporated ZnO onto nano clinoptilolite particles as the active centers in the photodegradation of phenylhydrazine. J. Ind. Eng. Chem. 2014, 20, 695–704. [Google Scholar] [CrossRef]
- Cabello-Alvarado, C.; Reyes-Rodríguez, P.; Andrade-Guel, M.; Cadenas-Pliego, G.; Pérez-Alvarez, M.; Cruz-Delgado, V.J.; Ávila-Orta, C.A. Melt-mixed thermoplastic nanocomposite containing carbon nanotubes and titanium dioxide for flame retardancy applications. Polymers 2019, 11, 1204. [Google Scholar] [CrossRef]
- Shen, D.; Liu, G.; Zhao, J.; Xue, J.; Guan, S.; Xiao, R. Thermo-chemical conversion of lignin to aromatic compounds: Effect of lignin source and reaction temperature. J. Anal. Appl. Pyrolysis 2015, 112, 56–65. [Google Scholar] [CrossRef]
- Brebu, M.; Tamminen, T.; Spiridon, I. Thermal degradation of various lignins by TG–MS/FTIR and Py–GC–MS. J. Anal. Appl. Pyrolysis 2013, 104, 531–539. [Google Scholar] [CrossRef]
- Bourbigot, S.; Le Bras, M.; Delobel, R.; Bréant, P.; Tremillon, J.M. 4A zeolite synergistic agent in new fame retardant intumescent formulations of polyethylenic polymers—The study of the effect of the constituent monomers. Polym. Degrad. Stabil. 1996, 54, 275–287. [Google Scholar] [CrossRef]
- Motsa, M.M.; Msagati, T.A.M.; Thwala, J.M. Polypropylene–zeolite polymer composites for water purification: Synthesis, characterisation and application. Desalination Water Treat. 2015, 53, 2604–2612. [Google Scholar] [CrossRef]
- Jakab, E.; Faix, O.; Till, F. Thermal decomposition of milled wood lignins studied by thermogravimetry/mass spectrometry. J. Anal. Appl. Pyrolysis 1997, 40–41, 171–186. [Google Scholar] [CrossRef]
