Synthesis of Thermoresponsive Chitosan-graft-Poly(N-isopropylacrylamide) Hybrid Copolymer and Its Complexation with DNA
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
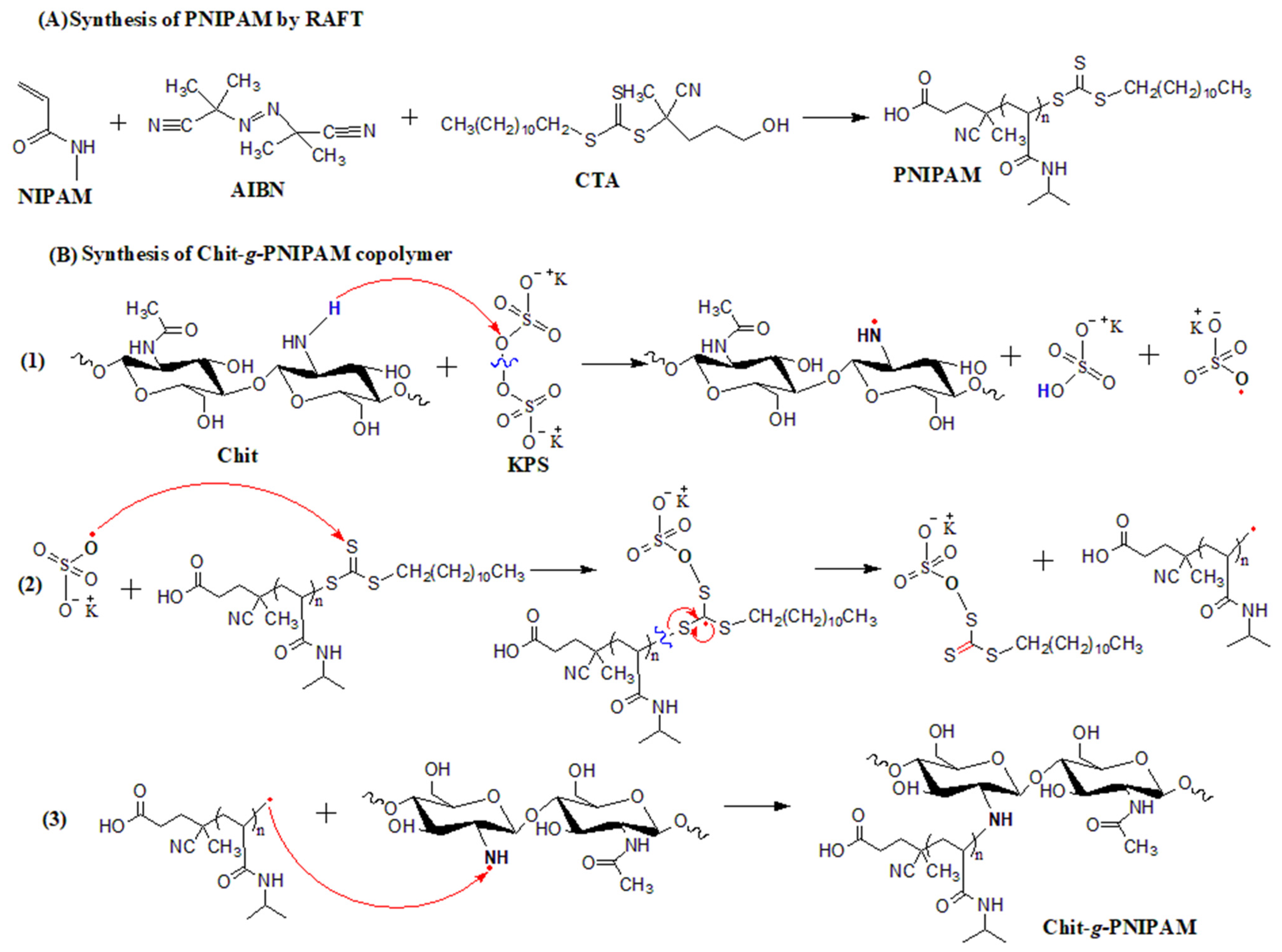
2.2. Synthesis of Chit-g-PNIPAM Copolymer
2.3. Preparation of Copolymer/DNA Polyplexes
2.4. ATR-FTIR Analysis
2.5. 1H NMR Spectroscopy
2.6. Thermogravimetric Analysis
2.7. Polyelectrolyte Titration
2.8. Conductometric Analysis
2.9. Particle Size Analysis
2.10. Electrophoretic Light Scattering-Zeta Potential
3. Results
3.1. Characterization of Chit-g-PNIPAM Copolymer
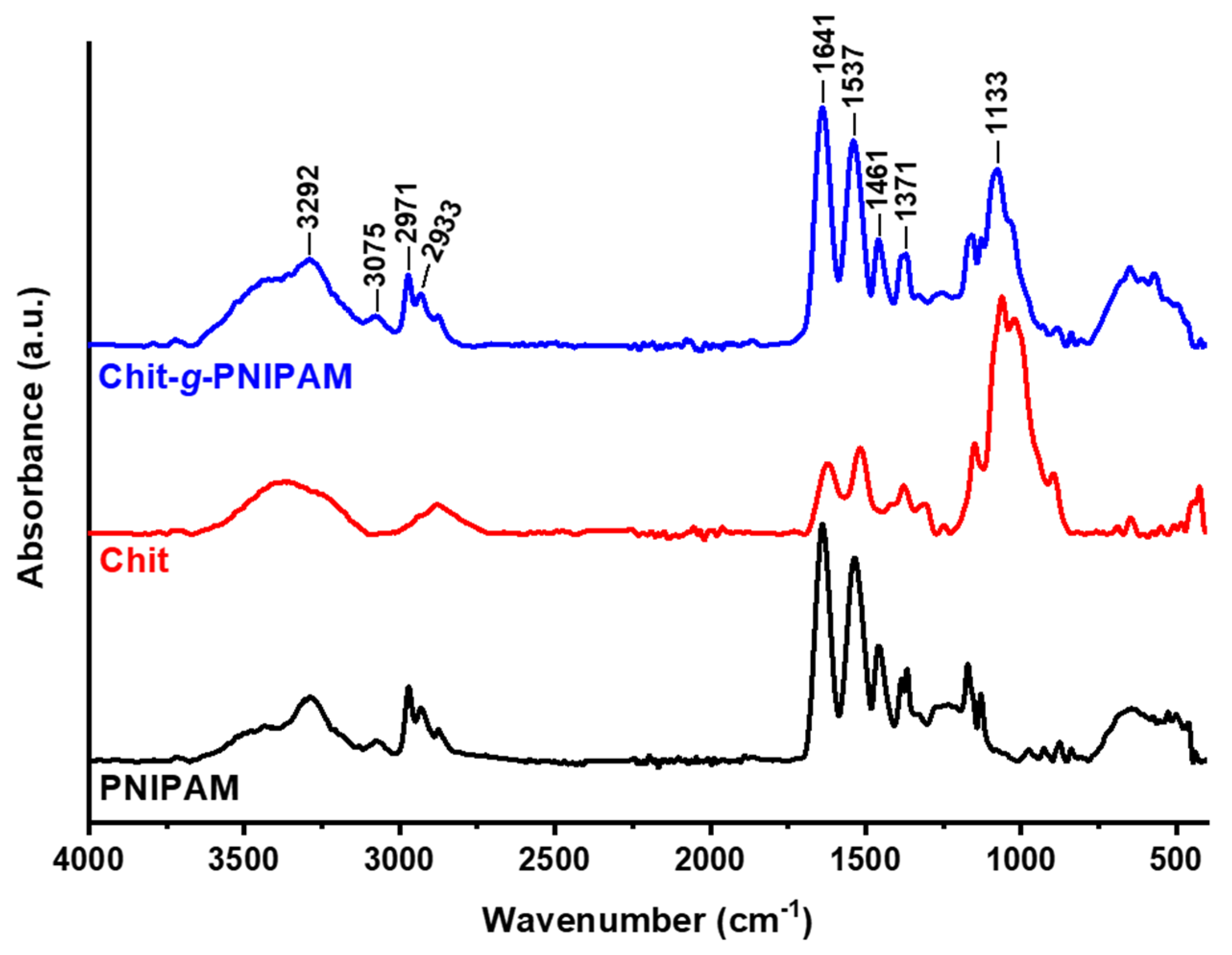
3.1.1. ATR-FTIR Spectroscopy
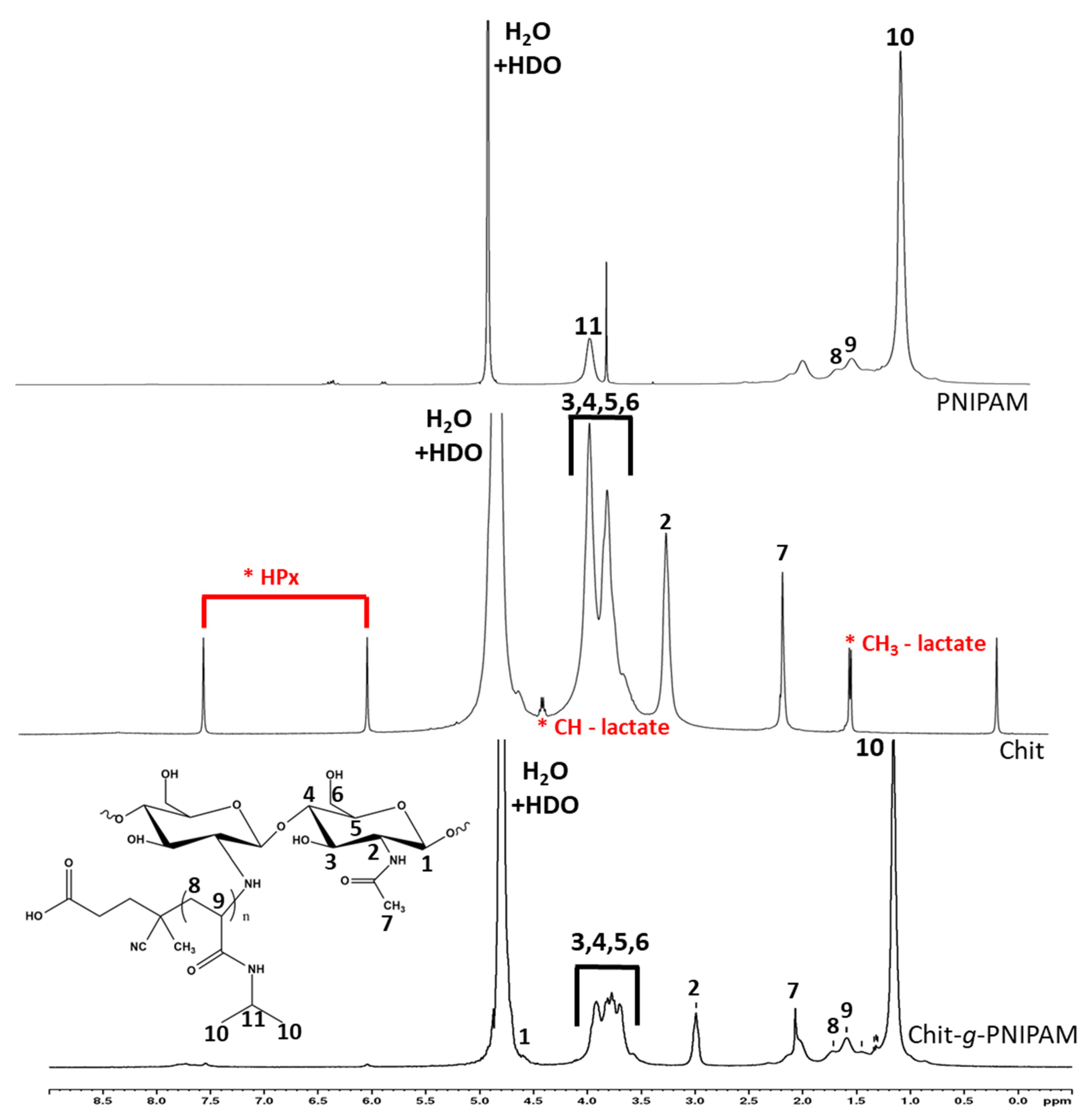
3.1.2. 1H NMR Spectroscopy
3.1.3. Thermogravimetric Analysis
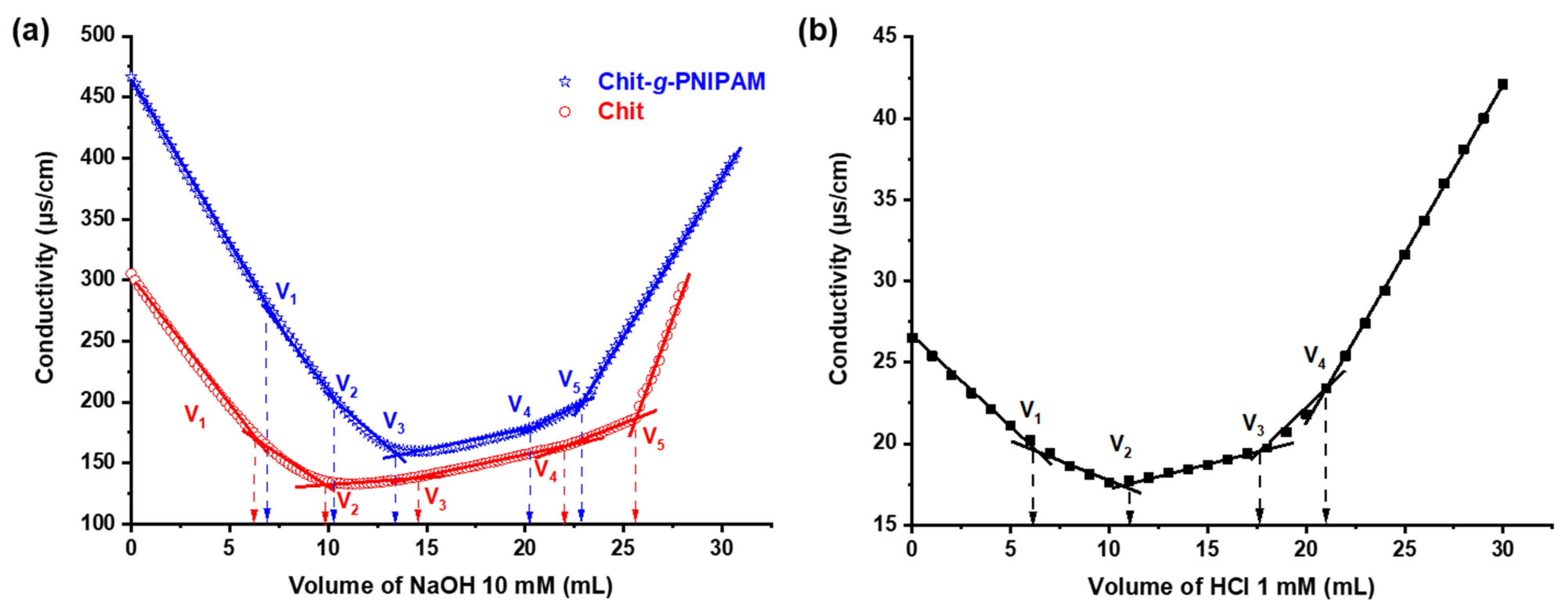
3.1.4. Polyelectrolyte Titrations
3.1.5. Back Conductometric Titrations
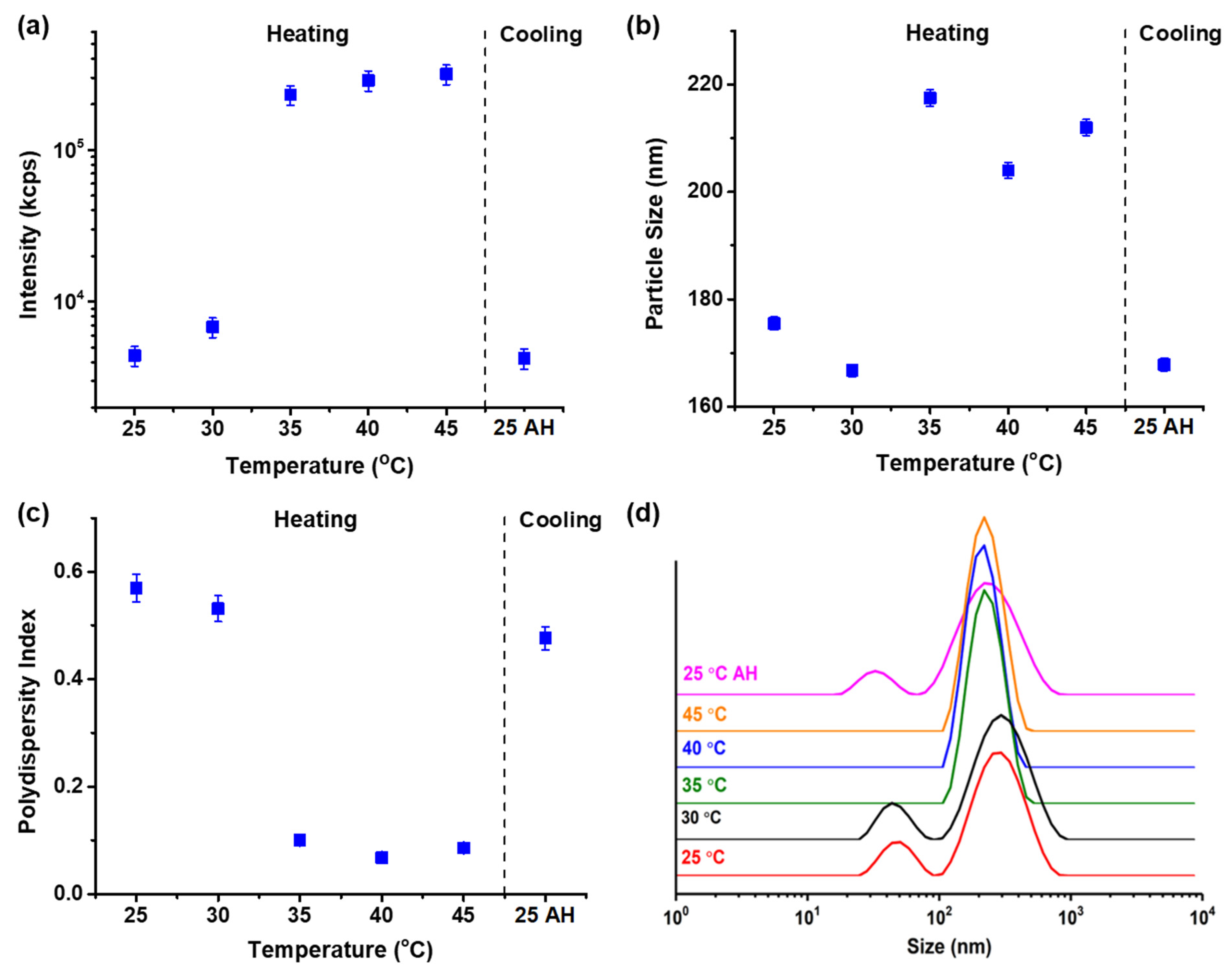
3.2. Aggregation State of Chit-g-PNIPAM on Temperature Changes
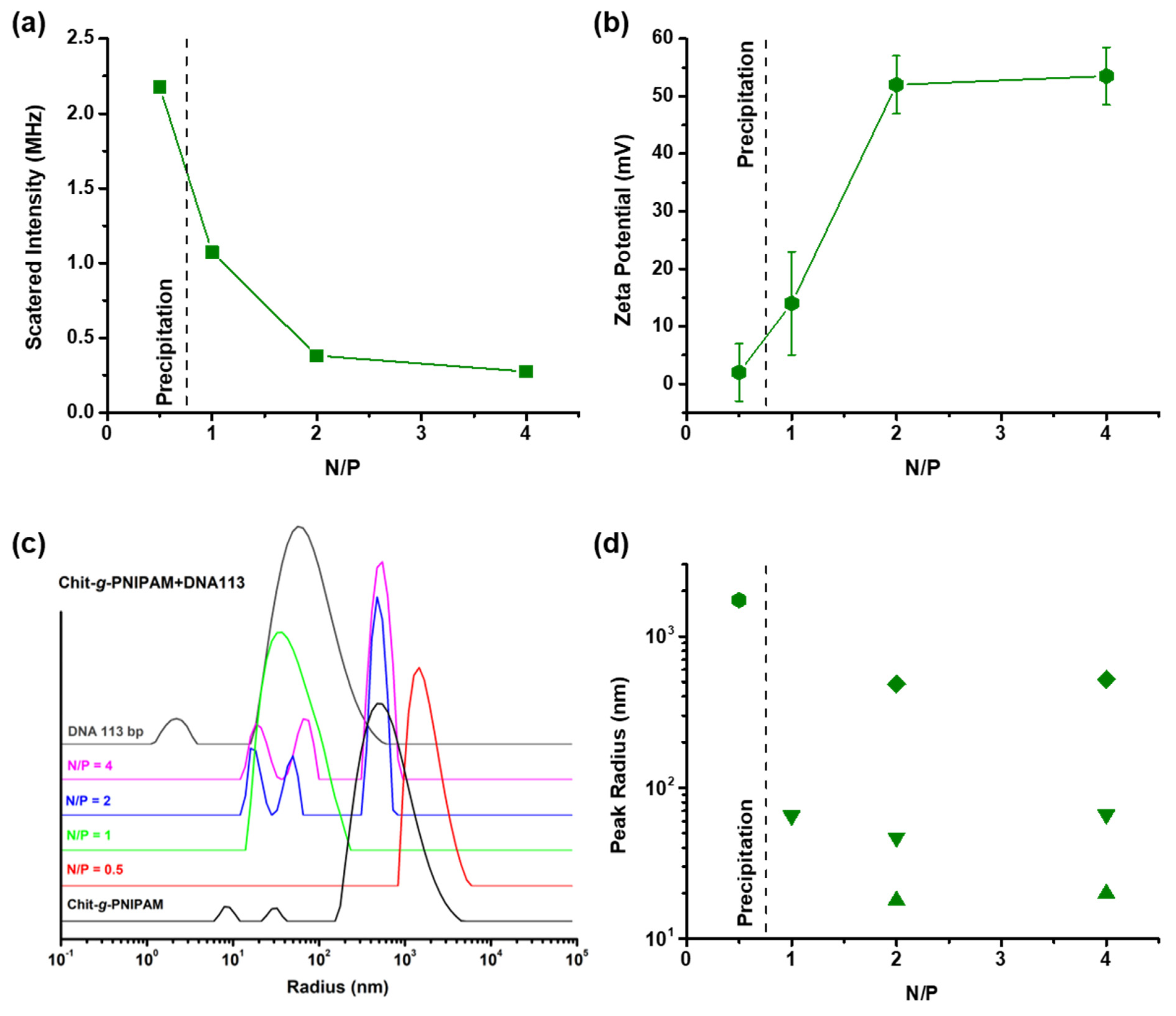
3.3. Complexation of Chit-g-PNIPAM Graft Copolymer with DNA
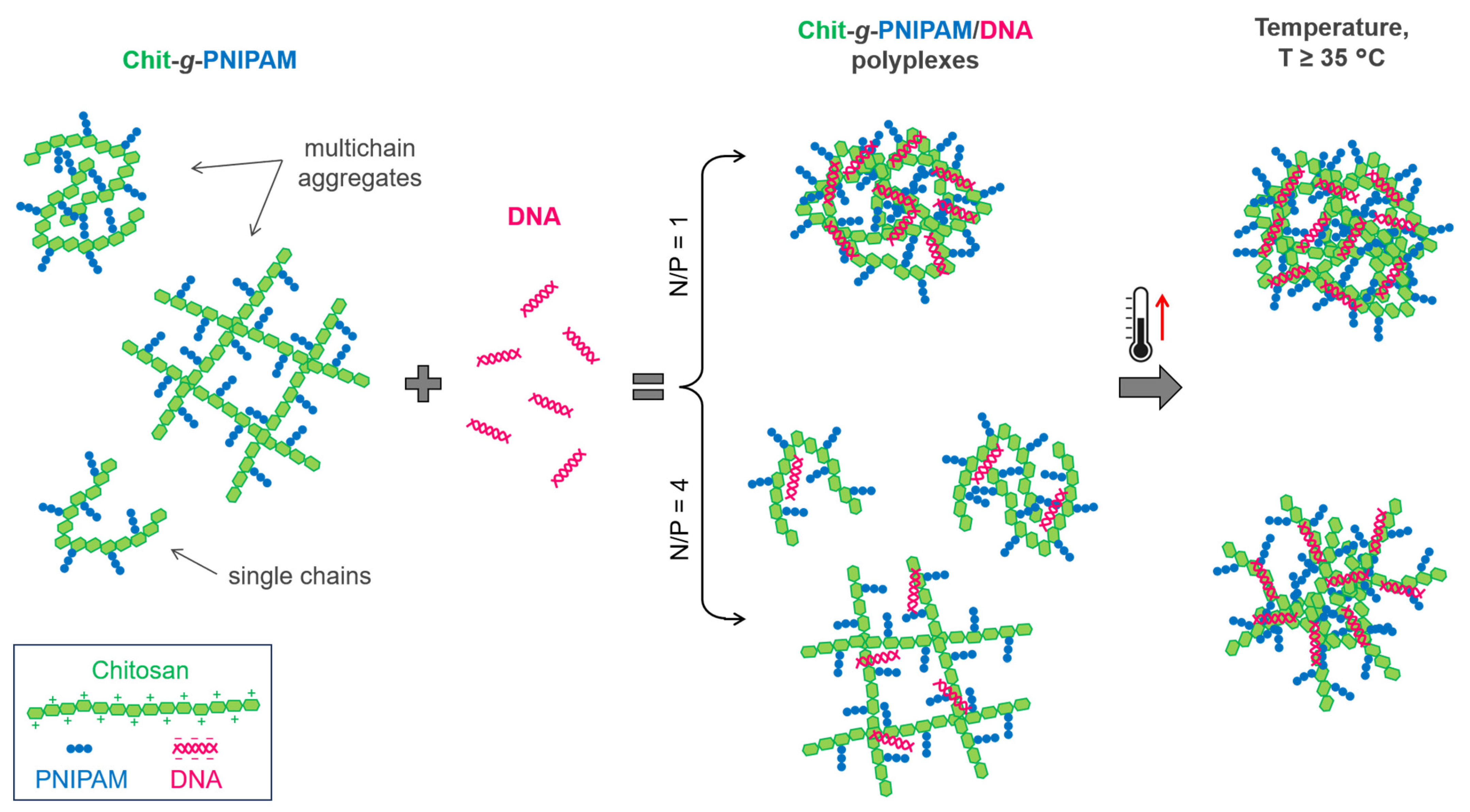
3.4. Thermal Response of the Chit-g-PNIPAM and DNA Complexes
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Guan, X.; Pei, Y.; Song, J. DNA-based nonviral gene therapy-challenging but promising. Mol. Pharm. 2024, 21, 427–453. [Google Scholar] [CrossRef]
- Harun-Or-Rashid, M.; Aktar, M.N.; Hossain, M.S.; Sarkar, N.; Islam, M.R.; Arafat, M.E.; Bhowmik, S.; Yusa, S.I. Recent advances in micro-and nano-drug delivery systems based on natural and synthetic biomaterials. Polymers 2023, 15, 4563. [Google Scholar] [CrossRef]
- Sun, Q.; Yang, Z.; Qi, X. Design and application of hybrid polymer-protein systems in cancer therapy. Polymers 2023, 15, 2219. [Google Scholar] [CrossRef]
- Kumar, R.; Santa Chalarca, C.F.; Bockman, M.R.; Bruggen, C.V.; Grimme, C.J.; Dalal, R.J.; Hanson, M.G.; Hexum, J.K.; Reineke, T.M. Polymeric delivery of therapeutic nucleic acids. Chem. Rev. 2021, 121, 11527–11652. [Google Scholar] [CrossRef]
