Lignin Nanoparticles Produced from Wheat Straw Black Liquor Using γ-Valerolactone
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Recovery of Lignin
2.3. Preparation of Lignin Nanoparticle
2.4. Morphology Characterization
2.5. Particle Size Distribution
2.6. NMR Analysis
2.7. FTIR Analysis
2.8. Thermal Stability
3. Results and Discussion
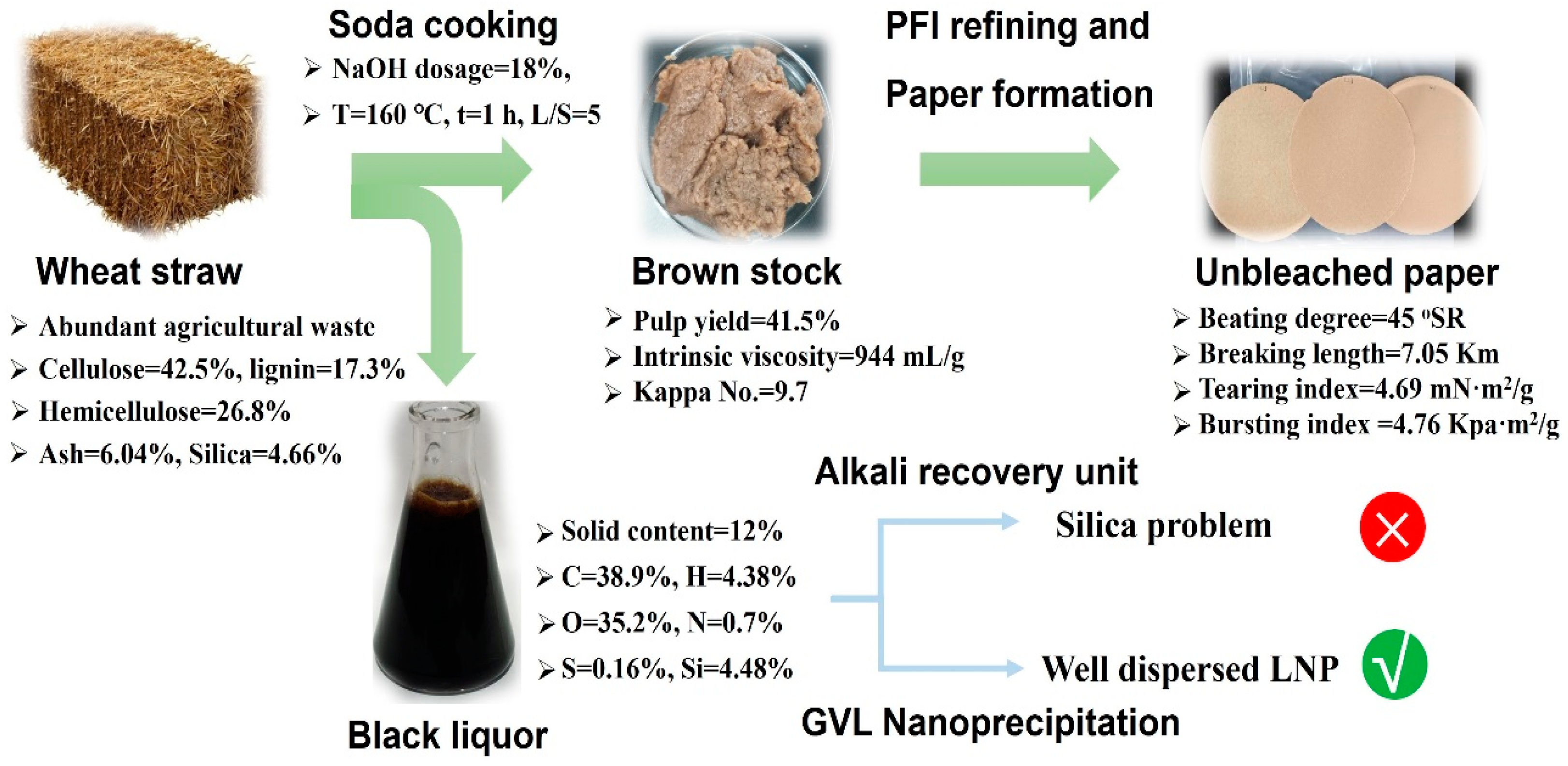
3.1. Valorization of Wheat Straw
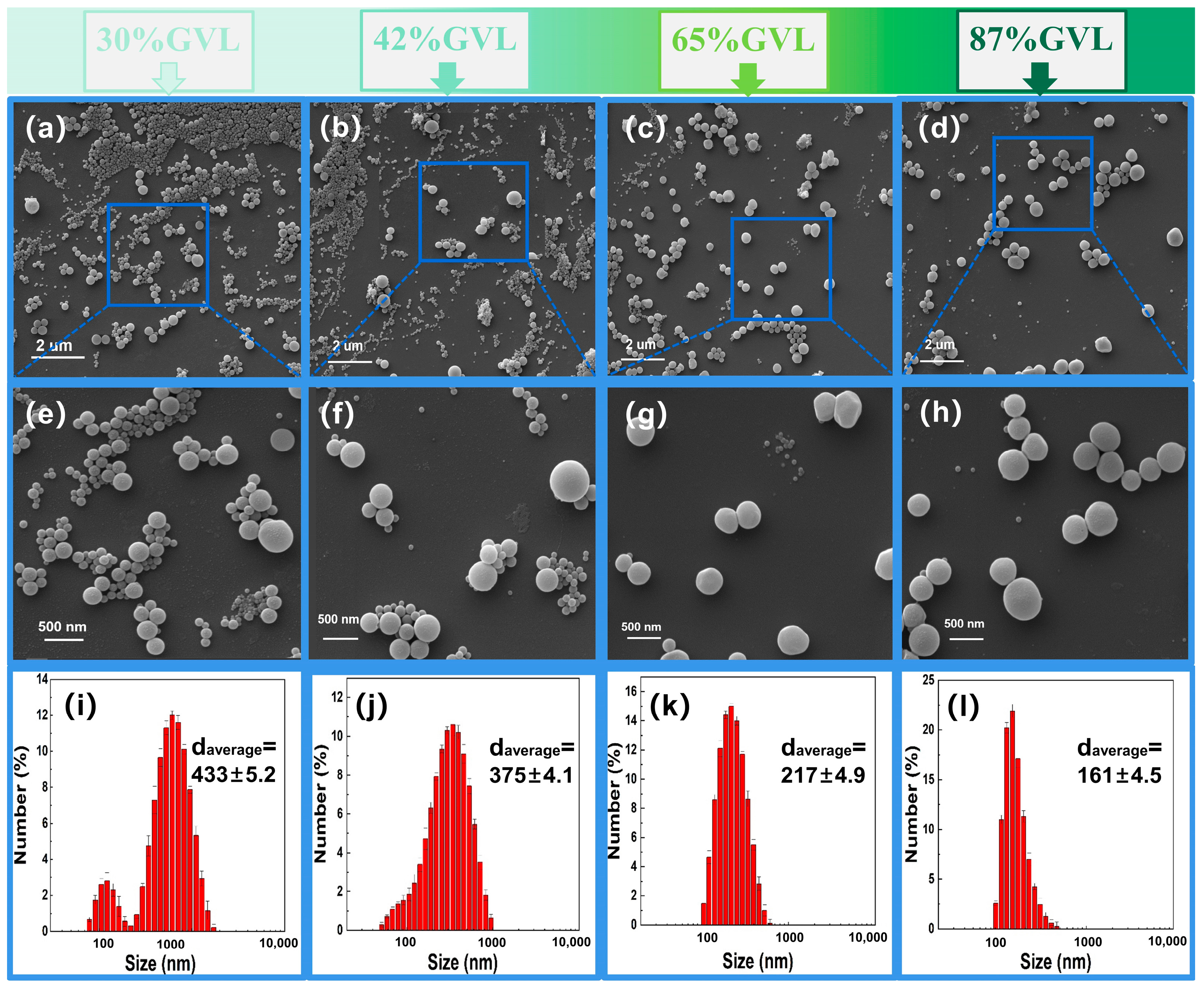
3.2. Morphology and Particle Size Distribution of LNP
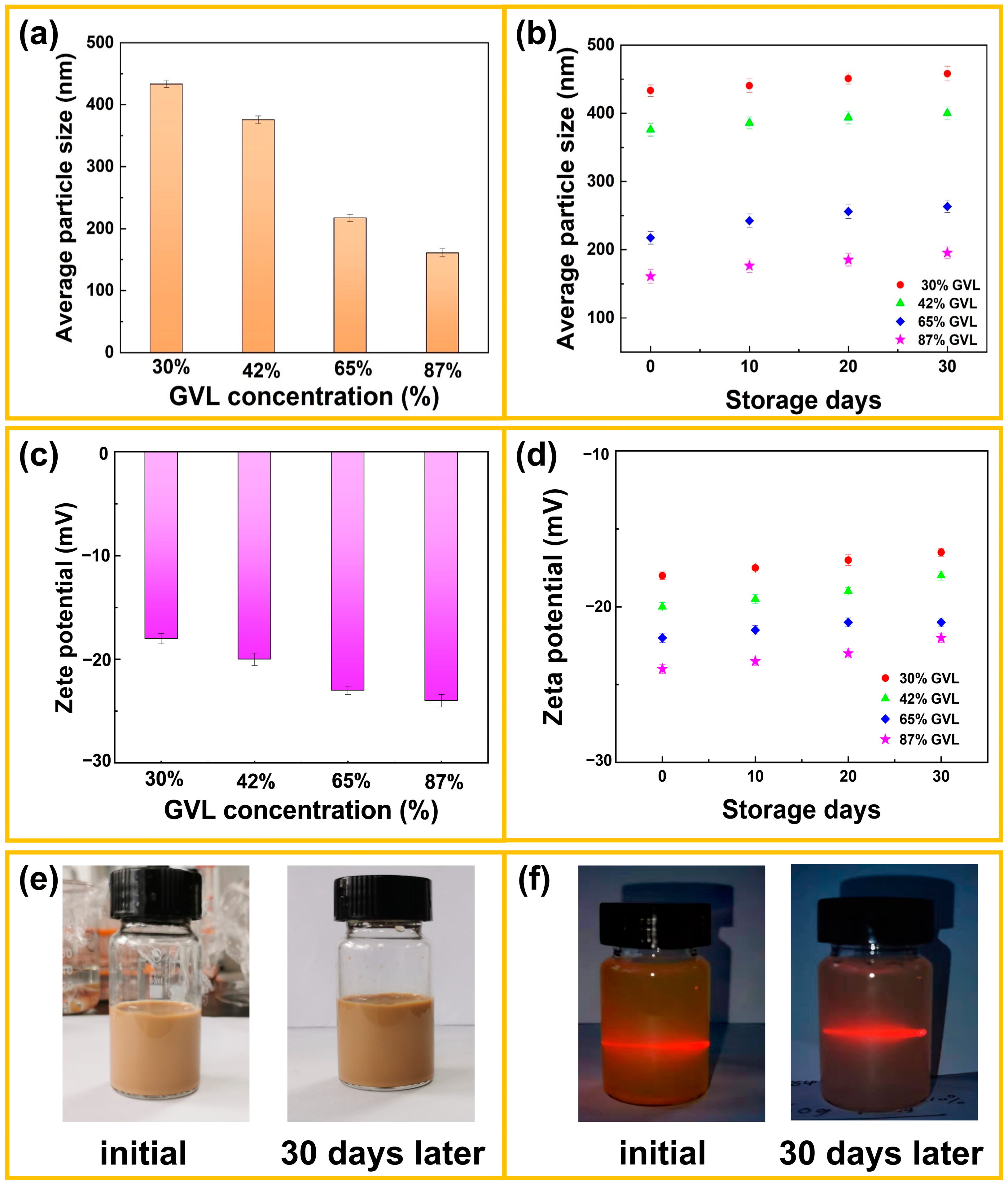
3.3. Dispersion Stability Analysis of LNPs
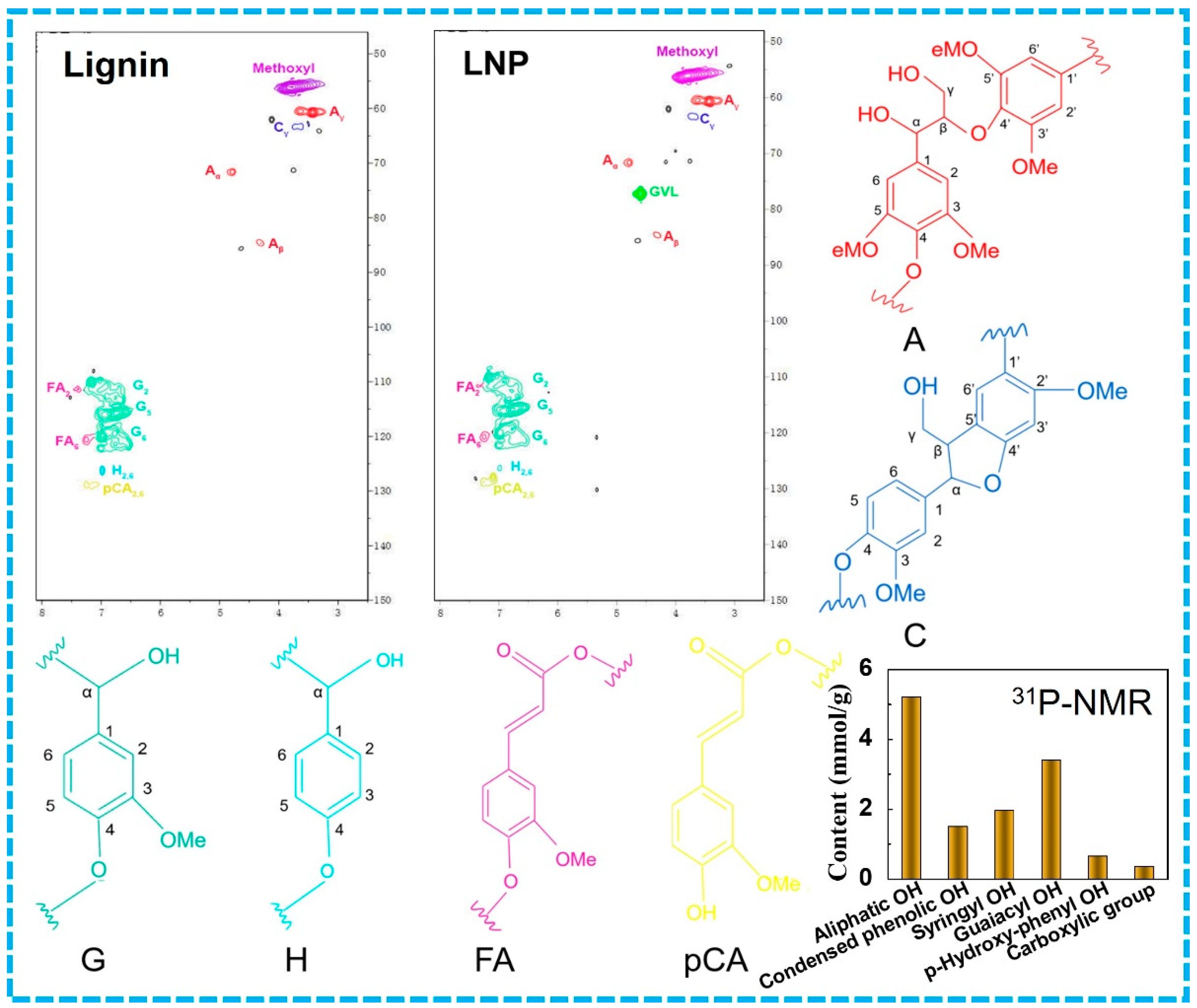
3.4. NMR Analysis of LNPs
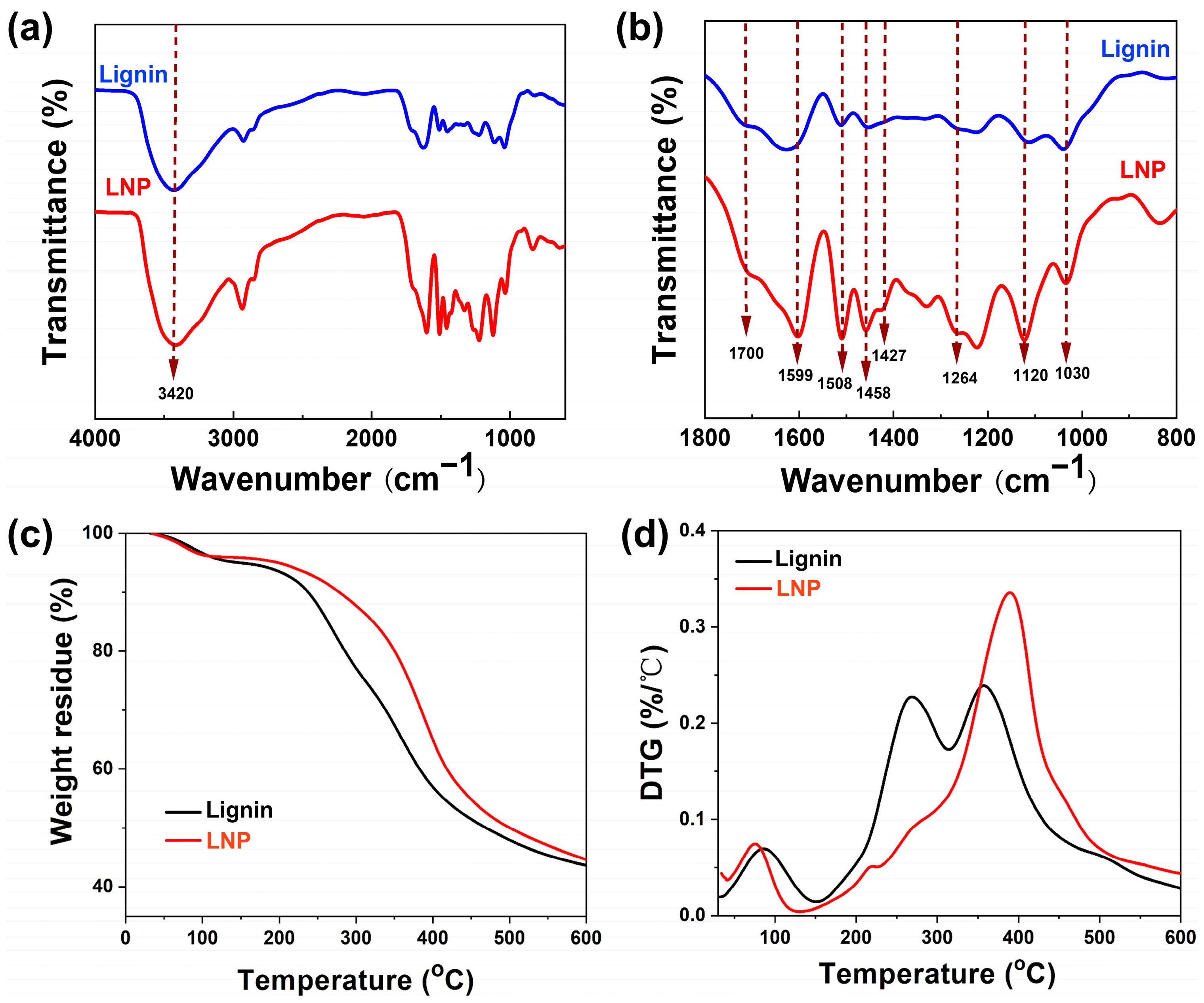
3.5. FTIR Analysis and Thermal Stability of LNPs
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Baicha, Z.; Salar-García, M.J.; Ortiz-Martínez, V.M.; Hernández-Fernández, F.J.; De los Ríos, A.P.; Labjar, N.; Lotfi, E.; Elmahi, M. A critical review on microalgae as an alternative source for bioenergy production: A promising low cost substrate for microbial fuel cells. Fuel Process. Technol. 2016, 154, 104–116. [Google Scholar] [CrossRef]
- Ibrahim, M.S.C.; Meng, T.H.; Ahmad, A.; Ghazali, M.S.M.; Abdullah, W.R.W.; Chuen, N.L. Potential of nanosilicon dioxide extraction from silicon-rich agriculture wastes as a plant growth promoter. Adv. Nat. Sci. Nanosci. 2022, 13, 033001. [Google Scholar] [CrossRef]
- Qi, G.; Xiong, L.; Li, H.; Huang, Q.; Luo, M.; Tian, L.; Chen, X.; Huang, C.; Chen, X. Hydrotropic pretreatment on wheat straw for efficient biobutanol production. Biomass Bioenergy 2019, 122, 76–83. [Google Scholar] [CrossRef]