- Yan, Q.; Li, J.; Zhang, J.; Cai, Z. Thermal Decomposition of Kraft Lignin under Gas Atmospheres of Argon, Hydrogen, and Carbon Dioxide. Polymers 2018, 10, 729. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Li, S.Y.; Zhang, M.; Zeng, H.Y.; Wu, K.; Tian, X.Y.; Chen, C.-R.; Pan, Y. Fabrication of green alginate-based and layered double hydroxides flame retardant for enhancing the fire retardancy properties of polypropylene. Carbohydr. Polym. 2020, 234, 115891. [Google Scholar] [CrossRef] [PubMed]
- Klapiszewski, Ł.; Bula, K.; Sobczak, M.; Jesionowski, T. Influence of processing conditions on the thermal stability and mechanical properties of PP/silica-lignin composites. Int. J. Polym. Sci. 2016, 2016, 1627258. [Google Scholar] [CrossRef]
- Bula, K.; Klapiszewski, Ł.; Jesionowski, T. A novel functional silica/lignin hybrid material as a potential bio-based polypropylene filler. Polym. Compos. 2015, 36, 913–922. [Google Scholar] [CrossRef]
- González Sánchez, C.; Alvarez, L.E. Micromechanics of lignin/polypropylene composites suitable for industrial applications. Die Angew. Makromol. Chem. 1999, 272, 65–70. [Google Scholar] [CrossRef]
- Zheng, Z.; Xia, Y.; Liao, C.; Liu, Y.; Chai, W.; Niu, E.; Hu, Z. Fabrication of starch-based multi-source integrated halogen-free flame retardant in improving the fire safety of polypropylene. J. Polym. Res. 2021, 28, 445. [Google Scholar] [CrossRef]
- Ramasamy, R.P.; Yang, K.; Rafailovich, M.H. Polypropylene–graphene–a nanocomposite that can be converted into a meta-material at desired frequencies. RSC Adv. 2014, 4, 44888–44895. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, W.; Chen, H.; Zhang, S.; Li, J. Synergistic effect of synthetic zeolites on flame-retardant wood-flour/polypropylene composites. Constr. Build. Mater. 2015, 79, 337–344. [Google Scholar] [CrossRef]