- Wadetwar, R.N.; Godbole, A.P. Nanocarriers: A tool for effective gene delivery. In Nanopharmaceutical Advanced Delivery Systems; Dave, V., Gupta, N., Sur, S., Eds.; Scrivener Publishing LLC: Beverly, MA, USA, 2021; pp. 161–185. [Google Scholar] [CrossRef]
- Zhang, H.; Vandesompele, J.; Braeckmans, K.; De Smedt, S.C.; Remaut, K. Nucleic acid degradation as barrier to gene delivery: A guide to understand and overcome nuclease activity. Chem. Soc. Rev. 2024, 53, 317–360. [Google Scholar] [CrossRef]
- Mendes, B.B.; Conniot, J.; Avital, A.; Yao, D.; Jiang, X.; Zhou, X.; Sharf-Pauker, N.; Xiao, Y.; Adir, O.; Liang, H.; et al. Nanodelivery of Nucleic acids. Nat. Rev. Methods Primers 2022, 2, 24. [Google Scholar] [CrossRef]
- Zu, H.; Gao, D. Non-viral vectors in gene therapy: Recent development, challenges, and prospects. AAPS J. 2021, 23, 78. [Google Scholar] [CrossRef]
- Ren, S.; Wang, M.; Wang, C.; Wang, Y.; Sun, C.; Zeng, Z.; Cui, H.; Zhao, X. Application of non-viral vectors in drug delivery and gene therapy. Polymers 2021, 13, 3307. [Google Scholar] [CrossRef]
- Szewczyk-Łagodzińska, M.; Plichta, A.; Dębowski, M.; Kowalczyk, S.; Iuliano, A.; Florjańczyk, Z. Recent advances in the application of ATRP in the synthesis of drug delivery systems. Polymers 2023, 15, 1234. [Google Scholar] [CrossRef]
- Chernikova, E.V.; Kudryavtsev, Y.V. RAFT-based polymers for click reactions. Polymers 2022, 14, 570. [Google Scholar] [CrossRef]
- Moad, G. RAFT Polymerization to form stimuli-responsive polymers. Polym. Chem. 2017, 8, 177–219. [Google Scholar] [CrossRef]
- Perrier, S. 50th Anniversary perspective: RAFT polymerization—A user guide. Macromolecules 2017, 50, 7433–7447. [Google Scholar] [CrossRef]
- Semsarilar, M.; Abetz, V. Polymerizations by RAFT: Developments of the technique and its application in the synthesis of tailored (Co) polymers. Macromol. Chem. Phys. 2021, 222, 2000311. [Google Scholar] [CrossRef]
- Roka, N.; Kokkorogianni, O.; Kontoes-Georgoudakis, P.; Choinopoulos, I.; Pitsikalis, M. Recent advances in the synthesis of complex macromolecular architectures based on poly (n-vinyl pyrrolidone) and the RAFT polymerization technique. Polymers 2022, 14, 701. [Google Scholar] [CrossRef] [PubMed]