- Deniz, I.; Kırcı, H.; Ates, S. Optimisation of wheat straw Triticum drum kraft pulping. Ind. Crop. Prod. 2004, 19, 237–243. [Google Scholar] [CrossRef]
- Chen, H.; Wang, F.; Zhang, C.; Shi, Y.; Jin, G.; Yuan, S. Preparation of nano-silica materials: The concept from wheat straw. J. Non-Cryst. Solids 2010, 356, 2781–2785. [Google Scholar] [CrossRef]
- Diaz-Baca, A.J.; Fatehi, P. Process development for tall oil lignin production. Bioresour. Technol. 2021, 329, 124891. [Google Scholar] [CrossRef] [PubMed]
- Tutus, A.; Eroglu, H. An alternative solution to the silica problem in wheat straw pulping. Appita J. 2004, 57, 214–217. [Google Scholar]
- Yuan, Z.; Wen, Y.; Kapu, N.S.; Beatson, R. Evaluation of an organosolv-based biorefinery process to fractionate wheat straw into ethanol and co-products. Ind. Crop. Prod. 2018, 121, 294–302. [Google Scholar] [CrossRef]
- Ma, M.; Dai, L.; Si, C.; Hui, L.; Liu, Z.; Ni, Y. A facile preparation of super long-term stable lignin nanoparticles from black liquor. ChemSusChem 2019, 12, 5239–5245. [Google Scholar] [CrossRef]
- Qian, Y.; Deng, Y.; Qiu, X.; Li, H.; Yang, D. Formation of uniform colloidal spheres from lignin, a renewable resource recovered from pulping spent liquor. Green Chem. 2014, 16, 2156–2163. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, W.; Liu, W.; Qiu, X. High performance PVA/lignin nanocomposite films with excellent water vapor barrier and UV-shielding properties. Int. J. Biol. Macromol. 2020, 142, 551–558. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.; Li, X.; Fan, J.; Chang, J. Hydrothermal conversion of lignin: A review. Renew. Sustain. Energy Rev. 2013, 27, 546–558. [Google Scholar] [CrossRef]