- Singh, A.K.; Yadav, P.; Bharagava, R.N.; Saratale, G.D.; Raj, A. Biotransformation and cytotoxicity evaluation of kraft lignin degraded by ligninolytic Serratia liquefaciens. Front. Microbiol. 2019, 10, 2364. [Google Scholar] [CrossRef] [PubMed]
- Intapun, J.; Rungruang, T.; Suchat, S.; Cherdchim, B.; Hiziroglu, S. The Characteristics of natural rubber composites with Klason lignin as a green reinforcing filler: Thermal stability, mechanical and dynamical Properties. Polymers 2021, 13, 1109. [Google Scholar] [CrossRef] [PubMed]
- Vahabi, H.; Kandola, B.K.; Saeb, M.R. Flame retardancy index for thermoplastic composites. Polymers 2019, 11, 407. [Google Scholar] [CrossRef]
- Movahedifar, E.; Vahabi, H.; Saeb, M.R.; Thomas, S. Flame retardant epoxy composites on the road of innovation: An analysis with flame retardancy index for future development. Molecules 2019, 24, 3964. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Q.; Li, P.; Liu, Y.; Zhu, P. Green flame-retardant flexible polyurethane foam based on polyphenol-iron-phytic acid network to improve the fire safety. Compos. Part B Eng. 2022, 239, 109958. [Google Scholar] [CrossRef]
- Ye, G.; Huo, S.; Wang, C.; Shi, Q.; Liu, Z.; Wang, H. One-step and green synthesis of a bio-based high-efficiency flame retardant for poly (lactic acid). Polym. Degrad. Stab. 2021, 192, 109696. [Google Scholar] [CrossRef]
- Wang, J.; Huo, S.; Wang, J.; Yang, S.; Chen, K.; Li, C.; Fang, D.; Fang, Z.; Song, P.; Wang, H. Green and facile synthesis of bio-based, flame-retardant, latent imidazole curing agent for single-component epoxy resin. ACS Appl. Polym. Mater. 2022, 4, 3564–3574. [Google Scholar] [CrossRef]
- Wang, N.; Chen, S.; Li, L.; Bai, Z.; Guo, J.; Qin, J.; Chen, X.; Zhao, R.; Zhang, K.; Wu, H. An environmentally friendly nanohybrid flame retardant with outstanding flame-retardant efficiency for polypropylene. J. Phys. Chem. C 2021, 125, 5185–5196. [Google Scholar] [CrossRef]