- Jafarzadeh, F.; Peyman, H.; Roshanfekr, H.; Azizi, S.; Idris, A.O.; Maaza, M. Fabrication of a nanomagnetic smart polymer carrier as a potential candidate for a drug delivery system. Arab. J. Sci. Eng. 2024. accepted. [Google Scholar] [CrossRef]
- Yao, Y.; Patel, C.; Vekariya, R.L.; Yusa, S.I.; Sangani, C.B.; Duan, Y.; Pillai, S.; Patel, H.; Kumar, N.S.; Khimani, M. Synthesis and aggregation behaviour of thermo-responsive-b-poly (ionic liquid) diblock copolymers in aqueous solution. J. Mol. Liq. 2021, 339, 116754. [Google Scholar] [CrossRef]
- Ansari, M.J.; Rajendran, R.R.; Mohanto, S.; Agarwal, U.; Panda, K.; Dhotre, K.; Manne, R.; Deepak, A.; Zafar, A.; Yasir, M.; et al. Poly (N-isopropylacrylamide)-based hydrogels for biomedical applications: A review of the state-of-the-art. Gels 2022, 8, 454. [Google Scholar] [CrossRef]
- Xie, Y.; Li, N.K.; Singh, A.; Deshmukh, S.A.; Yingling, Y.G. A Comparison between the lower critical solution temperature behavior of polymers and biomacromolecules. Physchem 2022, 2, 52–71. [Google Scholar] [CrossRef]
- Lanzalaco, S.; Armelin, E. Poly (N-isopropylacrylamide) and copolymers: A review on recent progresses in biomedical applications. Gels 2017, 3, 36. [Google Scholar] [CrossRef] [PubMed]
- Markandeywar, T.S.; Singh, D.; Narang, R.K. A Complete sojorum on thermosensitive hydrogels for wound healing: Recent developments and ongoing research. Curr. Drug Ther. 2024, 19, 151–177. [Google Scholar] [CrossRef]
- Otulakowski, Ł.; Trzebicka, B. Aggregation of thermoresponsive polymethacrylates in a dulbecco’s modified eagle medium and its salts. Polymers 2023, 15, 3587. [Google Scholar] [CrossRef]
- Ziminska, M.; Wilson, J.J.; McErlean, E.; Dunne, N.; McCarthy, H.O. Synthesis and evaluation of a thermoresponsive degradable chitosan-grafted PNIPAAm hydrogel as a “smart” gene delivery system. Materials 2020, 13, 2530. [Google Scholar] [CrossRef]