- Baurhoo, B.; Ruiz-Feria, C.A.; Zhao, X. Purified lignin: Nutritional and health impacts on farm animals-A review. Anim. Feed Sci. Technol. 2008, 144, 175–184. [Google Scholar] [CrossRef]
- Ugartondo, V.; Mitjans, M.; Vinardell, M.P. Comparative antioxidant and cytotoxic effects of lignins from different sources. Bioresour. Technol. 2008, 99, 6683–6687. [Google Scholar] [CrossRef] [PubMed]
- Duan, H.; Wang, D.; Li, Y. Green chemistry for nanoparticle synthesis. Chem. Soc. Rev. 2015, 44, 5778–5792. [Google Scholar] [CrossRef] [PubMed]
- Nair, S.S.; Sharma, S.; Pu, Y.; Sun, Q.; Pan, S.; Zhu, J.Y.; Deng, Y.; Ragauskas, A.J. High shear homogenization of lignin to nanolignin and thermal stability of Nanolignin-Polyvinyl alcohol blends. ChemSusChem 2014, 7, 3513–3520. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Liu, S.; Wu, C.; Liu, Y.; Yang, G.; Ni, Y. Super-stable, solvent-resistant and uniform lignin nanorods and nanospheres with a high yield in a mild and facile process. Green Chem. 2020, 22, 8734–8744. [Google Scholar] [CrossRef]
- Kerkel, F.; Markiewicz, M.; Stolte, S.; Müller, E.; Kunz, W. The green platform molecule gamma-valerolactone-ecotoxicity, biodegradability, solvent properties, and potential applications. Green Chem. 2021, 23, 2962–2976. [Google Scholar] [CrossRef]
- Choi, M.; Byun, J.; Park, H.; Jeong, K.; Kim, S.M.; Han, J. Economically-feasible “greener” transformation of gamma-valerolactone to nylon 6, 6. Biomass Bioenerg. 2022, 162, 106503. [Google Scholar] [CrossRef]