- Wu, K.; Xu, S.; Tian, X.Y.; Zeng, H.Y.; Hu, J.; Guo, Y.H.; Jian, J. Renewable lignin-based surfactant modified layered double hydroxide and its application in polypropylene as a flame retardant and smoke suppression. Int. J. Biol. Macromol. 2021, 178, 580–590. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Qian, M.; Song, P.A.; Huang, G.; Yu, Y.; Fu, S. Fabrication of green lignin-based flame retardants for enhancing the thermal and fire retardancy properties of polypropylene/wood composites. ACS Sustain. Chem. Eng. 2016, 4, 2422–2431. [Google Scholar] [CrossRef]
- Khodadadi, B.; Bordbar, M.; Yeganeh-Faal, A.; Nasrollahzadeh, M. Green synthesis of Ag nanoparticles/clinoptilolite using Vaccinium macrocarpon fruit extract and its excellent catalytic activity for reduction of organic dyes. J. Alloys Compd. 2017, 719, 82–88. [Google Scholar] [CrossRef]
- Qin, H.; Zhang, S.; Zhao, C.; Hu, G.; Yang, M. Flame retardant mechanism of polymer/clay nanocomposites based on polypropylene. Polymer 2005, 46, 8386–8395. [Google Scholar] [CrossRef]
- Zanetti, M.; Kashiwagi, T.; Falqui, L.; Camino, G. Cone calorimeter combustion and gasification studies of polymer layered silicate nanocomposites. Chem. Mater. 2002, 14, 881–887. [Google Scholar] [CrossRef]
- Jin, X.; Xiang, E.; Zhang, R.; Qin, D.; Jiang, M.; Jiang, Z. Halloysite nanotubes immobilized by chitosan/tannic acid complex as a green flame retardant for bamboo fiber/poly(lactic acid) composites. J. Appl. Polym. Sci. 2021, 138, e49621. [Google Scholar] [CrossRef]
- Bourbigot, S.; Bras, M.L.; Bréant, P.; Trémillon, J.M.; Delobel, R. Zeolites: New synergistic agents for intumescent fire retardant thermoplastic formulations—Criteria for the choice of the zeolite. Fire Mater. 1996, 20, 145–154. [Google Scholar] [CrossRef]