- Thulasisingh, A.; Venkatesan, S.A.; Kumar, S. Green biopolysaccharides and its utilisation as biodegradable material in diverse fields: A review. Polym. Bull. 2024, 81, 165–187. [Google Scholar] [CrossRef]
- Thambiliyagodage, C.; Jayanetti, M.; Mendis, A.; Ekanayake, G.; Liyanaarachchi, H.; Vigneswaran, S. Recent advances in chitosan-based applications—A review. Materials 2023, 16, 2073. [Google Scholar] [CrossRef]
- Farasati Far, B.; Naimi-Jamal, M.R.; Safaei, M.; Zarei, K.; Moradi, M.; Yazdani Nezhad, H. A review on biomedical application of polysaccharide-based hydrogels with a focus on drug delivery systems. Polymers 2022, 14, 5432. [Google Scholar] [CrossRef]
- Harugade, A.; Sherje, A.P.; Pethe, A. Chitosan: A review on properties, biological activities and recent progress in biomedical applications. React. Funct. Polym. 2023, 191, 105634. [Google Scholar] [CrossRef]
- Wang, W.; Meng, Q.; Li, Q.; Liu, J.; Zhou, M.; Jin, Z.; Zhao, K. Chitosan derivatives and their application in biomedicine. Int. J. Mol. Sci. 2020, 21, 487. [Google Scholar] [CrossRef]
- Ahmed, R.; Wang, M.; Qi, Z.; Hira, N.U.A.; Jiang, J.; Zhang, H.; Iqbal, S.; Wang, J.; Stuart, M.A.C.; Guo, X. Pickering emulsions based on the pH-responsive assembly of food-grade chitosan. ACS Omega 2021, 6, 17915–17922. [Google Scholar] [CrossRef]
- Benalaya, I.; Alves, G.; Lopes, J.; Silva, L.R. A review of natural polysaccharides: Sources, characteristics, properties, food, and pharmaceutical applications. Int. J. Mol. Sci. 2024, 25, 1322. [Google Scholar] [CrossRef]
- Zhang, W.; Khan, A.; Ezati, P.; Priyadarshi, R.; Sani, M.A.; Rathod, N.B.; Goksen, G.; Rhim, J.W. Advances in sustainable food packaging applications of chitosan/polyvinyl alcohol blend films. Food Chem. 2024, 443, 138506. [Google Scholar] [CrossRef]
- Elizalde-Cárdenas, A.; Ribas-Aparicio, R.M.; Rodríguez-Martínez, A.; Leyva-Gómez, G.; Ríos, C.; González-Torres, M. Advances in Chitosan and chitosan derivatives for biomedical applications in tissue engineering: An updated review. Int. J. Biol. Macromol. 2024, 262, 129999. [Google Scholar] [CrossRef]