- Alonso, D.M.; Wettstein, S.G.; Dumesic, J.A. Gamma-valerolactone, a sustainable platform molecule derived from lignocellulosic biomass. Green Chem. 2013, 15, 584–595. [Google Scholar] [CrossRef]
- Qi, L.; Horváth, I.T. Catalytic conversion of fructose to γ-valerolactone in γ-valerolactone. ACS Catal. 2012, 2, 2247–2249. [Google Scholar] [CrossRef]
- Soh, L.; Eckelman, M.J. Green solvents in biomass processing. ACS Sustain. Chem. Eng. 2016, 4, 5821–5837. [Google Scholar] [CrossRef]
- Dutta, S.; Iris, K.M.; Tsang, D.C.; Ng, Y.H.; Ok, Y.S.; Sherwood, J.; Clark, J.H. Green synthesis of gamma-valerolactone (GVL) through hydrogenation of biomass-derived levulinic acid using non-noble metal catalysts: A critical review. Chem. Eng. J. 2019, 372, 992–1006. [Google Scholar] [CrossRef]
- Shuai, L.; Questell-Santiago, Y.M.; Luterbacher, J.S. A mild biomass pretreatment using γ-valerolactone for concentrated sugar production. Green Chem. 2016, 18, 937–943. [Google Scholar] [CrossRef]
- Wu, L.; Liu, S.; Wang, Q.; Wang, Y.; Ji, X.; Yang, G.; Chen, J.; Li, C.; Fatehi, P. High strength and multifunctional polyurethane film incorporated with lignin nanoparticles. Ind. Crop. Prod. 2022, 177, 114526. [Google Scholar] [CrossRef]
- Gürbüz, E.I.; Gallo, J.M.R.; Alonso, D.M.; Wettstein, S.G.; Lim, W.Y.; Dumesic, J.A. Conversion of hemicellulose into furfural using solid acid catalysts in γ-valerolactone. Angew. Chem. Int. Ed. 2013, 52, 1270–1274. [Google Scholar] [CrossRef]
- Kong, X.; Xu, H.; Wu, H.; Wang, C.; He, A.; Ma, J.; Ren, X.; Jia, H.; Wei, C.; Jiang, M.; et al. Biobutanol production from sugarcane bagasse hydrolysate generated with the assistance of gamma-valerolactone. Process. Biochem. 2016, 51, 1538–1543. [Google Scholar] [CrossRef]