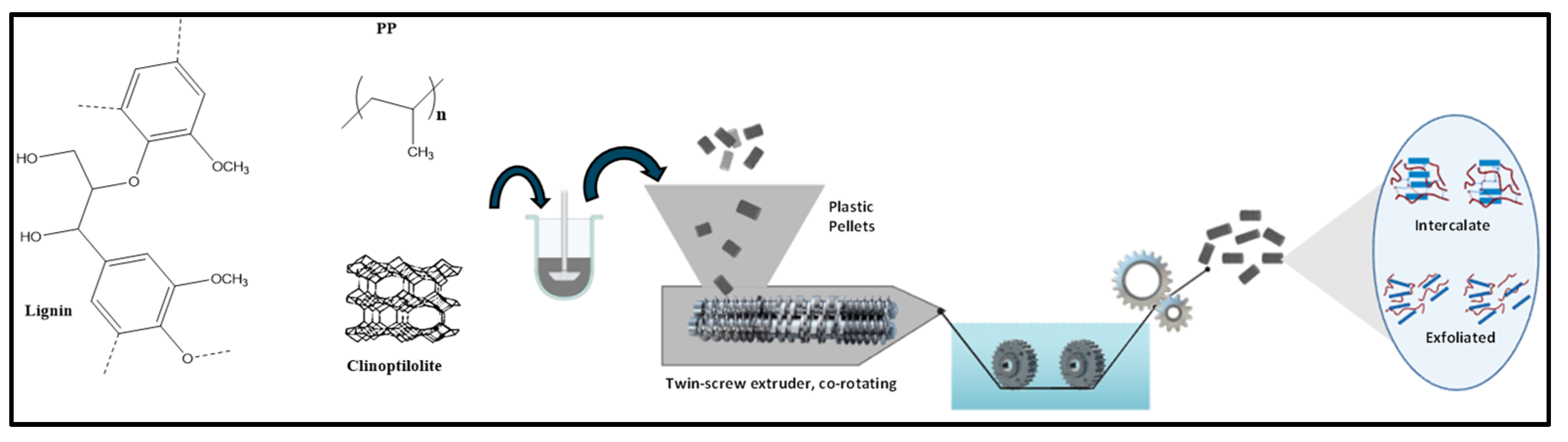
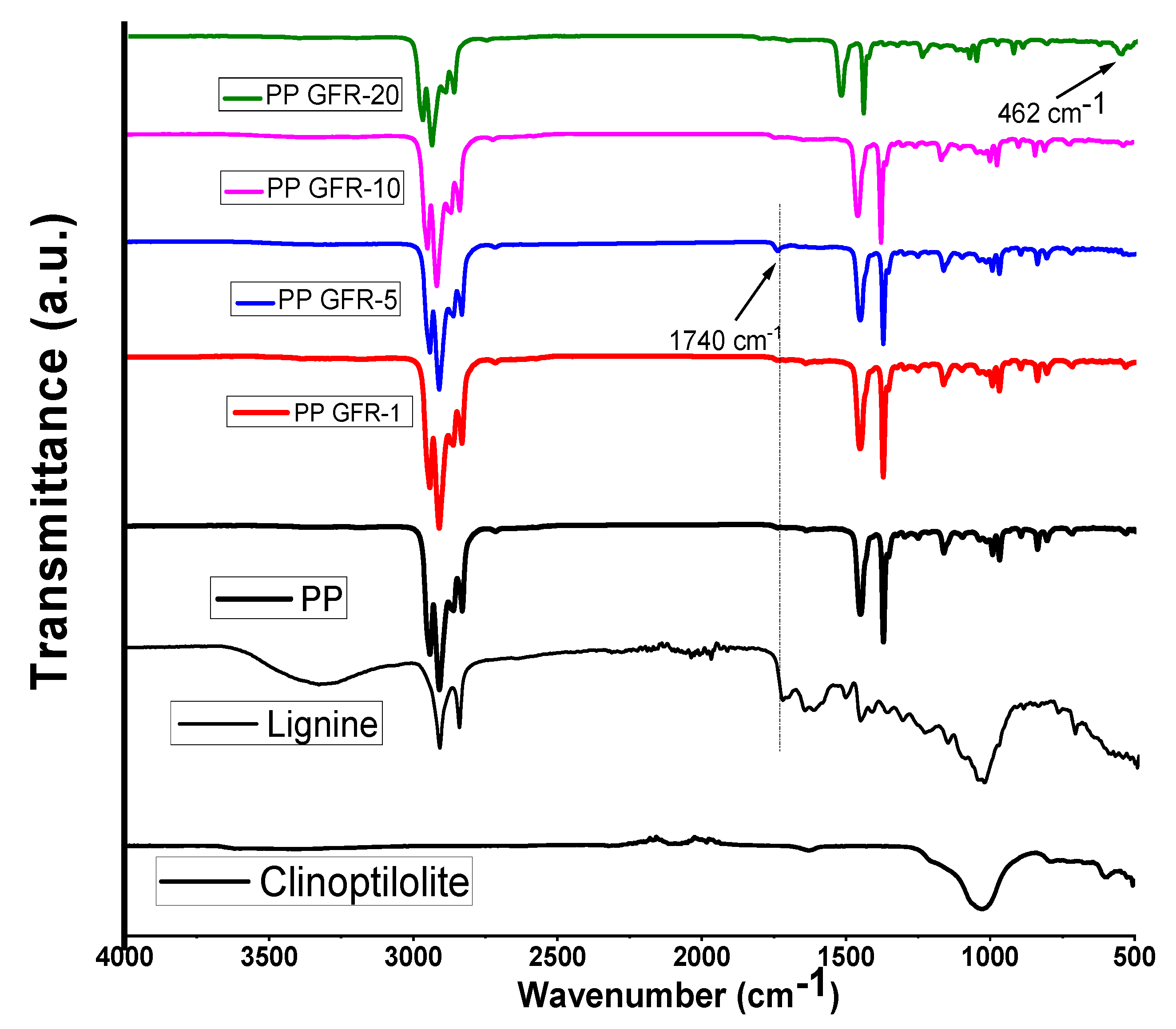

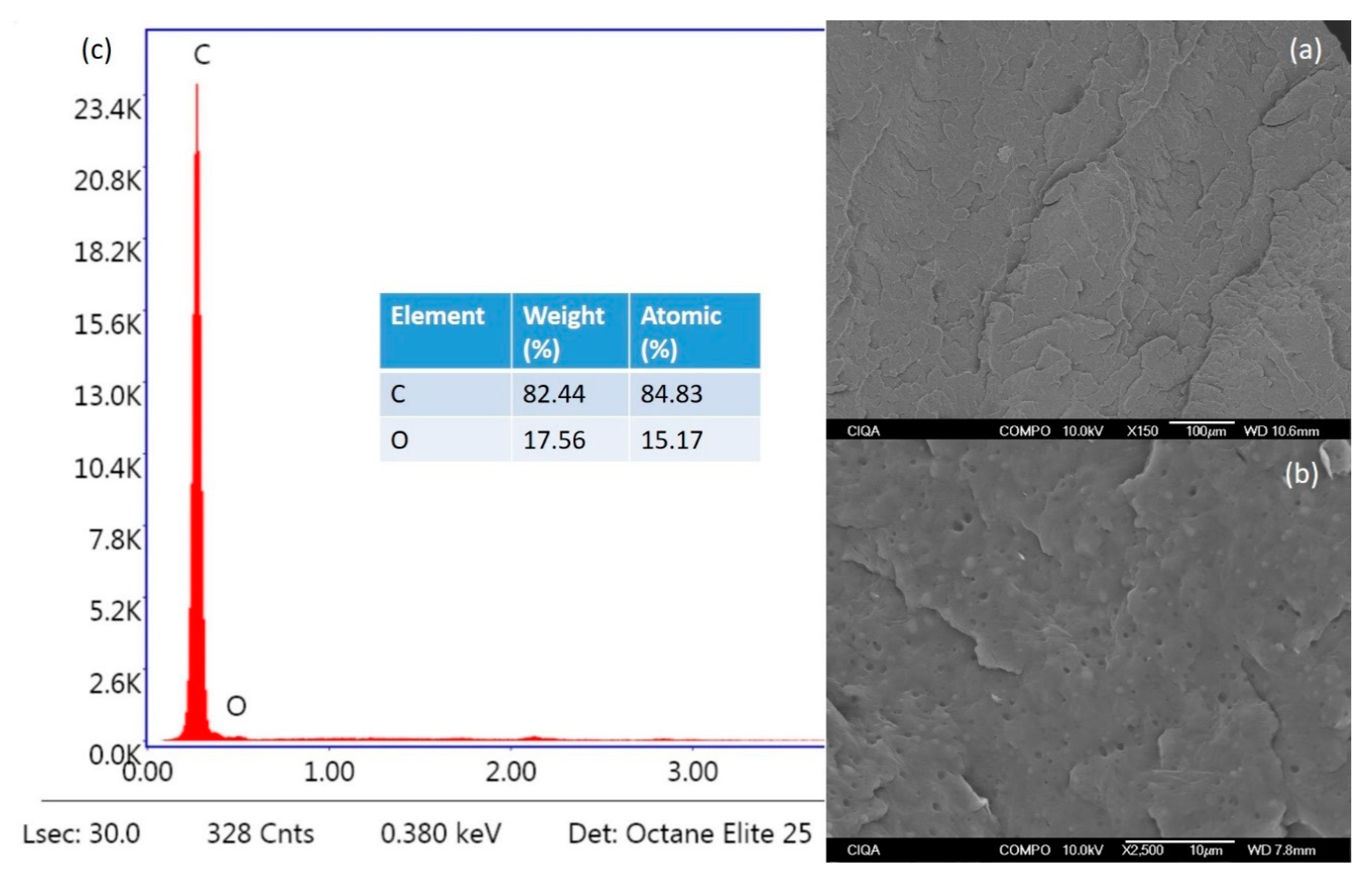

| Sample | PP (g) | Mix Green Flame Retardant (g) | Weight of Additive Clinoptilolite (g) | Weight of Additive Lignin (g) | Total Weight (g) |
|---|---|---|---|---|---|
| PP | 300 | 0 | 0 | 0 | 300 |
| PPGFR-1 | 297 | 3 | 2 | 1 | 300 |
| PPGFR-5 | 285 | 15 | 10 | 5 | 300 |
| PPGFR-10 | 270 | 30 | 20 | 10 | 300 |
| PPGFR-20 | 240 | 60 | 40 | 20 | 300 |
| Sample | Temperature at 5% of Weight Loss (T5%) (°C) | Temperature at 50% of Weight Loss (T50%) (°C) | Residue at 500 °C (%) |
|---|---|---|---|
| PP | 410 | 441 | 0 |
| PPGFR-1 | 368. | 428 | 1.3 |
| PPGFR-5 | 409 | 442 | 3.9 |
| PPGFR-10 | 408 | 447 | 5.6 |
| PPGFR-20 | 402 | 451 | 9.7 |
| Samples | Elongation at Break (%) | Tensile Strength (MPa) |
|---|---|---|
| PP | 247 ± 1.80 | 25.1 ± 2 |
| PPGFR-1 | 150 ± 1.25 | 24.4 ± 0.8 |
| PPGFR-5 | 117 ± 1.55 | 23.6 ± 5.7 |
| PPGFR-10 | 70 ± 2.7 | 23.3 ± 0.3 |
| PPGFR-20 | 76 ± 5.4 | 20.8 ± 0.4 |
| Sample | Peak HRR (kW/m2) | THR (MJ/m2) | Residue % | FRI | UL-94 |
|---|---|---|---|---|---|
| PP | 1961.28 ± 0.6 | 70.18 ± 2.3 | 0 | ------ | V-2 |
| PPGFR-1 | 1771.6 ± 0.4 | 71.62 ± 1.2 | 0.38 ± 0.1 | 0.80 | V-2 |
| PPGFR-5 | 1881.51 ± 1.3 | 71.89 ± 0.5 | 2.25 ± 0.2 | 0.75 | V-2 |
| PPGFR-10 | 1786.92 ± 0.6 | 70.78 ± 1.1 | 4.1 ± 0.1 | 0.66 | V-2 |
| PPGFR-20 | 1752.95 ± 1.0 | 67.87 ± 0.5 | 9.86 ± 0.5 | 1.15 | V-0 |
| Materials with Green Fire Retardants | UL-94 Classification | Tensile Strength (MPa) | References |
|---|---|---|---|
| PP and 30% hydrotalcite (LDHs-C) | V-0 | 16 ± 0.2 | [43] |
| Polyurethane + polyphenol-iron-phytic acid | V-0 | 0.08 | [54] |