- Zhao, W.; Zou, W.; Liu, F.; Zhou, F.; Altun, N.E. Molecular dynamics simulations of the solubility and conformation change of chitosan grafted polyacrylamide: Impact of grafting rate. J. Mol. Graph. Model. 2024, 126, 108660. [Google Scholar] [CrossRef]
- Kumar, D.; Gihar, S.; Shrivash, M.K.; Kumar, P.; Kundu, P.P. A review on the synthesis of graft copolymers of chitosan and their potential applications. Int. J. Biol. Macromol. 2020, 163, 2097–2112. [Google Scholar] [CrossRef]
- Argüelles-Monal, W.M.; Lizardi-Mendoza, J.; Fernández-Quiroz, D.; Recillas-Mota, M.T.; Montiel-Herrera, M. Chitosan Derivatives: Introducing New Functionalities with a Controlled Molecular Architecture for Innovative Materials. Polymers 2018, 10, 342. [Google Scholar] [CrossRef]
- Bhavsar, C.; Momin, M.; Gharat, S.; Omri, A. Functionalized and graft copolymers of chitosan and its pharmaceutical applications. Expert Opin. Drug Deliv. 2017, 14, 1189–1204. [Google Scholar] [CrossRef]
- Zhang, T.; Wu, Z.; Zhu, H.; Wang, Z.; Sun, S.; Hu, S. pH/temperature-responsive salt-tolerant Pickering emulsion formed by PNIPAM-modified chitosan particles. Colloids Surf. A: Physicochem. Eng. Asp. 2023, 657, 130548. [Google Scholar] [CrossRef]
- Moradi, S.; Najjar, R.; Hamishehkar, H.; Lotfi, A. Triple-responsive drug nanocarrier: Magnetic core-shell nanoparticles of Fe3O4@ poly (N-isopropylacrylamide)-grafted-chitosan, synthesis and in vitro cytotoxicity evaluation against human lung and breast cancer cells. J. Drug Deliv. Sci. Technol. 2022, 72, 103426. [Google Scholar] [CrossRef]
- Gade, S.S.; Pentlavalli, S.; Mishra, D.; Vora, L.K.; Waite, D.; Alvarez-Lorenzo, C.I.; Herrero Vanrell, M.R.; Laverty, G.; Larraneta, E.; Donnelly, R.F.; et al. Injectable depot forming thermoresponsive hydrogel for sustained intrascleral delivery of sunitinib using hollow microneedles. J. Ocul. Pharmacol. Ther. 2022, 38, 433–448. [Google Scholar] [CrossRef]
- Marsili, L.; Dal Bo, M.; Berti, F.; Toffoli, G. Chitosan-based biocompatible copolymers for thermoresponsive drug delivery systems: On the development of a standardization systems. Pharmaceuticals 2021, 13, 1876. [Google Scholar] [CrossRef]