- Wu, P.; Li, L.; Sun, Y.; Song, B.; Yu, Y.; Liu, H. Near complete valorisation of Hybrid pennisetum to biomethane and lignin nanoparticles based on gamma-valerolactone/water pretreatment. Bioresour. Technol. 2020, 305, 123040. [Google Scholar] [CrossRef]
- Jin, L.; Yu, X.; Peng, C.; Guo, Y.; Zhang, L.; Xu, Q.; Zhao, Z.; Xie, H. Fast dissolution pretreatment of the corn stover in gamma-valerolactone promoted by ionic liquids: Selective delignification and enhanced enzymatic saccharification. Bioresour. Technol. 2018, 270, 537–544. [Google Scholar] [CrossRef]
- Xue, Z.; Zhao, X.; Sun, R.T.; Mu, T. Biomass-derived γ-valerolactone-based solvent systems for highly efficient dissolution of various lignins: Dissolution behavior and mechanism study. ACS Sustain. Chem. Eng. 2016, 4, 3864–3870. [Google Scholar] [CrossRef]
- Fernández-Costas, C.; Gouveia, S.; Sanromán, M.A.; Moldes, D. Structural characterization of Kraft lignins from different spent cooking liquors by 1D and 2D Nuclear Magnetic Resonance spectroscopy. Biomass Bioenergy 2014, 63, 156–166. [Google Scholar] [CrossRef]
- Liu, S.; He, H.; Fu, X.; Wang, Y.; Wang, Q.; Yang, G.; Chen, J.; Ni, Y. Tween 80 enhancing cellulasic activation of hardwood kraft-based dissolving pulp. Ind. Crop. Prod. 2019, 137, 144–148. [Google Scholar] [CrossRef]
- Rasool, M.A.; Vankelecom, I.F.J. Use of γ-valerolactone and glycerol derivatives as bio-based renewable solvents for membrane preparation. Green Chem. 2019, 21, 1054–1064. [Google Scholar] [CrossRef]