| PLA+ phytic acid (PA) and furfuryl amine (FA) | V-0 | 44.7 ± 1.8 | [55] |
| Bisphenol-A-type epoxy resin Imidazole (IM), ethanol, and phytic acid | V-0 | ------------ | [56] |
| PP+ nano-graphitic lamellas | V-0 | 31.3 | [57] |
| PPGFR-20 | V-0 | 20.8 ± 0.4 | This study |
| Samples | Element Weight (%) | ||
|---|---|---|---|
| O | Al | Si | |
| PPGFR-1 | 12.83 | 29.98 | 57.19 |
| PPGFR-5 | 24.47 | 16.28 | 59.25 |
| PPGFR-10 | 14.49 | 17.40 | 68.11 |
| PPGFR-20 | 24.10 | 7.42 | 68.47 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cabello-Alvarado, C.J.; Andrade-Guel, M.; Pérez-Alvarez, M.; Cadenas-Pliego, G.; Bartolo-Pérez, P.; Martínez-Carrillo, D.; Quiñones-Jurado, Z.V. Green Flame-Retardant Blend Used to Improve the Antiflame Properties of Polypropylene. Polymers 2024, 16, 1317. https://doi.org/10.3390/polym16101317
Cabello-Alvarado CJ, Andrade-Guel M, Pérez-Alvarez M, Cadenas-Pliego G, Bartolo-Pérez P, Martínez-Carrillo D, Quiñones-Jurado ZV. Green Flame-Retardant Blend Used to Improve the Antiflame Properties of Polypropylene. Polymers. 2024; 16(10):1317. https://doi.org/10.3390/polym16101317
Chicago/Turabian StyleCabello-Alvarado, Christian J., Marlene Andrade-Guel, Marissa Pérez-Alvarez, Gregorio Cadenas-Pliego, Pascual Bartolo-Pérez, Diego Martínez-Carrillo, and Zoe V. Quiñones-Jurado. 2024. "Green Flame-Retardant Blend Used to Improve the Antiflame Properties of Polypropylene" Polymers 16, no. 10: 1317. https://doi.org/10.3390/polym16101317
APA StyleCabello-Alvarado, C. J., Andrade-Guel, M., Pérez-Alvarez, M., Cadenas-Pliego, G., Bartolo-Pérez, P., Martínez-Carrillo, D., & Quiñones-Jurado, Z. V. (2024). Green Flame-Retardant Blend Used to Improve the Antiflame Properties of Polypropylene. Polymers, 16(10), 1317. https://doi.org/10.3390/polym16101317