- Rana, M.M.; De la Hoz Siegler, H. Evolution of Hybrid Hydrogels: Next-Generation Biomaterials for Drug Delivery and Tissue Engineering. Gels 2024, 10, 216. [Google Scholar] [CrossRef]
- Wu, S.-W.; Liu, X.; Miller, A.L.; Cheng, Y.-S.; Yeh, M.-L.; Lu, L. Strengthening injectable thermo-sensitive NIPAAm-g-chitosan hydrogels using chemical cross-linking of disulfide bonds as scaffolds for tissue engineering. Carbohydr. Polym. 2018, 192, 308–316. [Google Scholar] [CrossRef]
- Debele, T.A.; Mekuria, S.L.; Tsai, H.C. Polysaccharide based nanogels in the drug delivery system: Application as the carrier of pharmaceutical agents. Mater. Sci. Eng. C 2016, 68, 964–981. [Google Scholar] [CrossRef]
- Xu, L.; Liang, X.; You, L.; Yang, Y.; Fen, G.; Gao, Y.; Cui, X. Temperature-sensitive poly (N-isopropylacrylamide)-chitosan hydrogel for fluorescence sensors in living cells and its antibacterial application. Int. J. Biol. Macromol. 2021, 189, 316–323. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Yu, W. Preparation and characterization of CS-g-PNIPAAm microgels and application in water vapor-permeable fabric. Carbohydr. Polym. 2015, 127, 11–18. [Google Scholar] [CrossRef]
- Cheaburu-Ylmaz, C.N. On the Development of chitosan-graft-poly(N-isopropylacrylamide) by raft polymerization technique. Cellul. Chem. Technol. 2020, 54, 1–10. [Google Scholar] [CrossRef]
- Giaouzi, D.; Pispas, S. Synthesis and self-assembly of thermoresponsive poly(N-isopropylacrylamide)-b-poly(oligo ethylene glycol methyl ether acrylate) double hydrophilic block copolymers. J. Polym. Sci. A Polym. Chem. 2019, 57, 1467–1477. [Google Scholar] [CrossRef]
- Babelyte, M.; Peciulyte, L.; Navikaite-Snipaitiene, V.; Bendoraitiene, J.; Samaryk, V.; Rutkaite, R. Synthesis and characterization of thermoresponsive chitosan-graft-poly(N-isopropylacrylamide) Copolymers. Polymers 2023, 15, 3154. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Xue, C.; Xue, Y.; Gao, R.; Zhang, X. Determination of the degree of deacetylation of chitin and chitosan by X-ray powder diffraction. Carbohydr. Res. 2005, 340, 1914–1917. [Google Scholar] [CrossRef]