- Wang, Q.; Gao, J.; Liu, S.; Wang, Y.; Wu, L. Lignin nanoparticle reinforced multifunctional polyvinyl alcohol/polyurethane composite hydrogel with excellent mechanical, UV-blocking, rheological and thermal properties. Int. J. Biol. Macromol. 2023, 232, 123338. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Zhou, M.; Jia, W.; Li, N.; Niu, M. Balancing the effect of pretreatment severity on hemicellulose extraction and pulping performance during auto-hydrolysis prior to kraft pulping of acacia wood. Biotechnol. Prog. 2019, 35, e2784. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Jing, S.; Zhang, X.; Chen, Z.; Zhuo, H.; Hu, Y.; Liu, Q.; Zhong, L.; Peng, X.; Sun, R. Strengthening effects of carboxymethylated hemicellulosic fractions on paper strength. Ind. Crop. Prod. 2018, 125, 360–369. [Google Scholar] [CrossRef]
- Liu, Z.; Hao, N.; Shinde, S.; Pu, Y.; Kang, X.; Ragauskas, A.J.; Yuan, J. Defining lignin nanoparticle properties through tailored lignin reactivity by sequential organosolv fragmentation approach (SOFA). Green Chem. 2019, 21, 245–260. [Google Scholar] [CrossRef]
- Zhao, W.; Simmons, B.; Singh, S.; Ragauskas, A.; Cheng, G. From lignin association to nano-/micro-particle preparation: Extracting higher value of lignin. Green Chem. 2016, 18, 5693–5700. [Google Scholar] [CrossRef]
- Lê, H.Q.; Zaitseva, A.; Pokki, J.P.; Ståhl, M.; Alopaeus, V.; Sixta, H. Solubility of organosolv lignin in γ-valerolactone/water binary mixtures. ChemSusChem 2016, 9, 2939–2947. [Google Scholar] [CrossRef]
- Marciniak, A. The Hildebrand solubility parameters of ionic liquids-part 2. Int. J. Mol. Sci. 2011, 12, 3553–3575. [Google Scholar] [CrossRef]
- Chen, L.; Shi, Y.; Gao, B.; Zhao, Y.; Jiang, Y.; Zha, Z.; Xue, W.; Gong, L. Lignin nanoparticles: Green synthesis in a γ-valerolactone/water binary solvent and application to enhance antimicrobial activity of essential oils. ACS Sustain. Chem. Eng. 2019, 8, 714–722. [Google Scholar] [CrossRef]
- Lepeltier, E.; Bourgaux, C.; Couvreur, P. Nanoprecipitation and the “Ouzo effect”: Application to drug delivery devices. Adv. Drug Deliv. Rev. 2014, 71, 86–97. [Google Scholar] [CrossRef] [PubMed]
- Figueiredo, P.; Lintinen, K.; Kiriazis, A.; Hynninen, V.; Liu, Z.; Bauleth-Ramos, T.; Rahikkala, A.; Correia, A.; Kohout, T.; Sarmento, B.; et al. In vitro evaluation of biodegradable lignin-based nanoparticles for drug delivery and enhanced antiproliferation effect in cancer cells. Biomaterials 2017, 121, 97–108. [Google Scholar] [CrossRef] [PubMed]
- Jiang, C.; He, H.; Jiang, H.; Ma, L.; Jia, D. Nano-lignin filled natural rubber composites: Preparation and characterization. Express Polym. Lett. 2013, 7, 480–493. [Google Scholar] [CrossRef]
- Richter, A.P.; Bharti, B.; Armstrong, H.B.; Brown, J.S.; Plemmons, D.; Paunov, V.N.; Stoyanov, S.D.; Velev, O.D. Synthesis and characterization of biodegradable lignin nanoparticles with tunable surface properties. Langmuir 2016, 32, 6468–6477. [Google Scholar] [CrossRef]
- Wang, G.; Liu, X.; Yang, B.; Si, C.; Parvez, A.M.; Jang, J.; Ni, Y. Using green γ-valerolactone/water solvent to decrease lignin heterogeneity by gradient precipitation. ACS Sustain. Chem. Eng. 2019, 7, 10112–10120. [Google Scholar] [CrossRef]