- Rwei, S.-P.; Tuan, H.N.A.; Chiang, W.-Y.; Way, T.-F.; Hsu, Y.-J. Synthesis and drug delivery application of thermo- and pH-Sensitive Hydrogels: Poly(-CD-co-N-Isopropylacrylamide-co-IAM). Materials 2016, 9, 1003. [Google Scholar] [CrossRef]
- Fathi, M.; Alami-Milani, M.; Geranmayeh, M.H.; Barar, J.; Erfan-Niya, H.; Omidi, Y. Dual thermo-and pH-sensitive injectable hydrogels of chitosan/(poly(N-isopropylacrylamide-co-itaconic acid)) for doxorubicin delivery in breast cancer. Int. J. Boil. Macromol. 2019, 128, 957–964. [Google Scholar] [CrossRef]
- Chalanqui, M.; Pentlavalli, S.; McCrudden, C.; Chambers, P.; Ziminska, M.; Dunne, N.; McCarthy, H. Influence of alginate backbone on efficacy of thermo-responsive alginate-g-P(NIPAAm) hydrogel as a vehicle for sustained and controlled gene delivery. Mater. Sci. Eng. C 2019, 95, 409–421. [Google Scholar] [CrossRef] [PubMed]
- Alves, N.M.; Mano, J.F. Chitosan derivatives obtained by chemical modifications for biomedical and environmental applications. Int. J. Boil. Macromol. 2008, 43, 401–414. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Zhuang, Y.; Mu, Q.; Wang, M.; Fang, Y. Preparation, characterization and aggregation behavior of amphiphilic chitosan derivative having poly (L-lactic acid) side chains. Carbohydr. Polym. 2008, 72, 60–66. [Google Scholar] [CrossRef]
- Sosnik, A.; Imperiale, J.C.; Vázquez-González, B.; Raskin, M.M.; Muñoz-Muñoz, F.; Burillo, G.; Cedillo, G.; Bucio, E. Mucoadhesive thermo-responsive chitosan-g-poly(N-isopropylacrylamide) polymeric micelles via a one-pot gamma-radiation-assisted pathway. Colloids Surf. B Biointerfaces 2015, 136, 900–907. [Google Scholar] [CrossRef] [PubMed]
- Petrila, L.-M.; Zaharia, M.-M.; Bucatariu, F.; Mihai, M.; Pispas, S. Exploring the remarkable properties of water soluble chitosans. Proc. Int. Conf. Prog. Org. Macromol. Compd. 2023, 102–104. Available online: https://icmpp.ro/macroiasi2023/files/volum%20proceedings.v6.pdf (accessed on 20 March 2024).