- Pang, T.; Wang, G.; Sun, H.; Wang, L.; Liu, Q.; Sui, W.; Parvez, A.M.; Si, C. Lignin fractionation for reduced heterogeneity in self-assembly nanosizing: Toward targeted preparation of uniform lignin nanoparticles with small size. ACS Sustain. Chem. Eng. 2020, 8, 9174–9183. [Google Scholar] [CrossRef]
- Agustin, M.B.; Penttila, P.A.; Lahtinen, M.; Mikkonen, K.S. Rapid and direct preparation of lignin nanoparticles from alkaline pulping liquor by mild ultrasonication. ACS Sustain. Chem. Eng. 2019, 7, 19925–19934. [Google Scholar] [CrossRef]
- Wang, Y.; Ji, X.; Wang, Q.; Tian, Z.; Liu, S.; Yang, G.; Liu, H. Recent advanced application of lignin nanoparticles in the functional composites: A mini-review. Int. J. Biol. Macromol. 2022, 222, 2498–2511. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, L.; Wang, Y.; Wang, Q.; Liu, S.; Ji, X. Lignin Nanoparticles Produced from Wheat Straw Black Liquor Using γ-Valerolactone. Polymers 2024, 16, 49. https://doi.org/10.3390/polym16010049
Zhao L, Wang Y, Wang Q, Liu S, Ji X. Lignin Nanoparticles Produced from Wheat Straw Black Liquor Using γ-Valerolactone. Polymers. 2024; 16(1):49. https://doi.org/10.3390/polym16010049
Chicago/Turabian StyleZhao, Lianjie, Yingchao Wang, Qiang Wang, Shanshan Liu, and Xingxiang Ji. 2024. "Lignin Nanoparticles Produced from Wheat Straw Black Liquor Using γ-Valerolactone" Polymers 16, no. 1: 49. https://doi.org/10.3390/polym16010049
APA StyleZhao, L., Wang, Y., Wang, Q., Liu, S., & Ji, X. (2024). Lignin Nanoparticles Produced from Wheat Straw Black Liquor Using γ-Valerolactone. Polymers, 16(1), 49. https://doi.org/10.3390/polym16010049