- Kumar, S.; Koh, J. Physiochemical, optical and biological activity of chitosan-chromone derivative for biomedical applications. Int. J. Mol. Sci. 2012, 13, 6102–6116. [Google Scholar] [CrossRef] [PubMed]
- Queiroz, M.; Melo, K.R.T.; Sabry, D.A.; Sassaki, G.L.; Rocha, H.A.O. Does the use of chitosan contribute to oxalate kidney stone formation? Mar. Drugs 2014, 13, 141–158. [Google Scholar] [CrossRef] [PubMed]
- Danaei, M.; Dehghankhold, M.; Ataei, S.; Davarani, F.H.; Javanmard, R.; Dokhani, A.; Khorasani, S.; Mozafari, M.R. Impact of particle size and polydispersity index on the clinical applications of lipidic nanocarrier systems. Pharmaceutics 2018, 10, 57. [Google Scholar] [CrossRef] [PubMed]
- Bao, H.; Li, L.; Leong, W.C.; Gan, L.H. Thermo-responsive association of chitosan-graft-poly(N-isopropylacrylamide) in aqueous solutions. J. Phys. Chem. B 2010, 114, 10666–10673. [Google Scholar] [CrossRef]
- Dimitrov, I.; Trzebicka, B.; Müller, A.H.E.; Dworak, A.; Tsvetanov, C.B. Thermosensitive water-soluble copolymers with doubly responsive reversibly interacting entities. Prog. Polym. Sci. 2007, 32, 1275–1343. [Google Scholar] [CrossRef]
- Pamies, R.; Zhu, K.; Kjøniksen, A.; Nyström, B. Thermal response of low molecular weight poly-(N-isopropylacrylamide) polymers in aqueous solution. Polym. Bull. 2009, 62, 487–502. [Google Scholar] [CrossRef]
- Karayianni, M.; Sentoukas, T.; Skandalis, A.; Pippa, N.; Pispas, S. Chitosan-Based Nanoparticles for Nucleic Acid Delivery: Technological Aspects, Applications, and Future Perspectives. Pharmaceutics 2023, 15, 1849. [Google Scholar] [CrossRef] [PubMed]


| Sample Name | CChit-g-PNIPAM (mg/mL) | CDNA (mg/mL) | CTotal (mg/mL) | N/P |
|---|---|---|---|---|
| Comp(1 + 0.25) | 0.02 | 0.07 | 0.09 | 4 |
| Comp(1 + 0.5) | 0.14 | 0.16 | 2 | |
| Comp(1 + 1) | 0.28 | 0.30 | 1 | |
| Comp(1 + 2) | 0.56 | 0.58 | 0.5 |
| Sample | Volume Titrant (mL) (Method 1) | Volume Titrant (mL) (Method 2) | meq/g | Initially Uncharged Functional Groups (%) |
|---|---|---|---|---|
| Chit | 1.89 | 1.88 | 1.65 | 33.58 |
| PNIPAM | 0.17 | - | 0.15 | 17.34 |
| Chit-g-PNIPAM | 2.95 | 3.03 | 2.18 | 39.47 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zaharia, M.-M.; Bucatariu, F.; Karayianni, M.; Lotos, E.-D.; Mihai, M.; Pispas, S. Synthesis of Thermoresponsive Chitosan-graft-Poly(N-isopropylacrylamide) Hybrid Copolymer and Its Complexation with DNA. Polymers 2024, 16, 1315. https://doi.org/10.3390/polym16101315
Zaharia M-M, Bucatariu F, Karayianni M, Lotos E-D, Mihai M, Pispas S. Synthesis of Thermoresponsive Chitosan-graft-Poly(N-isopropylacrylamide) Hybrid Copolymer and Its Complexation with DNA. Polymers. 2024; 16(10):1315. https://doi.org/10.3390/polym16101315
Chicago/Turabian StyleZaharia, Marius-Mihai, Florin Bucatariu, Maria Karayianni, Elena-Daniela Lotos, Marcela Mihai, and Stergios Pispas. 2024. "Synthesis of Thermoresponsive Chitosan-graft-Poly(N-isopropylacrylamide) Hybrid Copolymer and Its Complexation with DNA" Polymers 16, no. 10: 1315. https://doi.org/10.3390/polym16101315
APA StyleZaharia, M.-M., Bucatariu, F., Karayianni, M., Lotos, E.-D., Mihai, M., & Pispas, S. (2024). Synthesis of Thermoresponsive Chitosan-graft-Poly(N-isopropylacrylamide) Hybrid Copolymer and Its Complexation with DNA. Polymers, 16(10), 1315. https://doi.org/10.3390/polym16101315












